Abstract
In a previous study, we reported that overexpression of CDK4 in mouse epidermis results in epidermal hyperplasia, hypertrophy and severe dermal fibrosis. In this study, we have investigated the susceptibility to skin tumor formation by forced expression of CDK4. Skin tumors from transgenic mice showed a dramatic increase in the rate of malignant progression to squamous cell carcinomas (SCC) in an initiation-promotion protocol. Histopathological analysis of papillomas from transgenic mice showed an elevated number of premalignant lesions characterized by dysplasia and marked atypia. Interestingly, transgenic mice also developed tumors in initiated but not promoted skin, demonstrating that CDK4 replaced the action of tumor promoters. These results suggest that expression of cyclin D1 upon ras activation synergizes with CDK4 overexpression. However, cyclin D1 transgenic mice and double transgenic mice for cyclin D1 and CDK4 did not show increased malignant progression in comparison to CDK4 transgenic mice. Biochemical analysis of tumors showed that CDK4 sequesters the CDK2 inhibitors p27Kip1 and p21Cip1 suggesting that indirect activation of CDK2 plays an important role in tumor development. These results indicate that, contrary to the general assumption, the catalytic subunit, CDK4, has higher oncogenic activity than cyclin D1, revealing a potential use of CDK4 as therapeutic target.
Introduction
Studies in cell culture, human patients and mouse models have shown that numerous regulators of the cell cycle are targets for genetic alterations in cancer, or are disrupted secondarily by other oncogenic events (Malumbres et al., 2000; Roussel, 1999). In the last few years a consensus paradigm of the cell cycle has been developed (Nevins et al., 1991; Pines, 1995; Sherr, 1995a; Sherr, 1994). According to this paradigm, the master switch of the cell cycle is the Rb family of proteins. Proliferation is turned on by phosphorylation of these proteins by cyclin-dependent kinases 4, 6 and 2 (Sherr, 1995b; Sherr & Roberts, 1995). D-type cyclins bind and activate CDK4,6 whereas CDK2 is activated by binding cyclins A and E. In addition, CDKs are inhibited by two families of CDK-inhibitors (CKIs), the Ink (p16Ink4a, p15Ink4b, p18Ink4c, p19Ink4d) and Cip/Kip families (p21Cip1, p27Kip1 and p57Kip2) (Sherr & Roberts, 1995; Xiong, 1996). After Rb phosphorylation by CDKs, E2F proteins are released from pRb complexes and promote the transcription of genes essential for transition into the S phase of cell cycle (Nevins, 1992; Sherr, 1994).
In the last few years, work from our group, as well as other laboratories have shown that cyclins and CDK complexes are mechanistically involved in the development of human and experimental epidermal tumors (Jacks & Weinberg, 1998; Motokura & Arnold, 1993; Rodriguez-Puebla et al., 1999b; Weinberg, 1996). The inactivation of pRb is produced by direct mutation of the Rb protein, but this is a relatively rare event, except occurrences in retinoblastomas and osteosarcomas, a minority of breast carcinomas, and some other tumors (Hunter & Pines, 1994; Sherr, 1996). More frequent alterations of this pathway occur by functional inactivation of Rb by hyperphosphorylation. This is normally the result of elevated CDKs activities caused by overexpression of cyclins, CDKs, or the loss of function of CKIs, the most common being the deletion of p16Ink4a. The involvement of CDK4 in the neoplastic process is suggested by the observation that CDK4 amplification and/or overexpression occur in human gliomas, sporadic breast carcinomas (An et al., 1999) and sarcomas (Kanoe et al., 1998). In addition, an activating CDK4 mutation (CDK4 R24C) was identified in patients with familial melanoma (Wölfel et al., 1995; Zuo, 1996). A mouse model bearing that mutation displayed pancreatic hyperplasia and resulted in a wide spectrum of tumor development (Rane et al., 2002; Rane et al., 1999). These mice also developed invasive melanoma upon DMBA/TPA treatment (Sotillo et al., 2001b) and showed increased incidence of papillomas (Rane et al., 2002). It is clear that activating mutations of CDK4 are an important part of the study of the oncogenic effect of CDK4 (Wölfel et al., 1995; Zuo, 1996). However, amplification and/or overexpression of the wild type form of CDK4 has also been observed in human tumors, but a model for studying its effect in tumorigenesis has not been described yet. Thus, transgenic expression of CDK4 in mouse epidermis allows us to determine the role of CDK4 in a well-known model of tumor biology. The mouse skin carcinogenesis model has been extensively used in genetic toxicology, carcinogenesis (Berenblum, 1954; Boutwell, 1964; DiGiovanni, 1992; Slaga, 1989; Yuspa et al., 1990), and also studies using the pathobiology of squamous cell carcinomas (SCC) (Conti, 1992).
The first indication of cell cycle alterations in the mouse skin carcinogenesis system was the observation that cyclin D1 was overexpressed in mouse skin papillomas and carcinomas induced by the two stage protocol (Bianchi et al., 1993; Robles & Conti, 1995; Rodriguez-Puebla et al., 1998). Furthermore, we have provided evidence that cyclin D1 is essential for ras transformation of keratinocytes in three different carcinogenesis models (Robles et al., 1998; Rodriguez-Puebla et al., 1999c).
We have previously reported that forced expression of CDK4 in mouse epidermis led to phenotypic abnormalities, such as hyperplasia and hypertrophy and severe dermal fibrosis (Miliani de Marval et al., 2001). Here, we have investigated the susceptibility to chemically induced skin tumor formation. The CDK4 transgenic mice show enhanced squamous cell carcinomas (SCC) development compared to wild type controls when DMBA was used as initiator and 12-O-tetradecanoylphorbol-13-acetate (TPA) as a tumor promoter. Moreover, CDK4 overexpression results in tumor development in initiated but not promoted skin. Consistent with these data, we have recently shown that lack of CDK4 results in complete abrogation of chemically induced tumorigenesis (Rodriguez-Puebla et al., 2002). Histopathological analysis of benign tumors from K5-CDK4 transgenic mice showed an elevated number of premalignant lesions characterized by areas of dysplasia and marked atypia. These results demonstrate that in the early stages of tumor development in which overexpression of D-type cyclins is not detected, the elevated expression of CDK4 can cause a predisposition to tumor development. Hence, we have hypothesized that early stages of papilloma development are independent of D-type cyclins levels, although, later expression of cyclin D1 could synergize with the elevated level of CDK4. Contrary to this prediction, double transgenic mice for cyclin D1 and CDK4 did not show increased malignant conversion compared to CDK4 transgenic mice. Moreover, the oncogenic activity of CDK4 and the abrogation of CDK4 tumorigenesis in mice lacking CDK4 (Rodriguez-Puebla et al., 2002) suggest that this molecule has the properties to act as a target for therapeutic strategies.
Results
CDK4 overexpression results in enhanced malignant progression
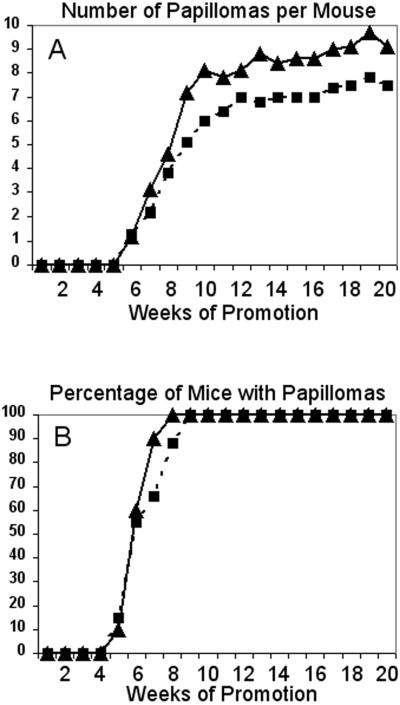
The Cyclin Dependent Kinase-4 (CDK4) has been found overexpressed or amplified in several human tumors, in which amplification or overexpression of cyclin D1 was not detected (Schmit et al., 1994; Sonoda et al., 1995). Therefore, we have used the mouse skin two-stage carcinogenesis model as an experimental approach to determine how CDK4 overexpression influences tumor development. We used the previously described K5-CDK4 transgenic lines which showed 2.5-fold (K5-CDK4-2305) and 7.5 fold (K5-CDK4-2303) increase expression of CDK4 in the basal cell layer of epidermis (Miliani de Marval et al., 2001). The dorsal skin of K5-CDK4 transgenic mice and wild type littermates were topically treated with a subcarcinogenic dose of the genotoxic carcinogen DMBA and later promoted with the phorbol ester TPA. Papilloma development began at five weeks of promotion in both transgenic lines and wild type littermates (Figure 1). The average number of papillomas per mouse (multiplicity) reached a plateau by 12 weeks. We observed a slight increase in the number of papillomas in K5-CDK4-2303 transgenic mice compared with wild type siblings, although it is not statistically significant (Figure 1A). Similarly, the transgenic line with lower expression (Line 2305) did not show differences in the number of papillomas per mouse between transgenic and wild type mice (Table 1). The incidence of papilloma development was 100% in both transgenic and wild type mice (Fig 1B, Table 1).
Figure 1.
Kinetics of papilloma formation in K5-CDK4 transgenic mice.
Groups of K5-CDK4 transgenic (Line 2303) and wild type sibling mice were initiated with DMBA and promoted with multiple applications of TPA on dorsal mouse skin. (A) Average number of papillomas per mouse as a function of weeks of study. (B) Percentage of mice with papillomas as a function of weeks of study. K5-CDK4 (▲), and Wild type (■) mice.
Table 1.
Summary of papilloma and carcinoma development in K5-CDK4 and normal sibling mice
| Papilloma Multiplicity3 |
Papilloma Incidence4 | SCC Multiplicity5 | SCC incidence6 | |
|---|---|---|---|---|
| K5-CDK4-2305 (n=28) | 7.5 | 100% | 0.9 | 85% |
| Wt-2305 (n=30) 1 | 8 | 100% | 0.2 | 21% |
| K5-CDK4-2303 (n-29) | 9 | 100% | 1.9 | 96% |
| Wt-2303 (n=30) 2 | 7.5 | 100% | 0.1 | 15% |
Wild type sibling mice from the K5-CDK4-2305 transgenic line.
Wild type sibling mice from the K5-CDK4-2303 transgenic line.
Average number of papillomas per mice
Percentage of mice with papilloma
Average number of SCC per mouse
Percentage of mice with SCC
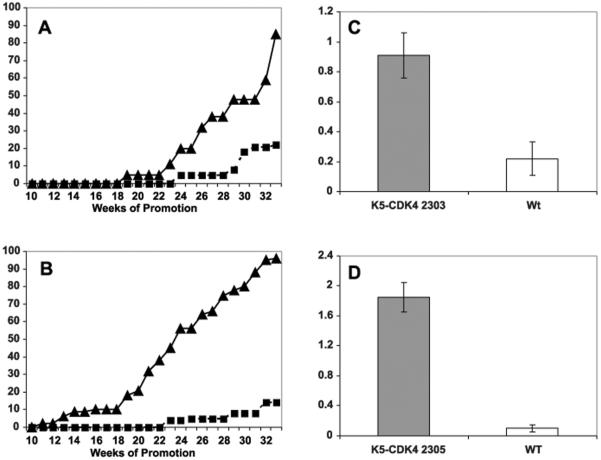
The fact that overexpression of CDK4 did not result in an elevated number of papillomas suggest that CDK4 expression does not enhance tumor development under conditions where ras protein is constitutively activated (ras mutation induced by DMBA treatment). On the other hand, forced expression of CDK4 results in dramatic changes in the number of tumors that progress to SCC. It is known that in SSIN mice, a markedly reduced number of papillomas progress to SCC after a long period of promotion (30 to 60 weeks) (Slaga, 1989). Figure 2 shows the average cumulative number of SCC per mouse (multiplicity) and the percentage of mice with SCC (incidence). After 24 weeks of promotion, 3% of wild type mice developed SCC whereas 20% of the transgenic mice developed SCC at the same time-point. The incidence of SCC in wild type sibling mice reached a plateau at 31 weeks of promotion where 21% of wild type mice developed SCC (Figure 2A). Over the same period of time 47% of the transgenic mice developed SCC and a plateau of 85% was reached at 33 weeks of promotion (Chi-square test, p<0.0001) (Figure 2A and Table 1). The average number of SCC per mouse at 33 weeks of promotion was also significantly different. In this regard, a significantly lower number of SCC was observed in wild type mice (0.2 per mouse) compare to a 0.9 SCC per mouse in K5-CDK4-2305 transgenic mice (t test, p<0.0001) (Figure 2C and Table 1). The two-stage carcinogenesis experiment was repeated with the transgenic line of higher CDK4 expression (line 2303). In this case, the changes observed in the malignant progression to SCC were much more dramatic. The number of carcinomas in wild type siblings was similar to the previous experiment. Strikingly, at 33 weeks of promotion 96% of the K5-CDK4-2303 transgenic mice developed SCC (Chi-square test, p<0.0001) (Figure 2B and Table 1). At the same time-point, the average number of SCC per mouse in these transgenic animals (1.9) was significantly higher than in normal siblings (0.1) (t test, p<0.0001) (Figure 2D and Table 1). This data clearly indicates that there is a dose-effect correlation between the level of CDK4 expression and the rate of malignant progression.
Figure 2.
Kinetics of carcinoma (SCC) formation in K5-CDK4 transgenic mice.
Groups of transgenic and wild type sibling mice were initiated with DMBA and promoted with multiple applications of TPA on dorsal mouse skin. (A) K5-CDK4 (line 2305), (B) K5-CDK4 (line 2303), percentage of mice with carcinomas as a function of weeks of study (incidence). (C) K5-CDK4 (line 2305), (D) K5-CDK4 (line 2303), average number of carcinomas per mice at 33 weeks of promotion (multiplicity).
(A, B), K5-CDK4 (▲) and Wild type (■) mice. (C, D), K5-CDK4 (solid bars) and wild type (empty bars) mice.
No spontaneous skin tumors developed in untreated skin from transgenic mice aged for 18 months. Strikingly, 6 out of 8 (75%) K5-CDK4 transgenic mice initiated with DMBA but not promoted, developed papillomas that later progressed to very aggressive squamous cell carcinomas. No tumors were observed in wild type siblings with the same protocol. This result is consistent with our previous observation that epidermis from K5-CDK4 transgenic mice expresses keratin 6 (a marker of hyperproliferation) (Miliani de Marval et al., 2001), suggesting that CDK4 could itself act as a tumor promoter.
Pathological analysis of K5-CDK4 tumors
The malignant conversion to SCC was determined by macroscopic observation of mouse skin tumors and further confirmed by histopathological analysis of paraffin sections. In order to determine whether overexpression of CDK4 induces early premalignant changes in the mouse papillomas, we have studied the histopathologic aspect of the papillomas at 30 weeks of promotion. We took into account the dysplastic and anaplastic changes that included disturbed cell polarity (mainly on the basal cell layer), basal cell hyperplasia, disturbed differentiation sequence, increased number of mitosis, mitosis in suprabasal layers, abnormal mitosis, nuclear hyperchromatism, prominent nucleoli, and increased nuclear/cytoplasmic ratio (Aldaz & Conti, 1989).
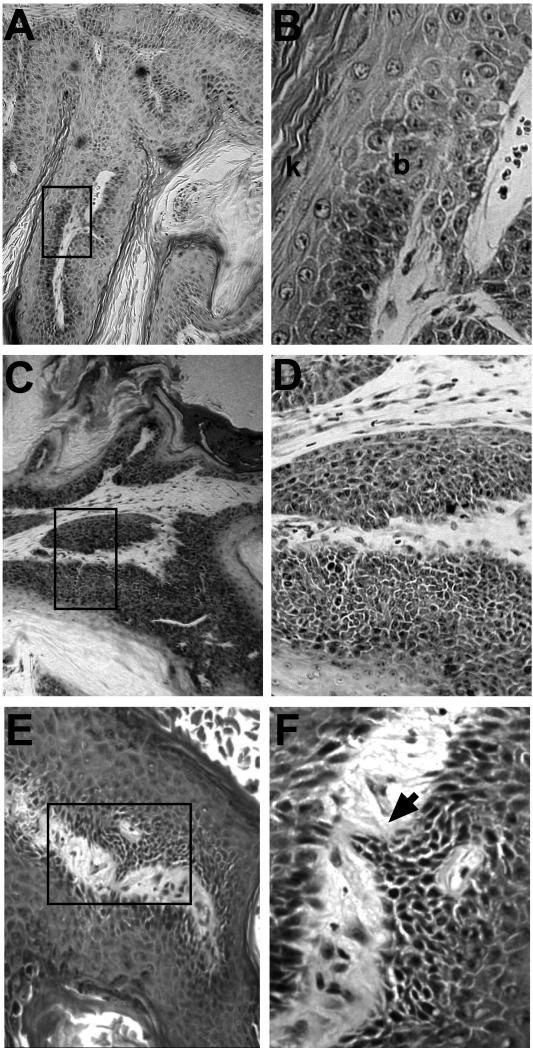
Strikingly, 53% of the papillomas from K5-CDK4-2305 mice presented marked atypia in all layers and lack of the differentiation pattern (intrapapilloma carcinoma and carcinoma in situ) with areas of microinvasion (Table 2). Also, 27% of the transgenic papillomas present atypia in basal and some suprabasal layers (severely dysplastic papilloma) (Figure 3, Table 2). In contrast, only 12% of wild type papillomas showed areas of intrapapilloma carcinoma (Table 2) whereas most of wild type papillomas were regular or moderately dysplastic (41% and 47%) (Figure 3 and Table 2). On the other hand, only 7% and 13% of the transgenic papillomas were classified on those categories (Table 2). We have also analyzed modifications in the pattern of keratin expressions as premalignant and malignant markers of tumorigenesis. Toftgard et al. have demonstrated that carcinomas contain very low levels of differentiation-associated keratins, i.e., K1 and K10 (Tofgard et al., 1985). We found reduced expression of these keratins in both transgenic and wild type papillomas correlating with the degree of transformation, although no significant differences were observed between transgenic and wild type mice (data not shown).
Table 2.
Percentage of papillomas with different degrees of atypia at 30 weeks of promotion
| Genotype | Regular Papillomas 1 |
Moderately dysplastic papillomas 2 |
Severely dysplastic papillomas 3 |
Intrapapilloma carcinoma 4 |
|---|---|---|---|---|
| K5-CDK4 (n=15) | 1/15 (7%) | 2/15 (13%) | 4/15 (27%) | 8/15 (53%) |
| Wild type (n=17) | 7/17 (41%) | 8/17 (47%) | 0/17 (0%) | 2/17 (12%) |
Chi-square, p=0.0013
No atypia in basal layer
Atypia in basal and some suprabasal layer
Atypia in all layers
Marked atypia in all layers and lack of differentiation pattern (carcinoma in situ). Areas of microinvasion.
Figure 3.
Histopathological analysis of papillomas at 30 weeks of promotion
Representative paraffin-sections of papillomas at 30 weeks of promotion from wild type (A, B) and K5-CDK4 (C, D, E, F) sibling mice. Panel A shows a papilloma of a wild type mouse at low magnification (10x). At higher magnification (panel B) it can be observed only moderate dysplasia in a well-differentiated tumor (b, stratum basale; k, stratum corneum). In contrast, panel C and D (higher magnification) shows a section of a papilloma from K5-CDK4 mice showing marked dysplasia with anaplastic area. Panel E and F (higher magnification) shows a papilloma from K5-CDK4 mice showing an area of microinvasion (arrowhead).
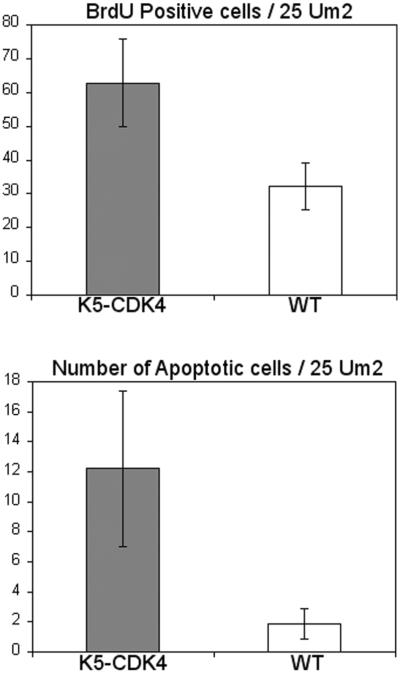
We have also studied the level of apoptosis and cell proliferation in papillomas of wild type and transgenic mice. To determine the number of apoptotic cells, TUNEL assay was performed on paraffin sections of papillomas at 30 weeks of promotion. The average number of apoptotic cells in wild type mice was 1.86/25 μm2 whereas K5-CDK4 (line 2303) papillomas showed an increased number of apoptotic cells (12/25 μm2) (t test, p=0.0002) (Figure 4). To determine if keratinocyte proliferation was also affected by overexpression of CDK4, we determined the number of cells in S-phase by BrdU incorporation. Immunohistochemical analysis has shown that the labeling index of K5-CDK4 (line 2303) papillomas increased two-fold compared to normal siblings, 62.8/25 μm2 and 32.2/25μm2 respectively (t test, p<0.0001) (Figure 4). These results show that even though the number of apoptotic cells increase in K5-CDK4 mice, the total number of proliferating cells are superior in transgenic compared with wild type mice favoring growth of tumors in transgenic mice.
Figure 4.
Apoptosis and proliferation indexes in papillomas of transgenic and wild type sibling mice.
BrdU and TUNEL assays were done on paraffin embedded section from papillomas of K5-CDK4 (line 2303) and wild type mice. The numbers of BrdU and TUNEL positive cells were determined per 25 μm2 of tumor area from K5-CDK4 (solid bars) and wild type siblings (empty bars) mice.
CDK4 complex formation and protein expression in mouse skin tumors
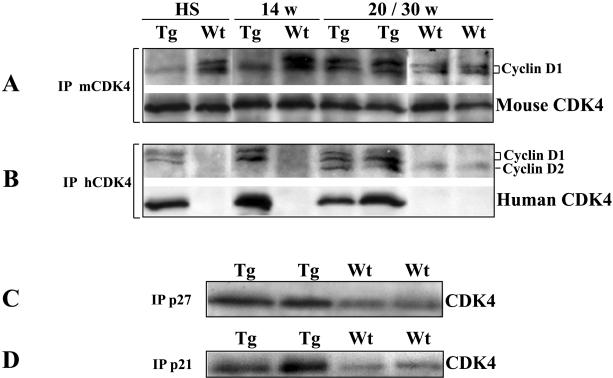
We have previously reported that overexpression of cyclin D1 correlated well with Ha-ras expression and non-random duplication of the chromosome bearing a mutated Ha-ras allele in mouse skin tumor (Bianchi et al., 1990; Rodriguez-Puebla et al., 1999a; Rodriguez-Puebla et al., 1998). Elevated cyclin D1 protein levels were observed in mouse skin papillomas, although the more relevant increase of cyclin D1 was detected after 20 weeks of promotion (Bianchi et al., 1993; Robles & Conti, 1995; Rodriguez-Puebla et al., 1998). In addition, elevated expression of cyclin D1 resulted in an increase CDK4/Cyclin D1 complex formation (Rodriguez-Puebla et al., 1998) in papillomas at 20 weeks of promotion, which correlated with the dysplastic phenotype observed in skin papillomas (Aldaz & Conti, 1989). Thus, we hypothesize that forced expression of CDK4 might result in increased CDK4/Cyclin D1 complex formation in transgenic mice in which CDK4 is not a limiting factor. Furthermore, the elevated level of CDK4 complexes might result in increased susceptibility to malignant progression. According to this hypothesis, cyclin D1 is the rate-limiting factor in early (10-15 weeks of promotion) but not in late tumors. In order to test this hypothesis, we analyzed the CDK4/Cyclin D1 complex formation from early and late papillomas of K5-CDK4 and wild type mice. Protein extracts of hyperplastic skin and papillomas at 14, 20 and 30 weeks of promotion from K5-CDK4 (line 2303) and normal sibling mice were immunoprecipitated (IP) with anti-human CDK4 (hCDK4) and anti-mouse CDK4 (mCDK4) antibodies and analyzed by western blot with anti-cyclin D1 and anti-mCDK4 antibodies. The antibody against human CDK4 (transgenic expression) did not IP the endogenous mouse CDK4 and it was only detected in K5-CDK4 samples (Figure 5B). On the contrary, the anti-mouse CDK4 immunoprecipitate only the endogenous CDK4 and its expression was observed in both transgenic and wild type mice (Figure 5A). Western blot analysis did not show changes in the level of human or endogenous CDK4 during tumor development of transgenic and wild type mice (data not shown). These results are consistent with our previously reported data where CDK4, CDK6 and CDK2 protein levels remain constant during the tumorigenesis process (Rodriguez-Puebla et al., 1998).
Figure 5.
Analysis of CDK4 complexes in mouse skin papillomas.
Fresh protein lysates of hyperplastic skin (HS), papillomas at 14 (14w), 20 (20w) and 30 (30w) weeks of promotion were immunoprecipitated (IP) with polyclonal antibodies against mouse CDK4 (A), human CDK4 (B), p27Kip1 (C) and p21Cip1 (D). Immunoblots were developed with polyclonal antibody against cyclin D1, human CDK4 and mouse CDK4. Protein lysates of papillomas of 30 weeks were used in panels C and D. Tg, K5-CDK4 (line 2303) transgenic mice; Wt, wild type mice.
In hyperplastic skin and early papillomas (14 weeks of promotion) of transgenic mice, hCDK4/Cyclin D1 but not mCDK4/Cyclin D1 complexes were clearly observed (Figure 5B, lines 1 and 3). This result indicates that overexpression of human CDK4 results in binding to most of the cyclin D1 available in early tumors and mouse epidermis in which cyclin D1 is the rate-limiting factor for complex formation. In contrast, mCDK4/Cyclin D1, but not hCDK4/Cyclin D1 complexes were observed in hyperplastic epidermis and early tumors of wild type mice (Figure 5A, lines 2 and 4). On the other hand, we determined elevated complex formation in late tumors (20-30 weeks of promotion) from K5-CDK4 (line 2303) mice in which both mCDK4/Cyclin D1 and hCDK4/Cyclin D1 complexes were detected (Figure 5, panels A and B, lines 5 and 6). This result suggests that overexpression of CDK4 results in greater increase in CDK4/Cyclin D1 complex formation in late tumors from transgenic mice, which could affect the malignant progression to SCC.
It is noteworthy that the antibody against cyclin D1 showed cross-reaction with cyclin D2. Thus, CDK4 binding to cyclin D2 was observed in late tumors from transgenic mice (Figure 5B). It is clear that overexpression of CDK4 can result not only in elevated complex formation with cyclin D1 but also with other D-type cyclins.
Cyclin D1 does not synergize with CDK4 in the development of SCC
To clarify whether the increased level of CDK4/Cyclin D1 complexes in late tumors of transgenic mice is responsible for the elevated level of malignant progression; we developed the double transgenic K5-CDK4/K5-Cyclin D1 overexpressing mice. It should be noted that K5-cyclin D1 transgenic mice did not show increased papilloma development nor malignant progression to SCC (Rodriguez-Puebla et al., 1999b).
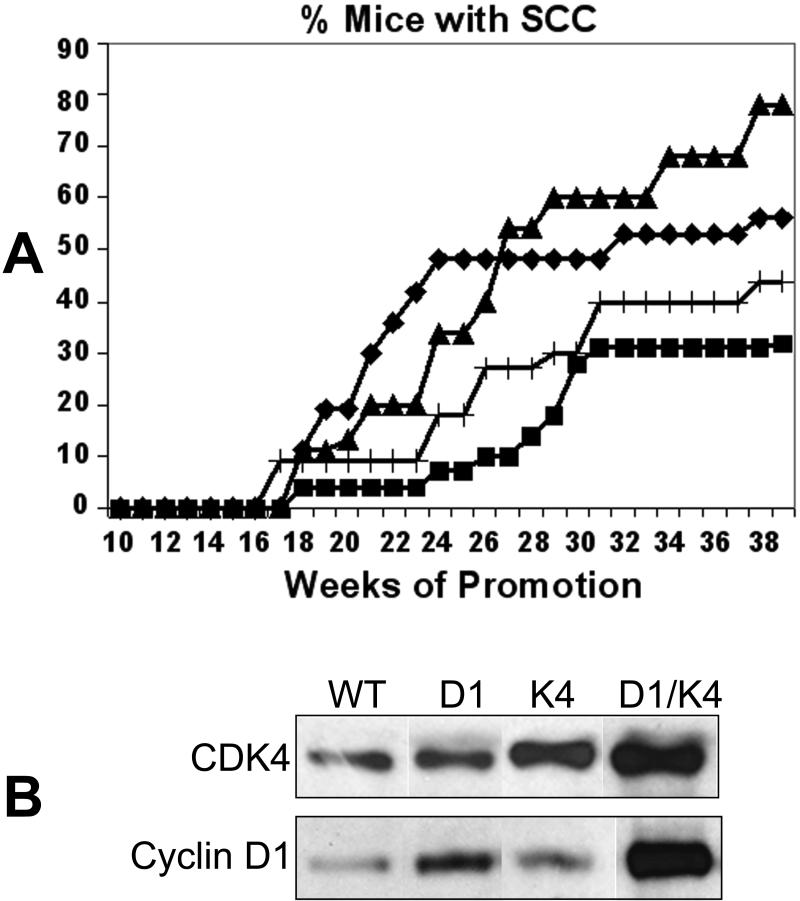
If the increased level of CDK4/cyclin D1 complexes in K5-CDK4 tumors is responsible for the increased progression to SCC, we expected that the double transgenic animals would show earlier development of SCC or a higher number of SCC compared to K5-CDK4 mice. To test this hypothesis, we performed a two-stage carcinogenesis experiment with the double transgenic K5-CDK4/K5-cyclin D1 mice. Interestingly, overexpression of CDK4 enhanced the expression of cyclin D1 in tumors from double transgenic mice (K5-CDK4/K5-Cyclin D1) compared to the cyclin D1 levels in papillomas from K5-cyclin D1 transgenic mice (Figure 6B, lines 2 and 4). We determined that even though the incidence of SCC was elevated in double transgenic mice (56% at 39 weeks of promotion) compared to wild type mice (30%) (Chi-square test, p=0.0229), this number was lower than those observed for K5-CDK4 mice (Line 2305) (78%) (Chi-square test, p=0.0020) (Figure 6 A). In addition, earlier development of SCC was not observed in the double transgenic mice. These results show that the effect of CDK4 in malignant progression is independent of the level of CDK4/Cyclin D1 complexes. In fact, cyclin D1 overexpression appears to attenuate the effect of CDK4 rather than synergize with its oncogenic function (Figure 6 A).
Figure 6.
Kinetics of carcinoma (SCC) formation in transgenic and double transgenic mice.
Groups of K5-CDK4 transgenic, K5-CDK4/K5-Cyclin D1double transgenic and wild type sibling mice were initiated with DMBA and promoted with multiple applications of TPA on dorsal mouse skin. (A), Percentage of mice with carcinomas as a function of weeks of study. (+) K5-Cyclin D1, (◆) K5-Cyclin D1/K5-CDK4 doubles transgenic, (▲) K5-CDK4 and (■) Wild type mice. (B), Western blot analysis of CDK4 and cyclin D1 expression in papillomas from K5-CDK4/K5-cyclin D1 double transgenic mice (K4/D1), K5-CDK4 single transgenic (K4), K5-cyclin D1 (D1) single transgenic and wild type mice (WT). Protein lysates of papillomas separated by SDS-PAGE and blotted to a nitrocellulose membrane. Primary antibodies against CDK4 and cyclin D1 were used in the immunoblotting analysis.
Redistribution of CKIs and cyclins in CDK4 complexes, particularly p27Kip1, causes marked activation or inhibition of CDK2. Thus, a second role of CDK4 sequestering CKIs has been established in several cell types (McConnell et al., 1999; Parry et al., 1999). According to this model, we have also demonstrated that CDK4 bind and sequester p27Kip1 and indirectly activates CDK2 in epidermis of K5-CDK4 mice (Miliani de Marval et al., 2001). In order to determine whether the sequestering activity of CDK4 also occurs in late papillomas, we immunoprecipitate p27Kip1 and p21Cip1 from papillomas at 30 weeks of promotion and analyze them for CDK4 content by western blotting. Figure 5 shows that the CDK4/p27Kip1 and CDK4/p21Cip1 complexes increased 2- and 3.7-fold respectively in K5-CDK4 compared with wild type tumors.
These data indicate that the non-catalytic or sequestering function of CDK4 in late tumors could also be responsible for the strong malignant progression through indirect activation of CDK2.
Discussion
The participation of CDK4 in the neoplastic process was suggested by its amplification and/or overexpression in human gliomas (He et al., 1994; Lam et al., 2000; Schmit et al., 1994; Sonoda et al., 1995), sporadic breast carcinomas (An et al., 1999), lipomatous tumors (Dei Tos et al., 2000) and sarcomas (Kanoe et al., 1998). In addition, a point mutation in cdk4 (R24C) that abrogates the binding of the product of the tumor suppressor gene, p16Ink4a, was identified in patients with familial melanoma (Wölfel et al., 1995; Zuo, 1996).
In this report, we have studied the effect of CDK4 overexpression in skin tumor development. We have used a transgenic mouse model that expresses human CDK4 in the basal cell layer of epidermis. These mice have shown increased CDK4 and CDK2 kinase activities, associated with hyperproliferation, hyperplasia and hypertrophy of the epidermis (Miliani de Marval et al., 2001). Here, we have determined that transgenic mice showed a mild increase in the number of benign lesions (papillomas) after topical application of the genotoxic carcinogen DMBA followed by multiple applications of the tumor promoter TPA. The increased number of apoptotic cells in papillomas of transgenic mice showed that CDK4 overexpression not only triggers a proliferative response in the affect tissue, but in fact, CDK4 transgenic mice showed a 7-fold increase in the rate of apoptosis compared with normal siblings. However, the total number of proliferating cells is superior to the total number of apoptotic cells in transgenic tumors, favoring a faster growth of tumors in transgenic mice. The size of benign tumors (papillomas) is worth mentioning, as they were similar in transgenic and wild type siblings, but the size of squamous cell carcinomas increased in transgenic mice (data non shown). However, several other phenomena must be involved determining the size of SCC because we detected much variability in this parameter.
Dramatic changes in the incidence of SCC were observed in K5-CDK4 mice compared with wild type siblings. Most of the K5-CDK4 mice developed carcinomas, while the incidence in wild type mice was only 20%. The fact that increased expression of CDK4 in our transgenic mice resulted in elevated CDK4/cyclin D1 complex formation in late tumors led us to hypothesize that the activity of this complex is involved in the observed malignant tumor progression. However, the double transgenic K5-CDK4/K5-cyclin D1 did not increase malignant progression nor did it develop earlier carcinomas. Thus, increased CDK4 expression may act in a fashion other than direct activation through the binding to cyclin D1. In fact, we have shown that CDK4 bind and sequester two inhibitors of CDK2 (p21Cip1 and p27Kip1). CDk4/p27Kip1 and CDK4/p21Cip1 complexes increased two and 3.7 times in transgenic compared with wild type papillomas. Thus, redistribution of these inhibitors from CDK2 to CDK4 can be an important step in malignant progression. These effects were also observed in K5-CDK4 mouse epidermis (Miliani de Marval et al., 2001) with increased kinase activity of CDK2. A direct evidence of activation of CDK2 in mouse skin tumors was difficult to assay because the non-epithelial components of these tumors unmask the potential increase in the CDK2 kinase activity in the epidermal compartment of transgenic papillomas. Altogether, these results show that the action of CDK4 is independent of the level of CDK4/cyclin D1 complexes although we cannot rule out that complexes with other D-type cyclins could also be involved in the increased rate of malignant progression. These results are consistent with several reports in which amplification or overexpression of CDK4 in human tumors has been observed without deregulation of D-type cyclins (He et al., 1995; Holland et al., 1998; Kanoe et al., 1998). Consistent with this study He et al. have showed that amplification of CDK4 and cyclin D1 in primary glial tumors are mutually exclusive (He et al., 1994). On the other hand, several pieces of evidence have suggested that CDK4 can act in an alternative unknown pathway in carcinogenesis. Kanoe et al (Kanoe et al., 1998) have shown that contrary to the prevailing theory that CDK4 amplification is an alternative mechanism for Rb gene mutation, the cdk4-gene is amplified in osteosarcomas that also showed loss of expression of RB protein. These redundancies of mutations indicate that CDK4 may have some roles other than inactivation of RB. In addition, CDK4 plays an important role in myogenesis, blocking the muscle differentiation by binding the MyoD transcription factor, showing again that alternative roles for CDK4 cannot be ruled out (Zhang et al., 1999). Interestingly, overexpression of CDK4 in astrocytes allow them to escape from senescence and convert to a tetraploid state (Holland et al., 1998). This data is consistent with the phenotype of our previously described K5-CDK4 transgenic mice which develop epidermal hypertrophy with the histopathological appearance of polyploid keratinocytes (Miliani de Marval et al., 2001).
Our results also showed histopathological evidence that CDK4 overexpression has a strong effect in early papilloma progression. We subdivided the papillomas into four groups according to the degree of atypia as was previously reported by Aldaz et al. (Aldaz & Conti, 1989). We observed foci of anaplastic cells in papillomas at 30 weeks of promotion. Clearly, the papillomas from K5-CDK4 mice showed increased foci of malignant progression whereas most of the papillomas occurring in wild type mice were regular or moderately dysplastic.
In a recent paper, Lazarov et al. (Lazarov et al., 2002) described the effect of combined expression of ras and CDK4 in human epidermal cells. They concluded that CDK4 expression can overcome the arrest induced by ras expression and results in rapid development of tumors. They also observed that co-transformation of CDK4 and cyclin D1 did not synergize in tumor development (Lazarov et al., 2002) supporting our results of the tumorigenic activity of CDK4 that is independent of the level of cyclin D1. Consistent with these results, our experimental in vivo model showed that CDK4 expression synergizes with ras mutation by increasing the rate of malignant progression. In addition, we have showed that CDK4 overexpression can also support keratinocyte hyperproliferation leading to SCC development without application of a tumor promoter. On the other hand, in the absence of ras mutation (no DMBA application), overexpression of CDK4 did not result in papilloma development demonstrating that CDK4 per se cannot induce tumorigenesis. Rane et al. have also shown that mice bearing a CDK4 activating mutation (R24C) did not develop skin tumors when DMBA application was not followed by application of a tumor promoter, however it is not reported whether SCC development was observed under those conditions (Rane et al., 2002). On the other hand, mice bearing the CDK4(R24C) mutation showed increased incidence in papilloma development after DMBA/TPA regimen (Rane et al., 2002). These data, suggest that lack of binding to p16Ink4a in the CDK4(R24C) mice affects mainly the stage of papilloma development. In contrast, our transgenic mice increased the malignant progression of papillomas to SCC. It is possible that overexpression of wild type CDK4 results in overactivation of CDK2 by sequestering of p27Kip1 and p21Cip1, and also favor the activity of CDK6 by sequestering p16Ink4a. The implications of lack of p16Ink4a binding warrant further investigation.
It is noteworthy that CDK4 overexpression can bypass the telomere-dependent replicative senescence in human primary culture of keratinocyte, but maintain their ability to differentiate (Ramirez et al., 2003). Supporting this fact, the epidermis of the K5-CDK4 mice showed a normal pattern of epidermal differentiation (Miliani de Marval et al., 2001). Analysis of papillomas at various time-points showed that forced expression of CDK4 did not affect the state of differentiation, in which the expression of keratins associated with proliferation (K5 and K14) and differentiation (K1 and K10) was normal (data not shown). Whether forced expression of CDK4 in mouse epidermis allows keratinocytes to escape the senescence resulting in elevated rates of malignant conversion is an intriguing issue that remains to be analyzed. In this sense, mouse embryo fibroblasts derived from CDK4(R24C) mice also displayed the escape of replicative senescence (Rane et al., 2002).
In the last few years, other mouse models have been developed to study the role of CDK4 in vivo. The CDK4(R24C) mice showed normal development although they developed a wide spectrum of tumors with the most common being lymphoma, endocrine tumors and hemangiosarcomas (Rane et al., 2002; Sotillo et al., 2001a). In addition, these mice developed invasive melanoma upon DMBA/TPA treatment (Sotillo et al., 2001b) and an increased incidence of papillomas (Rane et al., 2002). Our results showed that overexpression of wild type CDK4 has very different effects in comparison to the expression of the mutated form CDK4(R24C). Overexpression of CDK4 results in enhanced carcinoma development. In fact, both models represent different deregulation forms of the CDK4 gene in human tumors. While the CDK4(R24C) mutated form appears exclusively in some cases of sporadic and familial melanomas (Ohta et al., 1994; Soufir et al., 1998; Wölfel et al., 1995; Zuo, 1996), amplification and/or overexpression of CDK4 have been found in a wide spectrum of human tumors (He et al., 1995; Holland et al., 1998; Kanoe et al., 1998; Nikitakis et al., 2002). Surprisingly, Morris et al (Morris et al., 2002) have shown that the CDK4(R24C) produced no additional growth advantage compared with CDK4 overexpressing forms in human fibroblasts; indeed CDK4(R24C) was less effective than wild type CDK4 (Morris et al., 2002). These results support the observation that CDK4(R24C) mutation is restricted to melanoma development where escape of the p16Ink4a inhibitory effect appear to be indispensable, while overexpression of CDK4 may be a more general mechanisms for an acquired growth advantage (An et al., 1999; Dei Tos et al., 2000; He et al., 1994; Kanoe et al., 1998; Lam et al., 2000; Schmit et al., 1994; Sonoda et al., 1995).
We have previously shown that lack of CDK4 results in abrogation of tumor development in the mouse skin model (Rodriguez-Puebla et al., 2002). Also, disruption of CDK4 results in primary cells resistant to oncogenic transformation (He et al., 1995; Holland et al., 1998; Kanoe et al., 1998; Tsutsui et al., 1999; Zou et al., 2002). Altogether, these results demonstrate the potential use of CDK4 as therapeutic target and imply that the K5-CDK4 transgenic mouse is a valuable model to study the effect of CDK4 in malignant progression.
Materials and Methods
Mouse experiments
K5-CDK4 transgenic mice were developed as we described previously (Miliani de Marval et al., 2001). The mice were backcrossed onto the SSIN (Sencar) genetic background which was used for all the carcinogenesis experiments described here. Two K5-CDK4 transgenic lines were used for the two-stage carcinogenesis protocol. These transgenic lines were previously characterized (Miliani de Marval et al., 2001), showing 2.5- and 7.5-fold expression of CDK4 (K5-CDK4-2305 and K5-CDK4-2303 founder lines respectively) in mouse epidermis. K5-CDK4-2305 mice were crossed with K5-cyclin D1 (line 7111) (Robles et al., 1996; Rodriguez-Puebla et al., 1999b) to obtain the K5-CDK4/K5-cyclin D1 double transgenic mice. Mice experiments were performed with sibling animals in order to reduce the influence of the genetic background. The two-stage carcinogenesis protocol was performed with newborns mice as we previously described (Robles et al., 1998). Briefly, groups of 20 mice each were initiated at day 1 after birth by application of 50 μg of DMBA in 50 μl of acetone on each dorsal surface (dorsal mouse back). At day 14, mice received 2.5 μg of TPA in 200 μl of acetone twice a week for 10 weeks. The TPA regimen was subsequently changed to 1 μg of TPA in 200 μl of acetone twice a week for 10 weeks. Tumors were counted once a week until the end of the experiment at 40 weeks. Malignant transformations to SCC were determined by macroscopic observation and corroborated by histopathological analysis of paraffin embedded sections.
The incidence of SCC (percentage of mice with SCC) was calculated (A / Bx100) where A is the number of mice that developed SCC and B the total number of mice which have developed at least one papillomas. The multiplicity of SCC (average number of SCC per mouse) was calculated as (C / B) where C is the total number of SCC observed in each group of mice and B is the total number of mice that have developed at least one papilloma.
Immunohistochemical Staining
Epithelial cell proliferation was measured by intraperitoneal injection of BrdU (60 mg/g body weight) 30 minutes before the mice were sacrificed. BrdU incorporation was detected by immunohistochemical staining of paraffin-embedded sections of papillomas with mouse anti-BrdU monoclonal antibody (Becton Dickinson Immunocytometry System; Becton Dickinson, San Jose, CA). Apoptotic cells were determined by TUNEL assays as was previously described (Pierce et al., 1998). The reaction was visualized with a biotin-conjugated anti-mouse antibody (Vector Laboratories, Inc., Burlingame, CA) and an avidin-biotin-peroxidase kit (Vectastain Elite, Vector Laboratories, Inc.) with diaminobenzidine as chromogen. The number of BrdU- and apoptosis-positive cells and total cells were determined in sections of 200 μm2 with a reticule grid. Ten to 14 fields were counted per section on a total of 15 paraffin embedded sections representing 15 mice.
Histopathological analysis of papillomas at 30 weeks of promotion was carried out on hematoxylin and eosin staining paraffin-embedded sections as was previously described (Miliani de Marval et al., 2001).
Co-immunoprecipitation and Western Blotting Analysis
Mouse tumors were grounded with a mortar in liquid nitrogen and homogenized with homogenization buffer (25 mM Hepes, pH 7.5; 5 mM EDTA, 50 mM NaCl, 50 mM NaF, 10% (v/v) glycerol, 1% (v/v) Triton X-100) with a Polytron PT10 homogenizer (three 5-s bursts at setting 6 on ice). The homogenates were sonicated with a Branson sonifier 450 (two 8-s bursts on ice) and centrifuged at 12,000 rpm for 30 minutes in a microcentrifuge at 4°C. The supernatants were collected and used directly for western blot or co-immunoprecipitation analysis.
The protein concentration was measured with a Bio-Rad protein assay system (Bio-Rad Laboratories, Richmond, CA). Fresh protein preparations (250 μg per sample) were immunoprecipitated (IP) for 2 h at 4°C constant rotation with protein A-agarose beads and polyclonal anti-human CDK4 and anti-mouse CDK4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and washed three times with extraction buffer. Immunoprecipitates were electrophoresed through acrylamide gels and electrophoretically transferred onto nitrocellulose membranes. After blocking with 5% nonfat powdered milk in Dulbecco’s phosphate-buffered saline (Sigma Chemical Co.), the membranes were incubated with specific antibodies. The following antibodies used were: polyclonal anti-mouse CDK4 (C22) and anti-human CDK4 (H22) (Santa Cruz Biotechnology, Inc.) and mouse monoclonal anti-cyclin D1 (Zymed Laboratories, San Francisco, CA). Horseradish peroxidase-conjugated secondary antibody (Amersham Corp., Arlington Heights, IL), followed by enhanced chemiluminescense (ECL detection kit) (Amersham Corp.) were used for immunoblotting detection.
Statistic analysis
Chi-square and t test were performed using GraphPad InStat 3.05 (for Windows 95, GraphPad Software, San Diego California USA, www.graphpad.com) and SPSS software (SPSS, Inc., Chicago, Il).
Acknowledgements
We especially thank Dr. Dennis Johnston for assistance with the statistical analysis, Dr. Susan Fischer for helpful reading and discussion of this paper, April Weiss for helping with the mouse experiments, Cassie Bigbee for technical support, the Science Park animal facility personnel and the Science Park histology service for assistance with the immunohistochemical staining.
Supported by NIH Grants CA 42157, CA 57596 and CA 90864, Cancer Center grant CA 16672 to MDACC for the animal facility, Funds from the University Cancer Foundation at the University of Texas M. D. Anderson Cancer Center and NIEHS Center Grant ES07784.
Abbreviations
- CDK
cyclin-dependent kinases
- CKI
cyclin-dependent kinases inhibitor
- DMBA
7,12-dimethylbenz[a]anthracene
- TPA
12-O-tetradecanoylphorbol-13-acetate
- IP
immunoprecipitation
References
- Aldaz M, Conti C. In: Experimental and Clinical Aspects. Conti C, Slaga T, Klein-Szanto A, editors. Raven Press; New York: 1989. [Google Scholar]
- An H, Beckmann MW, Reifenger G, Bender HG, Niederacher D. Am J Pathology. 1999;154:113–118. doi: 10.1016/S0002-9440(10)65257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I. Adv Cancer Res. 1954;2:129–175. doi: 10.1016/s0065-230x(08)60493-5. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Aldaz MC, Conti CJ. Proc. Natl. Acad. Sci. USA. 1990;87:6902–6906. doi: 10.1073/pnas.87.17.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AB, Fischer SM, Robles AI, Rinchik EM, Conti CJ. Oncogene. 1993;8:1127–1133. [PubMed] [Google Scholar]
- Boutwell RK. Prog. Exp. Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- Conti C. Cancer Bulletin. 1992;54:62–128. [Google Scholar]
- Dei Tos A, Doglioni C, Piccini S, Sciot R, Furlanetto A, Boiocchi M, Dal Cin P, Maestro R, Fletcher C, Tallini G. J Pathol. 2000;190:531–536. doi: 10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J. Pharmacol. Theor. 1992;54:62–128. [Google Scholar]
- He J, Allen JR, Collins VP, Allalunis-Turner MJ, Godbout R, Day RS, James CD. Cancer Res. 1994;54:5804–5807. [PubMed] [Google Scholar]
- He J, Olson J, James C. Cancer Res. 1995;55:4833–4836. [PubMed] [Google Scholar]
- Holland EC, Hively WP, Gallo V, Varmus HE. Genes Dev. 1998;12:3644–3649. doi: 10.1101/gad.12.23.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Pines J. Cell. 1994;79:573–82. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Jacks T, Weinberg R. Science. 1998;280:1035–1036. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- Kanoe H, Nakayama T, Murakami H, Hosaka T, Yamamoto H, Nakashima Y, Tsuboyama T, Nakamura T, Sasaki M, Toguchida J. Anticancer Res. 1998;18:2317–2321. [PubMed] [Google Scholar]
- Lam P, Di Tomaso E, Ng H, Pang J, Roussel M, Hjelm N. Br J Neurosurg. 2000;14:28–32. doi: 10.1080/02688690042870. [DOI] [PubMed] [Google Scholar]
- Lazarov M, Kubo Y, Cai T, Dajee M, Tarutani M, Lin Q, Fang M, Tao S, FGreen C, Kharavi P. Nat Med. 2002;8:1105–1114. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Ortega S, Barbacid M. Biol Chem. 2000;38:827–838. doi: 10.1515/BC.2000.105. [DOI] [PubMed] [Google Scholar]
- McConnell B, Gregory F, Stott F, Hara E, Peters G. Mol. Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliani de Marval P, Gimenez-Conti I, LaCava M, Martinez L, Conti C, Rodriguez-Puebla M. Am J Pathology. 2001;159:369–379. doi: 10.1016/S0002-9440(10)61703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Hepburn P, Wynford-Thomas D. Oncogene. 2002;21:4277–4288. doi: 10.1038/sj.onc.1205492. [DOI] [PubMed] [Google Scholar]
- Motokura T, Arnold A. Bioch Biophys Acta. 1993;1155:63–78. doi: 10.1016/0304-419x(93)90022-5. [DOI] [PubMed] [Google Scholar]
- Nevins J, Chellappan S, Mudryj M, Hiebert S, Devoto S, Horowitz J, Hunter T, Pines J. Cold Spring Harb Symp Quant Biol. 1991;56:157–62. doi: 10.1101/sqb.1991.056.01.020. [DOI] [PubMed] [Google Scholar]
- Nevins JR. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nikitakis N, Drachenberg C, Papadimitriou J. Exp Mol Pathol. 2002;73:198–208. doi: 10.1006/exmp.2002.2465. [DOI] [PubMed] [Google Scholar]
- Ohta M, Nagai H, Shimizu M, Rasio D, Berd D, Mastrangelo M, Singh AD, Shields JA, Shields CJ, Croce CM. Cancer Res. 1994;54:5269–5272. [PubMed] [Google Scholar]
- Parry D, Mahony D, Wills K, Lees E. Mol. Cell. Biol. 1999;19:1775–1783. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AM, Gimenez-Conti IB, Schneider-Broussard R, Martinez LA, Conti CJ, Johnson DG. Proc. Natl. Acad. Sci. USA. 1998;95:8858–8863. doi: 10.1073/pnas.95.15.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Semin Cancer Biol. 1995;6:63–72. doi: 10.1006/scbi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Ramirez R, Herbert B, Vaughan M, Zou Y, Gandia K, Morales C, Wright W, Shay J. Oncogene. 2003;22:433–444. doi: 10.1038/sj.onc.1206046. [DOI] [PubMed] [Google Scholar]
- Rane SG, Cosenza S, Mettus RV, Reddy EP. Mol Cell Biol. 2002;22:644–656. doi: 10.1128/MCB.22.2.644-656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Premkumar Reddy E, Barbacid M. Nature Genetic. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Robles A, Rodriguez-Puebla M, Glick A, Trempus C, Hansen L, Sicinski P, Tennant R, Weinberg R, Yuspa S, Conti C. Genes & Dev. 1998;12:2469–2474. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles AI, Conti CJ. Carcinogenesis. 1995;16:781–786. doi: 10.1093/carcin/16.4.781. [DOI] [PubMed] [Google Scholar]
- Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, Jorcano JL, Conti CJ. P.N.A.S. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Puebla M, LaCava M, Bolontrade M, Rusell J, Conti C. Mol Carcinog. 1999a;26:150–156. [PubMed] [Google Scholar]
- Rodriguez-Puebla ML, LaCava M, Conti C. Cell growth Diff. 1999b;10:467–472. [PubMed] [Google Scholar]
- Rodriguez-Puebla ML, LaCava M, Gimenez-Conti IB, Jonhson DG, Conti CJ. Oncogene. 1998;17:2251–2258. doi: 10.1038/sj.onc.1202131. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Puebla ML, Miliani de Marval PL, LaCava M, Moons DS, Kiyokawa H, Conti CJ. Am. J. of Pathology. 2002;161:405–411. doi: 10.1016/S0002-9440(10)64196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Puebla ML, Robles AI, Conti CJ. Mol. Carcinogenesis. 1999c;24:1–6. [PubMed] [Google Scholar]
- Roussel MF. Oncogene. 1999;18:5311–5317. doi: 10.1038/sj.onc.1202998. [DOI] [PubMed] [Google Scholar]
- Schmit E, Ichimura K, Reifenberger G. Cancer Res. 1994;54:6321–6324. [PubMed] [Google Scholar]
- Sherr C. Proc Assoc Am Physicians. 1995a;107:181–6. [PubMed] [Google Scholar]
- Sherr CJ. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Trends Biochem. Sci. 1995b;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Slaga TJ. Carcinogenesis. In: Conti CJ, Slaga TJ, Klein-Szanto AJ, editors. A Comprehesive Survey. Vol. 11. Raven Press; New York: 1989. pp. 1–18. [Google Scholar]
- Sonoda Y, Yoshimoto T, Sekiya T. Oncogene. 1995;11:2145–2149. [PubMed] [Google Scholar]
- Sotillo R, Dubus P, Martin J, de la Cueva E, Ortega S, Malumbres M, Barbacid M. EMBO J. 2001a;20:6637–6647. doi: 10.1093/emboj/20.23.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R, Garcia J, Ortega S, Martin J, Dubus P, Barbacid M, Malumbres M. Proc Natl Acad Sci. 2001b;98:13312–13317. doi: 10.1073/pnas.241338598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufir N, Avril MF, Chompret A, Demenais F, Bombled J, Spatz A, Stoppa-Luonnet D, Bernard J, Bressac-de Paillerets R. Hum Mol Gen. 1998;7:209–216. doi: 10.1093/hmg/7.2.209. [DOI] [PubMed] [Google Scholar]
- Tofgard R, Roop D, Yuspa S. Carcinogenesis. 1985;6:655–657. doi: 10.1093/carcin/6.4.655. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Hesabi B, Moons DS, Pandolfi P, Hansel K, Koff A, Kiyokawa H. Mol. Cell. Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. Cytokines & Mol Ther. 1996;2:105–110. [PubMed] [Google Scholar]
- Wölfel T, Hauer M, Schneider J, Serrano M, Wölfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Büschenfelde KH, Beach D. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Biochim. Biophys. Acta. 1996;1288:1–5. doi: 10.1016/0304-419x(96)00012-1. [DOI] [PubMed] [Google Scholar]
- Yuspa S, Hennings H, Roop D, Strickland J, Greenhalgh D. Environ. Health Perspect. 1990;88:193–195. doi: 10.1289/ehp.9088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, Wei Q, Zhao X, Paterson B. EMBO J. 1999;18:926–933. doi: 10.1093/emboj/18.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Ray D, Aziyu A, Christov K, Boiko A, Gudko A, Kiyokawa H. Genes Dev. 2002;16:2923–2934. doi: 10.1101/gad.1033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L. Nature Genet. 1996;12:97–99. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]