Summary
Caveolins, components of the uncoated invaginations of plasma membrane, regulate signal transduction and vesicular trafficking. Loss of caveolin-3, resulting from dominant negative mutations of caveolin-3 causes autosomal dominant limb-girdle muscular dystrophy (LGMD) 1C and autosomal dominant rippling muscle disease (AD-RMD). Myostatin, a member of the muscle-specific transforming growth factor (TGF)-β superfamily, negatively regulates skeletal muscle volume. Herein we review caveolin-3 suppressing of activation of type I myostatin receptor, thereby inhibiting subsequent intracellular signaling. In addition, a mouse model of LGMD1C has shown atrophic myopathy with enhanced myostatin signaling. Myostatin inhibition ameliorates muscular phenotype in the model mouse, accompanied by normalized myostatin signaling. Enhanced myostatin signaling by caveolin-3 mutation in human may contribute to the pathogenesis of LGMD1C. Therefore, myostatin inhibition therapy may be a promising treatment for patients with LGMD1C. More recent studies concerning regulation of TGF-β superfamily signaling by caveolins have provided new insights into the pathogenesis of several human diseases.
Keywords: caveolin-3, limb-girdle muscular dystrophy 1C (LGMD1C), autosomal dominant rippling muscle disease (AD-RMD), myostatin, transforming growth factor-β (TGF-β)
Caveolins are primary components of caveolae
Caveolae, uncoated invaginations of the plasma membrane, are an abundant feature of many terminally differentiated cells, such as adipocytes, endothelial cells, and muscle cells. Caveolin family proteins, 21-24 kDa integral membrane proteins, are the principle components of caveolae, designated as caveolin-1, -2, and -3 (1, 2). Caveolin-1 and caveolin-2 are coexpressed and form heterooligomers in nonmuscle cells, whereas caveolin-3 is muscle specific and forms homooligomers in muscle cells (3, 4). De novo synthesized caveolins assemble to about 350 kDa oligomers in the endoplasmic reticulum, subsequently target to the plasma membrane via the trans-Golgi network, and play a crucial role in the formation of caveolae. These caveolin family proteins have been implicated in numerous cellular events including vesicular trafficking, lipid metabolism, and signal transduction (1–6). They directly bind to and regulate specific lipid and lipid-modified proteins including cholesterol, G-protein, G-protein coupled receptors, Src family kinase, Ha-Ras, and nitric oxide synthases (5–7). The interaction between caveolins and these molecules is mediated by a caveolin-binding motif on the target protein and a scaffolding domain in caveolin (7). The number of in vitro studies linking caveolins to signal transduction pathways has grown exponentially. To date, however, only a few studies have been concluded the exact roles of caveolins to signal transduction in vivo (3).
Dominant-negative mutations of caveolin-3 gene causes LGMD1C/AR-RMD
Many mutations in caveolin-3 gene have been detected in autosomal dominant limb-girdle muscular dystrophy (LGMD) 1C and autosomal dominant rippling muscle disease (AD-RMD) (8, 9). Mutations of the caveolin-3 gene cause a significant reduction in the cell surface level of caveolin-3 protein in a dominant-negative fashion and, to a lesser extent, mistargeting of the mutant caveolin-3 protein to the Golgi complex (8–10).
The loss of caveolin-3 by mutations of the caveolin-3 gene in LGMD1C/AD-RMD patients has resulted in subsequent abnormalities of caveolin-3-binding molecules. The enzymatic activity of neuronal nitric oxide synthase, which is strongly suppressed by caveolin-3, increases in the skeletal muscles from a transgenic mouse model of LGMD1C and LGMD1C/AD-RMD patients (11, 12). Consistently, cytokine-induced NO production increases in C2C12 myoblast cells transfected with LGMD1C/AD-RMD-type mutant caveolin-3 compared to ones transfected with wild-type caveolin-3 (9). Src tyrosine kinase, a membrane tyrosine kinase whose activation regulates the balance between cell survival and cell death, is extremely activated and accumulates not in the plasma membrane but in the perinuclear region in cells transfected in LGMD1C/AD-RMD mutant caveolin-3 (13). Muscle-specific phosphofruktokinase, an enzyme of central importance in the regulation of glycolytic metabolism is also significantly reduced in cells transfected with LDMD1C/AD-RMD mutant caveolin-3 probably through ubiquitin-proteasomal degradation (14). Noteworthy also is the finding that dysferlin, a membrane-repair molecule deficient in LGMD2B/Miyoshi myopathy, mistargets to the cytoplasm from sarcolemma in skeletal muscle from LGMD1C/AD-RMD patients, probably due to the caveolin-3’s delivery function to the correct targeting of plasma membrane (15–18).
Despite these findings, the underlying molecular mechanism leading to LGMD1C/AD-RMD in caveolin-3-deficient muscle remains to be elucidated.
Myostatin, a muscle-specific TGF-β superfamily member, is a therapeutic target of muscular dystrophy
Myostatin is a muscle-specific transforming growth factor (TGF)-β superfamily member and negatively regulates skeletal muscle growth and skeletal muscle volume (19). Overexpression of myostatin causes severe muscle atrophy, whereas targeted disruption of myostatin increases skeletal muscle mass in mice (19, 20). Like most members of the TGF-β superfamily, myostatin is synthesized as a precursor protein and undergoes proteolytic processing to generate an N-terminal prodomain and a biologically active, C-terminal disulfide-linked dimer (21). In the inactive state, the prodomain strongly inhibits the biological activity of the C-terminal dimer (22, 23), as do follistatin, and the follistatin-related gene (FLRG); which are collectively called natural inhibitors for myostatin (24). The circulating active form of myostatin directly binds to and phosphorylates the type II serine/threonine kinase receptor, namely activin receptor IIB (ActRIIB) (Fig. 1) (25). This, in turn, phosphorylates the type I serine/threonine kinase receptors, namely activin receptor-like kinase 4/5 (ALK4/5) at the plasma membrane (25–27). The activation of a heteromeric receptor complex consisting of phosphorylated type II and type I serine/threonine kinase receptors induces the phosphorylation of intracellular effectors, receptor-regulated Smads (R-Smads), namely Smad2/3 (26, 27). Phosphorylated R-Smads translocate to the nucleus from the cytoplasm, where it regulate the transcription of specific target genes inducing skeletal muscle atrophy (26–28).
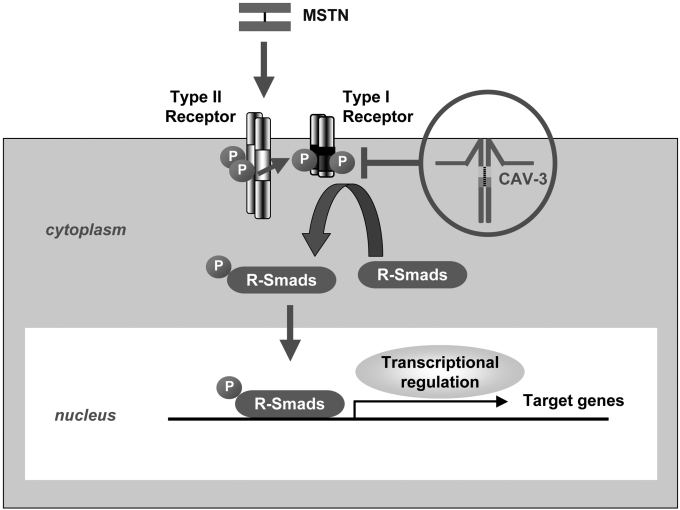
Figure 1.
Putative scheme of the regulation of myostatin signaling by caveolin-3. Myostatin (MSTN) signaling is propagated through the myostatin receptor, a heteromeric complex consisting with transmembrane receptor serine/threonine kinases. Myostatin binds to and phosphorylates its type II serine/threonine kinase receptor (Type II Receptor). Subsequently, its type I serine/threonine kinase receptor (Type I Receptor) is phosphorylated by Type II Receptor and is recruited into the heteromeric complex, which in turn phosphorylates receptor-regulated Smads (R-Smads), a family of transcription factor controlling the expression of specific target genes. Caveolin-3 (CAV-3) binds to and suppresses activation of the Type I Receptor of MSTN at the plasma membrane and suppresses intracellular myostatin signaling, including phosphorylation of R-Smads and transcription of specific target genes. Loss of caveolin-3 resulting from dominant negative mutations of the caveolin-3 genes in patients with LGMD1C could enhance intracellular myostatin signaling, and thereby result in muscle mass reduction. Type II Receptor, ActRIIB; Type I Receptor, ALK4/5; R-Smads, Smad2/3. P indicates phosphorylation.
Notably, administration of a blocking antibody against myostatin, myostatin vaccine, and myostatin prodomain, or genetic introduction of a follistatin-derivative ameliorates the pathophysiology of dystrophin-deficient mdx mice (29–32). In addition, a blocking antibody against myostatin improves the condition of young model mice with δ-sarcoglycan-deficient LGMD2F (33). An adeno-associated virus (AAV)-mediated myostatin prodomain has ameliorates the pathology of calpain-3-deficient LGMD2A model mice (34). Therefore, myostatin inhibition through different strategies has recently come to be considered for a therapeutic option for muscular dystrophies. However, the precise molecular mechanism by which myostatin inhibition improves the above dystrophic skeletal muscle is not fully understood; i.e. the molecular interaction of myostatin and the dystrophin-glycoprotein complex is unknown.
Caveolin-1 regulates TGF-β superfamily signaling in vitro
Recently, caveolin-1 has drawn attention as a regulator of TGF-β superfamily signaling. Caveolin-1 binds to and suppresses activation of the type I receptor of TGF-β1, which induces growth arrest in nonmuscle cells (35). Consistently, the binding affinity of caveolin-1 with type I TGF-β1 receptor decreases after stimulation with TGF-β1. In addition, caveolin-1 associates with the type II receptor of TGF-β1 (36–38). Caveolin-1 also facilitates ligand–bound TGF-β1 receptors internalization and degradation via the formation of endocytic vesicles with ubiquitin-ligase (39, 40). In addition, caveolin-1 interacts with type II and type I receptors of bone morphogenic proteins (BMPs) in vivo (41). These findings indicate that caveolin-1 regulates TGF-β superfamily signaling, including TGF-β1 and BMPs, at its receptor level.
Caveolin-3 suppresses myostatin signaling through its type I receptor in vitro
Upon consideration of molecular analogy and tissue distribution, we hypothesized that caveolin-3 inhibits myostatin signaling in a similar manner to that of inhibition of caveolin-1 to multiple TGF-β superfamily signaling in nonmuscle cells. We found several caveolin-3 binding motifs (7); ΦXΦXXXXΦXXΦX, where Φ indicates aromatic or aromatic-like amino acids in the cytoplasmic kinase domain of type I serine/threonine myostatin receptors, ALK4/5 (42). Therefore, we cotransfected caveolin-3 and these type I myostatin receptors in COS-7 monkey kidney cells and found that caveolin-3 colocalized with type I myostatin receptor. Immunoprecipitation and subsequent immunoblot analysis revealed that caveolin-3 associates with the type I myostatin receptor. In addition, phosphorylation level of the type I myostatin receptor decreased with the addition of caveolin-3 in cells cotransfected with constitutively active type I receptor and caveolin-3. Moreover, caveolin-3 eventually suppressed subsequent intracellular myostatin signaling; the phosphorylation level of an R-Smad of myostatin, Smad2 as well as the transcription level of the Smad-sensitive (CAGA)12-reporter gene. Therefore, caveolin-3 suppresses the myostatin signal at its type I receptor level, in a similar manner to caveolin-1 for TGF-β1 signaling in vitro.
Caveolin-3 deficient muscles exhibit enhanced intracellular myostatin signaling
We previously generated transgenic (Tg) mice overexpressing mutant caveolin-3 (CAV-3P104L) to develop a mouse model of LGMD1C/AD-RMD (11). The skeletal muscle phenotype of the transgenic mice showed severe myopathy with loss of caveolin-3. To determine whether caveolin-3 regulates myostatin signaling in vivo, we generated and characterized the double-transgenic mice showing myostatin deficiency and myostatin inhibition. Heterozygous mating of mutant caveolin-3 Tg mice with other Tg mice overexpressing myostatin prodomain (MSTNPro) (43), a potent inhibitor of myostatin signaling, gave rise to mice with four distinct phenotypes: wild-type, mutant caveolin-3 Tg, mutant MSTN Tg, and double-mutant Tg (CAV-3P104L/MSTNPro). Growth curves revealed that the double-mutant Tg mice were significantly larger than the mutant caveolin-3 Tg mice and similar in size to the wild-type mice beginning at 6 weeks until 16 weeks of age (42). The muscle atrophy seen in the mutant caveolin-3 Tg was reversed in the double-mutant Tg with increased myofiber size and myofiber number. Thus, myostatin inhibition reverses caveolin-3-deficient muscular atrophy in vivo.
Caveolin-3-deficient muscle from mutant caveolin-3 Tg mice showed hyperphosphorylation of an R-Smad of myostatin, Smad2 and significant upregulation of a myostatin target gene, p21. These in vivo findings were consistent with our in vitro study in which caveolin-3 suppresses myostatin signaling. In the double-mutant Tg mouse, the levels of phospho-Smad2 and p21 gene expression were significantly reduced compared to those in the mutant caveolin-3 Tg mice and were similar to those in the wild-type mice. Thus, myostatin inhibition by genetic introduction of myostatin inhibitor normalized enhanced myostatin signaling and also reversed muscular phenotype in the caveolin-3 deficient mouse.
Myostatin inhibition therapy reversed muscular atrophy in caveolin-3 deficiency
We injected a soluble form of the extracellular domain of type II myostatin receptor, ActRIIB, which can inhibit myostatin-its type II receptor binding (25, 44), into the mutant caveolin-3 Tg mice to develop myostatin inhibition through its type II receptor as a therapeutic strategy for patients with LGMD1C. Intraperitoneal injection of soluble ActRIIB four times significantly increased skeletal muscle mass and reversed myofiber hypotrophy accompanied with suppression of Smad2 phosphorylation and downregulation of p21. This finding, therefore, suggests that myostatin inhibition therapy may be a reasonable and promising therapy for caveolin-3-deficient muscular dystrophy associated with enhanced myostatin signaling.
Conclusions and prospective for future research
Caveolin-3 has been considered to regulate numerous signal pathways for maintaining the normal integrity of skeletal muscles, but the in vivo significance of signal alterations by loss of caveolin-3 in the pathogenesis of LGMD1C/AD-RMD has not been well delineated. As reviewed herein, caveolin-3 regulates myostatin signaling in vitro, and thus disrupted interaction between caveolin-3 and myostatin could contribute to the pathogenesis of caveolin-3-deficient muscular dystrophy (Fig. 1).
We could not conclude that activated intracellular signaling molecules, hyperphosphorylation of an R-Smad, Smad2, and upregulation of p21 in the caeveolin-3 deficient skeletal muscle result simply from enhanced myostatin signaling by loss of caveolin-3, because the myostatin prodomain or the soluble myostatin receptor suppresses not only myostatin, but also other TGF-β ligands including growth and differentiation factor 11 (GDF11) (22, 25, 44, 45). In fact, evidence of an unknown TGF-β ligand exists in the form of a similar negative regulator of muscle mass like myostatin (45, 46). Thus TGF-β ligands other than myostatin also could be involved in the pathogenesis of caveolin-3 deficieny via the Smad2-p21-mediated pathway. Crossing of mutant caveolin-3 mice with myostatin-null mice is a prospective project for obtaining straightforward evidence that hyperphosphorylation of Smad2 and upregulation of p21 in caveolin-3-deficient muscles is the simple result of enhanced myostatin signaling.
More recent studies have shown to be caveolins as an exact negative regulator of TGF-β superfamily signaling because the loss of caveolins has play important roles in the pathogenesis of human disorders. Mutations of the caveolin-1 gene or downregulation of caveolin-1 protein have been detected in some sporadic breast cancers (47) and epithelial cells derived from caveolin-1 null mice have shown hyperphosphorylation of Smad2 and epithelial mesenchymal transition, corresponding to premalignant status (48). In addition, loss of caveolin-1 has been strongly associates with idiopathic pulmonary fibrosis (49, 50). Caveolin-1 protein has been found to be reduced in the lung tissue from patients with idiopathic pulmonary fibrosis. TGF-β1-induced extarcellular matrix production, which is indicative of fibrosis, significantly increases in primary fibroblasts isolated from patients with idiopathic pulmonary fibrosis. Moreover, retroviral introduction of caveolin-1 ameliorates bleomycin-induced lung fibrosis in mice. Together with this review, it may be concluded that aberrant TGF-β superfamily signaling by loss of caveolins participate in the pathogenesis of some human diseases, including LGMD1C/AD-RMD, breast cancer, and idiopathic pulmonary fibrosis.
Myostatin inhibition therapy is effective, to some extent, with mouse models of several types of muscular dystrophies (29–34). Further investigation is needed to determine which types of myostatin inhibition therapy could be applied and to clarify the molecular mechanism by which myostatin-inhibition improves muscular dystrophy for prospective treatment of patients with muscular dystrophy. As reviewed herein, myostatin inhibition may be a potent therapy for caveolin-3-deficient muscular dystrophy with enhanced myostatin signaling.
Acknowledgments
We are grateful to Ms. N. Akazawa for critical reading of the manuscript. This work was supported by a Research Grant (14B-4) for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare; Grants (15131301 and 17231401) for Research on Psychiatric and Neurological Diseases and Mental Health from the Ministry of Health, Labor and Welfare of Japan and from the Japan Society for the Promotion of Science (JSPS) KAKENHI (14370212).
References
- 1. Parton RG. Caveolae-from ultrastructure to molecular mechanism. Nat Rev Mol Cell Biol 2003;4:162-7. [DOI] [PubMed] [Google Scholar]
- 2. Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci 2006;119:787-96. [DOI] [PubMed] [Google Scholar]
- 3. Parton GP, Simonds K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 2007;8:185-94. [DOI] [PubMed] [Google Scholar]
- 4. Monier S, Parton RG, Vogel F, et al. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell 1995;6:911-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Razani B, Schlegel A, Lisanti MP. Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy. J Cell Sci 2000;113:2103-9. [DOI] [PubMed] [Google Scholar]
- 6. Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell 2001;106:403-11. [DOI] [PubMed] [Google Scholar]
- 7. Couet J, Li S, Okamoto T, et al. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem 1997;272:6523-33. [DOI] [PubMed] [Google Scholar]
- 8. Minetti C, Sotgia F, Bruno C, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 1998;18:365-8. [DOI] [PubMed] [Google Scholar]
- 9. Betz RC, Schoser BG, Kasper D, et al. Mutations in CAV3 cause mechanical hyperirritability of skeletal muscle in rippling muscle disease. Nat Genet 2001;28:218-9. [DOI] [PubMed] [Google Scholar]
- 10. Galbiati F, Volonte D, Minetti C, et al. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the Golgi complex. J Biol Chem 1999;274:25632-41. [DOI] [PubMed] [Google Scholar]
- 11. Sunada Y, Ohi H, Hase A, et al. Transgenic mice expressing mutant caveolin-3 show severe myopathy associated with increased nNOS activity. Hum Mol Genet 2001;10:173-8. [DOI] [PubMed] [Google Scholar]
- 12. Kubisch C, Schoser BG, von Düring M, et al. Homozygous mutations in caveolin-3 cause a severe form of rippling muscle disease. Ann Neurol 2003;53:512-20. [DOI] [PubMed] [Google Scholar]
- 13. Smythe GM, Eby JC, Disatnik MH, et al. A caveolin-3 mutant that causes limb girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in skeletal myotubes. J Cell Sci 2003;116:4739-49. [DOI] [PubMed] [Google Scholar]
- 14. Sotgia F, Bonuccelli G, Minetti C, et al. Phosphofructokinase muscle-specific isoform requires caveolin-3 expression for plasma membrane recruitment and caveolar targeting: implications for the pathogenesis of caveolin-related muscle diseases. Am J Pathol 2003;163:2619-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 1998;20:31-6. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda C, Hayashi YK, Ogawa M, et al. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum Mol Genet 2001;10:1761-6. [DOI] [PubMed] [Google Scholar]
- 17. Bansal D, Miyake K, Vogal SS, et al. Defective membrane repair in dystrophin-deficient muscular dystrophy. Nature 2003;423:168-72. [DOI] [PubMed] [Google Scholar]
- 18. Hernández-Deviez DJ, Martin S, Laval SH, et al. Aberrant dysferlin trafficking in cells lacking caveolin or expressing dystrophy mutants of caveolin-3. Hum Mol Genet 2006;15:129-42. [DOI] [PubMed] [Google Scholar]
- 19. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997;387:83-90. [DOI] [PubMed] [Google Scholar]
- 20. Zimmers TA, Davies MV, Koniaris LG, et al. Induction of cachexia in mice by systemically administered myostatin. Science 2002;296:1486-8. [DOI] [PubMed] [Google Scholar]
- 21. Massagué J. TGF-beta signal transduction. Annu Rev Biochem 1998;67:753-91. [DOI] [PubMed] [Google Scholar]
- 22. Thies RS, Chen T, Davies MV, et al. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors 2001;18:251-9. [DOI] [PubMed] [Google Scholar]
- 23. Hill JJ, Davies MV, Pearson AA, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 2002;277:40735-41. [DOI] [PubMed] [Google Scholar]
- 24. Tsuchida K. Activins, myostatin and related TGF-beta family members as novel therapeutic targets for endocrine, metabolic and immune disorders. Curr Drug Targets Immune Endocr Metabol Disord 2004;4:157-66. [DOI] [PubMed] [Google Scholar]
- 25. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 2001;98:9306-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rebbapragada A, Benchabane H, Wrana JL, et al. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 2003;23:7230-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ríos R, Fernández-Nocelos S, Carneiro I, et al. Differential response to exogenous and endogenous myostatin in myoblasts suggests that myostatin acts as an autocrine factor in vivo. Endocrinology 2004;145:2795-803. [DOI] [PubMed] [Google Scholar]
- 28. Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 2000;275:40235-43. [DOI] [PubMed] [Google Scholar]
- 29. Bogdanovich S, Krag TO, Barton ER, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 2002;420:418-21. [DOI] [PubMed] [Google Scholar]
- 30. Tang L, Yan Z, Wan Y, et al. Myostatin DNA vaccine increases skeletal muscle mass and endurance in mice. Muscle Nerve 2007;36:342-8. [DOI] [PubMed] [Google Scholar]
- 31. Bogdanovich S, Perkins KJ, Krag TO, et al. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 2005;19:543-9. [DOI] [PubMed] [Google Scholar]
- 32. Nakatani M, Takehara Y, Sugino H, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J 2007 (in press). [DOI] [PubMed] [Google Scholar]
- 33. Parsons SA, Millay DP, Sargent MA, et al. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol 2006;168:1975-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartoli M, Poupiot J, Vulin A, et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther 2007;14:733-40. [DOI] [PubMed] [Google Scholar]
- 35. Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 2001;276:6727-38. [DOI] [PubMed] [Google Scholar]
- 36. Zhang XL, Topley N, Ito T, et al. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem 2005;280:12239-45. [DOI] [PubMed] [Google Scholar]
- 37. Schwartz EA, Reaven E, Topper JN, et al. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J 2005;390:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee EK, Lee YS, Han IO, et al. Expression of Caveolin-1 reduces cellular responses to TGF-beta1 through down-regulating the expression of TGF-beta type II receptor gene in NIH3T3 fibroblast cells. Biochem Biophys Res Commun 2007;359:385-90. [DOI] [PubMed] [Google Scholar]
- 39. Di Guglielmo GM, Roy CL, Goodfellow AF, et al. Distinct endocytic pathway regulates TGF-beta receptor signaling and turnover. Nat Cell Mol Biol 2003;5:410-21. [DOI] [PubMed] [Google Scholar]
- 40. Roy L, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signaling. Nat Rev Mol Cell Biol 2005;6:112-26. [DOI] [PubMed] [Google Scholar]
- 41. Nohe A, Keating E, Underhill TM, et al. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci 2005;118:643-50. [DOI] [PubMed] [Google Scholar]
- 42. Ohsawa Y, Hagiwara H, Nakatani M, et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest 2006;116:2924-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishi M, Yasue A, Nishimatu S, et al. A missense mutant myostatin causes hyperplasia without hypertrophy in the mouse muscle. Biochem Biophys Res Commun 2002;293:247-51. [DOI] [PubMed] [Google Scholar]
- 44. Lee SJ, Reed LA, Davies MV, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 2005;102:18117-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 2004;15:1-11. [DOI] [PubMed] [Google Scholar]
- 46. Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE 2007;2:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv 2003;3:445-64. [DOI] [PubMed] [Google Scholar]
- 48. Sotgia F, Williams TM, Schubert W, et al. Caveolin-1 deficiency (-/-) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am J Pathol 2006;168:292-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 2006;203:2895-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verma S, Slutsky AS. Idiopathic pulmonary fibrosis – new insights. N Engl J Med 2007;356:1370-2. [DOI] [PubMed] [Google Scholar]