Abstract
The costs of CT Colonography (CTC) are not yet established for screening use. In this study, we estimated the threshold costs for which CTC screening would be a cost-effective alternative to colonoscopy for colorectal cancer (CRC) screening in the general population. We used the MISCAN-Colon microsimulation model to estimate costs and life-years gained of screening persons aged 50 to 80 years for four screening strategies: 1) optical colonoscopy; and CTC with referral to optical colonoscopy of 2) any suspected polyp; 3) a suspected polyp ≥ 6 mm; and 4) a suspected polyp ≥ 10 mm. For each of the four strategies screen intervals of 5, 10, 15 and 20 years were considered. Subsequently, for each CTC strategy and interval, the threshold costs of CTC were calculated. We performed a sensitivity analysis to assess the effect of uncertain model parameters on the threshold costs. With equal costs ($662), optical colonoscopy dominated CTC screening. For CTC to gain similar life-years as colonoscopy screening every 10 years, it should be offered every 5 years with referral of polyps ≥ 6 mm. For this strategy to be as cost-effective as colonoscopy screening, the costs must not exceed $285, or 43% of colonoscopy costs (range in sensitivity analysis: 39-47%). With 25% higher adherence than colonoscopy, CTC threshold costs could be 71% of colonoscopy costs. Our estimate of 43% is considerably lower than previous estimates in literature, because previous studies only compared CTC screening to 10-yearly colonoscopy, where we compared to different intervals of colonoscopy screening.
Keywords: colorectal neoplasms, screening, cost-effectiveness, computer simulation, CT Colonography
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States, with almost 149,000 new diagnosed cases and 50,000 deaths in 2008.1 Screening can prevent many of these deaths, not only by detecting CRC in an early stage and thus improving prognosis, but also by detecting and removing its non-malignant precursor lesion, the adenoma, and thus preventing CRC incidence. Randomized controlled trials have shown that biennial and annual screening with Fecal Occult Blood Test (FOBT) can reduce CRC mortality by 15% to 33%.2-5 FOBT is a cheap and non-invasive test, but it still leaves many cancers undetected 3, 6-8 and there is therefore room for improvement. Endoscopy is highly sensitive for CRC and adenomas.9-13 Case-control studies have suggested that endoscopic screening is associated with a substantial reduction in CRC mortality.14-17 Nonetheless, its efficacy in screening is yet to be quantified by randomized controlled trials, several of which are currently underway.18-21 Limitations of endoscopy screening include cost, risk of severe complications, and hesitancy of patients to undergo these tests. Furthermore, there are currently insufficient well-trained gastroenterologists to meet projected screening endoscopy needs.22
Computed Tomographic Colonography (CTC) is a promising technique for CRC screening, combining high sensitivity for larger polyps and cancer 23, 24 with a less invasive procedure.25, 26 With CTC two- and three-dimensional images are constructed to investigate the presence of lesions in the colon and rectum. A serious potential drawback is that conventional (optical) colonoscopy is required to further evaluate and remove abnormalities detected through CTC. Several studies have shown that CTC is currently not a cost-effective option for average-risk CRC screening if all suspected polyps are followed-up by optical colonoscopy.27-29 CTC could be cost-effective if diagnostic follow-up is only recommended for patients with suspected polyps of 6 mm or larger.30, 31 However, CTC screening for CRC is still under development and therefore its costs have not yet been established. As a consequence, different cost-effectiveness estimates are often based on fairly different cost assumptions.
In the present study, we used the MISCAN-Colon microsimulation model to estimate life-years gained and costs of CTC screening for various screen intervals and polyp size thresholds for diagnostic follow-up for different levels of unit CTC costs and compared the cost-effectiveness to colonoscopy screening. Furthermore, we determined the threshold CTC unit costs for which CTC screening would be cost-effective compared to colonoscopy screening. Finally, we placed the results in the context of other studies in a literature overview of CTC cost-effectiveness analyses.
METHODS
MISCAN-Colon Microsimulation Model
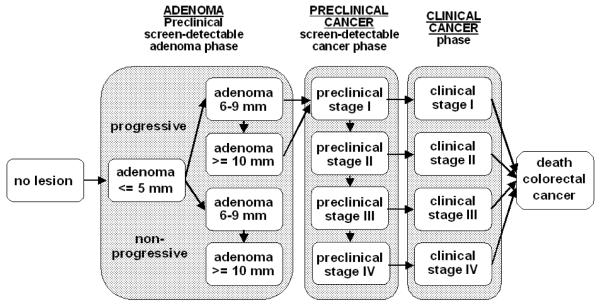
The Department of Public Health at Erasmus MC, the Netherlands, developed the MISCAN-Colon microsimulation model in collaboration with the National Cancer Institute to assess the effect of different interventions on CRC. The model and the data sources that inform the quantification of the model can be found in Appendix 1, in previous publications,32-34 and also in a standardized model profile.35 A graphical representation of the natural history in the model is given in Figure 1. In brief, the MISCAN-Colon model simulates the relevant biographies of a large population of individuals from birth to death, first without screening and subsequently with the changes that would occur under the implementation of a screening program. CRC arises in this population according to the adenoma-carcinoma sequence.36, 37 More than one adenoma can occur in an individual and each can independently develop into CRC. Adenomas progress in size from small (1-5 mm) to medium (6-9 mm) to large (10+ mm). Most adenomas will never grow into cancer in a lifetime (non-progressive adenomas). These adenomas either stay 6-9 mm in size or continue to grow to 10 mm or larger. Some adenomas (progressive adenomas) may eventually become malignant, transforming to a stage I cancer. The cancer may then progress from stage I to stage IV. In every stage there is a probability of the cancer being diagnosed because of symptoms versus alternatively progressing without symptoms into the next stage. However, a person may die of other causes at any time during the process before diagnosis. The survival after clinical diagnosis depends on the stage in which the cancer was detected. Once a life-history without screening is established, screening is simulated and whether it interrupts the development of CRC. With CTC screening, polyps of different sizes are detected. After a person is detected with polyps above the follow-up cut-off size, he is referred for follow-up optical colonoscopy for removal of adenomas and diagnosis of cancers. In this way, CRC incidence or CRC death can be prevented. The life-years gained by screening are calculated by comparing the model-predicted life-years lived in the population with and without screening.
Figure 1.
Adenoma and cancer stages in the MISCAN-Colon model. Cancer stages correspond to the American Joint Committee on Cancer / International Union Against Cancer staging system for CRC. Adenomas are categorized by size. The size-specific prevalence of adenomas as well as the proportion of adenomas that ever develop into cancer is dependent on age. It is assumed that the proportion of progressive adenomas increases from 16% at age 65 years to 37% at age 75 years, and 96% at age 100 years. It is assumed that 50% of non-progressive adenomas will remain 6-9 mm stage until death and that 50% will progress to the ≥10 mm stage. For progressive adenomas, it is assumed that 30% will develop through the sequence ≤5 mm adenoma → 6-9 mm adenoma → preclinical cancer stage I and that 70% will develop through the sequence ≤5 mm adenoma →6-9 mm adenoma → ≥10 mm adenoma → preclinical cancer stage I. The mean duration time for progressive adenoma is assumed to be 16.4 years (with an exponential distribution). The mean duration time for preclinical cancer is assumed to be 2 years (stage I), 1 year (stage II), 1.5 years (stage III), and 0.8 years (stage IV).
The validity of the model is based on observational data before the introduction of screening, such as clinical incidence and mortality from CRC 38 and the size and multiplicity distribution of adenomas in autopsy studies.39-48 The external validity has further been tested on the results of large (randomized) screening and surveillance studies, such as the CoCap sigmoidoscopy study,32 the Minnesota Colon Cancer Control Study 32 and the National Polyp Study.49 Finally, the model was able to explain observed incidence and mortality trends in the US when accounting for risk factor trends, screening practice and chemotherapy treatment.50
Screening characteristics
The assumptions for sensitivity and specificity of CTC (Table 1) were based on the meta-analysis of Mulhall.23 Mulhall conducted a meta-analysis for per-patient sensitivity. For the MISCAN-Colon model we needed per-adenoma sensitivity, so we repeated the meta-analysis for per-adenoma sensitivity based on the original studies included by Mulhall. Non-adenomatous polyps were not explicitly modeled. However, we did take into account the costs and complications incurred by the detection and removal of non-adenomatous polyps with CTC and colonoscopy. We adjusted the specificity estimates for not having polyps in the Mulhall study to an estimate for not having adenomas, by subtracting the proportion of patients with non-adenomatous polyps only from the specificities reported. This higher lack of specificity resulted in a larger number of patients being referred for colonoscopy and receiving polypectomy and pathology. This lack of specificity also ensured the possibility of detection and removal of co-existing smaller adenomas or even missed larger polyps, that were otherwise not referred for colonoscopy. In a screening population, 33.3% (CI 30.6% - 36.0%) of individuals only have non-adenomatous polyps, 8.8% (CI 7.3% - 10.6%) only have non-adenomatous polyps of 6 mm or larger and 2.0% (CI 1.3% - 3.0%) of 10 mm or larger.51
Table 1.
Screening test characteristics in the model for CTC and for optical colonoscopy
| Parameter | CTC | Optical Colonoscopy |
|---|---|---|
| Sensitivity adenomas 1-5 mm (%) | 29 (22 – 37) | 75 |
| Sensitivity adenomas 6-9 mm (%) | 66 (59 – 72) | 85 |
| Sensitivity adenomas 10+ mm (%) | 87 (82 – 93) | 95 |
| Sensitivity cancers (%) | 87 (82 – 93) | 95 |
|
| ||
| Specificity (%) | Cut off 0 mm: 53 (50 – 55) | 90 |
| Cut off 6 mm: 84 (83 – 86) | ||
| Cut off 10 mm: 95 (94 – 96) | ||
|
| ||
| Reach | 100% reach cecum | 95% reach cecum, reach of remaining 5% is distributed evenly over colorectum |
|
| ||
| Non-fatal perforation rate | n.a. | 2.4 per 1,000 |
|
| ||
| Fatal perforation rate | n.a. | 0.1 per 1,000 |
n.a.: not applicable
The sensitivity and specificity of optical colonoscopy were based on back-to-back colonoscopy studies (Table 1).11-13 The lack of specificity for optical colonoscopies reflected the fact that in 10% of persons without adenomas additional costs were incurred because of removal and pathology of non-adenomatous polyps. The rate of serious nonfatal complications was assumed to be 2.4 per 1,000 colonoscopies.52-55 The rate of fatal events was assumed 1 per 10,000 colonoscopies.56
Cost inputs
Colonoscopy costs were based on 2007 Medicare average payments (including beneficiary co-pays).57 CTC screening is currently not reimbursed by Medicare and hence no average payments are available. We therefore considered different cost levels for CTC: same as, half of and one third of colonoscopy costs. Finally, we varied the unit costs of CTC to determine the threshold costs for which it would be a cost-effective alternative to colonoscopy screening. Because these threshold costs are derived relative to the cost-effectiveness of colonoscopy under Medicare average payments, they include the same components as the colonoscopy costs.
The costs of complications requiring inpatient hospitalization were based on the relevant Diagnostic Related Group (DRG) codes.57 The phase-specific cost of CRC treatment was derived from comparison of costs for CRC cases relative to those of matched controls in the SEER-Medicare files.58 Treatment cost data were reported in 2004 dollars and subsequently updated to 2007 dollars using the medical care component of the Consumer Price Index. Appendix 2 contains a detailed overview of the derivation of the costs. The final cost inputs used in the model are summarized in Table 2.
Table 2.
Unit costs in 2007$ (confidence interval) for screening and CRC treatment, used as inputs for the MISCAN-Colon model
| Screening costs | CRC treatment costs | |||||
|---|---|---|---|---|---|---|
| Procedure | Cost | Stage | Initial* | Continuous* | Terminal care, death CRC* |
Terminal care, death other cause* |
| Colonoscopy | $662 | I | $ 28,668 | $ 2,395 | $ 51,935 | $ 12,703 |
| Colonoscopy with polypectomy | $846 | II | $ 39,700 | $ 2,237 | $ 51,712 | $ 11,035 |
| CTC | Varied | III | $ 48,951 | $ 3,249 | $ 54,776 | $ 14,708 |
| Treatment of perforation | $12,446 | IV | $ 64,801 | $ 10,419 | $ 73,522 | $ 39,679 |
| Treatment of serosal burn | $5,208 | |||||
| Treatment of bleed with transfusion | $5,208 | |||||
| Treatment of bleed without transfusion | $320 | |||||
Costs for care were divided into three clinically relevant phases of care – initial, continuing and terminal care. The initial phase was defined as the first 12 months following diagnosis, the terminal phase was defined as the final 12 months of life, and the continuing phase was defined as all months between the initial and last year of life phases of care. The terminal care phase of CRC patients was further subdivided into terminal care before CRC death and terminal care before death of other causes. Cause of death was identified by use of death certificate information in the SEER database. For patients surviving less than 24 months after diagnosis, the final 12 months of observation and costs of care were then allocated first to the last year of life phase, because the content of care for patients with short survival is more similar to the last year of life phase than the initial phase. The remainder of months of observation and costs were allocated to the initial phase, with no contribution to the continuing phase.
Screening strategies
We simulated 4 colonoscopy and 12 CTC screening strategies. In all strategies screening began at age 50 and was discontinued after age 80. Screen intervals of 20, 15, 10 and 5 years were considered. This corresponded with 2, 3, 4 and 7 screens offered in a lifetime respectively. With CTC screening, we simulated three different follow-up strategies:
intensive referral: any suspected polyp detected, irrespective of size
intermediate referral: suspected polyps of 6 mm or larger detected
minimal referral: suspected polyps of 10 mm or larger detected.
Persons without referral for diagnostic colonoscopy or without adenomas detected at (diagnostic) colonoscopy continued in the CTC screening program. If an adenoma was detected and thus removed at colonoscopy, surveillance was conducted according to the guidelines of the US Multi-Society Task Force on Colorectal Cancer.59 Adherence with screening, diagnostic follow-up and surveillance were assumed to be 100%.
Analysis
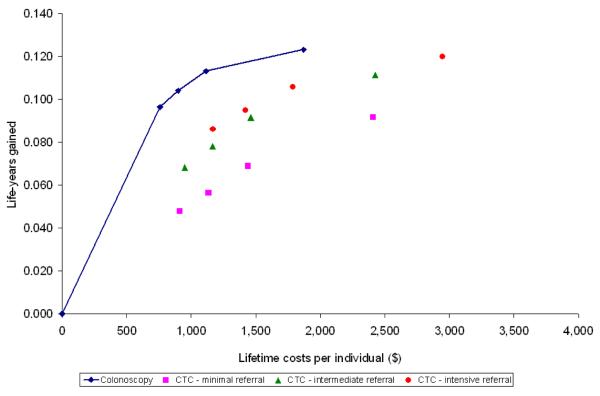
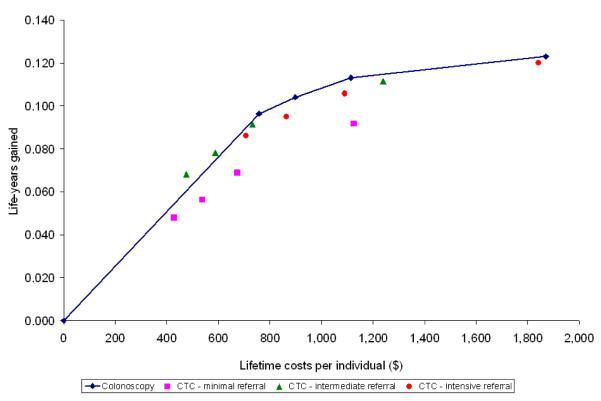
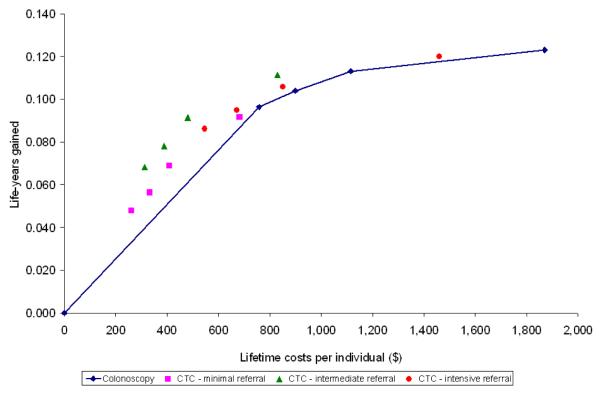
For each of the sixteen screening strategies, we calculated life-years gained, number of screen tests and costs. Future costs and life-years were discounted at an annual rate of 3%. We plotted the costs and life-years gained from the colonoscopy strategies on a graph and connected the strategies by a line, representing the colonoscopy cost-effectiveness frontier (Figures 2 and 3). For the CTC strategies to be a cost-effective alternative for colonoscopy screening, the strategies needed to be on or to the left of this line. We plotted the costs and life-years gained with the different CTC screening strategies in the same plot for different CTC cost levels: same as colonoscopy costs, half of colonoscopy costs and one-third of colonoscopy costs (figures 2A, B and C respectively). Finally, we determined the threshold costs for which each of the CTC screening strategies were on the colonoscopy cost-effectiveness frontier (Figure 3). There were three possible situations to consider: (1) the CTC strategy was less effective than the least effective colonoscopy strategy, (2) the CTC strategy was more effective than the most effective colonoscopy strategy, and (3) the effectiveness of the CTC test strategy was intermediate to the least effective and most effective strategies on the colonoscopy cost-effectiveness frontier. In the first case, the threshold costs of CTC were calculated such that the average costs per life-year gained for the CTC strategy were equal to those of the least effective colonoscopy strategy. In the second case, the threshold test costs were calculated such that the incremental costs per life-year gained for the CTC strategy compared with the most effective colonoscopy strategy were equal to $50,000 per life-year gained. In the third case, we identified the colonoscopy strategy with lowest life-years gained that would still have more life-years gained than the CTC strategy. Subsequently, the threshold costs were calculated such that the incremental costs per life-year gained of the CTC strategy were equal to those of that selected strategy.
Figure 2.
The net costs required over a lifetime and the life-years gained (3% discounted and compared to a situation without screening) for screening a cohort of 50-year olds according to different colonoscopy and CTC screening strategies varying with respect to screening interval and referral threshold. Lifetime costs include costs for screening, diagnostic and surveillance colonoscopy minus the savings from treatment compared to a situation without screening. The blue diamonds represent the colonoscopy strategies; the pink squares CTC strategies with minimal referral, the green triangles CTC with intermediate referral and the orange circles CTC strategies with intensive referral. From left to right the symbols per strategy represent intervals of 20, 15, 10 and 5 years. The solid line represents the cost-effectiveness of colonoscopy screening strategies, corresponding with Table 3. CTC costs are equal to colonoscopy costs in figure 2A, half of colonoscopy costs in figure 2B, and one third of colonoscopy costs in figure 2C.
Figure 2a: CTC costs equal to colonoscopy costs
Figure 2b: CTC costs half of colonoscopy costs
Figure 2c: CTC costs one third of colonoscopy costs
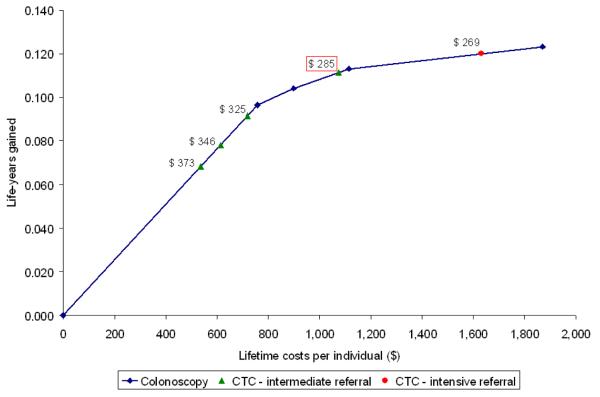
Figure 3.
The threshold costs of non-dominated CTC strategies for which the strategies are a cost-effective alternative to colonoscopy screening. The green triangles represent CTC screening with intermediate referral. From left to right the triangles represent screening intervals of 20, 15, 10 and 5 years. The orange circle represents CTC screening every 5 years with intensive referral. The costs next to the symbols per strategy indicate the threshold unit costs for CTC to be cost-effective compared to colonoscopy screening. The boxed cost is the threshold costs for 5-yearly CTC with intermediate referral, the strategy with similar life-years gained as 10-yearly colonoscopy.
We looked at the effect of differential adherence between colonoscopy and CT colonography on the threshold costs for CTC. We compared costs and effects of colonoscopy screening with current adherence 60 (75% of patients had colonoscopy at least once during their lifetime, 45% according to recommendation) to CT screening with adherence rates comparable to that of mammography screening 61 (90% had CT colonography at least once during their lifetime, 70% according to recommendation).
Finally, we performed a sensitivity analysis for uncertain model parameters to assess their effect on the threshold costs. CTC sensitivity and specificity estimates were set at the lower and higher range of their confidence intervals (Table 1). Natural history parameters varied were the average duration of the adenoma carcinoma sequences (base value: 20 years, values considered: 10, 30 and 40 years) and the variance of the duration, assessed as the percent of cancers that develop within 5 years (base value: 22%, values considered: 1%, 5%, 10% and 25%). All of the alternative natural history models were calibrated to age-specific SEER CRC incidence.
RESULTS
The colonoscopy strategies varied in life-years gained from 0.096 per individual entering the program with a screening interval of 20 years to 0.123 with a 5-year interval (Table 3, 3% discounted results). The costs for colonoscopies increased from $1,900 for 2 screening colonoscopies to $3,364 for 7 colonoscopies. This increase is smaller than one may have expected, because of surveillance colonoscopies. The savings increased from $1,142 to $1,494. The current screening recommendation of colonoscopy screening every 10 years saved 0.113 life-years. CTC screening resulted in comparable life-years gained when performed every 5 years with intermediate or intensive referral. The life-years gained with CTC screening varied from 0.048 to 0.120. CTC screening induced lower colonoscopy costs than with colonoscopy screening but required additional CTC screen tests varying from 1.40 to 3.88 per individual. (undiscounted results are presented in Appendix 3)
Table 3.
CTC tests, colonoscopy costs, treatments savings and life-years gained for different follow-up strategies of CTC compared to colonoscopy screening (3% discounted)
| Screening strategy 1 | # CTC tests | Colonoscopy costs2 ($) |
Treatment savings3 ($) |
Life-years gained3 |
|
|---|---|---|---|---|---|
| Colonoscopy | |||||
| Interval: | 20 years | 0 | 1,900 | 1,142 | 0.096 |
| 15 years | 0 | 2,137 | 1,238 | 0.104 | |
| 10 years | 0 | 2,467 | 1,352 | 0.113 | |
| 5 years | 0 | 3,364 | 1,494 | 0.123 | |
| CTC–minimal referral | |||||
| Interval: | 20 years | 1.458 | 438 | 492 | 0.048 |
| 15 years | 1.802 | 512 | 570 | 0.056 | |
| 10 years | 2.316 | 590 | 682 | 0.069 | |
| 5 years | 3.877 | 735 | 892 | 0.092 | |
| CTC–intermediate referral | |||||
| Interval: | 20 years | 1.432 | 769 | 767 | 0.068 |
| 15 years | 1.745 | 885 | 875 | 0.078 | |
| 10 years | 2.206 | 1,019 | 1,017 | 0.091 | |
| 5 years | 3.582 | 1,289 | 1,236 | 0.111 | |
| CTC–intensive referral | |||||
| Interval: | 20 years | 1.399 | 1,242 | 999 | 0.086 |
| 15 years | 1.685 | 1,410 | 1,103 | 0.095 | |
| 10 years | 2.102 | 1,625 | 1,230 | 0.106 | |
| 5 years | 3.341 | 2,141 | 1,406 | 0.120 | |
Minimal referral: referral of patients with findings at CTC of 10 mm or larger
Intermediate referral: referral of patients with findings at CTC of 6 mm or larger
Intensive referral: referral of patients with any findings at CTC
An interval of 20 years corresponds with 2 screens in a lifetime, 15 years with 3 screens, 10 years with 4 screens and 5 years with 7 screens.
Including costs for screening, diagnostic and surveillance colonoscopies and treatment of complications due to colonoscopy. CTC screening costs are not included.
Compared to situation without screening
With CTC unit costs equal to colonoscopy costs ($662), CTC screening was dominated by colonoscopy screening (Figure 2A). The CTC screening strategies saved fewer life-years than colonoscopy screening for the same costs or required more costs to save the same number of life-years. Of the CTC screening strategies, CTC with intensive referral was least dominated. With CTC costs of $331 (half of colonoscopy costs), intermediate referral was the most cost-effective CTC screening strategy. However, only the intermediate referral strategy offered every 15 or 20 years was a cost-effective alternative for colonoscopy screening (Figure 2B). With CTC costs of $221 (one third of colonoscopy costs), most CTC screening strategies were a cost-effective alternative to colonoscopy screening (figure 2C). Intermediate referral remained the preferred strategy.
Threshold analysis
Figure 3 shows the threshold costs for the non-dominated CTC screening strategies to have equal incremental costs per life-year gained as colonoscopy screening. CTC screening every 20 years with intermediate referral had the highest threshold costs of $373. However, the life-years gained with this strategy are much lower than with the current recommendation of colonoscopy every 10 years. With increasing screening intensity, the threshold costs of CTC decreased. A strategy that is comparable to the current recommendation with respect to life-years gained is CTC screening every 5 years with a referral threshold of 6 mm. For this strategy to have the same incremental costs per life-year gained as 10-yearly colonoscopy, the unit costs of CTC need to be $285 or lower. With 25% higher adherence for CTC than for colonoscopy, threshold costs of 5-yearly CTC with intermediate referral increased to $470.
Sensitivity analysis
The worst and best case scenarios for CTC test characteristics yielded a similar ordering of strategies as the base case analysis. The threshold costs for CTC every 5 years with a referral threshold of 6 mm were $313 for the best-case scenario and $264 for the worst-case analysis. Varying the mean duration of the adenoma-carcinoma sequence from 20 to 10, 30 or 40 years did not change the threshold costs much: the threshold costs for CTC every 5 years with intermediate referral were $284, $282 and $277 respectively. Changing the variation in the duration of the adenoma-carcinoma sequence had somewhat more influence. CTC screening every 5 years with intermediate referral remained the strategy with similar life-years gained as colonoscopy every 10 years and the threshold costs for this strategy were $260 if 1% of cancers progress within 5 years (base case 22%), $265 if 5%, $271 if 10% and $284 if 25% of cancers progress within 5 years.
DISCUSSION
In this study, we found that at costs of $285 per test, CTC screening would be a cost-effective alternative to and provide similar life-years gained as the currently recommended strategy of colonoscopy screening every 10 years, provided that CTC screening would be offered every 5 years with follow-up restricted to findings of 6 mm or larger. Our results were very robust for changes in CTC test characteristics and natural history assumptions. CTC with intermediate referral remained the most-cost-effective CTC screening strategy in the majority of sensitivity analyses. The threshold costs for this strategy varied from $260-$312, 53-61% lower than that of optical colonoscopy. Assuming differential adherence for CTC and colonoscopy, had more effect on threshold costs. In the extreme scenario in which CTC would be able to increase screening adherence with 25% compared to colonoscopy, the threshold costs for CTC still needed to be 30% lower than colonoscopy costs.
Our results support the expectations of clinicians that follow-up of small findings at CTC is not efficient.62 Small adenomas are common and most will never develop into CRC. Furthermore, small findings at CTC are often non-adenomatous polyps or even artifacts, negatively influencing the specificity of CTC. Of course, when ignoring small findings and because of the lower sensitivity of CTC for small adenomas, the preclinical screen-detectable period is shorter than with colonoscopy screening. We showed that with a mean dwelling time of 9.1 years for adenomas of 1-5 mm and of 7.3 years for an adenoma of 6 mm to preclinical cancer (which is less than the screening interval of 10 years), CTC should be offered at an interval of 5 years to be as effective as colonoscopy every 10 years. Some radiologists and gastroenterologists suggest that follow-up could be restricted to polyps of 10 mm or larger.62 They argue that dysplasia and malignancy occur too rarely in smaller adenomas to warrant diagnostic follow-up and polypectomy. The rate of malignant transformation may indeed be up to ten times higher in large polyps than in small polyps.63, 64 However, small and medium adenomas are almost ten times more prevalent than large adenomas,39, 48 making the CRC incidence from small and medium adenomas potentially as high as that from large adenomas.65, 66 This becomes clear from the results from our study: In none of the analyses was CTC screening cost-effective with only follow-up of lesions of 10mm or more.
The strength of CTC to be able to distinguish between low-risk and high-risk individuals for CRC, may also turn out to be its weakness. Despite the fact that small findings are ignored with CTC, they are present and will frequently be seen. Then questions of ethics arise: is it ethical not to register and/or to inform patients about this finding? Informing about these findings without taking action on them will induce anxiety in otherwise healthy individuals. The shorter screening interval in the strategy with a higher referral threshold takes away some of the concerns. The probability that adenomas less than 6 mm will grow into cancer within five years is small. The shorter interval further offers the possibility to detect previously missed lesions. Also, it should be noted that setting thresholds for further action is inherent to screening. Ignoring small findings with CTC is, in terms of risk management, not that different from the cut-off levels used for a positive test result with for example PSA testing or immunochemical FOBT. If, however, it would be decided that ignoring of small findings at CTC is unacceptable, this will further decrease the threshold costs to 33%-39% of colonoscopy costs, depending on the interval chosen.
In this analysis, we assumed that restriction of follow-up did not impair CTC sensitivity. However, radiologists may over- or underestimate the real size of an adenoma.67 When restricting follow-up to adenomas of 6 mm or 10 mm or larger, some lesions will be misclassified as smaller and wrongfully not be followed up. Some small lesions will also be overestimated in size and will be followed up, but the benefit of their removal is smaller than of removal of larger adenomas. Errors in size estimation are therefore likely to make intensive referral more favorable compared to intermediate or minimal referral.
CTC screening is a non-invasive alternative to colonoscopy screening, and is not associated with the major complications of colonoscopy such as perforations, serosal burns, and bleeds.52-54 A recent study comparing CTC and colonoscopy for CRC screening,68 reported seven serious adverse events in 3,163 people undergoing colonoscopy, and no complications in 3,120 people undergoing CTC. However, CTC is associated with exposure to radiation, which we did not consider in the current analysis. Brenner et al.69 estimated that the excess cancer risk from a pair of CTC scans using typical current scanner techniques is about 0.14% for a 50-year old and half that for a 70-year old. This estimate is controversial, because it was based on simulation calibrated to atomic bomb survivors. Multiple CTC screens will increase the radiation dose proportionally and most likely also the radiation risks. We found that CTC is only compatible to colonoscopy screening if offered seven times (every 5 years between ages 50 and 80), potentially leading to an excess cancer risk of approximately 0.47%. This will lead to life-years lost due to CTC which are not negligible compared to the life-years gained. We did not take these excess cancer cases into account, because there is good evidence that radiation dose with CTC can be reduced by at least a factor of 5 (and perhaps as much as 10), while still maintaining sensitivity and specificity for polyps larger than approximately 5 mm.69 With these dose reductions, excess risk of cancer from CTC becomes negligible.
Several other studies have been published on the cost-effectiveness of CTC screening in the general population (Table 4). In all these studies, the threshold costs for CTC screening were higher than the 43% of colonoscopy costs found in this study. An important reason for this is that we compared CTC screening with different intervals of colonoscopy screening, whereas the other studies compared CTC only to 10-yearly colonoscopy. Sonnenberg estimated that 10-yearly intensive CTC should cost 46% of colonoscopy costs to have the same costs per life-year gained.29 The same comparison in our study yields similar threshold costs (47%, footnote Table 4). The estimated threshold costs from Ladabaum were slightly higher (60%), but he assumed better CTC test sensitivity.28 Vijan et al. compared CTC every 5 years (referral of all lesions) with 10-yearly colonoscopy.70 They found threshold costs of 75% of colonoscopy costs. The same comparison in our study yields costs of 33% (footnote Table 4). This is explained by better test characteristics (especially for specificity) based on 3-dimensional (3D) CTC in Vijan’s assumptions. Using the performance characteristics of 2D CTC (which had slightly lower sensitivity than in this analysis, but still better specificity), Vijan found very low CTC threshold costs, which corresponds with our finding of 33%. Finally, Pickhardt compared 10-yearly CTC screening with a referral threshold of 6 mm to 10-yearly colonoscopy screening.30 He found that with CTC costs at 70% of colonoscopy costs, CTC screening with referral of lesions 6 mm or larger was cost-effective compared to colonoscopy. This is somewhat higher than the estimate from the same comparison in our study (62%). Our study adds that to compete with colonoscopy cost-effectiveness, one must consider different intervals of colonoscopy screening. This is necessary to ascertain that CTC screening is not dominated by colonoscopy screening. In Figure 3, this is represented by the (incremental cost-effectiveness) lines connecting the colonoscopy strategies. It is harder for 5-yearly intermediate CTC screening to achieve a level on the cost-effectiveness frontier line connecting 15-yearly colonoscopy and 10-yearly colonoscopy than to get on the line connecting no screening (the origin) to 10-yearly colonoscopy. The 43% threshold for the CTC costs relative to the colonoscopy costs would increase to 62% with this more relaxed criterion.
Table 4.
Literature overview of studies estimating the cost-effectiveness of CTC screening in the average-risk population
| Study | Comparator strategy |
Sensitivity CTC for adenomas |
Specificity CTC |
Threshold costs as % of cspy costs |
|---|---|---|---|---|
| Lansdorp- Vogelaar |
several cspy strategies |
Small: 29% | 0 mm: 53% | 42%, for 5-yearly CTC, referral 6 mm |
| Medium: 66% | 6 mm: 84% | |||
| Large: 87% | 10 mm: 95% | |||
| Sonnenberg 1999 | 10-yearly cspy | 80% | 95% | 46% for 10-yearly CTC, referral 0 mm1 |
| Ladabaum 2004 | 10-yearly cspy | Small: 87% | 85% | 60% for 10-yearly CTC, referral 0 mm1 |
| Medium: 87% | ||||
| Large: 94% | ||||
| Vijan 2007 | 10-yearly cspy | Small: 46% | 91% | 75% for 5-yearly CTC, referral 0 mm2 |
| Medium: 83% | ||||
| Large: 91% | ||||
| Pickhardt 2007 | 10-yearly cspy | Small: 48% | 86% | >70% for 10-yearly CTC, referral 6 mm3 |
| Medium: 70% | ||||
| Large: 85% |
Cspy: colonoscopy
Comparison of the same strategies in our model yielded threshold costs of 47%
Comparison of the same strategies in our model yielded threshold costs of 33%
Comparison of the same strategies in our model yielded threshold costs of 62%
CTC is still under development. The development of computer-assisted reading of the images and detection of lesions has potential for decreasing radiologists reading time and therewith reducing costs.71 Furthermore, the potential introduction of CTC without cathartic preparation will further reduce the inconvenience of patients, and therefore probably increase adherence with CTC.72, 73 These developments will have to be monitored for updating the comparative evaluation between CTC and other screening modalities. Our analysis shows that CTC can be a cost-effective alternative for CRC screening in the general population if offered every 5 years, diagnostic follow-up is restricted to those with polyps of 6 mm or larger and CTC costs less than 43% of colonoscopy costs. In view of the abovementioned developments, this level of CTC costs may be possible.
Supplementary Material
Acknowledgments
STUDY SUPPORT The National Cancer Institute U01 CA97426 and U01 CA115935 supported this work for the Cancer Intervention and Surveillance Modeling Network. The study sponsor has played no role in the study design, collection, analysis and interpretation of the data, nor in the writing of the report.
Abbreviations
- CTC
Computed Tomographic Colonography
- CRC
Colorectal Cancer
- FOBT
Fecal Occult Blood Test
- Cspy
Colonoscopy
Footnotes
POTENTIAL CONFLICT OF INTEREST Rob Boer has participated since 1989 in the screening research group at the Department of Public Health of the Erasmus MC. He is affiliated with RAND since 2000. Since 2007, he is a Director of Evidence Based Strategies - Disease Modeling and Economic Evaluation at Pfizer Inc, which develops and sells various medicines for cancer and other diseases. This research and article were not funded or supported by Pfizer.
Novelty and impact of the paper: We found that CTC costs should not exceed 43% of colonoscopy costs for CTC to be as cost-effective as colonoscopy screening. This estimate is considerably lower than previous estimates in literature, because previous studies only compared CTC screening to 10-yearly colonoscopy, while in this study we compared to different intervals of colonoscopy screening.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674–80. doi: 10.1053/j.gastro.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–37. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 6.Gyrd-Hansen D, Sogaard J, Kronborg O. Analysis of screening data: colorectal cancer. Int J Epidemiol. 1997;26:1172–81. doi: 10.1093/ije/26.6.1172. [DOI] [PubMed] [Google Scholar]
- 7.Church TR, Ederer F, Mandel JS. Fecal occult blood screening in the Minnesota study: sensitivity of the screening test. J Natl Cancer Inst. 1997;89:1440–8. doi: 10.1093/jnci/89.19.1440. [DOI] [PubMed] [Google Scholar]
- 8.Young GP, St John DJ, Winawer SJ, Rozen P. Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol. 2002;97:2499–507. doi: 10.1111/j.1572-0241.2002.06046.x. [DOI] [PubMed] [Google Scholar]
- 9.Levin TR. Flexible sigmoidoscopy for colorectal cancer screening: valid approach or short-sighted? Gastroenterol Clin North Am. 2002;31:1015–29. vii. doi: 10.1016/s0889-8553(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman DA, Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–60. doi: 10.1056/NEJMoa010328. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17–23. doi: 10.1016/s0016-5085(97)70213-0. [see comments] [DOI] [PubMed] [Google Scholar]
- 13.Hixson LJ, Fennerty MB, Sampliner RE, Garewal HS. Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125–27. doi: 10.1016/s0016-5107(91)70668-8. [DOI] [PubMed] [Google Scholar]
- 14.Hoff G, Sauar J, Vatn MH, Larsen S, Langmark F, et al. Polypectomy of adenomas in the prevention of colorectal cancer: 10 years’ follow-up of the Telemark Polyp Study I. Scand J Gastroenterol. 1996;31:1006–10. doi: 10.3109/00365529609003121. [DOI] [PubMed] [Google Scholar]
- 15.Muller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995;155:1741–8. doi: 10.1001/archinte.1995.00430160065007. [DOI] [PubMed] [Google Scholar]
- 16.Newcomb PA, Storer BE, Morimoto LM, Templeton A, Potter JD. Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. J Natl Cancer Inst. 2003;95:622–5. doi: 10.1093/jnci/95.8.622. [DOI] [PubMed] [Google Scholar]
- 17.Selby JV, Friedman GD, Quesenberry CP, Jr., Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–57. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 18.Atkin WS, Edwards R, Wardle J, Northover JM, Sutton S, Hart AR, Williams CB, Cuzick J. Design of a multicentre randomised trial to evaluate flexible sigmoidoscopy in colorectal cancer screening. J Med Screen. 2001;8:137–44. doi: 10.1136/jms.8.3.137. [DOI] [PubMed] [Google Scholar]
- 19.Bretthauer M, Gondal G, Larsen K, Carlsen E, Eide TJ, Grotmol T, Skovlund E, Tveit KM, Vatn MH, Hoff G. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia: attendance rates in the NORCCAP study (Norwegian Colorectal Cancer Prevention) Scand J Gastroenterol. 2002;37:568–73. doi: 10.1080/00365520252903125. [DOI] [PubMed] [Google Scholar]
- 20.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 21.Segnan N, Senore C, Andreoni B, Aste H, Bonelli L, Crosta C, Ferraris R, Gasperoni S, Penna A, Risio M, Rossini FP, Sciallero S, et al. Baseline findings of the Italian multicenter randomized controlled trial of “once-only sigmoidoscopy”--SCORE. J Natl Cancer Inst. 2002;94:1763–72. doi: 10.1093/jnci/94.23.1763. [DOI] [PubMed] [Google Scholar]
- 22.Seeff LC, Manninen DL, Dong FB, Chattopadhyay SK, Nadel MR, Tangka FK, Molinari NA. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–9. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 23.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635–50. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 24.van Dam J, Cotton P, Johnson CD, McFarland BG, Pineau BC, Provenzale D, Ransohoff D, Rex D, Rockey D, Wootton FT., 3rd AGA future trends report: CT colonography. Gastroenterology. 2004;127:970–84. doi: 10.1053/j.gastro.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Taylor SA, Halligan S, Saunders BP, Bassett P, Vance M, Bartram CI. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. AJR Am J Roentgenol. 2003;181:913–21. doi: 10.2214/ajr.181.4.1810913. [DOI] [PubMed] [Google Scholar]
- 26.Thomeer M, Bielen D, Vanbeckevoort D, Dymarkowski S, Gevers A, Rutgeerts P, Hiele M, Van Cutsem E, Marchal G. Patient acceptance for CT colonography: what is the real issue? Eur Radiol. 2002;12:1410–5. doi: 10.1007/s003300101082. [DOI] [PubMed] [Google Scholar]
- 27.Heitman SJ, Manns BJ, Hilsden RJ, Fong A, Dean S, Romagnuolo J. Cost-effectiveness of computerized tomographic colonography versus colonoscopy for colorectal cancer screening. Cmaj. 2005;173:877–81. doi: 10.1503/cmaj.050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact? Clin Gastroenterol Hepatol. 2004;2:554–63. doi: 10.1016/s1542-3565(04)00247-2. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenberg A, Delco F, Bauerfeind P. Is virtual colonoscopy a cost-effective option to screen for colorectal cancer? Am J Gastroenterol. 1999;94:2268–74. doi: 10.1111/j.1572-0241.1999.01304.x. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–21. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 31.Schoenfeld P. Small and diminutive polyps: implications for colorectal cancer screening with computed tomography colonography. Clin Gastroenterol Hepatol. 2006;4:293–5. doi: 10.1016/j.cgh.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Loeve F, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JDF. Final report MISCAN-COLON microsimulation model for colorectal cancer: report to the National Cancer Institute Project No. NO1-CN55186. Department of Public Health, Erasmus University; 1998. [Google Scholar]
- 33.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JDF. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32:13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 34.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92:557–63. doi: 10.1093/jnci/92.7.557. [see comments] [DOI] [PubMed] [Google Scholar]
- 35.Vogelaar I, van Ballegooijen M, Zauber AG, Boer R, Oortmarssen GJv, Loeve F, Habbema JDF. Model Profile of the MISCAN-Colon microsimulation model for colorectal cancer. Department of Public Health; Erasmus MC: 2004. Accessed at: https://cisnet.flexkb.net/mp/pub/cisnet_colorectal_sloankettering_profile.pdf. [Google Scholar]
- 36.Morson B. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451–57. [PMC free article] [PubMed] [Google Scholar]
- 37.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 38.Surveillance Research Program National Cancer Institute SEER*Stat Software. version 5.3.1. 2003.
- 39.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7:249–61. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 40.Blatt L. Polyps of the Colon and Rectum: Incidence and Distribution. Dis Colon Rectum. 1961;4:277–82. [Google Scholar]
- 41.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61:1472–6. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 42.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–6. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark JC, Collan Y, Eide TJ, Esteve J, Ewen S, Gibbs NM, Jensen OM, Koskela E, MacLennan R, Simpson JG, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36:179–86. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 44.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508–14. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24:799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 46.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847–57. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 47.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49:819–25. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 48.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835–42. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, Habbema JD. National Polyp Study data: Evidence for regression of adenomas. Int J Cancer. 2004;111:633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 50.Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, Zauber AG. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 51.Pickhardt PJ, Choi JR, Hwang I, Schindler WR. Nonadenomatous polyps at CT colonography: prevalence, size distribution, and detection rates. Radiology. 2004;232:784–90. doi: 10.1148/radiol.2323031614. [DOI] [PubMed] [Google Scholar]
- 52.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 53.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 54.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–73. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 55.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 56.Jentschura D, Raute M, Winter J, Henkel T, Kraus M, Manegold BC. Complications in endoscopy of the lower gastrointestinal tract. Therapy and prognosis. Surg Endosc. 1994;8:672–6. doi: 10.1007/BF00678564. [DOI] [PubMed] [Google Scholar]
- 57.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, Ballegooijen Mv, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. 2007 doi: 10.1059/0003-4819-153-6-201009210-00004. Accessed at: https://www.cms.hhs.gov/mcd/viewtechassess.asp?where=index&tid=52. [DOI] [PMC free article] [PubMed]
- 58.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 59.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH, Brooks D, et al. Guidelines for Colonoscopy Surveillance After Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 61.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 62.Ransohoff DF. CON: Immediate colonoscopy is not necessary in patients who have polyps smaller than 1 cm on computed tomographic colonography. Am J Gastroenterol. 2005;100:1905–7. doi: 10.1111/j.1572-0241.2005.50130_3.x. discussion 07-8. [DOI] [PubMed] [Google Scholar]
- 63.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–76. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 64.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Illstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–13. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 65.Koretz RL. Malignant polyps: are they sheep in wolves’ clothing? Ann Intern Med. 1993;118:63–68. doi: 10.7326/0003-4819-118-1-199301010-00011. [DOI] [PubMed] [Google Scholar]
- 66.DiSario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991;86:941–5. [PubMed] [Google Scholar]
- 67.Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology. 2005;236:872–8. doi: 10.1148/radiol.2363041534. [DOI] [PubMed] [Google Scholar]
- 68.Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, Gopal DV, Reichelderfer M, Hsu RH, Pfau PR. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 69.Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129:328–37. doi: 10.1053/j.gastro.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Vijan S, Hwang I, Inadomi J, Wong RK, Choi JR, Napierkowski J, Koff JM, Pickhardt PJ. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol. 2007;102:380–90. doi: 10.1111/j.1572-0241.2006.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hara AK. The future of colorectal imaging: computed tomographic colonography. Gastroenterol Clin North Am. 2002;31:1045–60. doi: 10.1016/s0889-8553(02)00060-2. [DOI] [PubMed] [Google Scholar]
- 72.Iannaccone R, Laghi A, Catalano C, Mangiapane F, Lamazza A, Schillaci A, Sinibaldi G, Murakami T, Sammartino P, Hori M, Piacentini F, Nofroni I, et al. Computed tomographic colonography without cathartic preparation for the detection of colorectal polyps. Gastroenterology. 2004;127:1300–11. doi: 10.1053/j.gastro.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 73.McFarland EG, Zalis ME. CT colonography: progress toward colorectal evaluation without catharsis. Gastroenterology. 2004;127:1623–6. doi: 10.1053/j.gastro.2004.09.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.