Summary
Autosomal dominant Progressive External Ophthalmoplegias are Mendelian disorders characterized by the accumulation of multiple deletions of mitochondrial DNA in critical tissues. Most of the Autosomal dominant Progressive External Ophthalmoplegias families carry heterozygous mutations in one of three genes: ANT1, encoding the muscle-heart specific mitochondrial adenine nucleotide translocator, Twinkle, encoding the mitochondrial DNA helicase, and POLG1, encoding the catalytic subunit of the mitochondrial DNA-specific polymerase. Mutations in both POLG1 alleles are also found in autosomal recessive Progressive External Ophthalmoplegias sibships with multiple affected members and in apparently sporadic cases. In addition, recessive POLG1 mutations are responsible for three additional diseases: Alpers-Huttenlocher hepatopathic poliodystrophy, Sensory-Ataxic Neuropathy Dysarthria and Ophthalmoplegia and juvenile SpinoCerebellar Ataxia-Epilepsy syndrome. Mitochondrial neuro-gastro-intestinal encephalomyopathy is an autosomal recessive disorder of juvenile onset, caused by mutations in the gene encoding Thymidine Phosphorylase. Thymidine Phosphorylase is involved in the control and maintenance of the pyrimidine nucleoside pool of the cell. Finally, mitochondrial DNA depletion syndrome is a heterogeneous group of disorders characterized by a reduction in mitochondrial DNA copy number. Clinically, they include a myopathic form, a more generalized encephalomyopathic form and a fatal infantile hepato-cerebral syndrome leading to rapidly progressive liver and brain failure. To date, eight genes have been associated with mitochondrial DNA depletion syndrome. Novel disease genes have recently been added to this list, including OPA1 and GFER, and new clinical variants add further complexity to this expanding area of mitochondrial medicine.
Keywords: Mitochondrial DNA, oxidative phosphorylation, mitochondrial disorders, MtDNA multiple deletions, MtDNA depletion
Introduction
The main role of mitochondria is to synthesize adenosine triphosphate (ATP), the universal energy “ currency” of the cell, through an intricate process involving cellular uptake and transport of substrates, metabolic conversion and regulatory control systems, many of which act in a cell, tissue, or system-dependent manner. The final biochemical pathway in ATP synthesis is oxidative phosphorylation (OXPHOS), carried out by the mitochondrial respiratory chain (RC). The RC is composed of 5 complexes encoded by both nuclear genome and mitochondrial genome (mitochondrial DNA – mtDNA). The mtDNA is found in high copy number in all cell-types. In humans, 13 of the approximately 85 proteins composing the five OXPHOS complexes are encoded by the 16.6 kb mtDNA. Of the mtDNA-encoded polypeptides, 7 are subunits of complex I, 3 are subunits of complex IV, 2 are subunits of the F0 domain of cV, and one, cytochrome b, is part of complex III. In addition, mtDNA encodes 2 rRNAs and 22 tRNAs that contribute to the autonomous translational apparatus of mitochondria, dedicated to the synthesis of the 13 mtDNA-encoded polypeptides. All other structural subunits, including all four components constituting complex II, assembly factors of the OXPHOS complexes, as well as all the proteins involved in replication, transcription and translation of mtDNA, are nuclear-coded and targeted either co- or post-translationally to mitochondria, through the intermembrane space.
A clinical-genetic classification of mitochondrial disorders due to mutations in nuclear genes includes: (i) disorders due to mutations in respiratory chain subunits; (ii) disorders due to mutations in ancillary proteins; (iii) disorders due to faulty intergenomic communication affecting the maintenance and expression of mtDNA; (iv) disorders due to defects in non-protein components of the respiratory chain; (v) disorders due to gene defects encoding proteins indirectly related to OXPHOS.
We shall focus on the Mendelian mitochondriopathies due to defects of intergenomic communication disorders affecting the maintenance and expression of mtDNA.
Mitochondrial mtDNA maintenance is a complex process. In the yeast Saccharomyces cerevisiae, over a hundred genes have been identified the deletion of which causes loss or instability of mtDNA (1). However, both factors and mechanisms regulating mtDNA stability are only beginning to be decoded in mammals. Two models of mtDNA replication have been proposed. According to the orthodox ‘strand displacement’ or ‘asynchronous’ model, there are two spatially and temporally separated origins of replication, one for each mtDNA strand. The process of mtDNA replication begins at the heavy strand origin (OH), located in the mtDNA non-coding region (displacement loop, D-loop), and proceeds in an anticlockwise direction for 2/3 of the mtDNA circle, where the origin of light-strand replication (OL) is exposed. This allows light-strand synthesis to proceed in the clockwise direction until the entire molecule is copied (2). A few years ago, Holt et al. (3) proposed a ‘strand-synchronous’ model of mtDNA replication. According to this model, replication is initiated from multiple origins, distributed across a 4 KB fragment 3’ from the noncoding D-loop and proceeds in both directions in replication ‘bubbles’.
Irrespective of the model, mtDNA replication requires a number of nuclear-encoded proteins, few of which have been identified in humans, including a DNA polymerase activity provided by pol-γ, the only DNA polymerase found in mitochondria, a mitochondrial single-strand binding-protein (mtSSB), a DNA-RNA primase/helicase, denominated Twinkle, a DNA ligase and several topoisomerases. Replication-initiation is provided by RNA primers that couple replication with transcription, a complex process which is, in turn, carried out by a set of factors that include the mtDNA-specific RNA polymerase, and several transcription factors and regulators: TFAM TFB1M, TFB2M, mTERF, etc. The supply of deoxy-nucleotides and ribonucleotides, the building blocks of mtDNA replication and transcription, is essential for both processes and is carried out by complex metabolic pathways partly distinct for purines and pyrimidines. Finally, a number of proteins, including some of those mentioned above, are involved in the formation of the mitochondrial nucleoid, a dynamic structure containing one or more copies of mtDNA, which represents the fundamental segregation unit of mtDNA inheritance.
In principle, any defective protein involved in mtDNA replication, maintenance, and integrity could precipitate loss or instability of mtDNA, causing either qualitative (multiple deletions) or quantitative (depletions) mtDNA molecular lesions. So far, only a few such proteins have been identified as responsible for human mitochondrial disease.
Clinical manifestations
The most frequent clinical presentations, as shown in Table 1, are:
Table 1. Defects of intergenomic communication: clinical and genetic classification. ANT, adenine nucleotide translocators; CK, creatine kinase; PEO, progressive external ophthalmoplegia; AD, autosomal dominant; AR, autosomal recessive; ADOA: autosomal dominat optic atrophy, SCAE: spinocerebellar ataxia and epilepsy; SANDO, sensory- ataxic neuropathy, dysarthria and ophtalmoparesis, mtDNA, mitochondrial DNA; IOSCA infantile onset spino-cerebellar ataxia TYMP, thymidine phosphorylase; TK, thymidine kinase; DGUOK, deoxyguanosine kinase; SMA, spinal muscular atrophy; OPA1, optic atrophy, RRF:ragged red fibres, COX cytochrome c oxidase.
| mtDNA multiple deletions | mtDNA multiple deletions/mtDNA depletion | mtDNA depletion | ||||||||||
| Genes | ANT1 | OPA1 | GEFER | POLG1 | TYMP | TWINKLE | DGUOK | MPV17 | RRM2B | SUCLA2 | TK2 | SUCLG1 |
| Inheritans | AD, AR | AD | AR | AD/AR | AR | AD/AR | AR | AR | AR | AR | AR | AR |
| Gene product | Muscle-heart-specific mitochondrial adenine nucleotide translocator | Dynamin-like GTPase | sulfhydryl oxidases | Catalytic subunit polymerase gamma | thymidine phosphorylase | mtDNA helicase | deoxyguanosine kinase | Unknown function | p53-inducible ribonucleotide reductase | B subunit of succinyl–coenzyme A lyase | Thymidine kinase | β-subunit of ADP forming succinyl–coenzyme A lyase |
| Clinical features | CPEO, myopathy and exercise intollerance hypertrophic cardiomyopathy | ADOA,PEO, peripheral neuropathy | Cataract , mental retardation,myopathy | Hepatic failure, PEO, Alpers syndrome, SCAE, SANDO | MNGIE | CPEO, IOSCA, Hepatic failure | Hepatic failure hyoglicemia, hypotonia, Nystagmus, dystonic movement. | Hepatic failure, hypoglicemia, hypotonia, Nystagmus,dystonic movements. Corneal scarring, mental retardation,scoliosis | Microcephalia and global development delay, hearing loss. Trunk hypotonia. Tubulopathy and nefrocalcinosis | Hepatomegalia, hyopotonia, lactic acidosis Dysmorphismsmethylmalonicaciduria Leigh like syndrome | Myopathy, SMA1,SMA3 like syndrome muscular dystrophy | Hyopotonia, lactic acidosis Dysmorphisms Deafness and methylmalonic aciduria |
| Muscle morphology | Scattered RRF, COX negative fibres | RRF, COX negative fibres | Scattered COX negative fibres | RRF, COX negative fibres | Neurogenic changes, sometimes RRFs, COX negative fibres | RRF, COX negative fibres | RRF, scattered COX negative fibres/Diffuse and severe COX deficiency/Normal | - | RRF, COX negative fibres, lipid storage | - | Neurogenic signs, diffuse COX deficiency +/- RRF | |
an adult-onset encephalomyopathy, defined clinically by Progressive External Ophthalmoplegias (PEO), genetically by the autosomal dominant (ad) or recessive (ar) transmission of the trait, and molecularly by the presence of multiple deletions of mtDNA;
an autosomal recessive multisystem disorder known as mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), characterized by combined accumulation of multiple deletions and partial depletion of mtDNA;
a spectrum of recessive neurological syndromes ranging from typical infantile hepatopathic poliodystrophy (Alpers-Huttenlocher syndrome) to juvenile onset sensory-ataxia neuropathy, dysarthria and ophthalmoplegia (SANDO) to a combination of spino-cerebellar ataxia and epilepsy (SCAE) with or without external ophthalmoplegia;
early-onset, organ-specific autosomal recessive syndromes associated with profound mtDNA depletion.
Adult onset encephalomyopathy, Progressive External Ophthalmoplegias
Autosomal dominant progressive external ophthalmoplegia (adPEO) is characterized by the accumulation of multiple mtDNA deletions in post-mitotic tissues. The typical clinical feature of adPEO is progressive muscle weakness, most severely affecting extra-ocular muscles, determining the progression of bilateral eyelid ptosis and severe limitation of eye movements. The disease has adult-onset between 20 and 40 years of age. Additional features vary among families; they may include ataxia, sensorineural hearing loss, cataracts, hypogonadism, parkinsonism, and psychiatric abnormalities consisting of severe depression and avoidant personality. Dysphagia, dysphonia, weakness of facial muscles, and peripheral neuropathy may be prominent symptoms in selected families (4, 5). At rest, elevated levels of plasma lactate are detected only in severely affected patients. Symptoms seem to progress with the age of the patients. Skeletal muscle shows Ragged Red Fibres (RRFs) and a mild reduction in the activities of respiratory chain enzymes (5, 6). Recently, autosomal dominant optic atrophy (ADOA) has been reported in association with CPEO and multiple mtDNA deletions; additional signs included deafness, ataxia, axonal sensory-motor polyneuropathy, and mitochondrial myopathy with cytochrome c oxidase negative and RRFs (7, 8).
Since 1989 (9, 10), multiple deletions of mtDNA have also been reported in numerous sporadic PEO cases or in other families in which PEO was clearly transmitted as a recessive trait (up to 11% in our series) (11, 12). Most of these cases are due to mutations in the gene encoding POLG1. Recently, Di Fonzo et al. described three siblings who presented with progressive myopathy, congenital cataract, sensorineural hearing loss, and developmental delay associated with multiple mtDNA deletions (13) and caused by a mutation in the GFER gene.
Mitochondrial neurogastrointestinal encephalomyopathy
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is an autosomal recessive disorder characterized by PEO, peripheral neuropathy, leukoencephalopathy, and severe gastrointestinal dysmotility leading to cachexia and early death. Gastrointestinal manifestations comprise the most prominent and debilitating features and include gastroparesis, frequent diarrhoea, and intestinal pseudo-obstruction (14). The peripheral neuropathy is predominantly demyelinating. Skeletal muscle biopsies reveal neurogenic changes and occasional RRFs and cytochrome c oxidase-deficient fibres. Histological studies have revealed abnormalities of both the intestinal smooth muscle and the enteric nervous system, thus accounting for the severe gastrointestinal problems, probably secondary to the accumulation of mtDNA defect in these cells (15). The disorder affects young adults but later-onset and longer survival MNGIE patients have also been reported (16). The disease is invariably associated with mutations in the gene encoding thymidine phosphorylase.
Alpers-Huttenlocher hepatopathic poliodystrophy; sensory-ataxia neuropathy, dysarthria and ophthalmoplegia and spino-cerebellar ataxia and epilepsy syndromes
Alpers-Huttenlocher syndrome is an autosomal recessive early onset, invariably fatal disease. Within the first few years of life, patients exhibit progressive spastic quadriparesis, progressive cerebral degeneration leading to mental deterioration and seizures, cortical blindness, deafness, liver failure, and eventual death (17). Liver dysfunction is usually progressive, evolving from microvesicular steatosis with bile duct proliferation, into cirrhosis and chronic liver failure. The use of valproic acid as a means of treatment for epilepsy can precipitate acute liver failure. Alpers-Huttenlocher syndrome, and a spectrum of other disorders also including childhood- or juvenile-onset autosomal recessive and progressive sensory-ataxic syndromes (SANDO) with or without epilepsy (SCAE) are due to specific mutations in POLG1 (18). Other recessive phenotypes associated with POLG1 mutations have been described in single cases, including fatal liver failure with peripheral neuropathy, liver failure with myopathy, precocious menopause in females and hypofertility in males, etc. More recently, recessive mutations in the Twinkle gene have been reported in Alpers-like infantile encephalopathy, and IOSCA, or infantile onset spino-cerellar ataxia, a nosological entity which is part of the Finnish disease heritage (19).
MtDNA depletion syndromes
MtDNA depletion syndromes (MDS) are caused by a marked decrease of mtDNA copy number, and are transmitted as phenotypically heterogeneous autosomal recessive traits (20).
Some MDS children present with myopathy, others with liver failure in infancy, and some others with multisystem involvement. Consistent with the different phenotypes, mtDNA depletion may affect either a specific tissue (most commonly muscle or liver) or multiple organs, including heart, brain, and kidney. Three syndromes have been outlined clinically: infantile hepatopathy, infantile myopathy, and infantile encephalomyopathy.
The hepatopathic, or more precisely hepato-cerebral, form is probably the most common MDS. The onset of symptoms is between birth and 6 months; death usually occurs within 1 year of age. The most common symptoms and signs include persistent vomiting, failure to thrive, hypotonia, and hypoglycaemia associated with progressive neurological symptoms. Histological changes on liver biopsy include fatty degeneration, bile duct proliferation, fibrosis, and collapse of lobular architecture. Reduced COX histochemistry and combined deficiency of mtDNA encoded RC complexes are found in the liver. A variant form of hepatocerebral MDS affects the Navajo people, with a prevalence of 1:1600 live births, hence the term Navajo Neuro-Hepatopathy (NNH). The major clinical features are hepatopathy, peripheral neuropathy, corneal anaesthesia and scarring, acral mutilation, cerebral leuko-encephalopathy, failure to thrive, and recurrent metabolic acidosis with intercurrent infections (21).
In the myopathic form, affected children are usually born after an uncomplicated pregnancy, but a few patients have been reported with arthrogryposis and clubfeet. The onset is usually in the first year of life, with feeding difficulty, failure to thrive, hypotonia, weakness, and occasionally PEO. Death is typically due to pulmonary insufficiency and infections, but some patients can survive into their teens (22, 23). The clinical spectrum includes: i) a spinal muscular atrophy type 3; ii) rigid spine syndrome; iii) a severe muscle weakness with marked dystrophic alterations, encephalopathy and seizures (22); iv) a milder myopathic phenotype with no motor regression and longer survival (24). A severe variant form that combines a floppy-infant syndrome with renal proximal tubulopathy and nephrocalcinosis has also been described (25, 26). The muscle biopsy can show RRFs and foamy fibres, which can increase with age, and patchy or diffuse COX deficiency. Biochemical defects of all mtDNA-related respiratory chain complexes are always present in muscle mitochondria. Serum creatine kinase (CK) levels may be variably elevated. This is an important clue for the diagnosis, because increased serum creatine kinase is relatively uncommon in patients with other mitochondrial myopathies. Two encephalomyopathic forms have been described, both caused by a block of Succinyl-CoA lyase, a Krebs-cycle enzyme activity. The first is characterized by high lactate in blood, severe psychomotor retardation with muscle hypotonia, impaired hearing and generalized seizures, followed by knee and hip contractures, finger dystonia and mild ptosis. Brain MRI is suggestive of Leigh syndrome. Moderate mtDNA depletion (about 30%) has been documented in skeletal muscle (27–29). The second, extremely severe form is associated with combined muscle and liver mtDNA depletion, dysmorphic features, connatal lactic acidosis, dystonia, defness and death in the first days of life (28). Both syndromes are hallmarked by moderate-to-severe methylmalonic aciduria.
Aetiology and pathophysiology
Autosomal dominant PEO is a genetically heterogeneous clinical entity. Most of the adPEO families carry heterozygous mutations in one of three genes: ANT1, encoding the muscle-heart-specific mitochondrial adenine nucleotide translocator (30), Twinkle, encoding a mtDNA helicase (31), and POLG1, encoding the catalytic subunit of the mtDNA-specific polymerase gamma (32). A mutation of POLG2, encoding the accessory subunit of polymerase-gamma, has been reported in a single adPEO family. Finally, OPA 1, the major gene involved in autosomal dominant optic atrophy (ADOA), can also cause PEO, in combination with ADOA (8).
ANT1 mutations
The gene responsible for the adPEO form linked to the 4p locus encodes the muscle-heart specific isoform of the mitochondrial adenine nucleotide translocator (30). Dominant missense mutations have been found in families with adPEO and in sporadic patients with mild, slowly progressive myopathy and little or no extra-muscular symptoms. In 2005, Palmieri et al. (33) reported the first recessive mutation in the ANT1 gene in a patient who presented with hypertrophic cardiomyopathy, mild myopathy with exercise intolerance, RRF and lactic acidosis, but no ophthalmoplegia. Southern blot analysis disclosed multiple deletions of muscle mitochondrial DNA and virtually no ATP uptake was measured in proteoliposomes reconstituted with protein extracts from muscle of this patient. ANT1 mutations are responsible for approximately 7% of the adPEO cases in our series. A patient has recently been reported with a clinical presentation characterized initially by PEO with mtDNA multiple deletions, which later evolved into a severe neurological syndrome, including sensory and cerebellar ataxia, peripheral neuropathy, parkinsonism, and depression (34). This complex phenotype is the result of mutations in two distinct proteins, ANT1 and POLG1, which cause additive, deleterious effects on mtDNA maintenance and integrity (34).
Twinkle mutations
PEO1, the gene responsible for an adPEO form linked to chromosome 10q locus, encodes a mtDNA helicase involved in replication termed Twinkle, from the starry appearance of its specific immunofluorescence signals in cell cultures (35). Twinkle mutations cluster in a region presumably involved in protein-to-protein interactions, and are associated with clinical presentations of variable severity, ranging from late-onset “ pure” PEO to PEO “ plus” syndromes, with proximal muscle and facial weakness, dysphagia and dysphonia, mild ataxia, and peripheral neuropathy (personal observation). Symptoms markedly worsen in a few homozygous mutant patients described in consanguineous families. In addition to dominant traits, a specific, recessive twinkle mutation causes Infantile Onset Spino-Cerebellar Ataxia (IOSCA) (35), which is part of the Finnish disease heritage. Onset is usually between 1 and 2 years. Patients suffer from a combination of ataxia, athetosis, muscle hypotonia and severe epilepsy. Other features such as ophthalmoplegia, hearing loss, and optic atrophy appear later in the course of the disease. Some patients show reduced mental capacity and hypergonadotropic hypogonadism may occur in girls. Interestingly, IOSCA brain does not harbour mtDNA deletions or increased amount of mtDNA point mutations. However, IOSCA shows mtDNA depletion in brain and liver; the largest neurons display complex I and IV deficiency (35), but surprisingly helicase activity, homohexamerization and nucleoid structure are unaffected. The latter result suggests that the IOSCA mutation in Twinkle affects mtDNA maintenance in a highly context and cell-type specific manner.
POLG1 mutations
The mitochondrial DNA polymerase (pol-γ) is essential for mitochondrial DNA replication and proofreading-based repair. It is composed of a 140-kDa catalytic subunit (pol γA) and a 55-kDa accessory subunit (pol γB), which functions as a DNA binding factor increasing the processivity of the polymerase holoenzyme. The holoenzyme works as an ab2 heterotrimer. The 140-kDa catalytic subunit is encoded by the POLG1 gene, on chromosome 15q25, while the 55-kDa accessory subunit is encoded by POLG2, on chromosome 17q. Mutations in POLG1 are a major cause of human mitochondrial disease. So far more than 100 mutations in pol-γ have been reported (http://dir-apps.niehs.nih.gov/polg/index.cfm). This gene is the most frequent cause of adPEO. In adPEO due to POLG1 mutations, prominent features are severe dysphagia and dysphonia and, occasionally, a movement disorder including parkinsonism, cerebellar dysfunction, or chorea (36). Mental retardation, endocrine alterations, such as hypogonadism with ovarian failure, and gastrointestinal dysmotility, may be additional findings (37, 38). The severity of the syndromes varies in relation to the type of mutation.
Recessive mutations of POLG1 are also responsible for a spectrum of other syndromes, including: i) most of autosomal recessive (11) or, ii) apparently sporadic PEO cases with multiple mtDNA deletions (12), which may or may not be associated with additional findings including parkinsonism, severe peripheral neuropathy, endocrine failure, or psychotic depression (39); iii) different recessive variant syndromes such as sensory ataxic neuropathy, dysarthria, ophthalmoplegia, SANDO (40), or cerebellar ataxia and epilepsy, SCAE; iv) infantile Alpers-Huttenlocher syndrome, characterized by liver insufficiency and spongiotic poliodystrophy (17, 41, 42). Two mutant alleles carrying mutations in the spacer region of the POLG1 protein (A467T and S748W) are recurrent in all these conditions (43, 44), which may help the diagnostic work-up in suspected cases.
The molecular basis of this clinical heterogeneity can be explained, in part, by the structural and functional complexity of the enzyme. Pol-γA, the 145 kDa catalytic subunit encoded by POLG1, comprises an N-terminal exonuclease domain, with predominantly proofreading functions, and a polymerase domain, which performs the template-directed synthesis of the nascent mtDNA strands.
Only one, heterozygous dominant mutation has been identified in POLG2, in a 60-year-old female with adult-onset PEO, cardiac conduction defect, and increased CK (45). However, the search for nuclear genes associated with PEO is not over, since several families or sporadic PEO cases with multiple mtDNA deletions have failed to show mutations in the above genes.
OPA1 mutations
OPA1 is a dynamin-like GTPase located in the inner mitochondrial membrane involved in mitochondrial fusion, cristae organization (46) and control of apoptosis (47). OPA1 is linked to non-syndromic autosomal dominant optic atrophy (ADOA) (48, 49), a condition characterized by slowly progressive visual loss starting in childhood, first described by the Danish ophthalmologist Paul Kjer in 1959 (50). However, a few missense mutations, clustered in the GTPase domain, are responsible for an ADOA “ plus” syndrome, consisting of a combination of ADOA with PEO, peripheral neuropathy, ataxia and deafness (7, 8). These patients have RRFs and cytochrome-c-oxidase negative muscle fibres, with paracrystalline inclusions filling abnormally shaped mitochondria. Remarkably, all patients harboured multiple mtDNA deletions in their skeletal muscle. Moreover, Milone et al. described a patient with a multisystemic disorder and multiple muscle mtDNA deletions, carrying an in-frame deletion in OPA1 in the absence of optic atrophy (51). The mechanisms leading to the accumulation of multiple mtDNA deletions in this condition are still unknown but indicate that mitochondrial shape and mtDNA integrity are linked, possibly through a mechanism controlling the structure and function of nucleoids (52).
GFER mutations (growth factor, augmenter of liver regeneration)
Mammalian GFER is part of a mitochondrial Disulfide Relay System (DRS) localized in the mitochondrial intermembrane space (IMS ). The rat and human GFER proteins act as sulfhydryl oxidases (53) presumably playing a role similar to that of yeast Erv1p (protein import from the cytosol to IMS). Recently, a mutation in the human GFER gene has been described in an inbred Moroccan family with three siblings affected by congenital cataract, progressive muscular hypotonia, sensori-neural hearing loss, and developmental delay. Muscle biopsy showed scattered COX-negative fibres and mtDNA multiple deletions detected by PCR techniques (13).
Thymidine phosphorylase mutations
The gene responsible for MNGIE, identified in 1999 (54), encodes the enzyme thymidine phosphorylase (TP), which is involved in the catabolism of pyrimidines, by promoting the phosphorolysis of thymidine into thymine and deoxyribose-phosphate. To date, more than 30 mutations in TYMP gene are known to be associated with MNGIE (55). Defects of thymidine phosphorylase result in systemic accumulation of thymidine and deoxyuridine (56, 57), which leads to deoxynucleotide pool imbalance (58) and mtDNA instability (59). The latter phenomenon is reflected by the presence of both multiple deletions and partial depletion of muscle mtDNA. Interestingly, there are potential treatments for MNGIE. Haemodialysis has been shown to transiently reduce thymidine levels in blood (60, 61). Allogeneic stem cell transplantation has had some success in restoring TYMP activity and lowering plasma thymidine levels (62). In addition, repeated platelet infusions can reduce thymidine levels in blood in MNGIE patients (63).
MtDNA Depletion syndromes
Pathogenically relevant gene mutations have been identified in only 15-20% of mtDNA depletion syndrome cases. Since 2001, MDS has been linked to mutations in 8 nuclear genes. Mutations in TK2 and RRM2B are associated with early-onset myopathy with or without renal proximal tubulopathy (63, 25, 26). Mutations in SUCLA2 and SUCLG1 encoding isoforms of succinyl-coenzyme A lyase (a Krebs-cycle enzyme), have been associated with encephalomyopathy (27, 28) while mutations in Twinkle (64, 65), POLG1 (17, 41), DGUOK (66–68) and MPV17 are associated with the hepatocerebral form of MDS (69–71). Remarkably, the same pathogenic mutation in MPV17 that was previously identified in an Italian family (69) was later found to be responsible for NNH (72) raising the possibility of a common founder effect. However, haplotype analysis of the MPV17 locus in the Italian MDS and in several NNH families demonstrated that the mutation occurred independently in the two populations (73).
Balance and control of the mitochondrial deoxynucleotide pools are essential for the maintenance of mtDNA copy number in both tissues and cells (74). Perturbation of this homeostatic control, as determined by defects of DUGOK and TK2, TYMP, RRM2B and ANT1 lead to mtDNA depletion or multiple deletions. These enzymes are involved in the salvage pathways of mitochondrial deoxynucleotides, which constitute the major source of mtDNA precursors in stable tissues such as liver, brain, and muscle.
A defect in the last step of the mitochondria dNTPs salvage pathway has also been postulated in the pathogenesis of the SUCLA2 and SUCLG1 mutations (27–29) since SCS-A and SCS-G are associated with nucleoside diphosphate kinase (NDPK), which contributes to the homeostasis of ribonucleotides and deoxyribonucleotides in mitochondria.
Very recently, it has been reported that dominant mitochondrial damages due to ANT1 pathogenic mutations are not caused by aberrant nucleotide transport, but by uncoupled mitochondrial respiration (75).
Finally, the function of MPV17 and its role in the pathogenesis of MDS is still unknown, but studies on its yeast ortholog, SYM1, suggest, for this protein, a role in the cellular response to metabolic stress (72). The availability of an MPV17 KO mouse provides an important tool to elucidate the function of this gene in mitochondrial homeostasis and to investigate the pathogenesis of the disease.
Conclusions
Defects of intergenomic communication are an expanding area of mitochondrial disorders that involve genes and enzymatic activity related to a spectrum of metabolic pathways: mtDNA replication, deoxynucleotide and ribonucleotide supply, organellar biogenesis, etc. The function of some of the factors associated with specific syndromes are still poorly understood, or unknown. This is the case for MPV17. In addition, the basis of the phenotypic variability of the clinical presentation is also obscure. Enzymes that are apparently related to the same pathway, for instance TK2 and dGK, both involved in the intra-mitochondrial recycling of deoxynucleotides, are responsible for different syndromes, such as myopathic vs. hepatocerebral MDS. The most striking case is that of POLG1, which depending on the type and association of different mutations (and perhaps polymorphisms), can determine different molecular lesions (e.g. multiple mtDNA deletions vs. mtDNA depletion) as well as clinical features and organ specificity. This observation is even more surprising if one considers that the only known target of pol g is mtDNA, that this enzyme is ubiquitously expressed, and essential for life. However, this is not the only example: above we have discussed the wide variability observed in association with Twinkle mutations, and even in dominant vs. recessive mutations of ANT1. The same is true for MPV17, SUCLA2 and SUCLG1. The only relatively monomorphic (and monogenic) disease is probably MNGIE, the features of which have been elucidated in some more detail, at the mechanistic level, compared to other genes.
Neither mtDNA multiple deletions nor mtDNA depletion syndromes benefit from an effective treatment, although in some cases, such as the liver insufficiency associated with MPV17 mutations can be improved, including life-threatening hypoglycaemic episodes, by careful and assiduous dietetic treatment and, in some cases, by liver transplantation. Even the cases responding to such measures, however, are prone to develop neurological symptoms that seem to occur and progress independently from compensation of the hepatic abnormalities. Again, the only, encouraging exception seems to be MNGIE, for which bone marrow transplantation, aiming at promoting the clearance of toxic levels of thymidine from the body fluids, has been proposed and, indeed, applied in a few cases. The first results are, in fact, quite promising, since a spectacular improvement has been recorded not only in the biochemical profile but also in the clinical conditions of the patients.
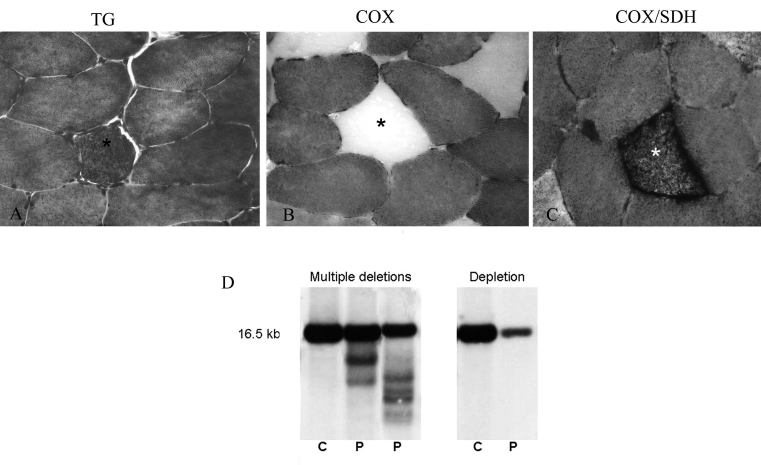
Figure 1.
Muscle morphology in patient with multiple mtDNA deletions: one RRF (asterisk) at TG (25x).
(A) showing absence of COX activity (40x) (B), confirmed with the double stain COX/SDH (40x) (C).
mtDNA multiple deletions and mtDNA depletion at Southern Blot(D).
References
- 1. Contamine V, Picard M. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol Mol Biol Rev 2000;64:281-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clayton DA. Vertebrate mitochondrial DNA - a circle of surprises. Exp Cell Res 2000;255:4-9. [DOI] [PubMed] [Google Scholar]
- 3. Holt IJ, Lorimer HE, Jacobs HT. Coupled leading- and lagging-strand synthesis of mammalian mitochondrial DNA. Cell 2000;100:515-24. [DOI] [PubMed] [Google Scholar]
- 4. Zeviani M, Servidei S, Gellera C, et al. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D loop region. Nature 1989;339:309-11. [DOI] [PubMed] [Google Scholar]
- 5. Servidei S, Zeviani M, Manfredi G, et al. Dominantly inherited mitochondrial myopathy with multiple deletions of mitochondrial DNA: clinical, morphologic, and biochemical studies. Neurology 1991;41:1053-9. [DOI] [PubMed] [Google Scholar]
- 6. Hirano M, Marti R, Ferreiro-Barros C, et al. Defects of intergenomic communication: autosomal disorders that cause multiple deletions and depletion of mitochondrial DNA. Semin Cell Dev Biol 2001;12:417-27. [DOI] [PubMed] [Google Scholar]
- 7. Amati-Bonneau P, Valentino ML, Reynier P, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain 2008;131:338-51. [DOI] [PubMed] [Google Scholar]
- 8. Hudson G, Amati-Bonneau P, Blakely EL, et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 2008;13:329-37. [DOI] [PubMed] [Google Scholar]
- 9. Yuzaki M, Ohkoshi N, Kanazawa I, et al. Multiple deletions in mitochondrial DNA at direct repeats of non-D-loop regions in cases of familial mitochondrial myopathy. Biochem Biophys Res Commun 1989;164:1352-7. [DOI] [PubMed] [Google Scholar]
- 10. Mizusawa H, Watanabe M, Kanazawa I, et al. Familial mitochondrial myopathy associated with peripheral neuropathy: partial deficiencies of complex 1 and complex 4. J Neurol Sci 1988;86:171-84. [DOI] [PubMed] [Google Scholar]
- 11. Lamantea E, Tiranti V, Bordoni A, et al. Mutations of mitochondrial DNA polymerase gammaA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol 2002;52:211-9. [DOI] [PubMed] [Google Scholar]
- 12. Agostino A, Valletta L, Chinnery PF, et al. Mutations of ANT1, Twinkle, and POLG1 in sporadic progressive external ophthalmoplegia (PEO). Neurology 2003;60:1354-6. [DOI] [PubMed] [Google Scholar]
- 13. Di Fonzo A, Ronchi D, Lodi T, et al. The mitochondrial disulfide relay system protein GFER is mutated in autosomal-recessive myopathy with cataract and combined respiratory-chain deficiency. Am J Hum Genet 2009;84:594-604. Epub 2009 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirano M, Silvestri G, Blake DM, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology 1994;44:721-7. [DOI] [PubMed] [Google Scholar]
- 15. Giordano C, Sebastiani M, De Giorgio R, et al. Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion. Am J Pathol 2008;174:1120-8. Epub 2008 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marti R, Verschuuren JJ, Buchman A, et al. Late-onset MNGIE due to partial loss of thymidine phosphorylase activity. Ann Neurol 200;58:649-52. [DOI] [PubMed] [Google Scholar]
- 17. Naviaux RK, Nyhan WL, Barshop BA, et al. Mitochondrial DNA polymerase gamma deficiency and mtDNA depletion in a child with Alpers’ syndrome. Ann Neurol 1999;45:54-8. [DOI] [PubMed] [Google Scholar]
- 18. Van Goethem G, Martin JJ, Dermaut B, et al. POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord 2003;13:133-42. [DOI] [PubMed] [Google Scholar]
- 19. Hakonen AH, Goffart S, Marjavaara S, et al. Infantile-onset spinocerebellar ataxia and mitochondrial recessive ataxia syndrome are associated with neuronal complex I defect and mtDNA depletion. Hum Mol Genet 2008;17:3822-35. [DOI] [PubMed] [Google Scholar]
- 20. Spinazzola A, Zeviani M. Disorders of nuclear-mitochondrial intergenomic signaling. Gene 2005;354:162-8. [DOI] [PubMed] [Google Scholar]
- 21. Vu TH, Tanji K, Holve SA, Bonilla E, et al. Navajo neurohepatopathy: a mitochondrial DNA depletion syndrome? Hepatology 2001;34:116-20. [DOI] [PubMed] [Google Scholar]
- 22. Moraes CT, Shanske S, Tritschler HJ, et al. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet 1991;48:492-501. [PMC free article] [PubMed] [Google Scholar]
- 23. Galbiati S, Bordoni A, Papadimitriou D. New mutations in TK2 gene associated with mitochondrial DNA depletion. Pediatr Neurol 2006;34:177-85. [DOI] [PubMed] [Google Scholar]
- 24. Oskoui M, Davidzon G, Pascual J, et al. Clinical spectrum of mitochondrial DNA depletion due to mutations in the thymidine kinase 2 gene. Arch Neurol 2006;63:1122-6. [DOI] [PubMed] [Google Scholar]
- 25. Bourdon A, Minai L, Serre V, et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 2007;39:776-80. [DOI] [PubMed] [Google Scholar]
- 26. Bornstein B, Area E, Flanigan KM, et al. Mitochondrial DNA depletion syndrome due to mutations in the RRM2B gene. Neuromuscul Disord 2008;18:453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elpeleg O, Miller C, Hershkovitz E, et al. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet 2005;76:1081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carrozzo R, Dionisi-Vici C, Steuerwald U, et al. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 2007;13:862-74. [DOI] [PubMed] [Google Scholar]
- 29. Ostergaard E, Hansen FJ, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain 2007;130:853-61. [DOI] [PubMed] [Google Scholar]
- 30. Kaukonen J, Juselius JK, Tiranti V, et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 2000;289:782-5. [DOI] [PubMed] [Google Scholar]
- 31. Spelbrink JN, Li FY, Tiranti V, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet 2001;28:223-31. [DOI] [PubMed] [Google Scholar]
- 32. Van Goethem G, Dermaut B, Lofgren A, et al. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 2001;28:211-2. [DOI] [PubMed] [Google Scholar]
- 33. Palmieri L, Alberio S, Pisano I, et al. Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum Mol Genet 2005;14:3079-88. [DOI] [PubMed] [Google Scholar]
- 34. Galassi G, Lamantea E, Invernizzi F, et al. Additive effects of POLG1 and ANT1 mutations in a complex encephalomyopathy. Neuromuscul Disord 2008;18:465-70. [DOI] [PubMed] [Google Scholar]
- 35. Nikali K, Suomalainen A, Saharinen J, et al. Infantile onset spinocerebellar ataxia is caused by recessive mutations in mitochondrial proteins Twinkle and Twinky. Hum Mol Genet 2005;14:2981-90. [DOI] [PubMed] [Google Scholar]
- 36. Luoma P, Melberg A, Rinne JO, et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 2004;364:875-82. [DOI] [PubMed] [Google Scholar]
- 37. Filosto M, Mancuso M, Nishigaki Y, et al. Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma. Arch Neurol 2003;60:1279-84. [DOI] [PubMed] [Google Scholar]
- 38. Invernizzi F, Varanese S, Thomas A, et al. Two novel POLG1 mutations in a patient with progressive external ophthalmoplegia, levodopa-responsive pseudo-orthostatic tremor and parkinsonism. Neuromuscul Disord 2008;18:460-4. [DOI] [PubMed] [Google Scholar]
- 39. Van Goethem G, Martin JJ, Dermaut B, et al. POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord 2003;13:133-42. [DOI] [PubMed] [Google Scholar]
- 40. Davidzon G, Mancuso M, Ferraris S, et al. POLG mutations and Alpers syndrome. Ann Neurol 2005;57:921-3. [DOI] [PubMed] [Google Scholar]
- 41. Ferrari G, Lamantea E, Donati A, et al. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-gammaA. Brain 2005;128:723-31. [DOI] [PubMed] [Google Scholar]
- 42. Van Goethem G, Luoma P, Rantamaki M, et al. POLG mutations in neurodegenerative disorders with ataxia but no muscle involvement. Neurology 2004;63:1251-7. [DOI] [PubMed] [Google Scholar]
- 43. Winterthun S, Ferrari G, He L, et al. Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology 2005;64:1204-8. [DOI] [PubMed] [Google Scholar]
- 44. Horvath R, Hudson G, Ferrari G, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain 2006;129:1674-84. [DOI] [PubMed] [Google Scholar]
- 45. Longley MJ, Clark S, Yu Wai Man C, et al. Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia. Am J Hum Genet 2006;78:1026-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duvezin-Caubet S, Jagasia R, Wagener J, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 2006;281:37972-9. [DOI] [PubMed] [Google Scholar]
- 47. Lee YJ, Jeong SY, Karbowski M, et al. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 2004;15:5001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alexander C, Votruba M, Pesch UE, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 2000;26:211-5. [DOI] [PubMed] [Google Scholar]
- 49. Delettre C, Lenaers G, Griffoin JM, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 2000;26:207-10. [DOI] [PubMed] [Google Scholar]
- 50. Kjer P. Infantile optic atrophy with dominant mode of inheritance: a clinical and genetic study of 19 Danish families. Acta Ophthalmol Scand 1959;37:1-146. [PubMed] [Google Scholar]
- 51. Milone M, Younge BR, Wang J, et al. Mitochondrial disorder with OPA1 mutation lacking optic atrophy. Mitochondrion 2009;9:279-81. [DOI] [PubMed] [Google Scholar]
- 52. Zeviani M. OPA1 mutations and mitochondrial DNA damage: keeping the magic circle in shape. Brain 2008;131:314-7. [DOI] [PubMed] [Google Scholar]
- 53. Mesecke N, Terziyska N, Kozany C, et al. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 2005;121:1059-69. [DOI] [PubMed] [Google Scholar]
- 54. Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 1999;283:689-92. [DOI] [PubMed] [Google Scholar]
- 55. Hirano M, Lagier-Tourenne C, Valentino ML, et al. Thymidine phosphorylase mutations cause instability of mitochondrial DNA. Gene 2005;354:152-6. [DOI] [PubMed] [Google Scholar]
- 56. Spinazzola A, Marti R, Nishino I, et al. Altered thymidine metabolism due to defects of thymidine phosphorylase. J Biol Chem 2002;277:4128-33. [DOI] [PubMed] [Google Scholar]
- 57. Marti R, Spinazzola A, Tadesse S, et al. Definitive diagnosis of mitochondrial neurogastrointestinal encephalomyopathy by biochemical assays. Clin Chem 2004;50:120-4. [DOI] [PubMed] [Google Scholar]
- 58. Ferraro P, Pontarin G, Crocco L, et al. Mitochondrial deoxynucleotide pools in quiescent fibroblasts: a possible model for mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). J Biol Chem 2005;280:24472-80. [DOI] [PubMed] [Google Scholar]
- 59. Pontarin G, Ferraro P, Valentino ML, et al. Mitochondrial DNA depletion and thymidine phosphate pool dynamics in a cellular model of mitochondrial neurogastrointestinal encephalomyopathy. J Biol Chem 2006;281:22720-8. [DOI] [PubMed] [Google Scholar]
- 60. Yavuz H, Ozel A, Christensen M, et al. Treatment of mitochondrial neurogastrointestinal encephalomyopathy with dialysis. Arch Neurol 2007;64:435-8. [DOI] [PubMed] [Google Scholar]
- 61. Hirano M, Martí R, Casali C, et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology 2006;67:1458-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lara MC, Weiss B, Illa I, et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology 2006;67:1461-3. [DOI] [PubMed] [Google Scholar]
- 63. Saada A, Shaag A, Mandel H, et al. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet 2001;29:342-4. [DOI] [PubMed] [Google Scholar]
- 64. Sarzi E, Goffart S, Serre V, et al. Twinkle helicase (PEO1) gene mutation causes mitochondrial DNA depletion. Ann Neurol 2007;62:579-87. [DOI] [PubMed] [Google Scholar]
- 65. Hakonen AH, Isohanni P, Paetau A, et al. Recessive Twinkle mutations in early onset encephalopathy with mtDNA depletion. Brain 2007;130:3032-40. [DOI] [PubMed] [Google Scholar]
- 66. Mandel H, Szargel R, Labay V, et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat Genet 2001;29:337-41. [DOI] [PubMed] [Google Scholar]
- 67. Salviati L, Sacconi S, Mancuso M, et al. Mitochondrial DNA depletion and dGK gene mutations. Ann Neurol 2002;52:311-16. [DOI] [PubMed] [Google Scholar]
- 68. Tadiboyina VT, Rupar A, Atkison P, et al. Novel mutation in DGUOK in hepatocerebral mitochondrial DNA depletion syndrome associated with cystathioninuria. Am J Med Genet 2005;135A:289-91. [DOI] [PubMed] [Google Scholar]
- 69. Spinazzola A, Viscomi C, Fernandez-Vizarra E, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet 2006; 38:570-5. [DOI] [PubMed] [Google Scholar]
- 70. Wong LJ, Brunetti-Pierri N, Zhang Q, et al. Mutations in the MPV17 gene are responsible for rapidly progressive liver failure in infancy. Hepatology 2007;46:1218-27. [DOI] [PubMed] [Google Scholar]
- 71. Spinazzola A, Santer R, Akman OH, et al. Hepatocerebral form of mitochondrial DNA depletion syndrome: novel MPV17 mutations. Arch Neurol 2008;65:1108-13. [DOI] [PubMed] [Google Scholar]
- 72. Karadimas CL, Vu TH, Holve SA, et al. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am J Hum Genet 2006;79:544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Spinazzola A, Massa V, Hirano M, et al. Lack of founder effect for an identical mtDNA depletion syndrome (MDS)-associated MPV17 mutation shared by Navajos and Italians. Neuromuscul Disord 2008;18:315-8. [DOI] [PubMed] [Google Scholar]
- 74. Ashley N, Adams S, Slama A, et al. Defects in maintenance of mitochondrial DNA are associated with intramitochondrial nucleotide imbalances. Hum Mol Genet 2007;16:1400-11. [DOI] [PubMed] [Google Scholar]
- 75. Wang X, Salinas K, Zuo X, et al. Dominant membrane uncoupling by mutant adenine nucleotide translocase in mitochondrial diseases. Hum Molec Genet 2008;17:4036-44. [DOI] [PMC free article] [PubMed] [Google Scholar]