Abstract
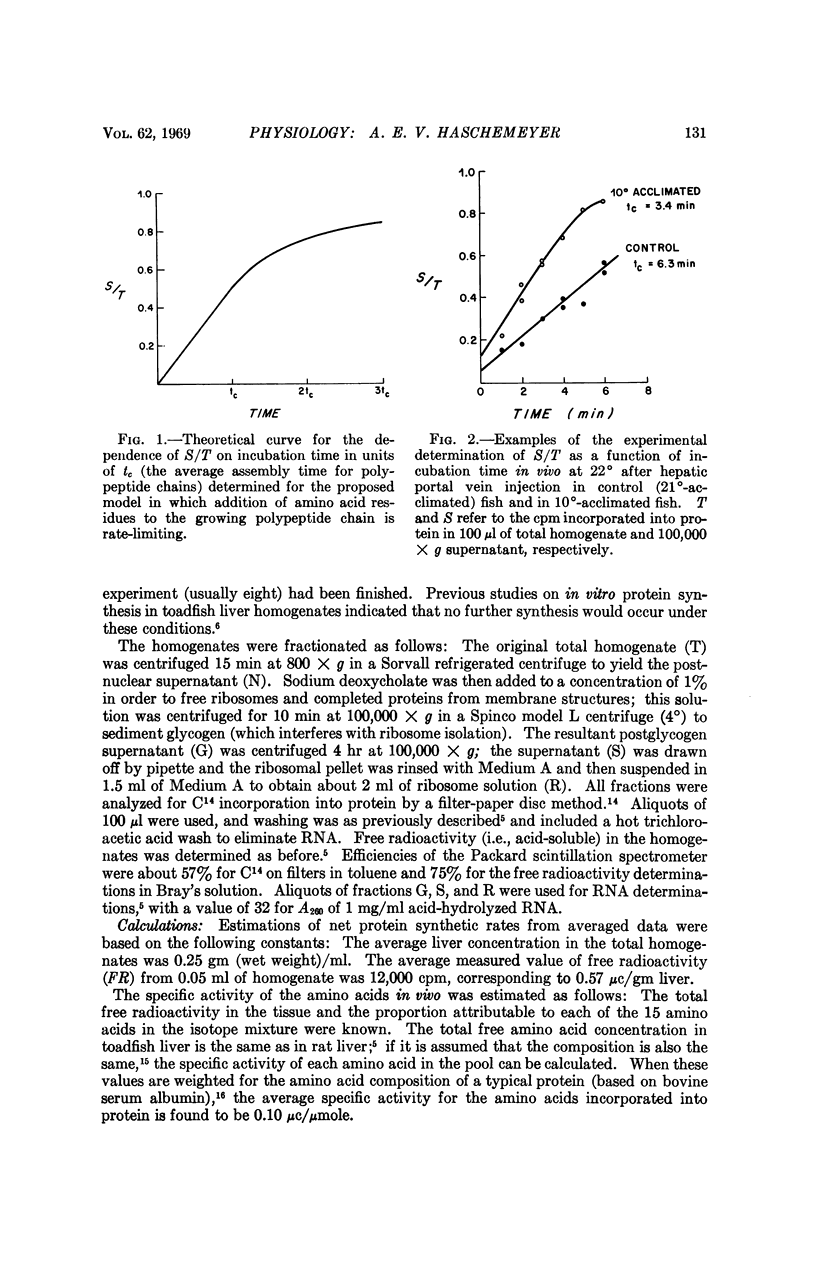
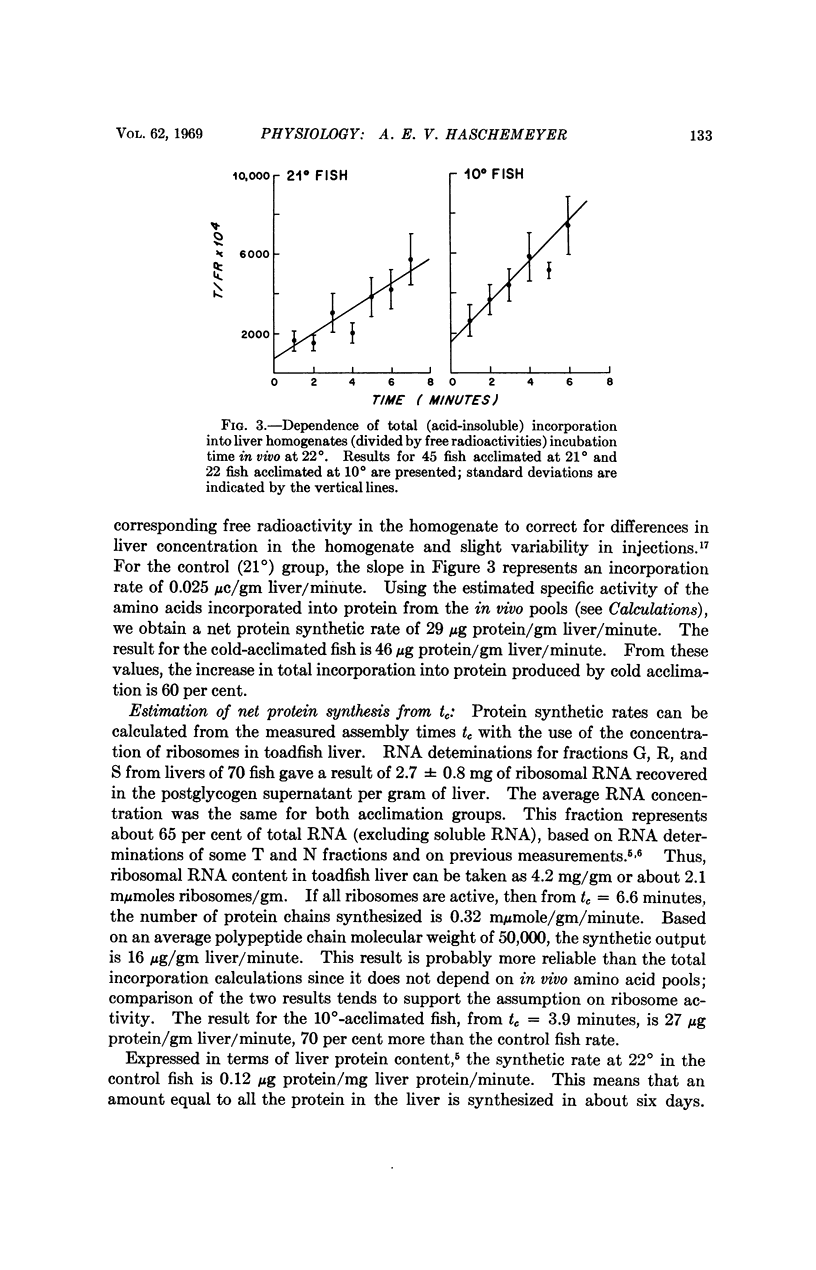
A simple translational model, present herein, permits experimental determination of the rates of protein synthesis in vivo in terms of a time constant representing the average assembly time of the polypeptide chains. The model has been used to interpret incorporation of radioactive amino acids into toadfish liver fractions as a function of time after hepatic portal vein injection. The results suggest that the increase in liver protein synthesis produced by cold acclimation is due to a more rapid rate of addition of amino acid residues to the growing polypeptide chains. The finding is consistent with the greater aminoacyl transferase activity in livers of cold-acclimated fish.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FESSENDEN J. M., MOLDAVE K. Studies on amino acyl transfer from soluble-RNA to rat liver ribonucleoprotein particles; effect of soluble and microsomal extracts. Biochemistry. 1962 May 25;1:485–490. doi: 10.1021/bi00909a019. [DOI] [PubMed] [Google Scholar]
- FESSENDEN J. M., MOLDAVE K. Studies on aminoacyl transfer from soluble ribonucleic acid to ribosomes. Resolution of two soluble transferring activities. J Biol Chem. 1963 Apr;238:1479–1484. [PubMed] [Google Scholar]
- FREED J. CHANGES IN ACTIVITY OF CYTOCHROME OXIDASE DURING ADAPTATION OF GOLDFISH TO DIFFERENT TEMPERATURES. Comp Biochem Physiol. 1965 Apr;14:651–659. doi: 10.1016/0010-406x(65)90252-5. [DOI] [PubMed] [Google Scholar]
- HOFMANN T., HARRISON P. M. The structure of apoferritin: degradation into and molecular weight of subunits. J Mol Biol. 1963 Apr;6:256–267. doi: 10.1016/s0022-2836(63)80087-x. [DOI] [PubMed] [Google Scholar]
- Haschemeyer A. E. Compensation of liver protein synthesis in temperature-acclimated toadfish, Opsanus tau. Biol Bull. 1968 Aug;135(1):130–140. doi: 10.2307/1539620. [DOI] [PubMed] [Google Scholar]
- Hunt T., Hunter T., Munro A. Control of haemoglobin synthesis: distribution of ribosomes on the messenger RNA for alpha and beta chains. J Mol Biol. 1968 Aug 28;36(1):31–45. doi: 10.1016/0022-2836(68)90217-9. [DOI] [PubMed] [Google Scholar]
- LOFTFIELD R. B., EIGNER E. A. The time required for the synthesis of a ferritin molecule in rat liver. J Biol Chem. 1958 Apr;231(2):925–943. [PubMed] [Google Scholar]
- Skogerson L., Moldave K. Evidence for aminoacyl-tRNA binding, peptide bond synthesis, and translocase activities in the aminoacyl transfer reaction. Arch Biochem Biophys. 1968 May;125(2):497–505. doi: 10.1016/0003-9861(68)90607-3. [DOI] [PubMed] [Google Scholar]



