Abstract
Purpose
We describe an ongoing study of enhanced continuity of pharmacy care following hospital discharge to assess its impact on quality and patient safety.
Methods
The Iowa Continuity of Care (COC) study is a randomized, prospective trial enrolling 1,000 patients with selected medical conditions admitted to one large Midwest hospital. All patients must agree to obtain medications from one community pharmacy of their choice for 90 days post-discharge. Patients will be randomized to a control group, minimal intervention, or an enhanced intervention. Patients in the control group will receive usual care. Patients in the minimal and enhanced intervention groups will receive admission medication verification with the patients’ community pharmacists, medication teaching, and discharge counseling from a pharmacist case manager (PCM). In addition, patients in the enhanced intervention group will have a discharge care plan faxed to their outpatient physician and community pharmacist and will receive a follow-up phone call from the PCM 3–5 days post-discharge. The PCM will continue to resolve medication problems and facilitate communication between the community providers for patients in the enhanced intervention group. A blinded research nurse will collect data, including adverse drug events (ADEs), at admission, 30 days post-discharge and 90 days post-discharge.
Outcome Measurements
The primary outcome measures include medication appropriateness, ADEs, emergency department visits, unscheduled office visits and re-hospitalizations. Data will be collected from the inpatient electronic medical record, outpatient physician medical records, community pharmacist records and directly from patients. A cost-effectiveness analysis will be performed.
Conclusion
The Iowa COC study will examine the effects of increased communication with a PCM on the incidence of serious ADE, hospitalizations, and unscheduled office visits in patients with cardiovascular disease, pulmonary disease, or diabetes. The study will address the value of a PCM to improve communication of care plans between the inpatient and community settings.
Index Terms: Clinical pharmacy, primary care, medication reconciliation, adverse reactions, research
Introduction
Poor communication between healthcare providers, healthcare settings and lack of shared patient information are common causes of under-treatment, sub-optimal therapy, adverse drug events (ADEs) and hospital admissions.1, 2 The hospital discharge can be a chaotic event and several types of errors can occur during or after discharge. Multiple medications may be changed during hospitalization, creating patient confusion and potential discrepancies between hospital and community records. Most health-care settings lack communication between the hospital setting and providers in the community. Community pharmacists and primary care physicians are often unaware of the complete list of medications for these discharged patients. Furthermore, many patients selectively fill discharge medications and one study found 32% of prescribed medications were not being taken by elderly patients two days after discharge.3, 4
Poor communication with community physicians and patient confusion following discharge may jeopardize proper therapy and increase the risk of ADEs. While various strategies exist to help solve these problems, a consistently effective approach has not been found. The Joint Commission’s 2008 National Patient Safety Goals are designed to address these issues.5 Goal number 8 is to “accurately and completely reconcile medications across the continuum of care”. The first requirement being that “there is a process for comparing the patient’s current medications with those ordered for the patient while under the care of the organization”. The second requirement is that “a complete list of the patient’s medications is communicated to the next provider of service when a patient is referred or transferred to another setting, service, practitioner, or level of care within or outside the organization”. The Joint Commission states that “patients are most at risk during transitions in care across settings, services, providers, or levels of care. The development, reconciliation and communication of an accurate medication list throughout the continuum of care is essential in the reduction of transition-related adverse drug events”.
Schnipper et al found medication discrepancies for 49% of 178 patients at discharge.6 These investigators found that discharge counseling and telephone follow-up by a clinical pharmacist reduced preventable ADEs (1% intervention group vs. 11% usual care, p=0.01) and preventable emergency department (ED) visits or readmissions (1% intervention group vs. 8% usual care, p=0.03). Another study evaluated a telephone call by a pharmacist two days after discharge in 221 patients.7 Fewer patients in the intervention group had a visit to the ED compared to usual care (10% vs. 24%, p = 0.005) and there was a trend for fewer hospitalizations (15% vs. 25%, p = 0.07). Both studies were relatively small, had short follow-up (4–6 weeks), did not provide data to community physicians and community pharmacists and neither study completely identified ADEs from multiple sources such as from the community pharmacy or outpatient physician. These limitations need to be addressed in future studies in order to completely identify ADEs and determine the extent that ADEs can be prevented by sharing information.
A recent study found that many recommended workups or treatment plans were not completed following discharge, especially when primary care physicians did not have access to discharge summaries.8 The transfer of information between inpatient and community pharmacies was considered likely to be beneficial with a “medium” implementation cost and complexity.9, 10 Two randomized, controlled studies have evaluated information transfer between hospitals and outpatient pharmacies.11, 12 Using facsimile transmissions at discharge from a community hospital to the community pharmacy, Kuehl et al found significantly more pharmacist interventions in either the hospital (47%, p < 0.001) or community (42%, p < 0/05) in the intervention group compared with patients who did not have the information transfer (14%, 0, respectively).12 Other studies that evaluated shared information found reduced medication problems.6, 7, 13
The Iowa COC study builds upon this previous research and is designed to evaluate an intervention to improve continuity of pharmacy care and target patients with specific cardiovascular diseases, pulmonary diseases or diabetes who are at high risk for ADEs. This intervention is designed to improve medication appropriateness, reduce ADEs associated with sub-optimal therapy and reduce costs due to hospitalizations or unscheduled office visits. The primary goal is to determine if this model can improve medication therapy by using a pharmacist case manager (PCM) to improve communication and continuity of care. Two PCMs (33% each) will be devoted to this study to ensure proper coverage and timely performance of all interventions. The PCMs have a Doctor of Pharmacy degree (Pharm.D.) and at least one year of residency and/or clinical practice experience including conducting admission medication histories, discharge education, medical team rounding and chronic disease management of patients. Previous studies have described the value of medication reconciliation, and studies have shown increased collaboration between pharmacists and physicians improved therapy, improved disease control and reduced ADEs.14–31 The proposed intervention is supported by small studies.11, 12, 32 The investigators conducted a pilot study, funded by the ASHP Research and Education Foundation, that helped establish the electronic communication links that will be performed in the present study. The present study is a five year trial, funded by the National Heart Lung and Blood Institute, which is designed to assess the success of a comprehensive COC model in a randomized, controlled clinical trial.
Research hypotheses
The study has four testable hypotheses stated here in the positive form:
Medication appropriateness during and after hospitalization will be improved in patients who receive the enhanced intervention from a pharmacist case manager compared to usual care.
Preventable ADEs during and after hospitalization will be lower for patients who receive the enhanced intervention compared to patients who receive usual care or minimal intervention.
The number of hospital readmissions, emergency department visits, and unscheduled office visits, and specifically the number of visits attributed to poor medication adherence, will be lower for patients who receive the enhanced intervention compared to usual care or minimal intervention.
Methods
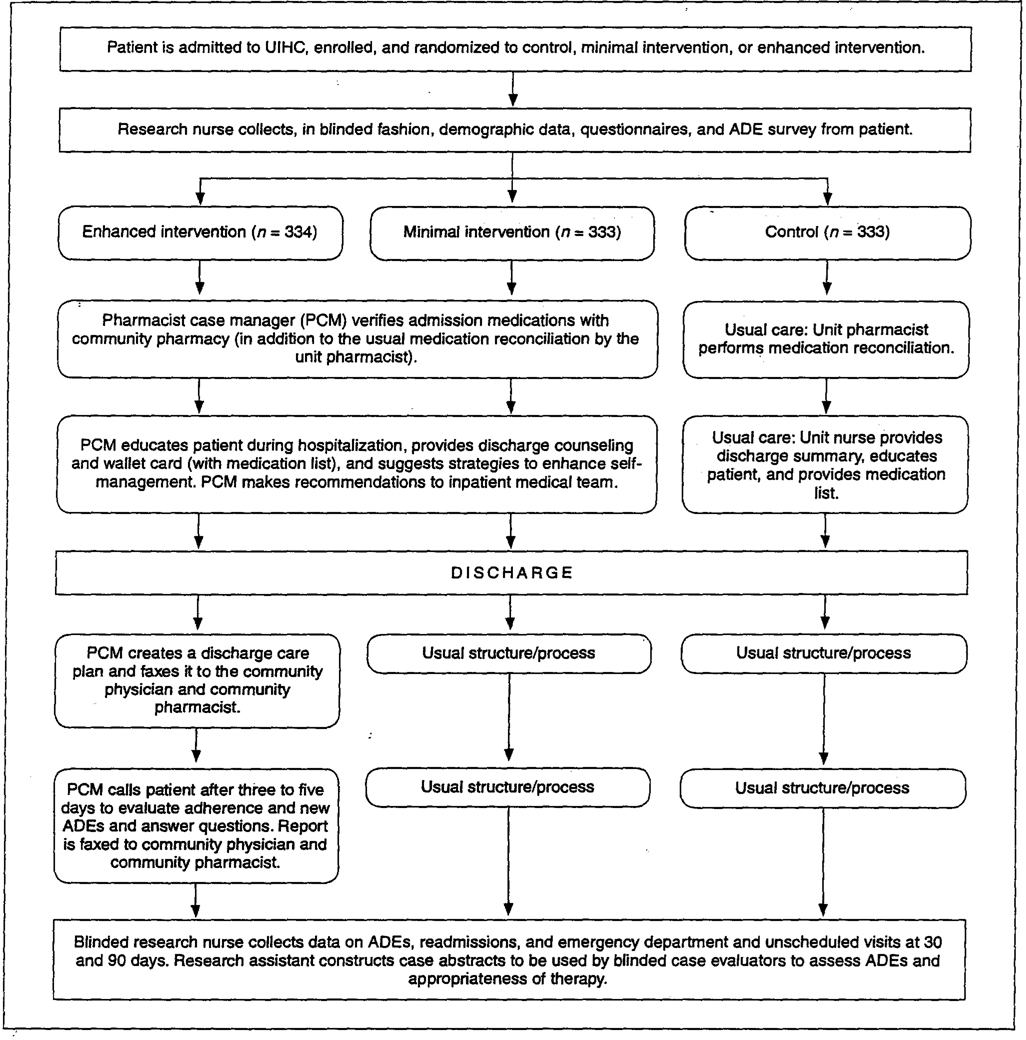
This randomized, controlled trail will enroll 1000 patients at the University of Iowa Hospitals and Clinics (UIHC), a tertiary academic health sciences center. The study has been approved by the University of Iowa committee on protection of human subjects. The overall study design is displayed in figure 1.
Figure 1.
Overall study design. UIHC = University of Iowa Hospitals and Clinics, ADE = adverse drug event.
Inclusion and exclusion criteria
To be eligible to be enrolled in the study, a patient must be an English or Spanish speaking male or female 18 years of age or older admitted to UIHC with one of the following diagnoses: hypertension, hyperlipidemia, heart failure, coronary artery disease, myocardial infarction, stroke, transient ischemic attack, asthma, COPD, diabetes, or patients receiving oral anticoagulation. The patient must be admitted to the general medicine, family medicine, cardiology, or orthopedics services. Patients initially admitted to an intensive care unit will only qualify if they are later transferred to one of the above services. The patients must also receive their medical care in the community and their prescriptions from a community pharmacy. Patients must also agree to obtain all chronic prescriptions from one community pharmacy of their choice during the 90 days post-hospital discharge.
Patients will be excluded from the study if they receive their primary medical care from any UIHC clinic with electronic medical records connected to the hospital. They will also be excluded if they receive their chronic prescriptions from the UIHC outpatient pharmacies. Patients will be excluded if they do not have a working telephone or have a hearing impairment that does not allow them to use a telephone, have a life expectancy estimated at less than 6 months, dementia or cognitive impairment, severe psychiatric or psychosocial factors, including substance abuse, that may impair their desire or ability to complete all aspects of the study, or if they are admitted to the psychiatric, surgery, or hematology/oncology services.
Patient selection and randomization
Electronic medical records will be reviewed daily by the project manager for all new patients admitted to each of the four medical services included in this study. In order to meet NHLBI goals for minority recruitment, we will over enroll African Americans and Hispanics. The project manager will first determine if the patient meets the inclusion criteria and whether they are a member of a minority group, and if so, they will be approached to participate if they meet the inclusion criteria. The project manager will next approach other patients. Once patients sign informed consent, randomization will occur. The study biostatistician created a blinded randomization scheme to stratify for the medical services and for minority versus nonminority patients. The randomization was developed using pseudo-random number generation via SAS statistical software to ensure the probabilities of assignment to each treatment group are equal. Once the patient has signed informed consent, the project manager will open a sealed envelope to determine the group to which the patient will be randomized. Patients will be randomized equally to one of three groups: enhanced intervention, minimal intervention, or control.
Next, a research nurse, who will be blinded to the group randomization, will collect demographic information including ability to pay for medications. She will also administer the baseline surveys including ADEs, health status, self-reported adherence, Pfeiffer mental status questionnaire,33 and Katz index of activities of daily living.34, 35 Medication management skills will be evaluated including ability to read a prescription label, ability to remove two tablets from a 7-dram prescription vial, ability to interpret directions and ability to differentiate tablet colors (developed by Ruscin and Semla),36 which are important predictors of adherence and ADEs. The blinded research nurse will verbally ask all questions on each survey for all study patients, therefore the procedure will be the same for English or Spanish speaking patients. For Spanish speaking patients, a translator will read each question and obtain the responses from the patient.
Once the research nurse has completed collection of all baseline information, one of the two pharmacy case managers (PCM) assigned to the study will be informed if the patient is randomized to one of the two intervention groups. However, to maintain blinding, the pharmacists will not be informed whether the patient is in the minimal or enhanced intervention group at this time.
Admission medication reconciliation
In alignment with recent Joint Commission National Patient Safety Goals, “usual care” at UIHC now consists of medication reconciliation within 24 hours of admission to the hospital for all patients. Hospital pharmacists obtain a list of medications the patient was taking prior to hospital admission from the patient or caregiver. The patient’s medication list is then reconciled with the previous, possibly outdated, medication list found in the electronic medical record. When a current medication list cannot be obtained from the patient or caregiver, one is obtained by calling the patient’s community pharmacy. The pharmacist then reconciles the newly obtained medication history with the hospital admission orders. If a change is deemed appropriate, consultation with the inpatient team occurs. This process will continue in the control or usual care group. The PCMs will augment usual care by calling the patient’s community pharmacy to verify the complete medication list for all patients randomized to the minimal or enhanced intervention groups. When possible, discrepancies will be clarified with the patient.
Medication Teaching and Discharge Counseling
Patients in the minimal and enhanced intervention groups will receive medication teaching throughout hospitalization. The PCM will visit the patient every 2–3 days to discuss probable discharge medications and their purpose, goals of therapy, dosages, administration, duration required for the medication, potential adverse effects, potential barriers to adherence, strategies to improve adherence and what to expect for future dosage changes and monitoring. The PCM will perform extensive patient medication teaching throughout hospitalization to allow patients a chance to ask follow-up questions. This approach also helps minimize the negative effect of a hurried discharge counseling session that might overwhelm the patient. The PCM will track the patient throughout their hospital stay to monitor for medication problems and regularly contact the medical service in charge of the patient’s care or the unit hospital pharmacist in order to determine timing of discharge and to identify a potential discharge care plan. The day the patient is to be discharged from the hospital the PCM will complete medication teaching about the patient’s discharge medications. The patient will receive a discharge medication list and a wallet card containing all discharge medications.
Patients in the control group will not receive medication teaching from the PCM, but will receive a discharge medication list and verbal information from a hospital nurse as per usual care in our hospital. After a patient in an intervention group is discharged from the hospital, the PCM will contact the project manager who will then inform the pharmacist if the patient is in the enhanced intervention group. Patients in the minimal intervention group will receive no further contact or intervention from the PCM.
Enhanced Intervention
For patients in the enhanced intervention group, the PCM will compile a discharge care plan containing the patient’s discharge medication list including purpose of all medications, plans for drug titration, duration of therapy, monitoring plan, recommendations for preventing adverse drug events, and anticipated timing of refills where applicable. The care plan will also include important points from discharge teaching (e.g. medication adherence issues, cost issues, patient anxiety about new diagnosis, etc). The discharge care plan will be faxed to the patient’s community physician and community pharmacist.
Patients in the enhanced intervention group will also receive a follow-up phone call from the PCM 3–5 days after hospital discharge. The purpose of the call is to identify if, and reasons why, the patient failed to fill any discharge medications, evaluate any administration difficulties or problems taking discharge medications, evaluate patient understanding of each medication, inquire about specific ADEs since discharge, reinforce patient education on medication purpose, administration and monitoring, encourage follow-up with their community physician, and encourage communication of ADEs or poor treatment response to the patient’s community physician and community pharmacist. Any problems identified during the follow-up phone call will be communicated to the patient’s community physician or to the inpatient medical team if the patient has not yet seen their community physician. Additionally, an electronic report of the follow-up call will be faxed to the community physician and community pharmacist. The PCM will continue to communicate with the patient and the patient’s community healthcare providers at least weekly until all identified problems are resolved.
Follow-up patient questionnaires
The blinded research nurse will contact all patients by telephone to administer the ADE survey, self-reported adherence and overall health status questionnaires by 30 and 90 days after discharge. The patients will be asked structured questions to determine if they have had any re-admissions, ED or urgent care visits.
Pharmacist and Physician Surveys
For each patient in the study, we will survey community physicians and community pharmacists using a survey instrument that was previously piloted and subsequently modified.37 Physicians will be surveyed to determine how much they value the interactions with the pharmacist. Physicians with a patient in the enhanced intervention group will receive questions for both the community pharmacist and the PCM (Appendix 1). Physicians with a patient in the usual care and minimal intervention groups will complete the survey using only the community pharmacist as the frame of reference.
Community pharmacists with patients in the control and minimal intervention groups will receive a patient-specific survey to determine if they valued interactions with physicians for each specific patient in the study. For patients in the enhanced intervention group, the community pharmacist will receive the same questionnaire plus a second part that asks their opinion on the value of data, information and recommendations communicated by the PCM. Community physicians and pharmacists will be compensated $50 per patient for their time and resources used to prepare copies of medical or pharmacy records and reimbursement for the time to complete questionnaires.
Data Collection and Analysis
Data Abstraction
For each patient, a blinded research assistant will obtain data from community pharmacy refill and inpatient records, outpatient physician records, and billing records for days 1–90 following discharge. The trained research assistant will take all collected data and compile it into a structured report for each patient. The intervention endpoints, data sources and timing of data collection are displayed in Table 1. Discharge summaries, ED summaries, and billing information will be obtained for any admission to the community hospital or clinic or UIHC. A structured, blinded case abstract will be created for each patient using methods developed by the principal investigator.38, 39 The case abstract will include any medication list available in the progress notes, the goals of therapy for each medication, and whether there is a monitoring plan documented. We will determine if this information is available on the following dates: admission and discharge (hospital records), and 30 and 90 days post-discharge (community pharmacy and outpatient physician records).
Table 1.
Study Endpoints, Data Sources, and Timing of Data Collectiona
| Item | Data Source | During Hospitalization | 30 Days after Discharge | 90 Days after Discharge |
|---|---|---|---|---|
| Primary Endpoints | ||||
| Medication appropriateness, guideline adherence | Community physician records, pharmacy records | X | X | X |
| Documented serious or life-threatening ADEs | Pharmacy and medical records, adjudicated by blinded panel | X | X | |
| Self-reported ADEs | Patient interview by research nurse | X | X | X |
| Hospital readmissions (university and local hospitals) | Medical record and patient interview by research nurse | X | X | |
| ED visits, all scheduled and unscheduled office visits (university, local hospital, clinics) | Medical record review and patient interview by research nurse | X | X | |
| Billing records for university and community hospital admissions, physician office visits | Medical/hospital records collected by the blinded research assistant | X | X | |
| All prescription costs | Pharmacy records | X | X | |
| Secondary Endpoints | ||||
| Adherence to medications (refill records) | Pharmacy prescription records | X | X | |
| Self-reported adherence to medications | Interview by research nurse | X | X | X |
| Value of pharmacist recommendations | Physician survey | X | ||
| Inappropriate medications in elderly | Medical record, patient interview, peer review | X | X | X |
| Up-to-date medication list in chart | Medical record, peer review | X | X | X |
| Covariates | ||||
| Number of active medications | Prescription records | X | X | X |
| Demographics | Patient interview, medical record | X | ||
| Comorbidity | Patient interview, medical record | X | X | |
| Pfeiffer mental status Katz index, medication management skills | Patient questionnaire and interview | X | ||
| Patient insurance and pharmacy benefit | Patient interview | X | X | |
| Source of discharge medications (hospital, community pharmacy, or both) | Patient interview/pharmacy records | X | X | |
| Inpatient service | Hospital record | X | ||
| Community physician | Patient interview or referral | X | ||
| Level of community pharmacy services | Pharmacist survey | X | ||
ADE = adverse drug event, ED = emergency department.
Medication Appropriateness
Blinded evaluators will score case abstracts using a modified Medication Appropriateness Index (mMAI).40 The blinded evaluators are all board certified clinical pharmacy specialists and faculty. The mMAI will be scored for each patient at discharge, 30 days and 90 days to evaluate changes in medication appropriateness. The blinded pharmacist evaluators will also 1) determine if there is a complete medication list at 30 and 90 days, 2) categorize medication errors at admission, discharge, 30 and 90 days, and 3) determine the severity and significance of each medication error.
Identification of ADEs
We will combine several previously developed methods to identify ADEs.1, 21, 41, 42 We use the method of Bates to identify inpatient ADEs.43 We will use other methods developed by Bates to identify ADEs during the outpatient period.6, 43, 44 Specifically, drug-related ADEs will be detected by examining: 1) the patient self-reported survey done on admission, 2) free text from the inpatient EMR, 3) the physician discharge summary, 4) the free text from the pharmacist case manager’s electronic discharge summary, 5) community pharmacy records, 6) patient self-report following discharge, and 7) outpatient physicians’ records. We have chosen to use ambulatory medical records because they yield many more ADEs than electronic indicators.1 We will also use patient self-report because it identifies ADEs not obtained by other methods and has been used in other recent studies of ADEs in ambulatory care.42, 44 One study in ambulatory care found that patient self-report resulted in a five-fold greater frequency of ADEs than clinician report, computerized indicators and computerized searching of electronic notes.1, 44 Multiple methods, including patient and clinician report and chart review, are complementary and most likely to result in more complete identification of ADEs.42 We expect that this combined approach will identify a higher frequency of ADEs than other studies.
Classification of possible drug-related ADEs
We will use blinded expert panels to separate serious and life-threatening ADEs from minor events and then determine if ADEs could have been prevented. Serious ADEs will be evaluated on admission, discharge and 90 days. The latter time point will include any event that occurs from 1–90 days after discharge. The clinical pharmacy evaluators will blindly evaluate each case abstract.38, 45, 46 The evaluators will review each reported ADE and determine if it is potentially related to one of the patient’s medications using the U.S. Pharmacopoeia and the American Hospital Formulary Service (AHFS) Drug Information. This strategy was used at our institution to evaluate ADEs in elderly patients and these ADEs were found to lead to greater health care utilization.47 All potential ADEs will be presented to blinded pairs of investigators that include one physician and one clinical pharmacy investigator. These investigators will be blinded to intervention or control groups and independently classify each ADE using a structured implicit review using the following criteria:1, 43, 48, 49 whether an ADE was present, the severity of the event, whether the event was preventable, and the effects the event had on the patient. The investigators will determine the probability that the event was related to the drug (Naranjo probability scale).50 Next, severity of an ADE will be categorized as significant, serious, life-threatening, or fatal.1, 43 Examples of each of these have been previously described.1 For the purpose of hypothesis 2, the primary outcomes of interest are serious, life-threatening or fatal ADEs classified into Type A (predictable, dose dependent and/or exaggerated pharmacologic effect of a drug) or Type B (idiosyncratic or due to an allergic reaction that is not predictable). ADEs will be considered preventable if they were due to an error that could have been prevented by any means including physician ordering, transcribing, dispensing (pharmacy), administration (nursing or patient in the home), monitoring or adherence. Preventability will be classified as preventable, probably preventable, probably not preventable, or absolutely not preventable by the pair of investigators.1, 43 The effects of the ADE will be classified as abnormal laboratory results without signs or symptoms, symptoms of less than one day in duration, symptoms of one day and longer in duration, nonpermanent disability, permanent disability, and death.1 Previous studies that used these techniques found good to excellent inter-rater agreement (kappa 0.66–0.89). Differences between the two reviewers’ classifications will be resolved by discussion to achieve consensus for each ADE.44
Evaluation of readmissions and unscheduled office visits
Schnipper found a rate of readmissions or ED visits in 1% of the intervention group and 8% with usual care (p=0.03).6 Other studies have found higher rates.7, 48, 51–53 We will use the same method to identify unscheduled visits.6, 44 Readmissions and unscheduled visits to EDs or urgent care facilities will be identified by the research nurse via patient self-report, outpatient physician notes and hospital records for readmissions. Patients will be questioned during the telephone survey conducted at 30 and 90 days concerning any healthcare visits. If they have visited any facility, the patient will be asked to describe the reason for the visit, the dates(s) and the outcome of the visit. The research nurse will attempt to confirm each visit by comparing the patient’s report with hospital, ED, clinic or physician records. Only visits related to an ADE or medication problem (including under use) as determined following adjudication by the senior investigators will be counted as an outcome and only one outcome will be counted per unscheduled office visit or hospitalization. The adjudication process will also determine if the ADE was preventable. If the patient subsequently has a different event during the study, the second event will also be counted.
Cost-Effectiveness
A cost-effectiveness analysis (CEA) will be performed. We assume that the decision to reimburse for our intervention in practice will be at the discretion of third-party payers so we will use this perspective as the basis for our CEA. We will focus on the differences in direct resource costs across the three study arms using two distinct CEAs. The first analysis will contrast the control and minimal intervention arms. The cost-effectiveness of the enhanced intervention arm will be assessed in the second analysis. The comparison intervention (either control or minimal intervention) used in the second analysis will depend on the results found in the first analysis. The enhanced intervention will be compared to a single intervention if a clear best choice emerges in the first analysis. If the choice is not clear in the first analysis the enhanced intervention will be compared to both.
Secondary Measures
Number of medications
Use of a greater number of medications has been shown to increase the risk for ADEs and drug interactions.20, 44, 54, 55 The intervention might reduce the number of unnecessary medications but it might increase total medications if there are untreated indications as we have observed in several other studies.15, 56, 57 It will be important to categorize the total number of medications and their association with the primary outcome variables (mMAI score, MEs, ADEs and visits to health care facilities). The number of active medications will be determined at admission, discharge and 30 and 90 days following discharge. Only non-topical medications will be counted. The number of regularly scheduled medications will be recorded separately from “as needed” medications. We have found that community pharmacists were more likely to provide an intervention for patients who were receiving greater numbers of medications.14 Therefore, the number of medications will be used as a covariate in the multiple regression model that examines the influence of the intervention on ADEs, re-admissions, ED and urgent care facility visits.
Complete medication list
The documentation of a complete medication list has been shown to reduce the risk of ADEs.58 Therefore, the peer review panel form will include a section that asks whether there was a complete medication list at the following index dates and locations: admission, discharge and 30 and 90 days post discharge. The outpatient medication lists will be determined from the community pharmacy and community physician records. The “gold standard” will be the medication list that has been generated by the PCMs.
Inappropriate medication use in elderly subjects
Many studies, including ours, have found very high use of inappropriate medications in the elderly as defined by Beer’s criteria.14, 59 We have found that 40% of elderly patients receiving Medicaid in the Iowa Pharmaceutical Case Management program were receiving an inappropriate medication.14 We expect to find that the intervention will reduce the number of inappropriate medications in the subgroup of patients over 65 years of age. These data will be used for descriptive purposes.
Barriers to adherence
The research nurse will administer baseline questionnaires on admission including adverse reaction symptoms, self-reported adherence, self-efficacy,60–62 cognitive impairment using the Pfeiffer Mental Status Questionnaire,33 and the Katz index of activities of daily living.34, 35 Medication management skills will be evaluated by assessing the ability to read a prescription label, ability to remove two tablets from a 7-dram prescription vial, ability to interpret medication directions and ability to differentiate tablet colors using an assessment instrument developed by Ruscin and Semla.36 We have selected these factors since they may be barriers to medication administration.
Medication adherence
Gurwitz et al found that 21% of preventable ADEs were due to errors in patient adherence.1 Two methods to evaluate adherence are preferred to a single assessment.63 We will estimate adherence by both refill history and self-report. Adherence scores from refill histories have been widely used to assess adherence including studies we conducted.64–71 We will determine timing of refills and calculate possession ratios. This evaluation will be performed at baseline and during the study at 30 and 90 days after discharge. Patient adherence will be defined as a score of 0.80 or greater, whereas, non-adherence will be a score below 0.80 which has been used by other investigators.65–67 We will test adherence as a predictor of ADEs and visits to health-care facilities as both a dichotomous and continuous variable. The second method will be a self-reported adherence tool developed by Morisky et al.72 This tool had item-to-total correlations of 0.48 to 0.56 and a Cronbach alpha of 0.61.72 When the tool was evaluated in patients with hypertension, the sensitivity was 0.81 and specificity was 0.44.73
Data Analysis
The main goal of the data analyses will be to make comparisons among the three groups (two intervention and one control) on primary outcome variables. These primary outcome variables will be collected at enrollment (pre-intervention), and at 30 and 90 days, as displayed in Table 1. These three distinct outcomes include: 1) MAI scores, 2) the occurrence of preventable serious ADEs; and 3) readmissions, ED visits, and unscheduled office visits. For each outcome, a longitudinal random-effects model will be used to incorporate the repeated-measures aspect of the data and, if deemed necessary by the data, to control for the effects of pharmacy and/or physician cluster. This model will be used for numeric outcomes, such as MAI scores.
Other outcomes are likely to be dichotomous in nature, such as whether a subject had at least one ADE or at least one hospital readmission. In these cases, the model above will be modified into a random-effects logistic regression model. If we find it to be relatively common that subjects have more than one of these types of outcomes (e.g., multiple readmissions or ER visits), then those outcomes will be treated as being discrete counts, and we will modify the model to perform random-effect Poisson regression analysis. SAS Proc NLMIXED can be used to fit all three of these types of regression models (continuous, dichotomous, and counts), although it is more straightforward to use SAS PROC MIXED for continuous data.
For each model that we fit, we will examine the appropriateness of the modeling assumptions and modify our approach via transformations or nonparametric alternatives, if needed.
Sample Size Approximations
For approximating the sample size necessary to achieve high power, we considered the following three distinct outcomes each evaluated separately in determining sample size and power: MAI score, serious ADE, and preventable hospitalization/ER visits. Although the actual analysis will incorporate random effects of subjects and physicians, we consider pair-wise comparisons of the three groups in order to facilitate power calculations. For MAI, we will have 80% power to detect between-group effect sizes of 0.22 standard deviations, and 90% power to detect between-group effect sizes of 0.25 standard deviations. Effect sizes of this order of magnitude are very small, and allow considerable overlap in the distribution of the observed outcomes. For example, a 0.25 standard deviation effect size between two groups would occur if the 50th percentile (median) of one group is at the 40th percentile or at the 60th percentile of the other group. With 1000 patients there should be excellent power to detect differences between the usual care group and the enhanced intervention group, for both the ADE’s or for preventable hospital/ED visits. We should also have reasonable power to distinguish the minimal intervention group from the usual care group or from the enhanced intervention group.
These sample size calculations assume a two-sided Type I error rate (α) of 5%, with a Bonferroni correction for performing all three pair-wise comparisons of proportions among groups. In the actual analyses, we will use more sophisticated statistical methods, including accommodating baseline patient characteristics, and the clustering within physician and pharmacy (as described above). However, since these clusters are crossed with, rather than nested within, treatment groups, the effect of ignoring this issue for power calculations should be minimal (and perhaps even conservative).
Discussion
Patient safety is one of the most important issues for hospitals and for ASHP. Medication reconciliation,29, 30 shared information and improved communication across levels of care6, 8 are critical activities needed to improve patient safety and optimize pharmacotherapy. Many hospitals are attempting to determine how to best implement these strategies and the Joint Commission will continue to require such programs. Hospital pharmacy departments are examining ways to include pharmacists in these activities and pharmacists are often the responsible individuals. The Iowa COC study is designed to evaluate the ability of a PCM to improve therapy and reduce ADEs, unscheduled office visits and re-admissions. This will be the first such study to include a comprehensive identification and assessment of ADEs and a cost-effectiveness evaluation. The study results are expected in 2012 and the knowledge gained will help hospital pharmacy departments in their goals to improve patient safety.
Limitations of the study include that one tertiary hospital may not be generalizable to other tertiary hospitals or smaller hospitals. Additionally, the study results may not be generalizable to other geographic areas or in different populations, especially large minority populations.
Conclusion
The Iowa COC study will examine the effects of increased communication with a PCM on the incidence of serious ADE, hospitalizations, and unscheduled office visits in patients with cardiovascular disease, pulmonary disease, or diabetes. The study will address the value of a PCM to improve communication of care plans between the inpatient and community settings.
Acknowledgments
This study is supported by the National Heart, Lung, and Blood Institute, (1RO1 HL082711). Funding for the COC pilot study (Paul Abramowitz, principal investigator) was supported by the ASHP Research and Education Foundation. Drs. Carter, Kaboli and Christensen are also supported by the Center for Research in Implementation in Innovative Strategies in Practice (CRIISP), Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (HFP 04-149). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Appendix
| Subject ID:_______________ | |
| Physician ID:_______________ | |
| Appendix 1. Survey questions for physicians with patients in the enhanced intervention group | |
| Part 1: First think about any interactions you had with the patient’s local community pharmacy within 90 days of the patient’s discharge from the hospital: | |
| 1. | Did the community pharmacist contact you by fax, telephone or other means about this patient? |
| □ Yes If yes, which method: FAX_______; Phone_______; Other, please specify______________ | |
| □ No (If no, go to question 13) | |
| 2. | Did the community pharmacist discuss a medication problem following discharge that could have resulted in an adverse drug reaction or poor disease control? |
| □ Yes, briefly describe____________________________________________________________ _ | |
| □ No | |
| Use the following scale for the remaining questions concerning the patient’s community pharmacist: 1=strongly agree; 2=agree; 3=neutral; 4=disagree; 5=strongly disagree: | |
| Strongly | Strongly | |
| Agree | Disagree | |
| 3. I approve of the pharmacist’s recommendation | 1 2 3 4 5 | |
| 4. This collaboration improved the quality of the patient’s drug therapy | 1 2 3 4 5 | |
| 5. The pharmacist’s recommendation was helpful | 1 2 3 4 5 | |
| 6. As a result of this study, my relationship with the pharmacist has improved | 1 2 3 4 5 | |
| 7. As a result of this study, the pharmacist and I communicate more | 1 2 3 4 5 | |
| 8. The contact procedure was satisfactory | 1 2 3 4 5 | |
| 9. The communication was time consuming | 1 2 3 4 5 | |
| 10. The recommendation had a major positive impact on the patient’s outcome | 1 2 3 4 5 | |
| 11. The patient’s adherence to therapy has improved with the recommendation | 1 2 3 4 5 | |
| 12. As a result of the study, my relationship with the patient has improved | 1 2 3 4 5 | |
| Part 2: Now think about the interactions you had with the UIHC hospital pharmacist following discharge from the hospital: | |
| 13. | Did the hospital pharmacist contact you by fax, telephone or other means about this patient (check all that apply)? |
| □ Yes If yes, which method: FAX_______; Phone_______; Other, please specify______________ | |
| □ No | |
| 14. | Did the hospital pharmacist discuss a medication problem following discharge that could have resulted in an adverse drug reaction or poor disease control? |
| □ Yes, briefly describe____________________________________________________________ | |
| □ No | |
| Use the following scale for the remaining questions concerning the UI hospital pharmacist: 1=strongly agree; 2=agree; 3=neutral; 4=disagree; 5=strongly disagree: | |
| Strongly | Strongly | |
| Agree | Disagree | |
| 15. I approve of the pharmacist recommendation | 1 2 3 4 5 | |
| 16. This collaboration improved the quality of the patient’s drug therapy | 1 2 3 4 5 | |
| 17. The pharmacist’s recommendation was helpful | 1 2 3 4 5 | |
| 18. As a result of this study, my relationship with the pharmacist has improved | 1 2 3 4 5 | |
| 19. As a result of this study, the pharmacist and I communicate more | 1 2 3 4 5 | |
| 29. The contact procedure was satisfactory | 1 2 3 4 5 | |
| 21. The communication was time consuming | 1 2 3 4 5 | |
| 22. The recommendation had a major positive impact on the patient’s outcome | 1 2 3 4 5 | |
| 23. The patient’s adherence to therapy has improved with the recommendation | 1 2 3 4 5 | |
| 24. As a result of the study, my relationship with the patient has improved | 1 2 3 4 5 | |
References
- 1.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003 Mar 5;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 2.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003 Feb 4;138(3):161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Himmel W, Tabache M, Kochen MM. What happens to long-term medication when general practice patients are referred to hospital. Eur J Clin Pharmacol. 1996;21:178–181. doi: 10.1007/s002280050103. [DOI] [PubMed] [Google Scholar]
- 4.Beers M, Sliwkowski J, Brooks J. Compliance with medication orders among elderly after hospital discharge. Hosp Formul. 1992;27:720–724. [PubMed] [Google Scholar]
- 5.The Joint Commission. National Patient Safety Goals Manual. 2008 Available at http://www.jointcommission.org/NR/rdonlyres/82B717D8-B16A-4442-AD00- CE3188C2F00A/0/08_HAP_NPSGs_Master.pdf.
- 6.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 7.Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. American Journal of Medicine. 2001;111(9B):26S–30S. doi: 10.1016/s0002-9343(01)00966-4. [DOI] [PubMed] [Google Scholar]
- 8.Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007 Jun 25;167(12):1305–1311. doi: 10.1001/archinte.167.12.1305. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal R, Bates DW. Evidence/technology assessment no. 43. Number 01-E058. Rockville, MD: 2001. The clinical pharmacist's role in preventing adverse drug events. Agency for Healthcare Research and Quality. Making healthcare safer: A critical analysis of patient safety; pp. 71–77. [Google Scholar]
- 10.Murff HJ, Bates DW. Evidence report/technology assessment no. 43. Number 01-E058. Rockville, MD: 2001. Information transfer between inpatient and outpatient pharmacies. Agency for Healthcare Research and Quality. Making health care safer: A critical analysis of patient safety; pp. 471–476. [Google Scholar]
- 11.Smith L, McGowan L, Moss-Barclay C, Wheater J, Knass D, Chrystyn H. An investigation of hospital generated pharmaceutical care when patients are discharged home from hospital. Br J Clin Pharmacol. 1997 Aug;44(2):163–165. doi: 10.1046/j.1365-2125.1997.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuehl AK, Chrischilles EA, Sorofman BA. System for exchanging information among pharmacists in different practice environments. Am J Health Syst Pharm. 1998 May 15;55(10):1017–1024. doi: 10.1093/ajhp/55.10.1017. [DOI] [PubMed] [Google Scholar]
- 13.Cromarty E, Downie G, Wilkinson S, Cromarty JA. Communication regarding the discharge medications of elderly patients: a controlled trial. Pharm J. 1998;260:62–64. [Google Scholar]
- 14.Chrischilles EA, Carter BL, Lund BC, et al. Evaluation of the Iowa Medicaid pharmaceutical case management program. J Am Pharm Assoc (Wash DC) 2004 May–Jun;44(3):337–349. doi: 10.1331/154434504323063977. [DOI] [PubMed] [Google Scholar]
- 15.Ellis SL, Billups SJ, Malone DC, et al. Types of interventions made by clinical pharmacists in the IMPROVE study. Impact of Managed Pharmaceutical Care on Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy. 2000;20(4):429–435. doi: 10.1592/phco.20.5.429.35055. [DOI] [PubMed] [Google Scholar]
- 16.Ellis SL, Carter BL, Malone DC, et al. Clinical and economic impact of ambulatory care clinical pharmacists in management of dyslipidemia in older adults: the IMPROVE study. Impact of Managed Pharmaceutical Care on Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy. 2000;20(12):1508–1516. doi: 10.1592/phco.20.19.1508.34852. [DOI] [PubMed] [Google Scholar]
- 17.Bond CA, Raehl CL, Franke T. Clinical pharmacy services and hospital mortality rates. Pharmacotherapy. 1999 May;19(5):556–564. doi: 10.1592/phco.19.8.556.31531. [DOI] [PubMed] [Google Scholar]
- 18.Bond CA, Raehl CL, Franke T. Interrelationships among mortality rates, drug costs, total cost of care, and length of stay in United States hospitals: summary and recommendations for clinical pharmacy services and staffing. Pharmacotherapy. 2001 Feb;21(2):129–141. doi: 10.1592/phco.21.2.129.34105. [DOI] [PubMed] [Google Scholar]
- 19.Bond CA, Raehl CL, Franke T. Clinical pharmacy services, hospital pharmacy staffing, and medication errors in United States hospitals. Pharmacotherapy. 2002 Feb;22(2):134–147. doi: 10.1592/phco.22.3.134.33551. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995 Oct 9;155(18):1949–1956. [PubMed] [Google Scholar]
- 21.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999 Jul 21;282(3):267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 22.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996 Apr;100(4):428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 23.Schumock GT, Butler MG, Meek PD, Vermeulen LC, Arondekar BV, Bauman JL. Evidence of the economic benefit of clinical pharmacy services: 1996–2000. Pharmacotherapy. 2003 Jan;23(1):113–132. doi: 10.1592/phco.23.1.113.31910. [DOI] [PubMed] [Google Scholar]
- 24.Wilt VM, Gums JG, Ahmed OI, Moore LM. Outcome analysis of a pharmacist-managed anticoagulation service. Pharmacotherapy. 1995;15(6):732–739. [PubMed] [Google Scholar]
- 25.McMullin ST, Hennenfent JA, Ritchie DJ, et al. A prospective, randomized trial to assess the cost impact of pharmacist-initiated interventions. Arch Intern Med. 1999 Oct 25;159(19):2306–2309. doi: 10.1001/archinte.159.19.2306. [DOI] [PubMed] [Google Scholar]
- 26.Malone DC, Carter BL, Billups SJ, et al. An economic analysis of a randomized, controlled, multicenter study of clinical pharmacist interventions for high-risk veterans: the IMPROVE study. Impact of Managed Pharmaceutical Care Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy. 2000;20(12):1149–1158. doi: 10.1592/phco.20.15.1149.34590. [DOI] [PubMed] [Google Scholar]
- 27.Malone DC, Carter BL, Billups SJ, et al. Can clinical pharmacists affect SF-36 scores in veterans at high risk for medication-related problems? Med Care. 2001;39(2):113–122. doi: 10.1097/00005650-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006 May 8;166(9):955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 29.Lizer MH, Brackbill ML. Medication history reconciliation by pharmacists in an inpatient behavioral health unit. Am J Health Syst Pharm. 2007 May 15;64(10):1087–1091. doi: 10.2146/ajhp060323. [DOI] [PubMed] [Google Scholar]
- 30.Varkey P, Cunningham J, O'Meara J, Bonacci R, Desai N, Sheeler R. Multidisciplinary approach to inpatient medication reconciliation in an academic setting. Am J Health Syst Pharm. 2007 Apr 15;64(8):850–854. doi: 10.2146/ajhp060314. [DOI] [PubMed] [Google Scholar]
- 31.Zarowitz BJ, Stebelsky LA, Muma BK, Romain TM, Peterson EL. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005 Nov;25(11):1636–1645. doi: 10.1592/phco.2005.25.11.1636. [DOI] [PubMed] [Google Scholar]
- 32.Murff HJ, Bates DW. Evidence report/technology assessment no. 43. Number 01-E058. Rockville, MD: 2001. Information transfer between inpatient and outpatient pharmacies. Agency for Healthcare Research and Quality. Making health care safer: A critical analysis of patient safety; pp. 471–476. [Google Scholar]
- 33.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975 Oct;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 34.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 35.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. Jama. 1963 Sep 21;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 36.Ruscin JM, Semla TP. Assessment of medication management skills in older outpatients. Ann Pharmacother. 1996 Oct;30(10):1083–1088. doi: 10.1177/106002809603001003. [DOI] [PubMed] [Google Scholar]
- 37.Simpson SH, Johnson JA, Farris KB, Lau TTY, Majumdar SR, Cave A, Tsuyuki RT, for the SCRIP Investigators Physician perceptions of enhanced community pharmacist care in cholesterol management: A survey of physicians contacted during the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP) Canadian Pharmacists Journal. 2005;138:33–39. [Google Scholar]
- 38.Carter BL, Helling DK, Jones ME, Moessner H, Waterbury CA., Jr Evaluation of family physician prescribing: influence of the clinical pharmacist. Drug Intell Clin Pharm. 1984;18(10):817–821. doi: 10.1177/106002808401801010. [DOI] [PubMed] [Google Scholar]
- 39.Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens (Greenwich) 2007 Feb;9(2):113–119. doi: 10.1111/j.1524-6175.2007.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992 Oct;45(10):1045–1051. doi: 10.1016/0895-4356(92)90144-c. [DOI] [PubMed] [Google Scholar]
- 41.Kaboli PJ, Hoth AB, Carter BL, Chrischilles EA, Shorr RI, Wagner P, Rosenthal GE. Lack of agreement between computerized medication profiles and structured medication histories. Journal of General Internal Medicine. 2002;17 Supplement 1:126. (abstract). [Google Scholar]
- 42.Kaboli P, Jaipaul C, Barry W, et al. Identification of Adverse Drug Events and Medication Errors: Poor Agreement Between Chart Review and Physician, Nurse, and Patient Report. Journal of General Internal Medicine. 2003;18 Supp 1:286. [Google Scholar]
- 43.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995 Jul 5;274(1):29–34. [PubMed] [Google Scholar]
- 44.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003 Apr 17;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 45.Carter BL, Barnette DJ, Chrischilles E, Mazzotti GJ, Asali ZJ. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997;17(6):1274–1285. [PubMed] [Google Scholar]
- 46.Chrischilles EA, Helling DK, Aschoff CR. Effect of clinical pharmacy services on the quality of family practice physician prescribing and medication costs. Drug Intell Clin Pharm. 1989;23(5):417–421. doi: 10.1177/106002808902300511. [DOI] [PubMed] [Google Scholar]
- 47.Chrischilles EA, Segar ET, Wallace RB. Self-reported adverse drug reactions and related resource use. A study of community-dwelling persons 65 years of age and older. Ann Intern Med. 1992 Oct 15;117(8):634–640. doi: 10.7326/0003-4819-117-8-634. [DOI] [PubMed] [Google Scholar]
- 48.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. American Journal of Medicine. 2000;109(2):87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 49.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22–29;277(4):307–311. [PubMed] [Google Scholar]
- 50.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 51.Manasse HR., Jr Medication use in an imperfect world: drug misadventuring as an issue of public policy, Part 2. Am J Hosp Pharm. 1989 Jun;46(6):1141–1152. [PubMed] [Google Scholar]
- 52.Manasse HR., Jr Medication use in an imperfect world: drug misadventuring as an issue of public policy, Part 1. Am J Hosp Pharm. 1989 May;46(5):929–944. [PubMed] [Google Scholar]
- 53.Ives TJ, Bentz EJ, Gwyther RE. Drug-related admissions to a family medicine inpatient service. Arch Intern Med. 1987 Jun;147(6):1117–1120. [PubMed] [Google Scholar]
- 54.Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992 Jul–Aug;2(4):356–367. doi: 10.2165/00002512-199202040-00008. [DOI] [PubMed] [Google Scholar]
- 55.Carter BL, Lund BC, Hayase N, Chrischilles E. The extent of potential antihypertensive drug interactions in a Medicaid population. Am J Hypertens. 2002 Nov;15(11):953–957. doi: 10.1016/s0895-7061(02)03026-1. [DOI] [PubMed] [Google Scholar]
- 56.Carter BL, Chrischilles EA, Scholz D, Hayase N, Bell N. Extent of services provided by pharmacists in the Iowa Medicaid Pharmaceutical Case Management program. J Am Pharm Assoc. 2003 Jan–Feb;43(1):24–33. doi: 10.1331/10865800360467015. [DOI] [PubMed] [Google Scholar]
- 57.Hoth AB, Carter BL, Ness J, et al. Development and reliability testing of the clinical pharmacist recommendation taxonomy. Pharmacotherapy. 2007 May;27(5):639–646. doi: 10.1592/phco.27.5.639. [DOI] [PubMed] [Google Scholar]
- 58.Knight EL, Avorn J. Quality indicators for appropriate medication use in vulnerable elders. Ann Intern Med. 2001 Oct 16;135(8 Pt 2):703–710. doi: 10.7326/0003-4819-135-8_part_2-200110161-00009. [DOI] [PubMed] [Google Scholar]
- 59.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997 Jul 28;157(14):1531–1536. [PubMed] [Google Scholar]
- 60.Bandura A, Jeffery RW, Wright CL. Efficacy of participant modeling as a function of response induction aids. J Abnorm Psychol. 1974;83(1):56–64. doi: 10.1037/h0036258. [DOI] [PubMed] [Google Scholar]
- 61.Bandura A. Self Efficacy Changing Societies. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 62.Bandura A. Self-efficacy: The Exercise of Control. New York, NY: W.H. Freeman and Company; 1997. [Google Scholar]
- 63.Grymonpre RE, Didur CD, Montgomery PR, Sitar DS. Pill count, self-report, and pharmacy claims data to measure medication adherence in the elderly. Ann Pharmacother. 1998 Jul–Aug;32(7–8):749–754. doi: 10.1345/aph.17423. [DOI] [PubMed] [Google Scholar]
- 64.Carter BL, Malone DC, Valuck RJ, Barnette DJ, Sintek CD, Billups SJ. The IMPROVE study: background and study design. Impact of Managed Pharmaceutical Care on Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Am J Health Syst Pharm. 1998;55(1):62–67. doi: 10.1093/ajhp/55.1.62. [DOI] [PubMed] [Google Scholar]
- 65.Billups SJ, Malone DC, Carter BL. The relationship between drug therapy noncompliance and patient characteristics, health-related quality of life, and health care costs. Pharmacotherapy. 2000;20(8):941–949. doi: 10.1592/phco.20.11.941.35266. [DOI] [PubMed] [Google Scholar]
- 66.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988 Aug;26(8):814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997 Jan;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 68.Farris KB, Kaplan B, Kirking DM. Examination of days supply in computerized prescription claims. J Pharmacoepidemiol. 1994;3:63–76. [Google Scholar]
- 69.Christensen DB, Williams B, Goldberg HI, Martin DP, Engelberg R, LoGerfo JP. Assessing compliance to antihypertensive medications using computer-based pharmacy records. Med Care. 1997 Nov;35(11):1164–1170. doi: 10.1097/00005650-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Hamilton RA, Briceland LL. Use of prescription-refill records to assess patient compliance. Am J Hosp Pharm. 1992 Jul;49(7):1691–1696. [PubMed] [Google Scholar]
- 71.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. Compliance with antihypertensive therapy among elderly Medicaid enrollees: the roles of age, gender, and race. Am J Public Health. 1996 Dec;86(12):1805–1808. doi: 10.2105/ajph.86.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health. 1992;82(12):1607–1612. doi: 10.2105/ajph.82.12.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]