Abstract
Background
Extensive experimental data in animals indicate that exposure to polychlorinated biphenyls (PCBs) during pregnancy leads to changes in offspring immune function during the postnatal period. Whether developmental PCB exposure influences immunologic development in humans has received little study.
Methods
The study population was 384 mother-infant pairs recruited from two districts of eastern Slovakia for whom prospectively collected maternal, cord, and 6-month infant blood specimens were available. Several PCB congeners were measured in maternal, cord, and 6-month infant sera by high-resolution gas chromatography with electron capture detection. Concentrations of IgG-specific anti-haemophilus influenzae type b, tetanus toxoid, and diphtheria toxoid were assayed in 6-month infant sera using ELISA methods. Multiple linear regression was used to estimate the relation between maternal, cord, and 6-month infant PCB concentrations and the antibody concentrations evaluated at 6-months of age.
Results
Overall, there was little evidence of an association between infant antibody concentrations and PCB measures during the pre- and early postnatal period. In addition, our results did not show specificity in terms of associations limited to a particular developmental period (e.g. pre- vs. postnatal), a particular antibody, or a particular PCB congener.
Conclusions
At the PCB concentrations measured in this cohort, which are high relative to most human populations today, we did not detect an association between maternal or early postnatal PCB exposure and specific antibody responses at 6-months of age.
Introduction
Polychlorinated biphenyls (PCBs) are ubiquitous compounds that were produced as complex mixtures for a variety of applications which included dielectric fluids for capacitors and transformers, and as additives to paint, adhesives, sealants, and carbonless copy paper.1 PCBs are chemically stable and lipophilic and as a result, are not easily degraded or metabolized, and their concentrations thereby tend to bioaccumulate up the food chain. Despite a general decline in PCB levels in human tissues worldwide,2 there is still concern about their potential health effects, particularly among populations exposed through environmental contamination3-5 or those populations whose diet includes consumption of PCB contaminated seafood.2, 6
Much of the concern over the potential health effects of PCBs stems from their structural similarity to 2,3,7,8-tetrachloro-dibenzodioxin (TCDD)—which demonstrates strong immunotoxic effects in experimental studies. Dioxin-like PCBs, or those with a planar or co-planar configuration, appear to affect the immune system by binding the aryl hydrocarbon (Ah) receptor.7-9 Less is known about the potential immunotoxic effects of non dioxin-like PCBs, or the so called “non-coplanar” PCBs. Since these compounds do not bind as strongly (if at all) to the Ah receptor, it is thought that they are significantly less immunotoxic. However, recent experimental work by Lyche and colleagues10, 11 demonstrated that neonatal immunity to several environmental microbes was reduced in goat kids following in utero exposure to PCB-153, a non-coplanar PCB.
T cell-dependent functional measures of immunity, such as vaccination response, serve as valuable biomarkers to assess potential developmental immunotoxicity.12, 13 Indeed, some epidemiologic evidence suggests that both dioxin-like and non dioxin-like PCBs affect responses to vaccinations in infants and children. For instance, in a cohort of Dutch infants environmentally-exposed to PCBs, antibody levels to measles, mumps, and rubella were assessed when children were 18- and 42-months of age and some associations between elevated maternal and cord PCBs and decreased antibody levels at 42-months were noted.14, 15 Other evidence comes from two cohorts of children followed in the Faroe Islands, which examined pre- and postnatal PCB exposures in relation to diphtheria and tetanus vaccine responses.16 In this study, the authors noted lower concentrations of diphtheria and tetanus antibodies in relation to higher pre- and postnatal PCB exposures, even after adjusting for several potential confounding factors. To our knowledge, the relation between antibody response and in utero and early postnatal PCB exposures in humans has only been examined in these two populations.
To better understand the role of PCBs on children's development, we initiated a cohort study in 2002 enrolling mother-infant pairs from two districts in eastern Slovakia: Michalovce, a district home to a chemical manufacturing facility that produced PCBs from 1959 to 1984 and resulted in significant environmental contamination; and Svidnik, an area approximately 70 km to the northwest with significantly less contamination. Exposure to PCBs in this population appears to occur, at least in part, through consumption of fats from locally-produced sources.17 In a previous analysis of mother-infant pairs from these districts, higher maternal PCB concentrations were associated with smaller thymus size in newborn infants,18 suggesting that infant immune development might be altered by in utero exposure to PCBs at concentrations present in this population. The current paper reports on pre- and postnatal PCB concentrations in relation to functional measures of immunity (post-vaccination antibody response) among 6-month-old infants in this eastern Slovak population.
Methods
Sample selection, specimen collection, and follow-up
Women were approached to participate in our study between 2002 and 2004 at the time they came to the local hospital in either Michalovce or Svidnik to deliver their child, and since each district has only one hospital, the vast majority of women delivering during this period delivered at these two hospitals. There, research nurses explained the details of the study, and answered any questions the mother might have about study procedures. The mother was then presented with a consent form which further described the details of the study and was asked to read it over. After having the opportunity to ask questions, she was asked to sign the consent form if she agreed to participate. All forms of communication were in Slovak. The protocol excluded 1) mothers with more than four previous births, 2) mothers less than 18 years of age, 3) mothers who had resided fewer than 5 years in their district, and 4) mothers with a major illness during pregnancy. Following birth, we also excluded mothers whose infants had severe birth defects. In total, 1134 women were enrolled (811 in Michalovce and 323 in Svidnik). The study protocol was approved by Institutional Review Boards at the University of Washington, the University of California at Davis, and the Slovak Medical University.
Once a woman provided written consent, a note was made in her medical chart to have maternal and cord blood specimens collected. Two 9-ml vacutainer tubes were used to collect serum for PCB and lipid determination. After collection, these tubes were refrigerated between 5 and 10°C, and within 2 hours of collection, transferred to the hospital's Department of Biochemistry for further processing. There, samples were allowed to clot, then centrifuged at 3000 rpm for 15 minutes to isolate serum. For the determination of PCBs, approximately 3 ml of the serum was pipetted into an 8 ml glass tube, and approximately 0.2 ml of serum was transferred to a 1.5 ml microcentrifuge tube for lipid determination. After the serum samples were aliquoted, samples were frozen at −20°C for future analyses.
Following delivery, cord blood was also collected for PCB and lipid analysis. The infant was held at the level of the introitus or mother's abdomen to prevent a significant shift of the infant's blood volume. As soon as possible after suctioning, the cord was clamped and cut to 5 cm from the infant's abdomen. After the infant was dried and stabilized and the umbilical base appeared normal, an umbilical clamp was secured to the cord 1 to 2 cm distal to the abdominal wall, and any excess length was cut. Blood from the cord was collected into vacutainers for further processing, and after centifugation serum was aliquoted in a manner similar to that of the maternal specimens.
When the child was 5 months of age, each mother was sent reminders and requests to schedule an appointment for the 6-month follow-up. The 6-month visit took place at the respective hospital in the Department of Pediatrics. During the visit, approximately 9 ml of blood was collected for PCB, lipid, and immune assays at 6-months, and samples were processed in a manner similar to the maternal and cord blood. For immune assays, approximately 500 μl of the serum was aliquoted into each microcentrifuge tube (2 tubes total), and stored frozen at −20°C. At age 6-months, 971 mother-infant pairs were still participating in the study (86%).
PCB measurement
From the maternal, cord, and 6-month infant serum samples, we determined the wet-weight concentrations (in ng/ml) of 15 PCB congeners [International Union of Pure and Applied Chemistry (IUPAC) numbers 28, 52, 101, 105, 114, 118, 123+149, 138+163, 153, 156+171, 157, 167, 170, 180, and 189]. PCB concentrations were determined at the Department of Toxic Organic Pollutants at the Slovak Medical University in Bratislava. This laboratory serves as the National Reference Laboratory for Dioxins and Related Compounds for the Slovak Republic, and regularly participates in interlaboratory comparison tests, such as the Intercomparison Programme (German External Quality Assessment Scheme) and the Interlaboratory Quality Assessment coordinated by the World Health Organization. The procedure for determination of PCB concentrations involved extraction, cleanup, and quantitation by high-resolution gas chromatrography with electron capture detection (GC-ECD), as described below.19, 20
First, samples were extracted on a solid-phase extraction column, using PCB-174 as an extraction standard. Samples were then extracted from the dry column with n-hexane—DCM (1:1, v/v), and concentrated under a nitrogen stream. Samples were then purified on a florisil-H2SO4/silica gel column. After washing the column, analytes were eluted with hexane. The eluate was concentrated by vacuum rotary evaporation. PCB-103 was added as an injection standard, and an aliquot was injected and analyzed on a chromatography system (HP 5890; Hewlett-Packard, Palo Alto, CA, USA) equipped with a Ni-63 electron capture detector using a 60-m DB-5 capillary column (J&W Scientific, Folsom, MA, USA). Quantification was based on the calibration curve generated by authentic PCB standard solutions at five different concentration levels. Quality control activities consisted of analyses of samples in batches of 10 run simultaneously with a blank sample and in-house reference material (spiked porcine serum). To check the daily response of the detector prior to batch sample analysis, response for a particular congener in a standard solution had to be in the range of 90–110%, or additional quality control procedures were initiated. We calculated the limit of detection (LOD) for each analyte using the ratio of background to noise (multiplied by three) and the peak height of the analyte in standard solution.
Lipid measurement
We estimated total serum lipid (TL) concentrations in maternal, cord, and infant serum samples using the enzymatic summation method proposed by Akins and colleagues.21 We measured serum total cholesterol (TC) and triglyceride (TG) concentrations at the Department of Clinical Biochemistry of TOPMED General Hospital Bratislava using a DuPont Automatic Clinical Analyzer III (DuPont, Jonesboro, AR, USA), and cholesterol oxidase without cholesterol esterase was used to detect free cholesterol (FC). The method by Takayama et al.22 was used to determine serum choline-containing phospholipids (PL). Total serum lipids were calculated using the formula:
Post-vaccination antibody response
During the first 6-months of life, Slovak infants receive several mandatory primary vaccinations which include haemophilus influenzae type b (HIB), tetanus toxoid (TT), and diphtheria toxoid (DT). The first dose for all three primary vaccinations is given concurrently at 3-4 months of age. A second dose is given at approximately 5-6 months of age, and the final dose of the primary series is given between 11 and 12 months of age. To determine the antibody response to these vaccinations at 6 months of age, we performed immunologic assays on a subset of the 971 infants still enrolled in the study at 6-months. Based on estimates of the partial correlation between antibody concentrations and PCBs from previous studies,14, 16 we selected 40% of the 6-month infant samples for analysis (n=384) to ensure adequate power to examine hypotheses related to post-vaccination antibody response and PCB concentrations. To select the subset of 384 infants, we randomly sampled mother-infant pairs within strata defined by total maternal PCB concentrations: 1) less than the 75th percentile; 2) between the 75th and 85th percentile; 3) between the 85th and 95th percentiles; and 4) greater than the 95th percentile. Within these 4 strata, we randomly sampled approximately 25, 75, 75, and 90% of subjects, respectively, to arrive at our targeted sample size. We sampled subjects based on maternal PCB concentrations (rather than cord or infant PCB concentrations) because the potential health effects of in utero PCB exposure was one of the main hypotheses of the study. This stratified random sampling maximized statistical power by oversampling mother-infant pairs with higher total maternal PCB concentrations. At the analysis stage, we adjusted for this design by applying normalized weights in all regression models (described below).
ELISA analyses were conducted at the Department of Immunology and Immunotoxiocology at the Slovak Medical University. Commercial ELISA kits were purchased from The Binding Site (Birmingham, UK) to quantify anti-tetanus toxoid (Kit # MK010), anti-diphtheria toxoid (Kit # MK014), and anti-haemophilus influenzae type b (Kit # MK016) antibodies. Each ELISA assayed anti-IgG levels specific to each antigen, since the IgG isotype 1) makes up approximately 75% of the immunoglobulin isotypes in blood,23 and 2) has been the outcome examined in other studies14-16 of PCB exposure and antibody response.
Data collection
At birth, mothers completed a questionnaire that included sociodemographic information, questions related to maternal health and medication use, family living environment, past pregnancies, and tobacco use. From the infant's medical record, we abstracted child's birth weight and gestational age, the latter based on last menstrual period reported in the medical records and the judgment made by the woman's physician. We also estimated a standardized measure of birth weight, which was expressed as a Z-score standardized for sex, parity, and gestational age based on all births in Slovakia in 2004. To collect information on the dates of any vaccinations during the first 6-months after birth, we abstracted data from 6-month pediatric medical records. At the child's 6-month visit, mothers completed another questionnaire to update information, with specific collection of questions about the home environment of the child, breastfeeding, illnesses the child had, and smoking habits of persons in the household. Data from this questionnaire were used to estimate the duration of breastfeeding up to age 6-months.
Statistical analyses
Selection of PCBs
In maternal, cord, and 6-month serum samples, we selected six, four, and four congeners, respectively, to evaluate in relation to antibody response. This decision was based on having <20% of samples below the limit of detection (LOD) for the congener. In maternal samples, these were PCB congeners 118, 138+163, 153, 156+171, 170, and 180. In both cord and 6-month infant samples, these were PCB congeners 138+163, 153, 170, and 180. When an individual value was <LOD, we assigned the value as the LOD divided by the square root of 2.24 For maternal, cord, and 6-month PCB concentrations, the corresponding “sum” variable is the sum of these six, four and four congeners, respectively.
Selection of potential confounding variables
To select potential confounding variables for our regression models, we employed Directed Acyclic Graphs (DAGs).25, 26 This method provides a graphical approach to causal modeling which allows one to identify a minimally sufficient set of adjustment variables that will adequately reduce confounding while avoiding adjustment for inappropriate variables, which may actually induce confounding. To do this, two separate DAGs [one for perinatal (maternal/cord) and one for postnatal exposure] were created. We did not consider separate DAGs for individual PCB congeners or antibodies as we regarded the causal structure of these models to be similar. Thus, we selected two different sets of confounders for our models. These were: 1) ethnicity (Roma vs. other), maternal smoking before or during pregnancy (yes/no), and maternal age at child's birth (years) for maternal and cord PCB models; and 2) sex, ethnicity (Roma vs. other), maternal smoking at 6 months (yes/no), maternal age at child's birth (years), and infant age at the time of 6-month blood draw (days). In secondary analyses, we also adjusted for: child's age (days) (maternal and cord PCB models), number of vaccine doses received (1 vs. 2), time since most recent vaccination (days), infant sex (maternal and cord PCB models), and Z-score birth weight, in addition to those variables already in our primary models formed by the DAG. Variables for our secondary models were selected from other potential confounders reported in the literature,16 and from variables noted in our DAGs to be predictors of the outcome, but which did not necessarily meet the criteria of a confounder, i.e., were not associated with PCB concentrations.
Multivariate methods
To estimate and test associations between PCB concentrations and post-vaccination antibody response, we fit linear regression models using the SURVEYREG procedure in SAS (version 9.1; SAS Institute, Inc., Cary, NC, USA). This procedure was chosen because the 384 6-month-old children in whom we measured antibody concentrations were not selected by simple random sampling from the full cohort at 6-months. Specifically, the SURVEYREG procedure allows for 1) the application of weights to the regression model (which are inversely proportional to the sampling probabilities of each stratum), and 2) valid measures of variance based on the stratified sampling design, which is necessary given the potentially greater homogeneity within our sampling strata. When weights are applied to the regression analysis, the results are as if the sample of 384 children was drawn completely at random from the 971 children participating at the 6-month follow-up. Thus, any potential bias created by the stratified sampling procedure is removed.
To reduce the potential influence of outlying values, exposure was the natural log transformation of the wet-weight concentration [in ng/ml (ppb)] of PCBs. In addition, all antibody response measures were transformed by the natural log to ensure homogeneity in error term variance. We excluded a small proportion of infants who did not receive (or for whom no report was found in their medical record) at least one vaccination of HIB, TT, or DT, or who were more than 7 months of age at the time of the 6-month blood draw. Results from multivariate analyses are expressed as the percent change in antibody concentration for a change in PCB concentration across the interquartile range, i.e. the 25th to the 75th percentile.
Results
Study subjects
Table 1 presents descriptive data for the subset of 384 mother-infant pairs selected for immunologic assessment and the 971 mother-infant pairs participating at the 6-month follow-up. In the 384 mother-infant pairs, slightly more male infants were in the study sample, and approximately 45% of women were nulliparous. Thirty-seven percent of women reported breastfeeding their child through the first 6 months of life, whereas only 4% of women reported no breastfeeding. Approximately 1 in 5 infants was of Romani ethnicity, and 74% of mothers were between 20 and 30 years of age at their child's birth. Additionally, 38% of women reported smoking before or during pregnancy, and at the 6-month follow-up, 24% of mothers reported smoking. Overall, characteristics for the 384 women and infants we selected were similar to those still participating in the overall cohort at 6 months.
Table 1.
Characteristics of infants and mothers in subset selected for immunologic analysis, and those in the full cohort followed to 6 months.
| Immunology subset (n=384) |
Full cohort at 6 months (n=971) |
|||
|---|---|---|---|---|
| Characteristic | N | %* | N | %* |
| Infant sex | ||||
| Male | 197 | 51.3 | 495 | 51.0 |
| Female | 187 | 48.7 | 476 | 49.0 |
| Birth weight (grams) | ||||
| <2500 | 21 | 5.5 | 43 | 4.4 |
| 2500-3499 | 213 | 55.5 | 550 | 56.6 |
| ≥3500 | 150 | 39.1 | 378 | 38.9 |
| Gestation length (weeks) | ||||
| <37 | 14 | 3.7 | 32 | 3.3 |
| 37-41 | 359 | 93.5 | 910 | 93.7 |
| ≥42 | 11 | 2.9 | 29 | 3.0 |
| Parity | ||||
| 0 | 173 | 45.1 | 401 | 41.3 |
| 1 | 117 | 30.5 | 322 | 33.2 |
| 2-4 | 83 | 24.2 | 246 | 25.3 |
| Missing | 1 | 0.3 | 2 | 0.2 |
| Breastfeeding duration (months) | ||||
| 0 | 17 | 4.4 | 33 | 3.4 |
| 1 - <6 | 196 | 51.0 | 457 | 47.1 |
| 6 | 141 | 36.7 | 402 | 41.4 |
| Missing | 30 | 7.8 | 79 | 8.1 |
| Ethnicity of child | ||||
| Romani | 69 | 18.0 | 189 | 19.5 |
| Slovak/eastern European | 315 | 82.0 | 782 | 80.5 |
| Maternal age at child's birth (years) | ||||
| 18- <20 | 26 | 6.8 | 83 | 8.6 |
| 20-30 | 285 | 74.2 | 716 | 73.7 |
| >30 | 73 | 19.0 | 172 | 17.7 |
| Maternal smoking before or during pregnancy | ||||
| Yes | 146 | 38.0 | 345 | 35.5 |
| No | 238 | 62.0 | 626 | 64.5 |
| Maternal smoking at 6-month follow-up | ||||
| Yes | 93 | 24.2 | 213 | 21.9 |
| No | 280 | 72.9 | 722 | 74.4 |
| Missing | 11 | 2.9 | 36 | 3.7 |
Percents may not sum to 100 because of rounding
PCB concentrations
Descriptive data for maternal, cord, and 6-month PCB concentrations are presented in Table 2 on a wet-weight basis (ng/ml), and to facilitate comparisons with other studies, on a per-lipid basis (ng/g lipid). In the selected subset of 384 mother-infant pairs, cord and 6-month-infant PCB measurements were available for 376, and 246 infants, respectively. Compared to the non dioxin-like PCBs (PCB congners 138+163, 153, 170, and 180) which were detected in nearly all maternal samples, the mono-ortho-substituted dioxin-like congeners PCB-118 and PCB-156+171 were below the limit of detection in 12% and 2.6% of samples, respectively. PCB-153, the congener with the highest concentration in our population, had a median concentration of 153, 122, and 138 ng/g lipid in maternal, cord, and 6-month samples, respectively.
Table 2.
Weighted* PCB concentrations in maternal, cord, and 6-month serum samples of mother-infant pairs in the study subset (n=384†).
| Wet-weight (ng/mL or ppb) |
Per-Lipid (ng/g or ppb) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB measure | N‡ | % < LOD | Mean | Min | P25 | P50 | P75 | Max | Mean | Min | P25 | P50 | P75 | Max |
| Maternal PCB | ||||||||||||||
| PCB 118 | 384 | 12.0 | 0.159 | 0.008 | 0.055 | 0.101 | 0.170 | 7.228 | 15.7 | 0.7 | 6.0 | 10.0 | 17.6 | 509.6 |
| PCB 138+163 | 384 | 0 | 1.360 | 0.192 | 0.625 | 0.944 | 1.431 | 39.786 | 135.5 | 19.4 | 64.2 | 95.2 | 148.8 | 2805.2 |
| PCB 153 | 384 | 0 | 2.085 | 0.343 | 1.023 | 1.533 | 2.237 | 56.132 | 208.8 | 34.6 | 108.4 | 152.6 | 227.5 | 3957.8 |
| PCB 156+171 | 384 | 2.6 | 0.196 | 0.003 | 0.090 | 0.137 | 0.216 | 4.889 | 19.5 | 0.2 | 9.3 | 13.8 | 21.5 | 451.4 |
| PCB 170 | 384 | 0 | 0.820 | 0.117 | 0.388 | 0.573 | 0.922 | 17.212 | 81.8 | 12.3 | 39.9 | 57.4 | 90.2 | 1361.7 |
| PCB 180 | 384 | 0 | 1.908 | 0.231 | 0.896 | 1.322 | 2.136 | 40.802 | 191.0 | 24.3 | 92.6 | 133.9 | 211.2 | 2876.9 |
| Sum of 6 | 384 | -- | 6.529 | 1.118 | 3.127 | 4.625 | 7.206 | 166.045 | 652.2 | 108.5 | 334.5 | 455.0 | 719.9 | 11707.5 |
| Cord PCB | ||||||||||||||
| PCB 138+163 | 376 | 0 | 0.288 | 0.026 | 0.128 | 0.202 | 0.323 | 5.494 | 119.1 | 9.5 | 56.6 | 85.6 | 125.5 | 2889.5 |
| PCB 153 | 376 | 0 | 0.398 | 0.034 | 0.181 | 0.286 | 0.449 | 6.389 | 164.4 | 13.8 | 79.8 | 121.8 | 184.0 | 3989.3 |
| PCB 170 | 376 | 0.8 | 0.138 | 0.004 | 0.062 | 0.094 | 0.161 | 2.302 | 56.4 | 1.5 | 25.6 | 39.6 | 62.4 | 1064.3 |
| PCB 180 | 376 | 0 | 0.342 | 0.042 | 0.160 | 0.236 | 0.395 | 4.869 | 140.9 | 12.2 | 69.1 | 98.3 | 155.0 | 3084.6 |
| Sum of 4 | 376 | -- | 1.166 | 0.143 | 0.537 | 0.829 | 1.332 | 18.829 | 480.7 | 41.0 | 224.3 | 343.1 | 534.8 | 11027.8 |
| 6-Month PCB | ||||||||||||||
| PCB 138+163 | 246 | 0.4 | 0.956 | 0.004 | 0.137 | 0.496 | 1.094 | 36.878 | 164.2 | 0.8 | 25.1 | 91.4 | 195.1 | 7608.0 |
| PCB 153 | 246 | 0.8 | 1.363 | 0.004 | 0.211 | 0.779 | 1.602 | 46.562 | 233.1 | 1.0 | 36.8 | 138.1 | 289.8 | 9605.9 |
| PCB 170 | 246 | 4.9 | 0.457 | 0.001 | 0.059 | 0.229 | 0.487 | 16.839 | 78.1 | 0.1 | 10.2 | 38.8 | 87.4 | 3473.9 |
| PCB 180 | 246 | 1.6 | 1.127 | 0.002 | 0.136 | 0.509 | 1.299 | 38.062 | 192.2 | 0.4 | 22.7 | 88.2 | 228.6 | 7852.3 |
| Sum of 4 | 246 | -- | 3.903 | 0.015 | 0.546 | 2.035 | 4.577 | 138.341 | 667.6 | 3.5 | 93.7 | 360.7 | 786.7 | 28540.1 |
Sampling weights applied to reflect the PCB distributions among the 971 mother-infant pairs participating at 6-months.
Numbers for maternal, cord, and 6-month concentrations on a per-lipid basis were 380, 358, and 243 due to missing lipid values.
Note that the values in the table reflect the distributions following imputation of values below the LOD.
As expected, (natural log) PCB concentrations were strongly associated both within sampling periods (i.e. maternal, cord, and 6-month) and across sampling periods. In maternal samples, PCB congeners 138 and 153 were strongly associated (r=0.99, p<0.0001) as were PCB congeners 170 and 180 (r=0.99, p<0.0001); a similarly strong association was noted for these congeners in cord and 6-month infant samples. Maternal PCB-118 showed the weakest association with other maternal congeners and total maternal PCBs—although not insignificant—with correlation coefficients between 0.72 and 0.79 (p<0.0001). Examining the associations between PCB concentrations across sampling periods, total maternal PCBs were associated with total cord PCBs (r=0.91, p<0.0001), but associations of total maternal PCBs with 6-month total PCB concentrations were more moderate (r=0.51, p<0.0001), and similarly of total cord with total 6-month PCB concentrations (r=0.50, p<0.0001).
Post-vaccination antibody concentrations
Of the 384 infants selected for immunologic analysis, 350 (91%) had blood specimens collected before 7 months of age; of these, 334 received the first dose each for HIB, TT, and DT prior to the 6-month blood draw for this study, and are included in our analysis. For 14 infants, the date of the first vaccination was later than the 6-month blood draw, and for 2 infants, the date of the first vaccination was not known, or the infant wasn't immunized. The first two doses of all three vaccines were completed for 284 children (85%); 63 children were given the second dose after blood was drawn at age 6-months, and for 3 children either the date of secondary vaccination was missing or the child was not immunized. In those 284 infants who received two doses of vaccine, protective levels of anti-haemophilus influenza type b (≥1 μg/ml), anti-tetanus toxoid (≥0.1 IU/ml), and anti-diphtheria toxoid (≥0.1 IU/ml) were reached in 79, 98, and 88% of children, respectively. Post-vaccination antibody concentrations were moderately correlated across specific vaccines both in infants who received 1 dose of each vaccination (rHIB and TT=0.65; rHIB and DT=0.43; rTT and DT=0.58, p<0.01 for all) and in infants who received 2 doses of each vaccination (rHIB and TT=0.55; rHIB and DT=0.35; rTT and DT=0.43, p<0.0001 for all).
Adjusted associations between PCBs and post-vaccination antibody concentrations
Overall, there was not strong evidence for an association between pre- or postnatal PCB levels and antibody concentrations measured at 6-months of age, and confidence intervals were wide (Table 3). Furthermore, the results lacked any consistent pattern that would indicate specificity of an association for a particular window of exposure, congener, or a particular antibody.
Table 3.
Adjusted* percent change in post-vaccination antibody concentrations for an increase in wet-weight maternal, cord, and 6-month PCB concentrations across the interquartile range.
| haemophilus influenzae type b |
tetanus toxoid |
diphtheria toxoid |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB Measure | N | % change | 95% CI | p-value | N | % change | 95% CI | p-value | N | % change | 95% CI | p-value |
| Maternal PCB | ||||||||||||
| PCB 118 | 316 | 4.7 | −13.9, 27.4 | 0.64 | 329 | −2.2 | −19.3, 18.6 | 0.82 | 306 | 2.9 | −12.8, 21.4 | 0.73 |
| PCB 138+163 | 316 | −5.6 | −22.6, 15.0 | 0.56 | 329 | 1.4 | −16.0, 22.5 | 0.88 | 306 | −2.3 | −16.0, 13.5 | 0.76 |
| PCB 153 | 316 | −3.0 | −21.0, 19.1 | 0.77 | 329 | 2.5 | −15.3, 24.1 | 0.80 | 306 | −1.5 | −15.7, 15.0 | 0.84 |
| PCB 156+171 | 316 | −0.9 | −16.9, 18.1 | 0.92 | 329 | −2.9 | −19.3, 16.8 | 0.75 | 306 | −10.5 | −22.7, 3.6 | 0.14 |
| PCB 170 | 316 | −8.6 | −24.6, 10.8 | 0.36 | 329 | −4.2 | −21.9, 17.6 | 0.68 | 306 | −7.0 | −21.4, 10.1 | 0.40 |
| PCB 180 | 316 | −8.7 | −24.7, 10.9 | 0.36 | 329 | −4.9 | −22.7, 17.1 | 0.64 | 306 | −6.1 | −20.8, 11.3 | 0.47 |
| Sum of 6 | 316 | −6.8 | −23.4, 13.3 | 0.48 | 329 | −1.7 | −19.5, 20.1 | 0.87 | 306 | −4.5 | −18.9, 12.5 | 0.58 |
| Cord PCB | ||||||||||||
| PCB 138+163 | 309 | −5.1 | −22.7, 16.6 | 0.62 | 322 | 4.9 | −14.0, 27.9 | 0.64 | 300 | −3.7 | −18.1, 13.2 | 0.64 |
| PCB 153 | 309 | −1.3 | −20.8, 23.1 | 0.91 | 322 | 4.9 | −14.7, 29.1 | 0.65 | 300 | −2.0 | −17.3, 16.1 | 0.82 |
| PCB 170 | 309 | −5.3 | −18.8, 10.5 | 0.49 | 322 | 1.2 | −14.1, 19.2 | 0.89 | 300 | −7.4 | −19.5, 6.5 | 0.28 |
| PCB 180 | 309 | −3.9 | −20.2, 15.7 | 0.67 | 322 | 0.7 | −18.0, 23.6 | 0.95 | 300 | −5.3 | −20.3, 12.5 | 0.53 |
| Sum of 4 | 309 | −4.3 | −20.9, 15.9 | 0.66 | 322 | 2.6 | −16.2, 25.6 | 0.81 | 300 | −4.5 | −19.4, 13.0 | 0.59 |
| 6-Month PCB | ||||||||||||
| PCB 138+163 | 211 | −8.3 | −31.7, 23.0 | 0.56 | 217 | 6.1 | −18.2, 37.6 | 0.66 | 198 | −15.2 | −34.8, 10.1 | 0.21 |
| PCB 153 | 211 | −7.0 | −29.6, 22.7 | 0.60 | 217 | 7.7 | −16.1, 38.3 | 0.56 | 198 | −16.6 | −35.5, 8.0 | 0.17 |
| PCB 170 | 211 | −8.1 | −28.9, 18.9 | 0.52 | 217 | −0.5 | −21.2, 25.6 | 0.96 | 198 | −14.5 | −32.0, 7.4 | 0.18 |
| PCB 180 | 211 | −2.5 | −27.2, 30.6 | 0.87 | 217 | 2.8 | −20.6, 33.2 | 0.83 | 198 | −15.3 | −34.4, 9.5 | 0.20 |
| Sum of 4 | 211 | −6.4 | −30.0, 25.1 | 0.65 | 217 | 5.4 | −18.8, 36.9 | 0.69 | 198 | −16.6 | −35.9, 8.7 | 0.18 |
Maternal and cord PCB analyses adjusted for: Romani ethnicity (vs. other), maternal smoking before or during pregnancy (yes/no), and maternal age at child's birth (years). Infant PCB analyses adjusted for: sex, Romani ethnicity (vs. other), maternal smoking at 6 months (yes/no), maternal age at child's birth (years), and infant's age at the time of blood draw (days).
We also fit models for maternal, cord, and infant 6-month PCBs where we adjusted for child's age at the time of blood draw (maternal and cord PCB models), number of vaccine doses received, time since most recent vaccination, infant sex (maternal and cord PCB models), and Z-score birth weight, in addition to the variables in our primary model. Overall, our results did not change appreciably with the addition of these variables, and no noteworthy associations were observed after the additional covariates were included into the model.
Discussion
We observed little evidence of an association between measures of PCB exposure during the prenatal and early postnatal period and post-vaccination antibody concentrations measured at 6-months of age. These null findings were consistent across the various sampling periods of PCB concentration (maternal, cord, and 6-month infant), and our results showed no consistent pattern with regard to a particular congener or the various antibodies assayed.
Much of the experimental work concerning the potential effects of TCDD and PCBs on the immune system has shown these compounds to be immunosuppressive, both in vivo and in vitro (though there are notable exceptions27). For TCDD, these findings include thymic atrophy and suppression of B-cell responsiveness,28, 29 and most relevant, decreases in antibody response in relation to gestational exposure to TCDD.30 Existing epidemiologic evidence also supports the potential immunosuppressive effects of PCBs. For instance, in the same cohort of Slovak infants, Park and colleagues18 found lower thymic indices among newborns with higher total maternal PCB concentrations. In a study of Dutch infants environmentally-exposed to PCBs, 207 infants were followed longitudinally with immunologic assessments of antibody levels to measles, mumps, and rubella at 18 and 42 months of age.14, 15, 31 At age 18 months, these authors found no association between pre- or postnatal PCB exposure and any of the antibody levels. However at 42-months, antibody levels to mumps were inversely associated with total maternal PCB concentrations (r= -0.17), and antibody levels to rubella were inversely associated with cord total PCB concentrations (r= -0.19). A recent study from the Faroe Islands also examined preand postnatal PCB exposures in relation to antibody levels among two cohorts of 129 and 119 children.16 Results across the two cohorts were relatively consistent, and suggested that PCB exposures, particularly during the early postnatal period, were associated with lower antibody responses to diphtheria and tetanus vaccines. The authors estimated a 24% lower diphtheria toxoid antibody level at age 18 months for each doubling of prenatal PCB exposure, and a 17% lower level of tetanus toxoid antibody at 7 years of age for each doubling of prenatal PCB exposure. An inverse association was also observed for maternal dioxin-like exposures and diphtheria antibody concentration, but not for tetanus.16 Given the consistency of these immunosuppressive findings across studies, how can our null findings be reconciled?
One possibility concerns the age at which we assessed antibody response. At 6 months of age, the infant immune system is developing rapidly, but it is still immature. For instance, although infants are able to produce IgG-specific antibodies from the time of birth (and even in utero), adult-like IgG repertoires and antibody concentrations do not develop until slightly later in infancy, beginning at approximately 8 months of age.32 As a result, it may be difficult to detect a “signal” at a time when the immune response lacks the same quality and magnitude of a response later in infancy. Considering age at assessment in terms of the existing literature on PCBs and antibody response, in both the Dutch and Faroe Islands cohorts, infants and children were examined later in development: at 18 and 42 months in the Dutch study, and at 18 months and 7 years of age in the Faroe Islands cohort. Immunosuppressive associations were observed at both 18 months and 7 years in the Faroe Islands study, and at 42 months in the Dutch study, but not at 18 months, suggesting the possibility that age of assessment may play a role in the ability to detect an association between PCB exposure and antibody response.
A second consideration is that comparisons between our study and the results of the Faroese and Dutch cohorts must take into account the levels of maternal PCB exposure among participants. In our study, we observed maternal PCB-153 concentrations of 153 ng/g lipid; concentrations similar to those in the United States during the 1950's and 1960's when PCBs were still being produced.33, 34 In comparison, PCB-153 concentrations in the Faroe Islands cohort (where immunosuppressive associations were observed) were approximately 3 times greater (450 ng/g lipid).16, 34 When a benchmark dose analysis was performed by Heilmann and colleagues,16 they estimated the lower 95% confidence limit for prenatal PCB exposure to be 1.14 μg/g lipid for diphtheria antibody measured at age 18 months. If we estimate prenatal PCB exposure based on the formula used by Heilmann and colleagues (2-times the sum of maternal PCB-138, 153, and 180), a concentration of 1.14 μg/g lipid corresponds to approximately the 75th percentile of maternal PCB exposure in our population. Thus to the extent that there is a threshold for prenatal PCB exposure and early postnatal effects on antibody response, the concentrations in our study are unlikely to be sufficiently high to observe such an association. A threshold hypothesis is also supported by the early postnatal antibody findings from the Dutch cohort. In that study, PCB-153 concentrations in maternal serum were approximately 100 ng/g lipid,34 and no statistically significant associations were observed between pre- or postnatal PCB/dioxin exposures and antibody concentrations to measles, mumps, or rubella at age 18 months.
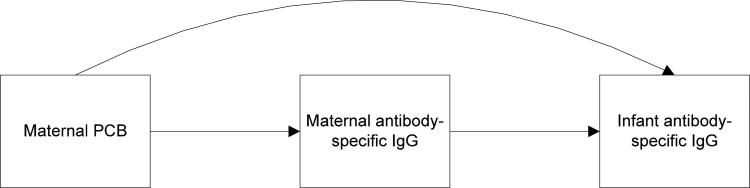
A final point to consider may be the potential effects of maternal antibody transfer on the infant antibody response at 6 months of age. During the 2nd trimester of gestation, placental transfer of maternal IgG begins, reaching a maximum during the 3rd trimester. After birth, maternally-derived infant IgG concentrations decrease throughout the first year of life, and the infant's own IgG synthesis begins.35 Thus, maternally-derived antigen-specific IgG is already circulating through the infant's immune system during the period of primary vaccination. This issue is illustrated graphically in Figure 1, which depicts the potential pathways by which maternal PCBs could affect infant antibody response. In the first path (curved, top arrow), maternal PCB concentration has a direct effect on infant antibody concentration. In this scenario, one would hypothesize that higher maternal PCB concentrations are associated with lower infant antibody concentrations. In the second pathway, maternal antibody concentrations (i.e. transplacental transfer) are on the causal pathway between maternal PCBs and infant antibody response. In this pathway, one could hypothesize that higher maternal PCBs lead to lower maternal antibody-specific IgG levels, which then result in higher antibody-specific IgG concentrations in the infant after vaccination (since priming with maternal antibodies in the infant serves to suppress the infant immune response to vaccination36-42). In fact, this scenario has been observed in goats and their offspring who were exposed to PCB-153 during fetal development. Specifically, maternal doses of PCB-153 (during pregnancy) led to decreases in maternal antibodies to influenza virus, which in turn resulted in increased influenza antibody concentrations in the goat kid following vaccination with influenza.11 Taken as a whole, increased maternal PCB concentrations could be exerting opposite effects on antibody response in the infant (in one case decreasing infant antibody response through a direct effect, and in a second case increasing infant antibody concentrations through another pathway—transplacental transfer).
Figure 1.
Pathways by which maternal PCBs may alter infant antibody response at 6 months of age.
There are however a few factors which would argue against the “competing” effects of maternal antibody-specific IgG concentrations in this study. First, 85% of the infants in our study received 2 doses of vaccine at the time of the 6-month blood draw. This is relevant since the suppressive effects of maternal antibodies tend to attenuate with subsequent doses of vaccine.37, 41 Further, when we stratified our results by the number of doses received, we saw no difference in the association between maternal PCBs and antibody response comparing infants who received 1 versus 2 doses of vaccine. If maternal antibody transfer did play a role in altering infant antibody response, we would have observed different associations in each group defined by the number of doses received (since the effect of transplacental transfer on antibody response tends to decrease with dose).
The lack of immunosuppressive results observed in this study would appear to contradict findings from a previous study done in this cohort. In that study, an increase in total maternal PCB concentration across the interquartile range was associated with a 3% decrease in thymus volume.18 In a secondary data analysis, we examined the association between infant thymus size at birth and 6-months of age in relation to the 6-month IgG antibody measures reported here and found no association. This finding, that size of thymus does not predict antibody response during early infancy is consistent with the results from prospective studies of infants undergoing thymectomy, who do not show differences in subsequent antibody response compared to controls that did not undergo the procedure.43, 44
Despite the size of our study, some limitations of our study deserve attention. First, we were limited by the number of PCB determinations that could be carried out in 6-month infant serum samples. As a result, the statistical power to detect some associations between 6-month PCB concentrations and antibody response was limited. This may be particularly important given that results from the study by Heilmann and colleagues16 suggest that postnatal PCB exposures are most strongly associated with children's antibody response. Finally, we were unable to quantify coplanar dioxin-like congeners in maternal, cord, and 6-month samples. This limitation was the result of the lower concentrations of serum lipids in cord and 6-month serum samples (compared to maternal serum), the low volume of blood collected from 6-month-old infants, and the GC-ECD method we used to quantify PCBs, which was more efficient and cost-effective given the number of specimens we analyzed, compared to high-resolution mass spectrometry.
At the PCB concentrations present in this population, we observed little evidence for an association between PCB exposures measured during the prenatal and early postnatal period and antibody response to anti-haemophilus influenzae type b, tetanus toxoid, and diphtheria toxoid at 6 months of age. Though our findings are null, our results add needed information concerning the role of developmental PCB exposure on T-cell dependent immune responses—which in human populations, is particularly limited. The possibility of other immunologic alterations in relation to PCBs later in development remains.
Acknowledgements
The authors wish to thank Dr. Dana Jureckova who abstracted all the infant medical records to obtain dates of vaccination. In addition, we wish to thank Dr. Allen Silverstone who served on the scientific advisory committee for this study and who encouraged us to examine T-cell dependent measures of immunity. We also wish to thank Dr. Troy Torgerson for helpful discussions about the developing immune system, and Jan Petrik, Zhiwei Yu, and Hye-Youn Park who were instrumental in getting the early parts of this project organized.
Grant information: This research received support from National Institutes of Health grants T32-ES007262, T32-RR023256, U01-ES016127, and R01-CA096525 and from the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. Support was also provided by a dissertation award from the University of Washington Department of Epidemiology and a Fulbright Grant from the US State Department. This work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ATSDR . Toxicological Profile for Polychlorinated Biphenyls (PCBs) Agency for Toxic Substances and Disease Registry; Atlanta, GA: Nov, 2000. 2000. [PubMed] [Google Scholar]
- 2.Fangstrom B, Strid A, Grandjean P, Weihe P, Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health. 2005;4:12. doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen L, DeCaprio A, Nisbet I. PCB congener comparisons reveal exposure histories for residents of Anniston, Alabama, USA. Fresenius Envir Bull. 2003;12:181–90. [Google Scholar]
- 4.Kocan A, Petrik J, Jursa S, Chovancova J, Drobna B. Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere. 2001;43(4-7):595–600. doi: 10.1016/s0045-6535(00)00411-2. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Linderholm L, Charles MJ, et al. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBS) in pregnant women from eastern Slovakia. Environmental health perspectives. 2007;115(1):20–7. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallaire F, Dewailly E, Muckle G, et al. Acute infections and environmental exposure to organochlorines in Inuit infants from Nunavik. Environmental health perspectives. 2004;112(14):1359–65. doi: 10.1289/ehp.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Critical reviews in toxicology. 1994;24(2):87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- 8.Silkworth JB, Antrim L, Kaminsky LS. Correlations between polychlorinated biphenyl immunotoxicity, the aromatic hydrocarbon locus, and liver microsomal enzyme induction in C57BL/6 and DBA/2 mice. Toxicol Appl Pharmacol. 1984;75(1):156–65. doi: 10.1016/0041-008x(84)90086-3. [DOI] [PubMed] [Google Scholar]
- 9.Tryphonas H, Feeley M. Polychlorinated Biphenyl-induced immunomodulation and human health effects. In: Robertson LW, Hansen LG, editors. PCBs: Recent advances in environmental toxicology and health effects. The University Press of Kentucky; Lexington, KY: 2001. [Google Scholar]
- 10.Lyche J, Larsen H, Skaare JU, et al. Effects of perinatal exposure to low doses of PCB 153 and PCB 126 on lymphocyte proliferation and hematology in goat kids. Journal of toxicology and environmental health. 2004;67(11):889–904. doi: 10.1080/15287390490443740. [DOI] [PubMed] [Google Scholar]
- 11.Lyche JL, Larsen HJ, Skaare JU, Tverdal A, Johansen GM, Ropstad E. Perinatal exposure to low doses of PCB 153 and PCB 126 affects maternal and neonatal immunity in goat kids. Journal of toxicology and environmental health. 2006;69(1-2):139–58. doi: 10.1080/15287390500259418. [DOI] [PubMed] [Google Scholar]
- 12.Dietert RR, Holsapple MP. Methodologies for developmental immunotoxicity (DIT) testing. Methods. 2007;41(1):123–31. doi: 10.1016/j.ymeth.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Luster MI, Johnson VJ, Yucesoy B, Simeonova PP. Biomarkers to assess potential developmental immunotoxicity in children. Toxicology and Applied Pharmacology. 2005;206(2):229–36. doi: 10.1016/j.taap.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Weisglas-Kuperus N, Patandin S, Berbers GA, et al. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environmental health perspectives. 2000;108(12):1203–7. doi: 10.1289/ehp.001081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisglas-Kuperus N, Sas TC, Koopman-Esseboom C, et al. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr Res. 1995;38(3):404–10. doi: 10.1203/00006450-199509000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jorgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3(8):e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonneborn D, Park HY, Babinska K, et al. Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. Journal of exposure science & environmental epidemiology. 2008;18(6):581–7. doi: 10.1038/jes.2008.1. [DOI] [PubMed] [Google Scholar]
- 18.Park HY, Hertz-Picciotto I, Petrik J, Palkovicova L, Kocan A, Trnovec T. Prenatal PCB exposure and thymus size at birth in neonates in Eastern Slovakia. Environmental health perspectives. 2008;116(1):104–9. doi: 10.1289/ehp.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conka K, Drobna B, Kocan A, Petrik J. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. Journal of chromatography. 2005;1084(1-2):33–8. doi: 10.1016/j.chroma.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Kocan A, Petrik J, Drobna B, Chovancova J. Levels of PCBs and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. I. Blood. Chemosphere. 1994;29(9-11):2315–25. doi: 10.1016/0045-6535(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 21.Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clinica chimica acta; international journal of clinical chemistry. 1989;184(3):219–26. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 22.Takayama M, Itoh S, Nagasaki T, Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clinica chimica acta; international journal of clinical chemistry. 1977;79(1):93–8. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- 23.Janeway C. Immunobiology : the immune system in health and disease. 6th ed. Garland Science; New York: 2005. [Google Scholar]
- 24.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental health perspectives. 2004;112(17):1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 26.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 27.Vorderstrasse BA, Bohn AA, Lawrence BP. Examining the relationship between impaired host resistance and altered immune function in mice treated with TCDD. Toxicology. 2003;188(1):15–28. doi: 10.1016/s0300-483x(02)00749-7. [DOI] [PubMed] [Google Scholar]
- 28.Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. International immunopharmacology. 2002;2(2-3):277–91. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence BP, Kerkvliet NI. Immune modulation by TCDD and related polyhalogenated aromatic hydrocarbons. In: Luebke RW, House RV, Kimber I, editors. Immunotoxicology and immunopharmacology. Third ed. CRC Press; Boca Raton, FL: 2006. pp. 239–58. [Google Scholar]
- 30.Vorderstrasse BA, Cundiff JA, Lawrence BP. Developmental Exposure to the Potent Aryl Hydrocarbon Receptor Agonist 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Impairs the Cell-Mediated Immune Response to Infection with Influenza A Virus, but Enhances Elements of Innate Immunity. Journal of immunotoxicology. 2004;1(2):103–12. doi: 10.1080/15476910490509244. [DOI] [PubMed] [Google Scholar]
- 31.Weisglas-Kuperus N, Vreugdenhil HJ, Mulder PG. Immunological effects of environmental exposure to polychlorinated biphenyls and dioxins in Dutch school children. Toxicol Lett. 2004;149(1-3):281–5. doi: 10.1016/j.toxlet.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19(25-26):3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 33.James RA, Hertz-Picciotto I, Willman E, Keller JA, Charles MJ. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the child health and development study cohort, 1963-1967. Environmental health perspectives. 2002;110(7):617–24. doi: 10.1289/ehp.02110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longnecker MP, Wolff MS, Gladen BC, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environmental health perspectives. 2003;111(1):65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stiehm ER, Ochs HD, Winkelstein JA. Immunologic disorders in infants & children. 5th ed. W.B. Saunders; Philadelphia, Pa.: 2004. [Google Scholar]
- 36.Englund JA, Glezen WP. Maternal immunization with Haemophilus influenzae type b vaccines in different populations. Vaccine. 2003;21(24):3455–9. doi: 10.1016/s0264-410x(03)00350-5. [DOI] [PubMed] [Google Scholar]
- 37.Bjorkholm B, Granstrom M, Taranger J, Wahl M, Hagberg L. Influence of high titers of maternal antibody on the serologic response of infants to diphtheria vaccination at three, five and twelve months of age. The Pediatric infectious disease journal. 1995;14(10):846–50. doi: 10.1097/00006454-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Sarvas H, Kurikka S, Seppala IJ, Makela PH, Makela O. Maternal antibodies partly inhibit an active antibody response to routine tetanus toxoid immunization in infants. The Journal of infectious diseases. 1992;165(5):977–9. doi: 10.1093/infdis/165.5.977. [DOI] [PubMed] [Google Scholar]
- 39.Nohynek H, Gustafsson L, Capeding MR, et al. Effect of transplacentally acquired tetanus antibodies on the antibody responses to Haemophilus influenzae type b-tetanus toxoid conjugate and tetanus toxoid vaccines in Filipino infants. The Pediatric infectious disease journal. 1999;18(1):25–30. doi: 10.1097/00006454-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Kurikka S, Olander RM, Eskola J, Kayhty H. Passively acquired anti-tetanus and anti-Haemophilus antibodies and the response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infancy. The Pediatric infectious disease journal. 1996;15(6):530–5. doi: 10.1097/00006454-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Barington T, Gyhrs A, Kristensen K, Heilmann C. Opposite effects of actively and passively acquired immunity to the carrier on responses of human infants to a Haemophilus influenzae type b conjugate vaccine. Infection and immunity. 1994;62(1):9–14. doi: 10.1128/iai.62.1.9-14.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Englund JA. The influence of maternal immunization on infant immune responses. Journal of comparative pathology. 2007;137(Suppl 1):S16–9. doi: 10.1016/j.jcpa.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Eysteinsdottir JH, Freysdottir J, Haraldsson A, et al. The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life. Clinical and experimental immunology. 2004;136(2):349–55. doi: 10.1111/j.1365-2249.2004.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torfadottir H, Freysdottir J, Skaftadottir I, Haraldsson A, Sigfusson G, Ogmundsdottir HM. Evidence for extrathymic T cell maturation after thymectomy in infancy. Clinical and experimental immunology. 2006;145(3):407–12. doi: 10.1111/j.1365-2249.2006.03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]