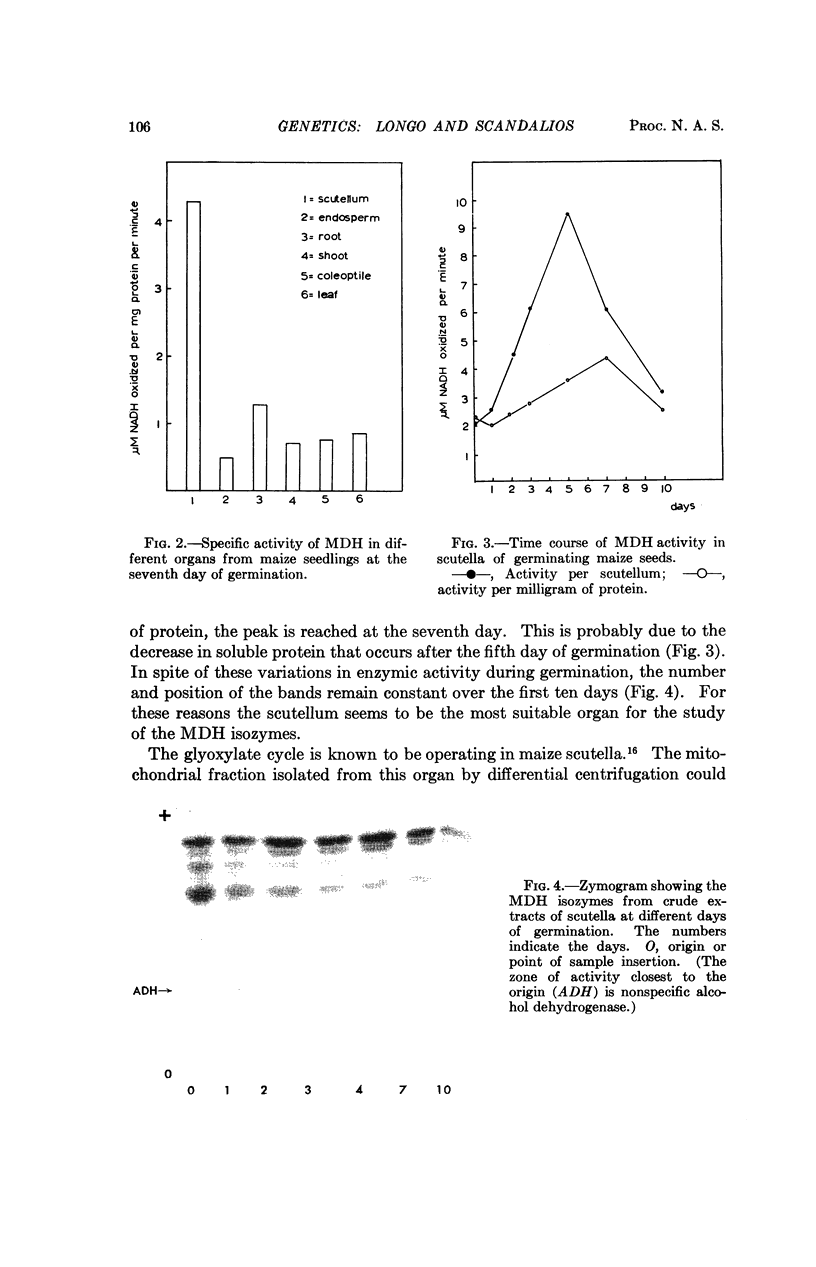
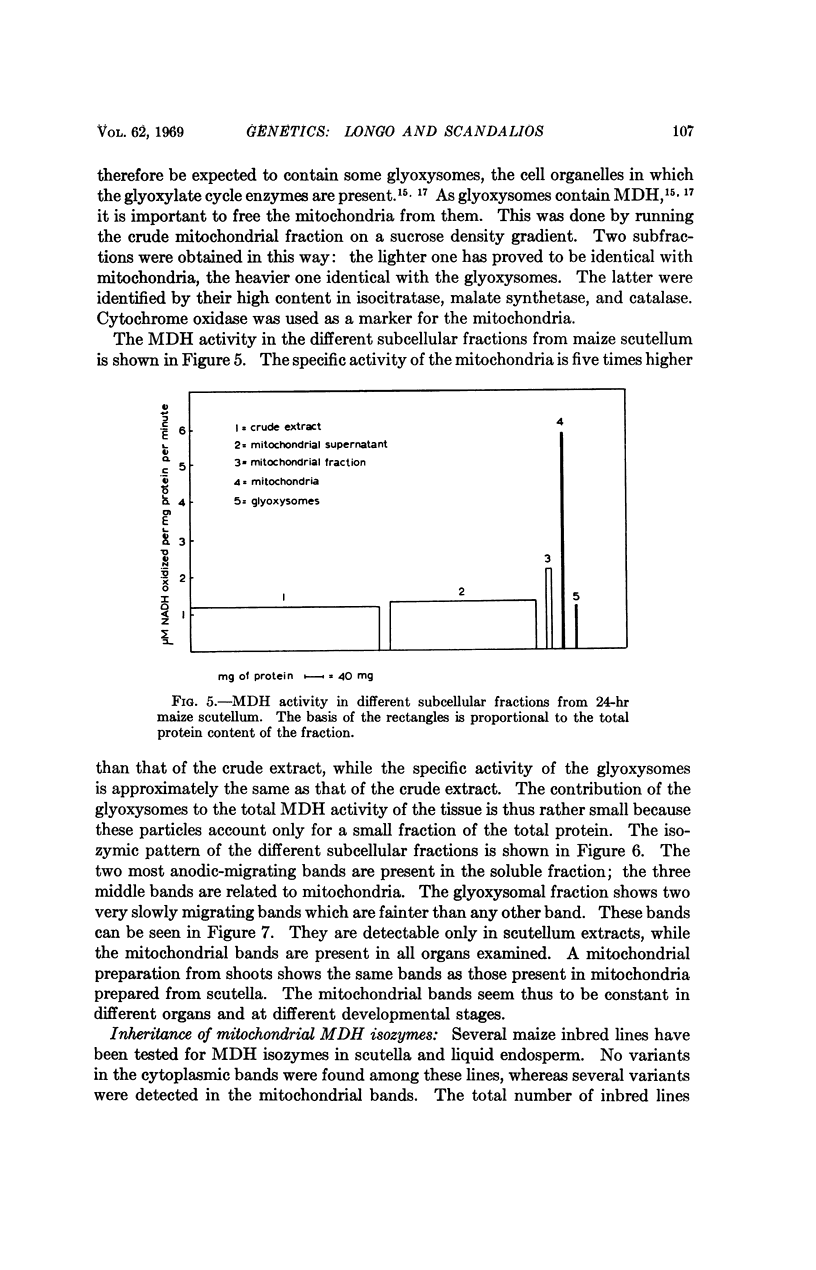
Abstract
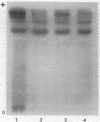
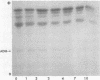
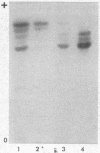
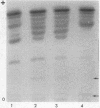
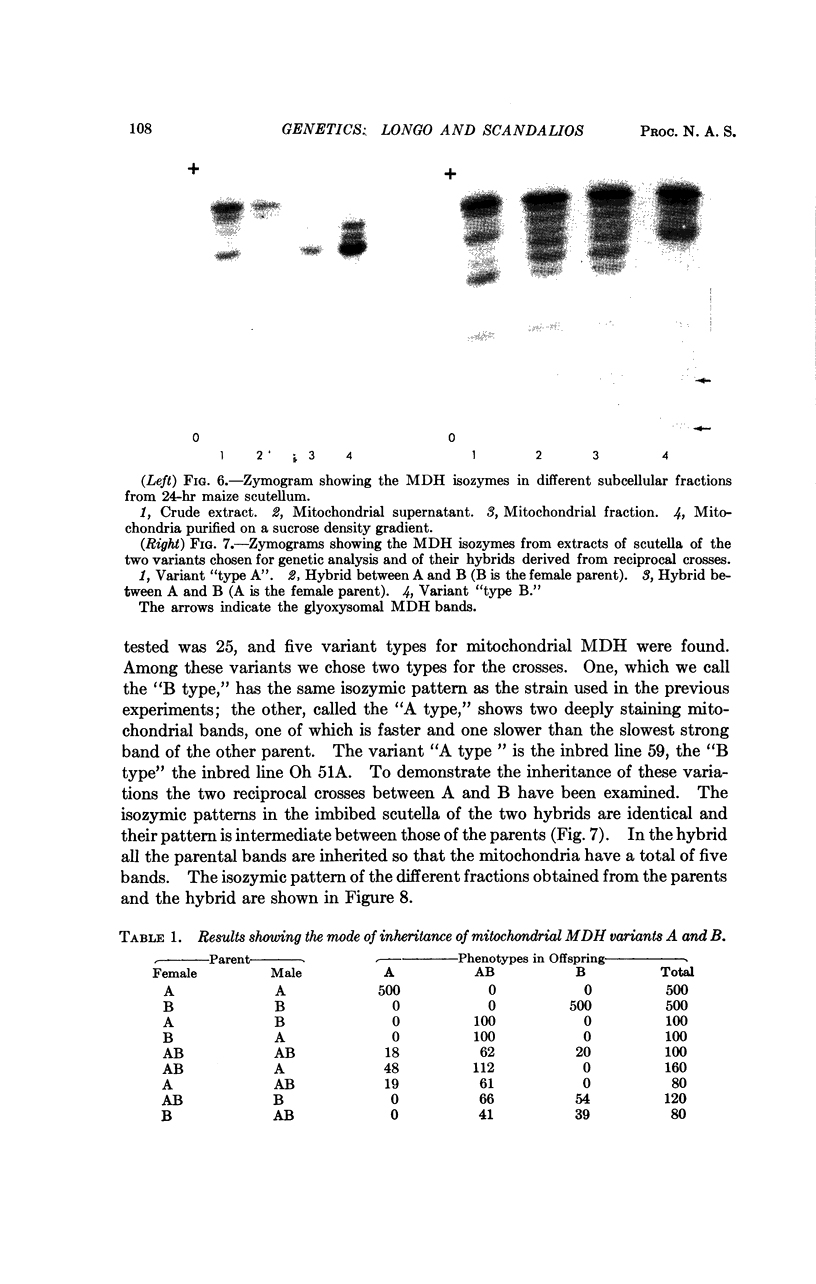
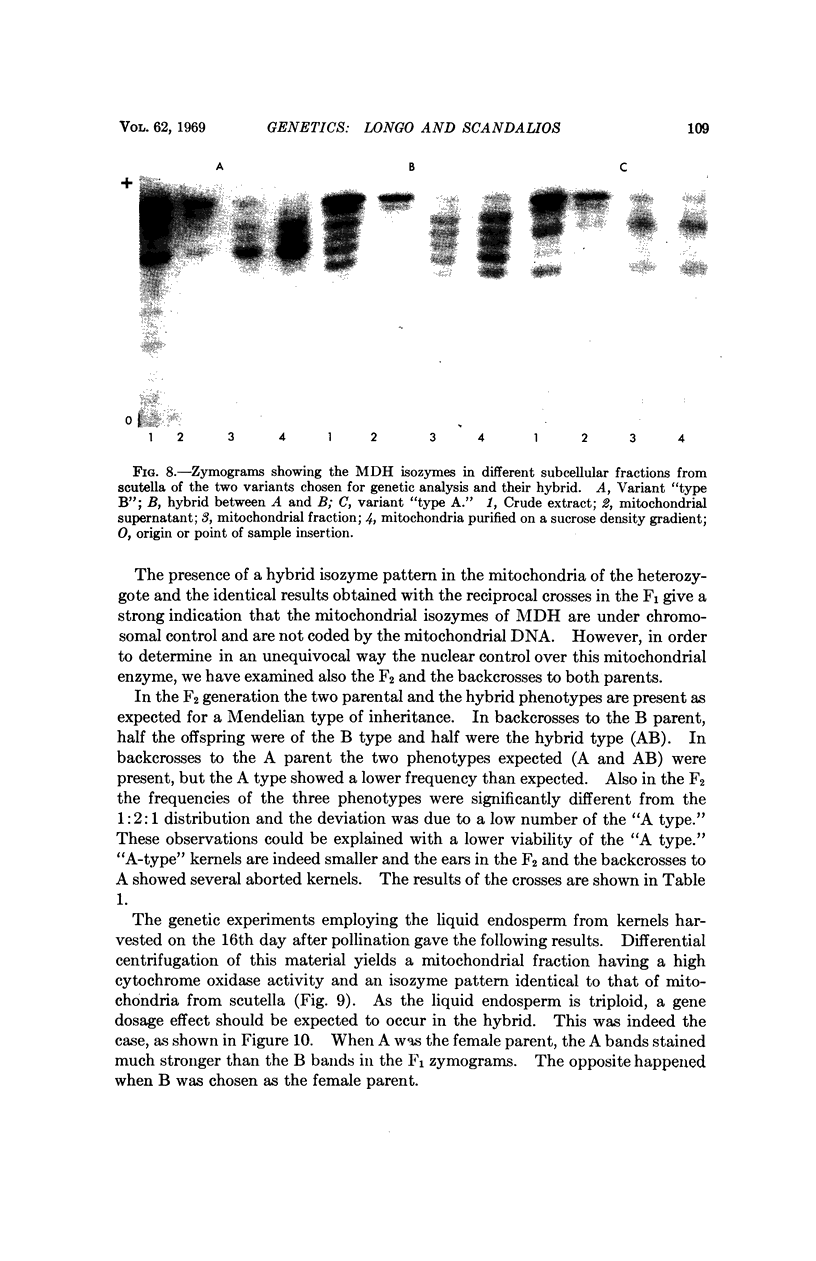
The isozymes of malic dehydrogenase (MDH) of maize have been separated by starch gel zone electrophoresis. The MDH isozyme pattern was the same in different organs and developmental stages. The subcellular distribution of the different isozymes was established by means of differential centrifugation. The MDH isozymes were found to be differentially distributed in the cytoplasm, the mitochondria, and the glyoxysomes. The latter proved the only exception to the pattern constancy among organs insofar as they are present only in the scutella. Among several inbred maize strains examined, only variants of the mitochondrial isozymes were found. Crosses between strains with different isozyme patterns gave Mendelian inheritance: thus, these mitochondrial isozymes are under nuclear-gene control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie D. S., Basford R. E., Koritz S. B. Studies on the biosynthesis of mitochondrial protein components. Biochemistry. 1966 Mar;5(3):926–930. doi: 10.1021/bi00867a018. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. G., Cortner J. A. Mitochondrial malate dehydrogenase: a new genetic polymorphism in man. Science. 1967 Sep 29;157(3796):1569–1571. doi: 10.1126/science.157.3796.1569. [DOI] [PubMed] [Google Scholar]
- GRIMM F. C., DOHERTY D. G. Properties of the two forms of malic dehydrogenase from beef heart. J Biol Chem. 1961 Jul;236:1980–1985. [PubMed] [Google Scholar]
- Kitto G. B., Lewis R. G. Purification and properties of tuna supernatant and mitochondrial malate dehydrogenases. Biochim Biophys Acta. 1967 May 16;139(1):1–15. doi: 10.1016/0005-2744(67)90107-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NASS S., NASS M. M., HENNIX U. DEOXYRIBONUCLEIC ACID IN ISOLATED RAT-LIVER MITOCHONDRIA. Biochim Biophys Acta. 1965 Mar 15;95:426–435. doi: 10.1016/0005-2787(65)90189-9. [DOI] [PubMed] [Google Scholar]
- Oaks A., Beevers H. The Glyoxylate Cycle in Maize Scutellum. Plant Physiol. 1964 May;39(3):431–434. doi: 10.1104/pp.39.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. III. Comparative studies of some properties of M-malic dehydrogenase and S-malic dehydrogenase. Biochim Biophys Acta. 1962 Oct 8;64:101–110. doi: 10.1016/0006-3002(62)90763-1. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. Genetic control of alcohol dehydrogenase isozymes in maize. Biochem Genet. 1967 Jun;1(1):1–9. doi: 10.1007/BF00487731. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., GROSSMAN L. I., KAPLAN N. O. Starch-gel electrophoresis of malate dehydrogenase. Biochim Biophys Acta. 1963 Jun 11;73:193–203. doi: 10.1016/0006-3002(63)90303-2. [DOI] [PubMed] [Google Scholar]
- Tait A. Genetic control of beta-hydroxybutyric dehydrogenase in Paramecium aurelia. Nature. 1968 Aug 31;219(5157):941–941. doi: 10.1038/219941a0. [DOI] [PubMed] [Google Scholar]
- Tapley D. F., Kimberg D. V., Buchanan J. L. The mitochondrion. N Engl J Med. 1967 May 18;276(20):1124–contd. doi: 10.1056/NEJM196705182762006. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Zinkham W. H. Malate dehydrogenase in Ascaris suum: characterization, ontogeny, and genetic control. Arch Biochem Biophys. 1968 Aug;126(2):574–584. doi: 10.1016/0003-9861(68)90444-x. [DOI] [PubMed] [Google Scholar]