Abstract
Over the last years, genetic studies have greatly improved our knowledge on the receptor subtypes mediating various pharmacological effects of positive allosteric modulators at GABAA receptors. This stimulated the development of new benzodiazepine (BZ)-like ligands, especially those inactive/low-active at GABAA receptors containing the α1 subunit, with the aim of generating more selective drugs. Hereby, the affinity and efficacy of four recently-synthesized BZ site ligands: SH-053-2’N, SH-053-S-CH3-2’F, SH-053-R-CH3-2’F and JY-XHe-053 were assessed. They were also studied in behavioral tests of spontaneous locomotor activity, elevated plus maze, and water maze in rats, which are considered predictive of, respectively, the sedative, anxiolytic, and amnesic influence of BZs. The novel ligands had moderately low to low affinity and mild to partial agonistic efficacy at GABAA receptors containing the α1 subunit, with variable, but more pronounced efficacy at other BZ-sensitive binding sites. While presumably α1 receptor-mediated sedative effects of GABAA modulation were not fully eliminated with any of the ligands tested, only SH-053-2’N and SH-053-S-CH3-2’F, both dosed at 30 mg/kg, exerted anxiolytic effects. The lack of clear anxiolytic-like activity of JY-XHe-053, despite its efficacy at α2- and α3-GABAA receptors, may have been partly connected with its preferential affinity at α5-GABAA receptors coupled with weak agonist activity at α1-containing subtypes. The memory impairment in water-maze experiments, generally reported with BZ site agonists, was completely circumvented with all four ligands. The results suggest that a substantial amount of activity at α1 GABAA receptors is needed for effecting spatial learning and memory impairments, while much weaker activity at α1- and α5-GABAA receptors is sufficient for eliciting sedation.
Keywords: GABAA subtype, benzodiazepine, anxiolytic, sedation, memory
INTRODUCTION
The benzodiazepines (BZs) have been widely used for half a century for several neuropsychiatric disorders. They are positive allosteric modulators of the BZ binding site at GABAA receptors in the central nervous system. This site is located at the interface of an α and a γ2 subunit of a GABAA receptor usually composed of 1γ and 2α and 2β subunits. BZs act through those populations of GABAA receptors which contain an α1, α2, α3 or α5 subunit adjacent to the γ2 subunit (α1 receptors, α2 receptors, etc), and by that exert anxiolytic, sedative, hypnotic, muscle relaxant, anticonvulsive and amnesic effects (reviewed in Sieghart and Ernst, 2005). Although the second α subunit of the receptor might be of the same or a different subunit type, according to current knowledge it does not play a role in benzodiazepine pharmacology since it does not form the interface with the γ2 subunit (Minier and Sigel, 2004).
In regard to the treatment of anxiety disorders, which has been the most extensive field of application of BZs, their continuous use as first-choice anxiolytics has been repeatedly discouraged in the pertinent guidelines (e.g. Baldwin et al., 2005; Bandelow et al., 2008), mainly due to untoward effects (most notably, sedation, amnesia, tolerance and dependence). However, the currently available non-BZ drugs, preferred for long-term treatment, are also far from completely meeting the needs imposed on contemporary pharmacotherapy of anxiety disorders (Garner et al., 2009). On the other hand, the results of exquisite genetic studies, with mice carrying a point mutation (‘knock-in’) that changes histidine to arginine in α1, α2, α3 or α5 subunits, which renders the respective GABAA receptors selectively insensitive to effects of BZ site modulators (Wieland et al., 1992), have supported the highly desirable possibility of separation of the diverse behavioral effect of BZs. Principally, the sedative and ataxic effects of BZs have been attributed to α1-containing GABAA receptor subtypes, anxiolytic actions to the α2/α3-containing receptors, anterograde amnesic effects to the α1/α5-subtypes, anticonvulsant activity, in part, to all the α1/α2/α3 containing receptors, muscle relaxant effects largely to α2-subtypes, and tolerance to sedative effects to α5-containing receptors (reviewed in Rudolph and Möhler, 2006). This has stimulated new interest in the synthesis of novel subtype selective ligands which act through BZ binding site, aimed to selectively bind and/or activate specific GABAA receptor subtype(s) presumably involved in the desired pharmacological effect (Sieghart and Ernst, 2005; Whiting, 2006). If the concept holds true, an ideal anxioselective anxiolytic, selectively acting through α2/α3-containing receptors, would be devoid of sedative, ataxic, amnesic and tolerance adverse effects. However, despite the notable successes of the studies of several research groups published to date (reviewed in Whiting, 2006; also Mirza et al., 2008), including the encouraging safety records for an α2/α3-subtype selective partial modulator in healthy volunteers (de Haas et al., 2007), it appears the ultimate goal of translating breakthroughs in preclinical research into new clinical therapies is still far from realization. The awareness that a similar conclusion may apply to the whole field of pharmacological modulation of central nervous system disorders (Markou et al., 2009) calls for rethinking the clear-cut hypotheses on receptor subtypes and their roles. In this vein, it is interesting to note that some newer BZ binding-site agonists, such as ocinaplon (Lippa et al., 2005) and DOV 51892 (Popik et al., 2007), were reportedly devoid of overt sedative effects, despite the fact that they did not display compelling selectivity for either of the GABAA receptor subtypes (cf. Berezhnoy et al., 2008). In pursuit of the approach of pharmacological testing of four subtypes of BZ-sensitive GABAA receptors to further elucidate their native role, a series of BZ site ligands, presumably inactive/low-active at α1-containing GABAA receptors, was recently synthesized at the University of Wisconsin-Milwaukee. Specific portions of the characterization of six of them, with code names SH-053-S-CH3, SH-053-R-CH3, SH-053-2’N, JY-XHe-053, SH-053-S-CH3-2’F and SH-053-R-CH3-2’F, have been already published (Rivas et al., 2009; Savić et al., 2008a; 2008b). In the present paper, the affinity and efficacy data of four of these ligands: SH-053-2’N, SH-053-S-CH3-2’F, SH-053-R-CH3-2’F and JY-XHe-053, as well as their behavioral actions in the tests of spontaneous locomotor activity, elevated plus maze and water maze, which are considered primarily predictive of, respectively, the sedative, anxiolytic, and amnesic influence of BZs, will be described. Diazepam, a standard non-selective positive allosteric modulator, and zolpidem, a moderately α1-subtype selective positive modulator (Hadingham et al., 1993; Sanna et al., 2002), were used as reference ligands, where applicable. The aim of the study was to elucidate whether the expected subtle affinity and efficacy differences at four BZ-sensitive GABAA receptor subtypes among the tested BZ-site modulators reflect themselves in dissimilarities in behavioral responses of Wistar rats, with possible implications for further research. Concurrently, the data were to be compared with findings from the knock-in approach (reviewed in Rudolph and Möhler, 2006) as well as with results from classical studies in the same behavioral tests, investigating antagonism of the effects of non-selective positive modulators (diazepam or midazolam) with the preferential α1-subunit affinity-selective antagonist, ß-CCt (Savić et al., 2004; 2009).
2. EXPERIMENTAL PROCEDURES
Drugs
The SH-053-2’N (8-ethynyl-6-(2’-pyrydine)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester), SH-053-S-CH3-2’F and SH-053-R-CH3-2’F (the (S) and (R) stereoisomer, respectively, of 8-ethynyl-6-(2-fluorophenyl)-4-methyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester), as well as JY-XHe-053 (8-ethynyl-6-(2-fluorophenyl)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester) were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin - Milwaukee. Zolpidem for behavioral studies was purchased from Toronto Research Chemicals (North York, Canada), and diazepam was obtained from Galenika (Belgrade, Serbia).
Competition Binding Assays
Competition binding assays were performed in a total volume of 0.5 mL at 4 °C for 1 hour using [3H] flunitrazepam as the radiolabelled ligand. A total of 6μg of cloned human GABAA receptor DNA containing desired α subtype along with β2 and γ2 subunits were used for transfecting HEK 293T cell line using Fugene 6 (Roche Diagnostic) transfecting reagent. Cells were harvested 48 hrs after transfection, washed with Tris-HCl buffer (pH 7.0) and Tris Acetate buffer (pH 7.4) and resulting pellets were stored at −80C until assayed. On the day of the assay, pellets containing 20-50 μg of GABAA receptor protein were resuspended in (50 mM Tris-acetate pH 7.4 at 4 degree) and incubated with the radiolabel as previously described (Choudhary et al., 1992). Nonspecific binding was defined as radioactivity bound in the presence of 100 μM diazepam and represented less than 20% of total binding. Membranes were harvested with a Brandel cell harvester followed by three ice-cold washes onto polyethyleneimine-pretreated (0.3%) Whatman GF/C filters. Filters were dried overnight and then soaked in Ecoscint A liquid scintillation cocktail (National Diagnostics; Atlanta, GA). Bound radioactivity was quantified by liquid scintillation counting. Membrane protein concentrations were determined using an assay kit from Bio-Rad (Hercules, CA) with bovine serum albumin as the standard.
Electrophysiological experiments
The cloning of rat GABAA receptor subunits α1, β3 and γ2 into pCDM8 expression vectors (Invitrogen, CA) has been described elsewhere (Fuchs et al., 1995). The rat cDNAs for subunits α2, α3 and α5 were gifts from P. Malherbe and were subcloned into pCI-vector. After linearizing the cDNA vectors with appropriate restriction endonucleases, capped transcripts were produced using the mMessage mMachine T7 transcription kit (Ambion, TX). The capped transcripts were polyadenylated using yeast poly(A) polymerase (USB, OH) and were diluted and stored in diethylpyrocarbonate-treated water at –70°C.
The methods used for isolating, culturing, injecting and defolliculating of the oocytes were identical as described previously (Sigel, 1987; Sigel et al., 1990). Briefly, mature female Xenopus laevis (Nasco, WI) were anaesthetized in a bath of ice-cold 0.17 % Tricain (Ethyl-m-aminobenzoat, Sigma, MO) before decapitation and removal of the frogs ovary. Stage 5 to 6 oocytes with the follicle cell layer around them were singled out of the ovary using a platinum wire loop. Oocytes were stored and incubated at 18°C in modified Barths’ Medium (MB, containing 88 mM NaCl, 10 mM HEPES-NaOH (pH 7.4), 2.4 mM NaHCO3, 1 mM KCl, 0.82 mM MgSO4, 0.41 mM CaCl2, 0.34 mM Ca(NO3)2) that was supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. Oocytes with follicle cell layers still around them were injected with a total of 2.25 ng of cRNA. cRNA ratio used was 1:1:5 for the α subunits, β3 and γ2, respectively. After injection of cRNA, oocytes were incubated for at least 36 hours before the enveloping follicle cell layers were removed. To this end, oocytes were incubated for 20 min at 37°C in MB that contained 1 mg/ml collagenase type IA and 0.1 mg/ml trypsin inhibitor I-S (both Sigma). This was followed by osmotic shrinkage of the oocytes in doubly concentrated MB medium supplied with 4 mM Na-EGTA and manually removing of the follicle cell layer. After peeling off the follicle cell layer, the cells were allowed to recover overnight before being used in electrophysiological experiments.
For electrophysiological recordings, oocytes were placed on a nylon-grid in a bath of Xenopus Ringer solution (XR, containing 90 mM NaCl, 5 mM HEPES-NaOH (pH 7.4), 1 mM MgCl2, 1 mM KCl and 1 mM CaCl2). The oocytes were constantly washed by a flow of 6 ml/min XR which could be switched to XR containing GABA and/or drugs. Drugs were diluted into XR from DMSO-solutions resulting in a final concentration of 0.1 % DMSO perfusing the oocytes. Drugs were preapplied for 30 sec before the addition of GABA, which was coapplied with the drugs until a peak response was observed. Between two applications, oocytes were washed in XR for up to 15 min to ensure full recovery from desensitization. For current measurements the oocytes were impaled with two microelectrodes (2–3 mΩ) which were filled with 2 mM KCl. All recordings were performed at room temperature at a holding potential of –60 mV using a Warner OC-725C two-electrode voltage clamp (Warner Instruments, Hamden, CT). Data were digitised, recorded and measured using a Digidata 1322A data acquisition system (Axon Instruments, Union City, CA). Results of concentration response experiments were graphed using GraphPad Prism 4.00 (GraphPad Software, San Diego, CA). Data were graphed as mean ± SEM of at least four oocytes from at least two batches.
Behavioral experiments
Experiments were carried out on male Wistar rats (Military Farm, Belgrade, Serbia), weighing 220-250 g. All procedures in the study conformed to EEC Directive 86/609 and were approved by the Ethical Committee on Animal Experimentation of the Faculty of Pharmacy in Belgrade. The rats were housed in transparent plastic cages, six animals per cage, and had free access to food pellets and tap water. The temperature of the animal room was 22±1°C, the relative humidity 40-70%, the illumination 120 lux, and the 12/12 h light/dark period (light on at 6:00 h). All handling and testing took place during the light phase of the diurnal cycle. Separate groups of animals were used for three behavioral paradigms. Care was taken to counterbalance the test order across treatment conditions. The differences in treatments in individual paradigms (vide infra) are related to the fact that some pertinent parts of experiments have been done previously (Savić et al. 2004; 2008b; 2009). The behavior was recorded by a ceiling-mounted camera and analyzed by the ANY-maze Video Tracking System software (Stoelting Co., Wood Dale, IL, USA). The drugs were dissolved/suspended with the aid of sonication in a solvent containing 85% distilled water, 14% propylene glycol, and 1% Tween 80, and were administered intraperitoneally in a volume of 2 ml/kg, 20 min before behavioral testing. The selection of dose ranges and the injection-test interval was based on previous experiments (Rivas et al., 2009; Savić et al., 2004; 2008b), as well as on observations in preliminary experiments, avoiding, where applicable, testing of lower doses in case of lack of the expected behavioral effect at a higher dose.
Measurement of locomotor activity
Twenty minutes after receiving the appropriate treatment, single rats were placed in a clear Plexiglas chamber (40 × 25 × 35 cm). Activity under dim red light (20 lux) was recorded for a total of 30 min or 45 min, without any habituation period, using ANY-maze software. Besides the total distance travelled, behavior was analyzed by dividing the locomotor activity data into 5-min bins. For purposes of improving data analysis, the central 20% of the chamber (200 cm2) was virtually set as a central zone. An entry into a zone was counted when 70% of the animal's body had crossed the zone border. An exit from the zone was counted when more than 50% of the animal's body had left the zone.
Three experiments were performed. In the first study, locomotor influences of SH-053-2’N, dosed at 30 mg/kg, were assessed in comparison with diazepam at 1.25 and 2.5 mg/kg. In the second experiment, the dose response curve for JY-XHe-053 (0; 2.5; 5; 10; 20 and 40 mg/kg), and in the third, for SH-053-R-CH3-2’F (0; 10; 20 and 30 mg/kg, in comparison with 2 mg/kg diazepam), were determined. The characterisation of SH-053-S-CH3-2’F in this test has been published previously (Savić et al., 2008b).
Behavior in the elevated plus maze
The apparatus was constructed of sheet metal, with a black rubber floor. It consisted of a maze elevated to a height of 50 cm with two open (50 × 10 cm) and two enclosed arms (50 × 10 × 40 cm), connected by a junction area (central platform) measured 10 × 10 cm. A ledge of sheet metal (0.3 cm high) surrounding the open arms was added. The illumination in the experimental room consisted of one red neon tube fixed on the ceiling, giving light intensity of 10 lux on the surface of the closed arms. At the beginning of the experiment, single rats were placed in the center of the maze, facing one of the enclosed arms, and their behavior was recorded for 5 min. An entry into an open or closed arm was scored when 90% of the animal crossed the virtual line separating the central square of the maze from the arm, whereas an exit occurred when more than 90% of the animal left the respective arm. After each trial, the maze was cleaned with dry and wet towels.
Three experiments were performed, in which the dose response curves for SH-053-2’N (0; 10; 20 and 30 mg/kg, in comparison with 2.0 mg/kg diazepam), JY-XHe-053 (0; 2.5; 5; 10; 20 and 40 mg/kg), and SH-053-R-CH3-2’F (0; 10; 20 and 30 mg/kg) were determined. The characterisation of SH-053-S-CH3-2’F in this test has been published previously (Savić et al., 2008b).
Behavior in the Morris water maze
The water maze consisted of a black cylindrical pool (diameter: 200 cm, height: 60 cm), with a uniform inner surface. The pool was filled to a height of 30 cm with 23°C (±1°C) water. The escape platform made of black plastic (15×10 cm) was submerged 2 cm below the water surface. The platform was made invisible to rats by having it painted the same color as the pool wall (Terry, 2000). There were many distal cues in the testing room (doors, pipes on the walls and the ceiling, cupboards). An indirect illumination in the experimental room was provided by white neon tubes fixed on the walls near the pool.
The rats received the appropriate treatment 20 min before a swimming block, each day for 5 consecutive days of spatial acquisition. Each block consisted of 4 trials, lasting a maximum time of 120 s, the intertrial interval being 60 s. For each trial the rat was placed in the water facing the pool at one of four pseudorandomly determined starting positions. As during spatial learning the platform was hidden in the middle of the NE quadrant, the four distal start locations were chosen: S, W, NW and SE. Once the rat found and mounted the escape, it was permitted to remain on the platform for 15 s. The rat was guided to the platform by the experimenter if it did not locate the escape within 120 s. To assess the long-term spatial memory at the end of learning, a probe trial for 60 s, with the platform omitted, was given 24 h after the last acquisition day. The probe trial, starting from the novel, most distant SW location, was performed without any pre-treatment. The tracking software virtually divided the pool into four quadrants, three concentric annuli and a target region consisting of the intersection of the platform quadrant and the platform (middle) annulus, as graphically represented in Savić et al., 2009. The central annulus was set up to 10% of the whole area; the platform annulus equaled 40%, whereas the area of the peripheral annulus was 50% of the whole.
Dependent variables chosen for tracking during the acquisition trials were: latency to platform (time from start to goal), total distance swam (path length), average swim speed and path efficiency (the ratio of the shortest possible path length to actual path length). All these indices are, to a lesser or greater degree, related to goal-directed behavior, i.e. spatial learning (Vorhees and Williams, 2006). As thigmotaxis (the tendency to swim or float near the pool wall) represents a factor which accounts for much of the variance in the water maze performance, and normally weakens during consecutive trials (Vorhees and Williams, 2006), we quantified the persistence of the thigmotaxis in the target (NE) quadrant. The loss of thigmotaxis is related to the procedural component of acquisition, and the percent of the distance swum in the target region (away from the wall) of the target quadrant may be seen as a measure of procedural learning. The indices of memory, assessed during the probe trial, included the distance and time in the platform (target) quadrant, platform ring and target region, as well as the number of entries and distance swum in the area where the platform used to be during training. In addition, the distance swum during 60 sec in the probe trial was taken as a measure of overall activity, while peripheral ring parameters (distance and time) were connected to the thigmotaxic behavior.
Three experiments in the water maze were performed. In the first study, the dose response curve for zolpidem (0; 0.5; 1 and 2 mg/kg) was found. In the second experiment, water-maze activity of SH-053-2’N, SH-053-S-CH3-2’F and SH-053-R-CH3-2’F, all dosed at 30 mg/kg, was assessed. Finally, the influence of JY-XHe-053 (0; 5; 20 and 40 mg/kg) on the water-maze behavior was determined.
Statistical analysis
All numerical data presented in the figures were given as the mean ± SEM. For electrophysiological data Student's t-test was used. Data sets were checked for homogeneity of variance and normality prior to analysis by a one-way ANOVA (the activity assay and elevated plus maze), or a two-way ANOVA with repeated measures (the water maze test). Where applicable, Student-Newman-Keuls or Dunnett's test (post hoc comparisons) and analysis of covariance were also used. Statistical analyses were performed with ANY-maze Video Tracking System software (Stoelting Co., Wood Dale, IL, USA) and SigmaStat 2.0 (SPSS, Inc., Chicago, IL, USA).
3. RESULTS
Competition binding assays
In vitro binding data for SH-053-2’N, SH-053-S-CH3-2’F, SH-053-R-CH3-2’F and JY-XHe-053, in parallel with those for diazepam and zolpidem, are presented in Table 1. Broadly speaking, these ligands were binding with biologically relevant nanomolar-to micromolar affinity to the BZ-sensitive recombinant human GABAA receptors, while were devoid of binding and activity in approximately 40 other receptor and enzyme assays (NIMH Psychoactive Drug Screening Program, UNC, available at https://kidbdev.med.unc.edu/pdsp). No major selectivity in binding at one over the other receptors was noticed (JY-XHe-053 was the most selective ligand, with approximately 18-fold selectivity for GABAA receptors containing the α5 subunit, while zolpidem exerted an approximately 5-fold selectivity for α1-GABAA receptors). It is of interest that none of the novel ligands had a relatively high affinity for the α1-containing receptors (once more, the highest α1-subtype affinity, the Ki of 22 nM, was possessed by JY-XHe-053).
Table 1.
Binding affinity at α×β3 γ2 GABAA/BZ site subtypes. Measurements were made in duplicate. Ki values are reported in nM.
| Compound | α1 | α2 | α3 | α4 | α5 | α6 |
|---|---|---|---|---|---|---|
| diazepam | 14.0 | 7.8 | 13.9 | NDa | 13.4 | NDa |
| zolpidem | 29.6 | 160.0 | 380.0 | NDa | >10000 | NDa |
| SH-053-2'N | 300.0 | 160.0 | 527.0 | NDb | 82.0 | >5000 |
| SH-053-2'Nc | 118.0 | 148.0 | 365.0 | >5000 | 77.0 | >5000 |
| SH-053-R-CH3-2'F | 759.1 | 948.2 | 768.8 | NDb | 95.2 | NDb |
| SH-053-S-CH3-2'F | 468.2 | 33.3 | 291.5 | NDb | 19.2 | >5000 |
| JY-XHe-053 | 22.0 | 12.3 | 34.9 | NDb | 0.7 | NDb |
ND, not determined
Binding at α4 and α6 subtypes have not been determined, but since the 6-phenyl group is present, the ligand will not bind to α4 and α6 subtypes
The second independent set of experiments with SH-053-2'N
Electrophysiological experiments
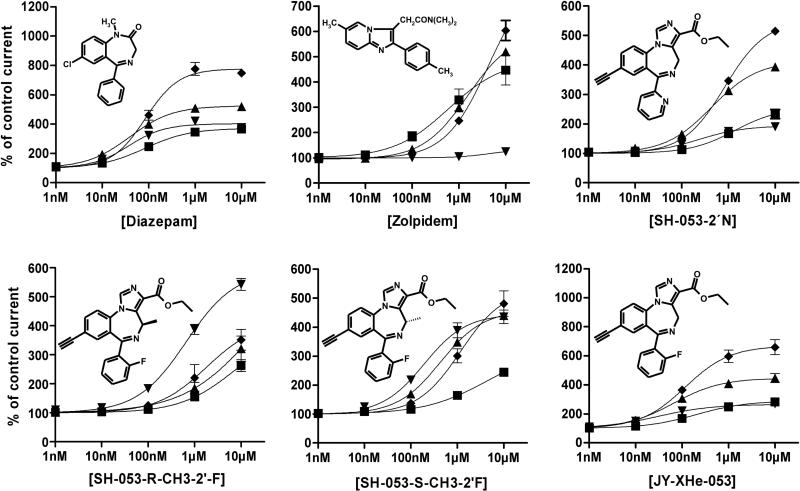
The in vitro concentration-effect curves for the four novel ligands, in parallel with those for zolpidem and diazepam, are presented in Figure 1, together with explicit data on percent potentiation of a within- and between- day stable EC3 GABA response at rat recombinant GABAA receptors. The efficacy data for SH-053-2’N and JY-XHe-053 have been recently published (Rivas et al., 2009), and all data are shown here together for comparison purposes. All four novel ligands exerted the lowest positive modulation at α1-containing subtypes of receptors relative to efficacy at the other subtypes. In regard to the other three receptor subtypes, the greatest separation of activity was noticed with SH-053-R-CH3-2’F, which shows relatively low efficacies at α2 and α3, in addition to the α1-containing GABAA receptors. The other ligands exerted substantial potentiation of an EC3 GABA response at α2 and α3-containing receptors. At 100 nM concentration, which lies well in the range of typical brain levels of BZ site ligands achievable in vivo (cf. Pike et al., 2007; Wang et al., 2003), this potentiation was slightly above (SH-053-2’N and JY-XHe-053) or slightly below (SH-053-S-CH3-2’F) that one elicited through the α5-containing subtypes.
Figure 1.
Concentration-effect curves for diazepam, zolpidem, SH-053-2'N, SH-053-S-CH3-2'F SH-053-R-CH3-2'F and JY-XHe-053 on α1β3γ2 (■), α2β3γ2 (▲), α3β3γ2 (◆), and α5β3γ2 (▼) GABAA receptors, using an EC3 GABA concentration. Data points represent means ±SEM from at least four oocytes from ≥ 2 batches. As explained in the Results section, the efficacy data for SH-053-2’N and JY-XHe-053 have been published in Rivas et al. (2009). A concentration of 100 nM of diazepam resulted in 246 ± 16%, 400 ± 22%, 461 ± 34%, and 322 ± 7% of control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively. A concentration of 100 nM of zolpidem resulted in 180±14%, 132±4%, 121±3%, and non-significant changes relative to control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively. A concentration of 100 nM of SH-053-2'N resulted in 113 ± 2%, 165 ± 2%, 149 ± 3%, and 130 ± 3% of control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively. A concentration of 100 nM of SH-053-S-CH3-2'F resulted in non-significant changes relative to control current, 169 ± 5%, 138 ± 5%, and 218 ± 4% of control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively. The effect of 1 μM of SH-053-S-CH3-2'F at α1β3γ2 receptors was significant relative to control (164 ± 6%). A concentration of 100 nM of SH-053-R-CH3-2'F resulted in 111 ± 2%, 124 ± 9%, 125 ± 8%, and 183 ± 7% of control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively. A concentration of 100 nM of JY-XHe-053 resulted in 169 ± 10%, 307 ± 14%, 345 ± 26%, and 220 ± 2% of control current in α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors, respectively. All values given were significantly different from the respective control currents (p < 0.01, Student's t-test).
Motor activity assay
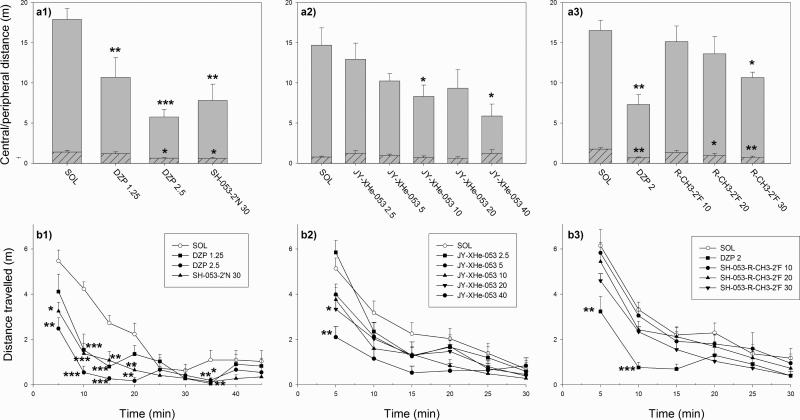
In the experiment with 1.25 and 2.5 mg/kg diazepam and 30 mg/kg SH-053-2’N, ANOVA showed a significant effect of treatment on total distance travelled during 45 min of monitoring (F(3,27) = 8.33, p<0.001) (Fig. 2, graph a1). According to Dunnett's test, the activity-depressing effect of all three treatments was significant compared with solvent control. The effect was more pronounced in the peripheral zone (F(3,27) = 8.56, p<0.001), than in the central zone (F(3,27) = 4.11, p=0.016). When the analysis of distance travelled was developed into 5-min bins (Fig. 2, graph b1), it turned out that hypolocomotion was significant during the first 20 min of monitoring, and again in the period 30-35 min, the effect of 30 mg/kg SH-053-2’N being somewhere in the middle between the effects of two tested doses of diazepam.
Fig. 2.
The effects of diazepam (DZP 1.25 and 2.5 mg/kg) and SH-053-2’N 30 mg/kg (left graphs, a1 and b1), JY-XHe-053 (2.5, 5, 10, 20 and 40 mg/kg) (middle graphs, a2 and b2) and DZP 2 mg/kg and SH-053-R-CH3-2’F (10, 20 and 30 mg/kg) (right graphs, a3 and b3) on distance travelled in the central (hatched bars) and peripheral (open bars) zone of the activity chamber during 45 or 30 min of recording (total activity corresponds to the height of the whole bar) (upper graphs, a1, a2 and a3) as well as on distance travelled in 5-min intervals (lower graphs, b1, b2 and b3). *,** and ***, P<0.05; 0.01 and 0.001, respectively, compared to solvent (SOL) group in each of three experiments. Numbers of animals per treatment, for consecutive groups on each of panels, were 8, 8, 7, 8 (left); 8, 6, 6, 8, 8, 6 (middle) and 7, 5, 7, 8, 8 (right).
The dose-response study with JY-XHe-053 showed a significant effect on total locomotor activity during 30 min of recording (F(5,36) = 2.85, p=0.029) (Fig. 2, graph a2). The effective doses of JY-XHe-053 were 10 and 40 mg/kg. The effect on central zone activity was not discernible, while statistical analysis of peripheral zone distance revealed that the three higher doses of JYXHe-053 were significantly different from control (not shown). The analysis of 5-min bins showed that during the first 5 min of recording the effect of JY-XHe-053 at 20 and 40 mg/kg was significant (Fig. 2, graph b2).
In the third experiment, an ANOVA for total distance was also significant (F(4,30) = 4.50, p=0.006) (Fig. 2, graph a3). According to Dunnett's test, 30 mg/kg SH-053-R-CH3-2’F, besides 2 mg/kg diazepam, significantly depressed locomotion. Notably, central zone activity was affected more profoundly (F(4,30) = 4.27, p=0.007) than peripheral locomotion (F(4,30) = 3.74, p=0.014), and SH-053-R-CH3-2’F at 20 and 30 mg/kg decreased central, but not peripheral locomotion. The analysis of 5-min bins (Fig. 2, graph b3) revealed that SH-053-R-CH3-2’F did not significantly decrease locomotion in neither of 6 intervals of recording, far different from diazepam (2 mg/kg), which induced overt sedation during the first 10 min of recording.
Our recent study with SH-053-S-CH3-2’F at 30 mg/kg has shown a significant decrease of locomotor activity, which was significantly depressed in the time period 5-20 min, and only in the peripheral, but not central, zone (Savić et al., 2008b).
Elevated plus maze
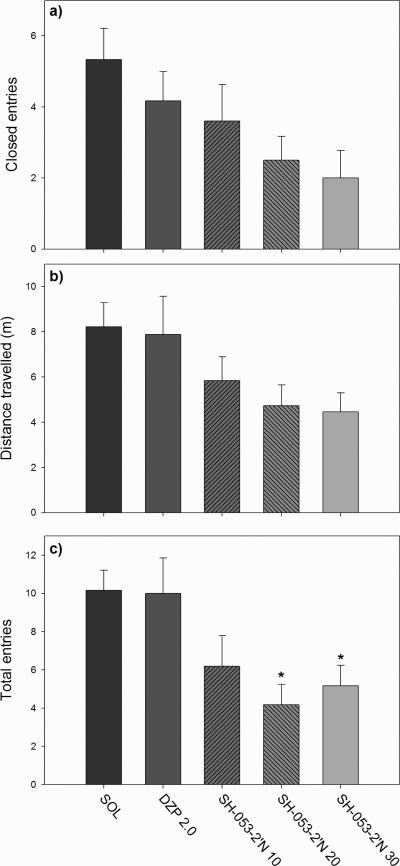
In regard to influence of SH-053-2’N on activity-related parameters (Fig. 3) in the plus maze, the overall effect of treatment did not reach statistical significance when closed arm entries (F(4,24) = 2.62, p=0.060) (Fig. 3a) and total distance travelled (F(4,24) = 2.33, p=0.085) (Fig. 3b) were analyzed. However, statistical analysis by ANOVA showed a significant effect of treatment on total arm entries (F(4,24) = 4.34, p=0.009). Dunnett's test revealed the significant effect of the two higher doses of SH-053-2’N in comparison to solvent group (p=0.014 and 0.037, respectively) (Fig. 3c).
Fig. 3.
The effects of diazepam (DZP 2.0 mg/kg) and SH-053-2’N (10, 20 and 30 mg/kg) on the a) closed arm entries, b) total arm entries and c) total distance travelled in the EPM. *P<0.05 compared to solvent (SOL) group. Number of animals per treatment (Fig. 3- 4, for SOL through SH-053-2’N 30 mg/kg, respectively): 6, 6, 5, 6, 6.
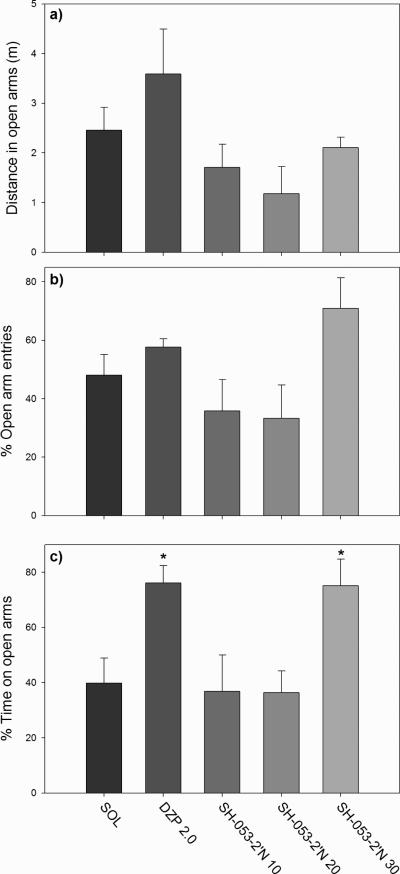
The influence of SH-053-2’N on anxiety-related parameters was presented in Fig. 4. There was no significant effect of treatment on distance travelled in the open arms (F(4,24) = 2.56, p=0.065) (Fig. 4a). Although the overall influence of treatment on the percentage of open arm entries had reached statistical significance (F(4,24) = 3.04, p=0.037) (Fig. 4b), neither of the single doses was significant on its own (post hoc test). The influence on the percentage of time on open arms was significant (F(4,24) = 5.04, p<0.001) (Fig. 4c), with diazepam and SH-053-2’N at 30 mg/kg as effective treatments (p-values of 0.032 in both post hoc comparisons). Because the parameter of total entries could not be seen as a relatively pure index of locomotor activity, i.e. sedation (Cruz et al. 1994; Rodgers and Johnson, 1995), an analysis of covariance in the anxiety-related parameters using the number of total arm entries as covariate was not necessary (cf. Savić et al. 2004). However, we opted to conduct a new analysis of anxiety-related parameters, without data for diazepam as positive control. This revealed the preserved significance of effects of SH-053-2’N on the percentage of open arm entries and the percentage of time spent in open arms when an analysis of covariance was performed using the number of closed arm entries as covariate (respective F values: [F(3,18) = 3.276, p=0.045)] and [F(3,18) = 6.027, p=0.005]). This indicates that the anxiolytic-like effects of SH-053-2’N were not directly related with a trend of hypolocomotion in rats treated with this ligand.
Fig. 4.
The effects of diazepam (2.0 mg/kg) and SH-053-2’N (10, 20 and 30 mg/kg) on the a) distance travelled on open arms, b) percentage of entries in open arms and c) percentage of time spent on open arms of the EPM. *P<0.05 compared to solvent (SOL) group.
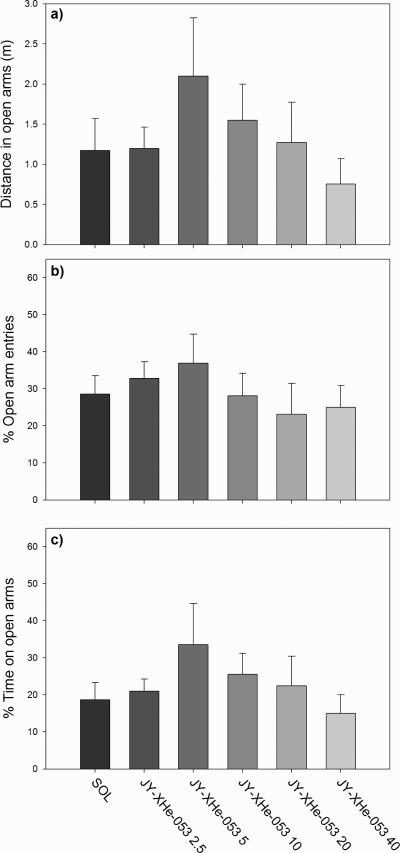
JY-XHe-053, administered in a wide range of doses, was devoid of significant behavioral activity in the elevated plus maze, when analyzed both, general activity (data not shown) and anxiety-related parameters. In the Fig. 5 are presented changes under this treatment of three anxiety-related parameters: distance in open arms (Fig. 5a), percent of open arm entries (Fig. 5b) and percent of time spent on open arms (Fig. 5c). Despite a hint of an inverted U-shape activity and behavioral disinhibition at 5 mg/kg, discernible changes of behavior were lacking.
Fig. 5.
The influence of JY-XHe-053 (0; 2.5; 5; 10; 20 and 40 mg/kg) on the a) distance travelled on open arms, b) percentage of entries in open arms and c) percentage of time spent on open arms of the EPM. Number of animals per treatment (for SOL through JY-XHe-053 40 mg/kg) was 7.
The SH-053-R-CH3-2’F in doses up to 30 mg/kg was completely devoid of changes of general activity- or anxiety-related parameters in the elevated plus maze, and hence this set of data was not presented.
Our recent study with SH-053-S-CH3-2’F has shown a significant effect of the 30 mg/kg dose on the percentage of open arm entries and the percentage of time on open arms, without concomitant effects on general activity-related parameters (Savić et al., 2008b).
Morris water maze
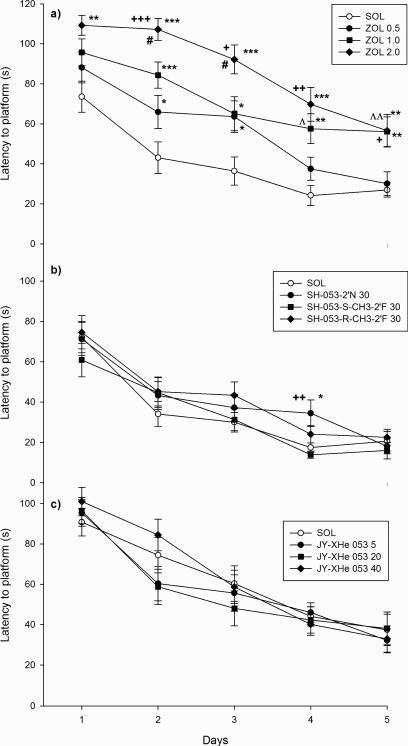
For the dose response study of zolpidem, the factors, treatment and days, but not the interaction treatment × days, were statistically highly significant for the latency to find the platform (treatment effect, F(3,124) = 21.01, p< 0.001; day effect, F(4,496) = 42.87, p< 0.001; and treatment × day interaction, F(12,496) = 1.31, p = 0.209) (shown in Fig. 6a), the distance swum before finding the platform (treatment effect, F(3,124) = 4.71, p = 0.004; day effect, F(4,496) = 32.48, p< 0.001; and treatment × day interaction, F(12,496) = 1.21, p = 0.270), average swim speed (treatment effect, F(3,124) = 18.08, p< 0.001; day effect, F(4,496) = 10.95, p< 0.001; and treatment × day interaction, F(12,496) = 1.57, p = 0.097) and path efficiency (treatment effect, F(3,124) = 7.75, p< 0.001; day effect, F(4,496) = 23.68, p< 0.001; and treatment × day interaction, F(12,496) = 1.68, p = 0.067). It is worth noting that maximum speed was also significantly different among treatments, but in such a counterintuitive way that zolpidem-treated animals tended to swim the faster (in the maximum) the higher the dose of zolpidem was applied (data not shown). Significant differences among treatments during training days, obtained by oneway ANOVA applied to that day's latency to find platform, are presented in Fig. 6a. The results of the post hoc analysis of overall influences for the factor treatment are summarized in Table 2. The analysis showed that the lowest tested dose of zolpidem, 0.5 mg/kg, was different related to control, but also that rats treated with higher doses of the drug (1 and 2 mg/kg) were behaviorally impaired related to 0.5 mg/kg zolpidem.
Fig. 6.
The effects of a) zolpidem (ZOL 0.5, 1 and 2 mg/kg), b) SH-053-2’N, SH-053-S-CH3-2’F and SH-053-R-CH3-2’F (all dosed at 30 mg/kg) and c) JY-XHe-053 (5; 20 and 40 mg/kg), on latency to platform. *,** and ***, P<0.05; 0.01 and 0.001, respectively, compared to solvent (SOL) group in each of three experiments. In graph a): +, ++, and +++, P<0.05; 0.01 and 0.001, respectively compared to ZOL 0.5 group; #, P<0.05 compared to ZOL 1.0 group; ^ and ^^, P<0.05 and 0.01, ZOL 1 group compared to ZOL 0.5 group. In graph b): ++, P<0.01 compared to SH-053-S-CH3-2’F 30group. Number of animals per each treatment was 8 in a) and b), and 7 in c).
Table 2.
Significant differences among overall influences (averaged for five days of acquisition) on the water maze learning parameters: latency to find the platform (L), distance swam before finding the platform (D), mean swim speed (S) and path efficiency (E) in the dose-response study of zolpidem (ZOL, mg/kg).
| ZOL 0.5 | ZOL 1 | ZOL 2 | |
|---|---|---|---|
| Solvent | L: p=0.009 S: p=0.009 E: p=0.010 |
L: p<0.001 D: p=0.010 S: p<0.001 E: p<0.001 |
L: p<0.001 D: p=0.004 S: p<0.001 E: p<0.001 |
| ZOL 0.5 | - | L: p=0.018 S: p=0.011 |
L: p<0.001 S: p<0.001 |
| ZOL 1 | - | - | L: p=0.014 |
The results of the probe trial showed an unexpected inconsistency in the data from the control group. Hence, it was decided to perform statistical analysis with zolpidem on its own (Table 3). The incapacitating influences of the previous treatment with zolpidem were discernible during the probe trial as well, when a number of indices of memory were dose-dependently adversely affected (number of entries in platform zone close to significantly, time in the target region significantly). Concomitantly, a significant increase in the peripheral ring distance, i.e. pronounced thygmotaxis, has shown that zolpidem impaired learning the required water maze skills and strategies.
Table 3.
The representative parameters of water maze performance in the probe trial of the Zolpidem (ZOL) dose-response experiment. The key to regions used in the analysis is given in Fig. 1.
| ZOL 0.5 | ZOL 1.0 | ZOL 2.0 | ANOVA, F(2,21) | P | |
|---|---|---|---|---|---|
| Whole water maze parameters | |||||
| Distance (m±SEM) | 10.93±0.97 | 11.50±0.96 | 12.75±0.56 | 1.187 | 0.325 |
| Platform quadrant (NE) parameters | |||||
| Distance (m±SEM) | 2.41±0.29 | 2.18±0.51 | 2.22±0.44 | 0.083 | 0.920 |
| Time (s±SEM) | 11.86±1.44 | 9.64±1.92 | 9.75±2.06 | 0.471 | 0.631 |
| Peripheral ring parameters | |||||
| Distance (m±SEM) | 4.16±0.61 | 6.09±0.89 | 7.85±0.82* | 5.564 | 0.012 |
| Time (s±SEM) | 33.48±3.68 | 39.86±1.67 | 42.80±3.35 | 2.473 | 0.109 |
| Platform ring parameters | |||||
| Distance (m±SEM) | 5.27±0.68 | 4.48±0.31 | 4.00±0.65 | 1.247 | 0.308 |
| Time (s±SEM) | 21.46±3.05 | 17.31±1.38 | 14.50±2.60 | 2.049 | 0.154 |
| Target region parameters | |||||
| Distance (m±SEM) | 1.51±0.27 | 1.12±0.14 | 0.99±0.22 | 1.520 | 0.242 |
| Time (s±SEM) | 7.26±1.36 | 4.03±0.52* | 3.51±0.76* | 4.596 | 0.022 |
| Platform parameters | |||||
| Number of entries (±SEM) | 1.25±0.41 | 0.38±0.18 | 0.50±0.19 | 2.813 | 0.083 |
| Distance (m±SEM) | 0.097±0.031 | 0.047±0.028 | 0.053±0.023 | 0.945 | 0.405 |
P<0.05 compared to ZOL 0.5 group.
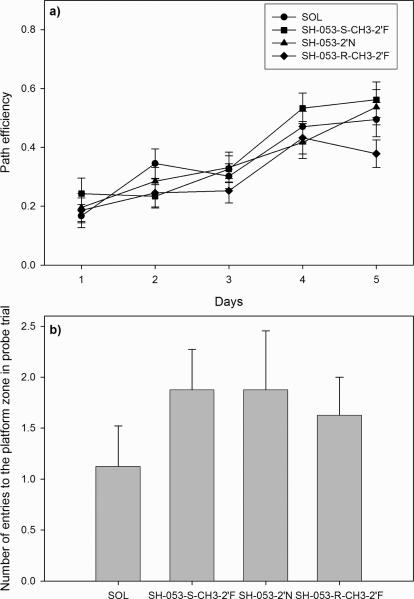
In regard to the two other experiments in the water maze, the results were generally dissimilar in relation to those obtained with zolpidem. In the experiment with SH-053-S-CH3-2’F, SH-053-RCH3-2’F and SH-053-2’N, all dosed at 30 mg/kg, only the factor days, but not the treatment or the interaction treatment × days, was statistically significant for the latency to find the platform (treatment effect, F(3,124) = 1.40, p = 0.248; day effect, F(4,496) = 54.45, p< 0.001; and treatment × day interaction, F(12,496) = 0.84, p = 0.611), the distance swum before finding the platform (treatment effect, F(3,124) = 0.95, p = 0.419; day effect, F(4,496) = 41.92, p< 0.001; and treatment × day interaction, F(12,496) = 0.44, p = 0.947), swim speed (treatment effect, F(3,124) = 2.24, p = 0.087; day effect, F(4,496) = 9.13, p< 0.001; and treatment × day interaction, F(12,496) = 0.96, p = 0.489) and path efficiency (treatment effect, F(3,124) = 0.32, p = 0.812; day effect, F(4,496) = 7.90, p< 0.001; and treatment × day interaction, F(12,496) = 1.31, p = 0.207). Similar results were obtained for the latency to find the platform (treatment effect, F(3,108) = 0.45, p = 0.718; day effect, F(4,432) = 90.88, p< 0.001; and treatment × day interaction, F(12,432) = 1.03, p = 0.417), the distance swum before finding the platform (treatment effect, F(3,108) = 0.32, p = 0.812; day effect, F(4,432) = 56.12, p< 0.001; and treatment × day interaction, F(12,432) = 1.64, p = 0.079), swim speed (treatment effect, F(3,108) = 1.13, p = 0.340; day effect, F(4,432) = 7.50, p< 0.001; and treatment × day interaction, F(12,432) = 1.11, p = 0.347) and path efficiency (treatment effect, F(3,108) = 0.90, p = 0.445; day effect, F(4,432) = 28.58, p< 0.001; and treatment × day interaction, F(12,432) = 1.44, p = 0.144) of rats treated with JY-XHe-053 in the 5 to 40 mg/kg dose range. The graphs representative of the latter two experiments, with latency to find the platform, are given in Fig. 6b and 6c, respectively. The one-way ANOVA applied to latency to find the platform showed a significantly longer search time on the fourth day in rats treated with 30 mg/kg SH-053-2’N in comparison with control animals or those treated with 30 mg/kg SH-053-S-CH3-2’F (Fig 6b). However, this result should be seen as an isolated case, since other learning parameters, such as path efficiency presented in Fig. 7a, were not affected by SH-053-2’N on either of the learning days. Furthermore, probe trial parameters were close to control throughout the water maze experiments performed with the newly-synthesized ligands. The lack of incapacitating consequences of the previous five-day treatment with SH-053-S-CH3-2’F, SH-053-R-CH3-2’F and SH-053-2’N is illustrated in Fig. 7b, with the parameter of platform zone entries during the probe trial presented.
Fig. 7.
The effects of SH-053-2’N, SH-053-S-CH3-2’F and SH-053-R-CH3-2’F, all dosed at 30 mg/kg, on a) path efficiency during 5-day acquisition sessions and b) number of entries to the zone of previous days’ position of platform in probe trial.
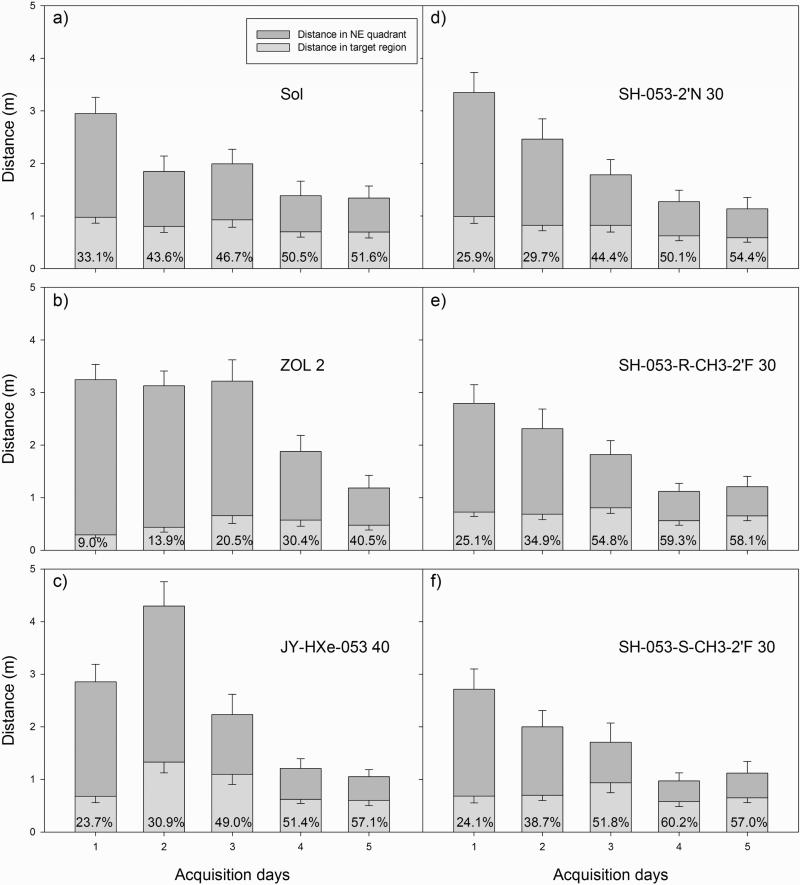
In Fig. 8, the distances the rats swam in the platform quadrant (NE) during acquisition trials are presented alongside the respective distance in the portion of NE quadrant lying in the platform annulus of the maze (“the target region”), for the chosen treatments from three experiments. Providing the rats have no preference for any part of a quadrant, the chance percent of distance swam in the target region would be 40%. The influence of four novel ligands tested at relatively high doses on this parameter was comparable to control values. On the contrary, the rats treated with 2 mg/kg zolpidem strikingly lacked the preferential activity in that part of the NE quadrant in which finding the platform was possible; even on day 5, only 40.5% of the overall distance they travelled in the NE quadrant was in the target region.
Fig. 8.
The effects of b) zolpidem (ZOL 2), c) JY-XHe-053 30, d) SH-053-2’N 30, e) SH-053-RCH3-2’F and f) SH-053-S-CH3-2’F 30 (all doses in mg/kg) on the distance rats travelled in the NE quadrant and target region during 5-day acquisition trials in the water maze. The numbers inside the columns are the percent of the distance swam inside the target (NE) quadrant which was travelled in the target region. Control values (SOL) given in a) are taken from the experiment with ZOL. Numbers of animals are as in Figure 6.
4. DISCUSSION
A substantial body of evidence from both, genetic (reviewed in Rudolph and Möhler, 2006) and pharmacological studies (e.g. Savić et al., 2009), supports the search for a new generation of positive allosteric modulators at GABAA receptors, which would mostly be devoid of the adverse effects of current BZ drugs. Most notably, GABAA receptors which contain the α1 subunit are believed to be responsible for most of the untoward effects of BZs, and silencing the activity at this subunit has appeared as the most attractive approach (Whiting, 2006). The present experiments focused on the recently-synthesized ligands: SH-053-2’N, SH-053-S-CH3-2’F, SH-053-R-CH3-2’F and JY-XHe-053, which as a common trait have moderately low to low affinity and mild to partial agonistic efficacy at α1-containing GABAA receptors. Positive efficacy is more pronounced at the other BZ-sensitive binding sites in comparison to the α1-subtype. Putting aside for the moment the subtle differences in the in vitro and in vivo pharmacology of novel ligands, it was observed that even with ligands that demonstrated efficacy “lower at the α1 subtypes than at the rest of the subunits”, the sedative-like potential of such GABAA modulation was not fully eliminated, while the spatial learning and memory impairments, assessed in the water maze and usually seen with BZ site agonists, were completely circumvented; the latter finding represents the single most important result from the present study.
The decreased locomotion induced by all four novel ligands (the effect of 30 mg/kg SH-053-SCH3-2’F was presented in Savić et al. 2008b) which were tested may be rationalized in two ways. Firstly, although the lack of occupancy (in vivo potency) data for distinct receptor subtypes precludes drawing firm conclusions about the actual activity at α1-containing GABAA receptors, which are predominantly involved in sedative actions of BZs according to genetic (Rudolph et al., 1999) and pharmacological antagonism studies (Savić et al., 2009), it cannot be excluded that, even at presumably low fractional occupancy, moderately low to moderate positive modulation (partial agonism) at these α1-receptors may be sufficient for eliciting weak sedation (cf. Grimwood and Hartig, 2009). Secondly, growing neuroanatomical evidence points to the possible role of α5-containing GABAA receptors in motor control (Bohlhalter et al., 1996; Pirker et al., 2000; Yamada et al., 2007), and we recently suggested that any locomotor activity changes induced by ligands possessing a substantial α5-agonist efficacy may be contributed by modulation at GABAA receptors containing this subunit (Savić et al., 2008b). GABAA receptors containing the α5-subunit are functionally interrelated in some manner with those containing the α1-subunit. Importantly, knock-in mice harboring the α5 subunit insensitive to diazepam are refractory to development of tolerance to the sedative effects of diazepam dosed subchronically (van Rijnsoever et al., 2004). Moreover, the α5-selective antagonist XLi093, at a dose presumably causing a complete antagonism of the effects of diazepam at α5-containing GABAA receptors, has potentiated sedation induced by diazepam (Savić et al., 2009). Hence, we further hypothesized that positive modulation at α5 GABAA receptors may exert a dual action: to limit sedative effects elicited by supra-physiological stimulation of α1-containing receptors (e.g. by full agonists), and, oppositely, to enhance low activation of α1 GABAA receptors (e.g. by partial agonists), and, hence, induce mild sedation (Savić et al., 2009). Such a modulation might be achieved if neurons involved in sedative actions are modulated strongly by α1 as well as more weakly by α5 receptors, and if the synapse or neuron mediating the α1 modulation is at the same time weakly regulated by α5 receptors. Then low activity at α1 receptors can overall be enhanced by a direct α5 receptor activity on the same neuron, but strong α5 activity would downregulate the strong sedative action of α1 activity via its indirect effect on the synapse or neuron mediating the α1 modulation. Such a scenario could, at least partly, explain the unexpected finding that the mice lacking the α1-containing GABAA receptors were more sensitive to hypolocomotion and loss of righting reflex induced by diazepam, compared to wild-type controls (Kralic et al., 2002).
The differences in the capability to induce anxiolytic effects, observed with the ligands evaluated here, are not easily reconciled. Genetic studies have indicated the α2 subunit was predominantly involved in the anxiolytic-like effects of positive modulation at GABAA receptors (Low et al., 2000), while results with some novel selective ligands pointed to the role of the α3 subunit, as well (Dias et al., 2005). The ligand SH-053-R-CH3-2’F was devoid of anxiolytic potential, in line with results of a congener with a similar profile of α5 GABAA functional selectivity, SH-053-RCH3 (Savić et al., 2008b). However, JY-HXe-053, a ligand with relatively high affinity and efficacy at α2 and α3-containing GABAA receptors, was devoid of discernible anxiolytic activity, too. When comparing this result with the anxiolytic-like properties of SH-053-2’N and SH-053-S-CH3-2’F (the latter is published in Savić et al., 2008b), it was found that JY-HXe-053 has a greater affinity at α1-, and especially α5-containing GABAA receptors, with substantial potentiation at these receptors achieved already at concentration of 100 nM (169% and 220%, respectively). Since JY-HXe-053 has induced a hypolocomotor effect in the SLA at doses (10 mg/kg and above) lower than those observed with SH-053-2’N or SH-053-S-CH3-2’F (30 mg/kg), it is possible that its activity at α1 and, in parallel, α5 receptors may have masked the expected anxiolytic-like effects mediated by the other two, α2 and α3-containing, subtypes of GABAA receptors. When one compares the in vitro profile of JY-XHe-053 with that of diazepam, it is clear that JY-XHe-053, but not diazepam, exhibits a preferential affinity for α5-GABAA receptors. The consequent ‘priority in activity’ at α5-GABAA receptors may have endowed this population of receptors with a relatively more distinct role in influencing the overall behavior of rats treated with JY-XHe-053, rather than with diazepam. Finally, it is notable that the hypolocomotor influence of JY-XHe-053 in the SLA test was evident only when analyzing activity in the peripheral, but not in the central zone of the chamber, and could be seen as a pure measure of sedation (cf. Savić et al., 2006; 2008b). On the contrary, the hypolocomotion induced by SH-053-R-CH3-2’F was due to the inactivity in the central, but not the peripheral zone, which suggests that the sedation-like actions exerted through α1 and α5 GABAA receptors are qualitatively different.
We have previously shown that, when observable, the anxiolytic-like effects of zolpidem, the preferential α1-subunit selective agonist with intermediate activity at α2 and α3-containing GABAA receptors, are statistically dependent on the concomitant decrease of closed arm entries in the elevated plus maze, and hence confounded by general activity changes (Savić et al., 2004). Analysis of covariance for the current results with SH-053-2’N did not reveal analogous dependence of its anxiolytic-like effects on the concomitant signs of sedation. Nevertheless, it is possible the clinical separation of wanted anxiolytic from unwanted sedative effects would be lower for SH-053-2’N than for SH-053-S-CH3-2’F, having in mind that the latter ligand was devoid of influence on the parameters related to general activity in the elevated plus maze (cf. Savić et al., 2008b).
The effects of zolpidem on the acquisition and retention of place learning in the water maze have not been previously assessed. Zolpidem dose-dependently impaired the declarative component of both, learning the task, as assessed by path efficiency, the latency and distance before finding the platform across acquisition trials, and recalling the previous platform position, as assessed by time in the target region during the probe trial. It also induced a decrease in average swim speed, accompanied by increase in maximum swim speed, which may be interpreted as ‘bursts’ of behavioral disinhibition (activation) interspersed among background sedative effect. By use of the treatment switch, McNamara and Skelton (1991) have shown that the reduced swim speed cannot account for deficits in platform localization induced by BZs. Namely, the rats acquiring the task under saline treatment continued to perform normally despite the switch to diazepam, which nonetheless elicited a decrease in swim speed (McNamara and Skelton, 1991). Hence, the data suggest that neither non-specific performance impairment, nor any sensorimotor deficit (according to our preliminary findings with zolpidem in the task of finding the visible platform) was responsible for the observed acquisition deficit (cf. McNamara and Skelton, 1991).
Zolpidem also negatively affected the procedural component of the water maze task. Namely, suppression of an instinct to swim thigmotaxically appears to be necessary to effectively accomplish this task (Cain, 1998), and zolpidem at 2 mg/kg induced an increase of peripheral annulus, and a decrease of platform annulus parameters throughout the test. We have recently shown that both, the α1-subunit affinity-selective antagonist ß-CCt, and the α5-subunit affinity- and efficacy-selective antagonist XLi093, may, at least partially, prevent diazepam-induced impairment in the declarative and procedural component of the water maze task; however, the antagonistic effect of ß-CCt was manifestly more profound (Savić et al., 2009). The present results show, first, that the effects of zolpidem, a ligand essentially inactive at α5 GABAA receptors, mirror those induced by diazepam, and secondly, that three novel ligands, with moderately low to low activity at α1 GABAA receptors, do not disturb water maze behavior. The conclusion is straightforward: a substantial amount of activity at α1 GABAA receptors is needed if spatial learning and memory incapacitation is to be induced by positive allosteric modulation at BZ site. The positive modulation at α5 GABAA receptors may add to the incapacitating action effected through the α1 subunit, but is neither necessary (cf. zolpidem), nor sufficient (cf. SH-053-R-CH3-2’F) for spatial cognition impairment in the water maze.
The present data and the above conclusions may thus significantly change the common knowledge on the function of individual receptor subtypes (Rudolph and Möhler, 2006) and reflect a more realistic view that more than one GABAA receptor subtype is modulating the same behavioral parameter. We suggest a tentative hypothesis based on the existence of the fine equilibrium between the α1- and α5- GABAA receptors on the one hand, and α2- and α3- GABAA receptors on the other side, in terms of the influence of positive allosteric modulation on the crucial behavioral endpoints of sedation, anxiolysis and amnesia. A substantial amount of positive modulation is needed by the α1, coordinated in some manner with α5- GABAA receptors, if sedation and amnesia are to be elicited, while for the anxiolytic-like actions the α2 and/or α3 GABAA receptors must be activated. The other pair of receptors, not primarily involved in mediation of the respective behavioral effect, may counteract, mask or in another way hinder the action of the responsible pair.
CONCLUSIONS
Between the novel positive allosteric modulators presented in this and the previous paper (Savić et al., 2008b), it appears that SH-053-S-CH3-2’F may possess a pharmacological profile appropriate for further fine tuning, by diminishing the potential of sedation, probably through an additional decrease of activity at α1- and α5- GABAA receptors. A future, more elaborate analysis of the substrate of behavioral effects of BZ site modulators shall take into account target site occupancy, quantitative neuroanatomical distribution of distinct receptor subtypes, differences among animal species, receptor reserve (if any) of the behavioral effect under investigation (cf. Grimwood and Hartig, 2009) as well as different settings and methods of behavioral assessment.
ACKNOWLEDGEMENTS
This work was supported in part by NIMH 46851 (JMC) and by The Ministry of Science, R. Serbia – Grant No. 145022B (MMS).
We acknowledge the support of this work by the Research Growth Initiative of the University of Wisconsin-Milwaukee and the Lynde and Harry Bradley Foundation.
Abbreviations
- ß-CCt
beta-carboline-3-carboxylate-t-butyl ester
- BZ
benzodiazepine
- cDNA
complementary deoxyribonucleic acid
- cRNA
complementary ribonucleic acid
- E
east
- EC3
molar concentration of an agonist that produces 3% of the maximal possible effect of that agonist
- EPM
Elevated plus maze
- GABAA
gamma-aminobutyric acid type A receptor
- HEK
human embryonic kidney cell
- HEPES
2-[4-(2-hydroxyethyl)-l-piperazinyl]-ethanesulfonic acid
- Ki
equilibrium dissociation constant of a ligand determined in inhibition studies
- MB
Barths’ Medium
- NW
North-west
- S
South
- W
West
- XR
Xenopus Ringer solution
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19:567–96. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Zohar J, Hollander E, Kasper S, Möller HJ. WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disoders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders – first revision. World J Biol Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- Berezhnoy D, Gravielle MC, Downing S, Kostakis E, Basile AS, Skolnick P, et al. Pharmacological Properties of DOV 315,090, an ocinaplon metabolite. BMC Pharmacol. 2008;8:11. doi: 10.1186/1471-2210-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Möhler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–97. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DP. Testing the NMDA, long-term potentiation, and cholinergic hypotheses of spatial learning. Neurosci Biobehav Rev. 1998;22:181–93. doi: 10.1016/s0149-7634(97)00005-5. [DOI] [PubMed] [Google Scholar]
- Choudhary MS, Craigo S, Roth BL. Identification of receptor domains that modify ligand binding to 5-hydroxytryptamine2 and 5-hydroxytryptamine1c serotonin receptors. Mol Pharmacol. 1992;42:627–33. [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoemaker RC, Rijnbeek B, et al. Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J Psychopharmacol. 2007;21:374–83. doi: 10.1177/0269881106072343. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, et al. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–8. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs K, Zezula J, Slany A, Sieghart W. Endogenous [3H]flunitrazepam binding in human embryonic kidney cell line 293. Eur J Pharmacol. 1995;289:87–95. doi: 10.1016/0922-4106(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Garner M, Möhler H, Stein DJ, Mueggler T, Baldwin DS. Research in anxiety disorders: From the bench to the bedside. Eur Neuropsychopharmacol. 2009;19:381–90. doi: 10.1016/j.euroneuro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Hartig PR. Target site occupancy: Emerging generalizations from clinical and preclinical studies. Pharmacol Ther. 2009;122:281–301. doi: 10.1016/j.pharmthera.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–5. [PubMed] [Google Scholar]
- Kralic JE, O'Buckley TK, Khisti RT, Hodge CW, Homanics GE, Morrow AL. GABA(A) receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology. 2002;43:685–94. doi: 10.1016/s0028-3908(02)00174-0. [DOI] [PubMed] [Google Scholar]
- Lippa A, Czobor P, Stark J, Beer B, Kostakis E, Gravielle M, et al. Selective anxiolysis produced by ocinaplon, a GABA(A) receptor modulator. Proc Natl Acad Sci U S A. 2005;102:7380–5. doi: 10.1073/pnas.0502579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Diazepam impairs acquisition but not performance in the Morris water maze. Pharmacol Biochem Behav. 1991;38:651–8. doi: 10.1016/0091-3057(91)90028-z. [DOI] [PubMed] [Google Scholar]
- Minier F, Sigel E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:7769–74. doi: 10.1073/pnas.0400220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, et al. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–68. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- Pike A, Cook SM, Watt AP, Scott-Stevens P, Rosahl TW, McKernan RM, et al. Contribution of specific binding to the central benzodiazepine site to the brain concentrations of two novel benzodiazepine site ligands. Biopharm Drug Dispos. 2007;28:275–82. doi: 10.1002/bdd.553. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, et al. The anxioselective agent 7-(2-chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha1 subunit-containing GABAA receptors. J Pharmacol Exp Ther. 2006;319:1244–52. doi: 10.1124/jpet.106.107201. [DOI] [PubMed] [Google Scholar]
- Rivas FM, Stables JP, Murphree L, Edwankar RV, Edwankar CR, Huang S, et al. Antiseizure activity of novel gamma-aminobutyric acid (A) receptor subtype-selective benzodiazepine analogues in mice and rat models. J Med Chem. 2009;52:1795–8. doi: 10.1021/jm801652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–10. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugrešić ND, Cook JM, Yin W, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacol Biochem Behav. 2004;79:279–90. doi: 10.1016/j.pbb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugrešić ND, Cook JM, Yin W, Van Linn M, et al. Benzodiazepine site inverse agonists and locomotor activity in rats: bimodal and biphasic influence. Pharmacol Biochem Behav. 2006;84:35–42. doi: 10.1016/j.pbb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardžić J, Savić S, et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;a1208:150–9. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Huang S, Furtmüller R, Clayton T, Huck S, Obradović DI, et al. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008b;33:332–9. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- Savić MM, Milinković MM, Rallapalli S, Clayton T, Joksimović S, Van Linn M, et al. The differential role of alpha1- and alpha5-containing GABAA receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. Int J Neuropsychopharmacol. 2009;12:1179–93. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Ernst M. Heterogeneity of GABAA receptors: revived interest in the development of subtype-selective drugs. Curr Med Chem Cent Nerv Syst Agents. 2005;5:217–42. [Google Scholar]
- Sigel E. Properties of single sodium channels translated by Xenopus oocytes after injection with messenger ribonucleic acid. J Physiol. 1987;386:73–90. doi: 10.1113/jphysiol.1987.sp016523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Möhler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–11. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Terry AV., Jr . Spatial navigation (water maze) tasks. In: Buccafusco JJ, editor. Behavioral Methods in Neuroscience. CRC Press; Boca Raton: 2000. pp. 153–66. [PubMed] [Google Scholar]
- van Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Möhler H, et al. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785–90. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shen X, Fenyk-Melody J, Pivnichny JV, Tong X. Simple and sensitive liquid chromatography/tandem mass spectrometry method for the determination of diazepam and its major metabolites in rat cerebrospinal fluid. Rapid Commun Mass Spectrom. 2003;17:519–25. doi: 10.1002/rcm.942. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol. 2006;6:24–9. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Lüddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–9. [PubMed] [Google Scholar]
- Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex. 2007;17:1782–7. doi: 10.1093/cercor/bhl087. [DOI] [PubMed] [Google Scholar]