Abstract
The N-termini of bacterial lipoproteins are acylated with a (S)-(2,3-bisacyloxypropyl)cysteinyl residue. Lipopeptides derived from lipoproteins activate innate immune responses by engaging Toll-like receptor 2 (TLR2), and are highly immunostimulatory and yet without apparent toxicity in animal models. The lipopeptides may therefore be useful as potential immunotherapeutic agents. Previous structure-activity relationships in such lipopeptides have largely been obtained using murine cells and it is now clear that significant species-specific differences exist between human and murine TLR responses. We have examined in detail the role of the highly conserved Cys residue as well as the geometry and stereochemistry of the Cys-Ser dipeptide unit. (R)-diacylthioglycerol analogues are maximally active in reporter gene assays using human TLR2. The Cys-Ser dipeptide unit represents the minimal part-structure, but its stereochemistry was found not to be a critical determinant of activity. The thioether bridge between the diacyl and dipeptide units is crucial, and replacement by an oxoether bridge results in a dramatic decrease in activity.
Keywords: Sepsis, septic shock, lipoteichoic acid, peptidoglycan, lipopeptides, innate immunity
Introduction
The link between immune stimulation resulting from acute infection and the resolution of tumors has long been recognized,1 and the phenomenon of infection-related “spontaneous regression” of cancer has been documented throughout history, beginning as early as 2600 B.C.2;3 The foundations of modern immunotherapy for cancer were laid in 1891 by William B. Coley, who, in 1891, injected live streptococcal organisms into a long-bone sarcoma lesion and observed shrinkage of the tumor.4 More than a century has elapsed since Coley’s empirical findings were first reported, but the active principle(s) in Coley’s toxin remain undetermined. This long lag-period is perhaps not altogether surprising because the recognition of innate immune system is, in itself, recent,5–7 and our understanding of the structure and function of the family of Toll-like receptors (TLRs)8;9 that recognize “pathogen-associated molecular patterns (PAMPs)”,10 and the effector mechanisms that mediate innate immune responses11–13 are yet nascent. Nonetheless, there exists considerable potential in harnessing innate immune responses in a wide range of disease states, notably cancer, as evidenced by the number of TLR agonists already in clinical trials,14 and the resurgence of interest in the immunotherapy of neoplastic states.15–17
The exoskeleton of the Gram-positive organism, similar to that of Gram-negative bacteria, is comprised of underlying peptidoglycan (PGN), a polymer of β-1→4-linked N-acetylglucosamine-N-acetylmuramic acid glycan strands that are cross-linked by short peptides.18–20 Unlike Gram-negative bacteria which bear lipopolysaccharide on the outer leaflet of the outer membrane, the external surface of the peptidoglycan layer is decorated with lipoteichoic acids (LTA).21;22 LTA are anchored in the peptidoglycan substratum via a diacylglycerol moiety and bear a surface-exposed, polyanionic, 1–3-linked polyglycerophosphate appendage 23;24 which varies in its subunit composition in LTAs from various Gram-positive bacteria; in S. aureus, the repeating subunit contains D-alanine and α-D-N-acetylglucosamine25. Lipoproteins are found in the bacterial cytoplasmic membrane and are also common constituents of the cell wall of both Gram-negative and Gram-positive bacteria.25–27 The free amine of the N-terminus of lipoproteins is acylated with a (S)-(2,3-bisacyloxypropyl)cysteinyl residue which has previously been demonstrated to be immunostimulatory28;29 as shown by studies on synthetic peptides containing the bis-acylthioglycerol unit.25;30;31 In contradistinction to enterobacterial LPS which is recognized by Toll-like receptor-4 (TLR4), PGN, 32;33 LTA,34;35 lipopeptides,36;37 as well as some non-enterobacterial LPS38;39 signal via TLR2.40;41
In comparing the various constituents of the Gram-positive cell wall, we have found lipopeptides to be extremely potent TLR2 agonists, and yet without apparent toxicity in animal models.42 In murine cells, PAM2CSK4 (S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-R-cysteinyl-S-seryl-S-lysyl-S-lysyl-S-lysyl-S-lysine), a commercially-available, synthetic model lipopeptide was equipotent in its NF-κB inducing activity relative to LPS, but elicited much lower proinflammatory cytokines, while both LPS and the lipopeptide potently induced the secretion of a similar pattern of chemokines suggestive of the engagement of downstream TRIF pathways.42 Furthermore, we have found that while LPS, as expected, elicited a robust fever response in rabbits at a dose of 100 ng/kg, a 200 μg/kg dose (2000 X) of the lipopeptide was without any discernible pyrogenic or other apparent acute toxic effect (manuscript in preparation). These observations suggest that the lipopeptides may be remarkably potent, yet nontoxic immunotherapeutic agents. Indeed, in a Phase I/Phase II clinical trial43 on a single, intraoperative 20–30 μg intratumoral injection of MALP-228 (a diacyl lipopeptide similar in its core structure to PAM2CSK4) in patients with inoperable carcinoma of the pancreas, the lipopeptide was well-tolerated and proved efficacious in prolonging survival.43
Considerable structure-activity relationships dictating TLR2 binding and subsequent stimulation of innate immune responses have been documented for a range of synthetic lipopeptides. It should be pointed out, however, that the majority of earlier SAR studies had been performed with murine cells, many even before the TLRs had been discovered, and it is now clear that significant species-specific differences exist between human and murine TLR responses36;44 as a consequence of subtle structural differences in the ligand binding pocket within TLR2.45
The minimal structure for biological activity is the Cys-Ser lipodipeptide46 with the lipoaminoacid Cys-OH derivative being almost inactive;47 the remainder of the peptidic unit appears, in large part, not to be critical for activity since a variety of analogues of different lengths with different amino acid sequences were found to be equipotent.46;48–50 The (R)-configuration at the asymmetric glyceryl carbon confers maximum activity,30;51 and the threshold of the acyl chain length of the two ester-bound fatty acids is 8 carbons, with shorter acyl analogues being inactive.36 Furthermore, the recent high-resolution crystal structure of lipopeptides complexed to human- as well as murine-derived TLR1/TLR2 heterodimers45 raises additional questions since the binding site in TLR2 is highly unusual, being located in the convex region formed at the border of the central and C-terminal domains with the peptidic unit interacting with interfacial residues at the neck region of the binding pocket.45 It was of interest to examine whether the chirality of the highly conserved Cys residue (L-Cys versus D-Cys) and the thioether bridge connecting the diacylglycerol unit to the peptide chain are critical determinants of activity, and if the thioether could be bioisosterically substituted by an oxoether linkage. The geometry of the Cys-Ser dipeptide unit also appears to be crucial, in that there are key interactions with conserved Tyr316 and Pro315 residues at the neck of the binding pocket in the crystal structure.45 It is to be noted that the carboxylic acid of Cys is in amide linkage with the amine of serine, and we wished to test whether inversion of the orientation of Ser, achieved by the coupling the carboxyl group of serine to the amino group of cysteamine could perhaps yield analogues that were TLR2 receptor antagonists.
Results and Discussion
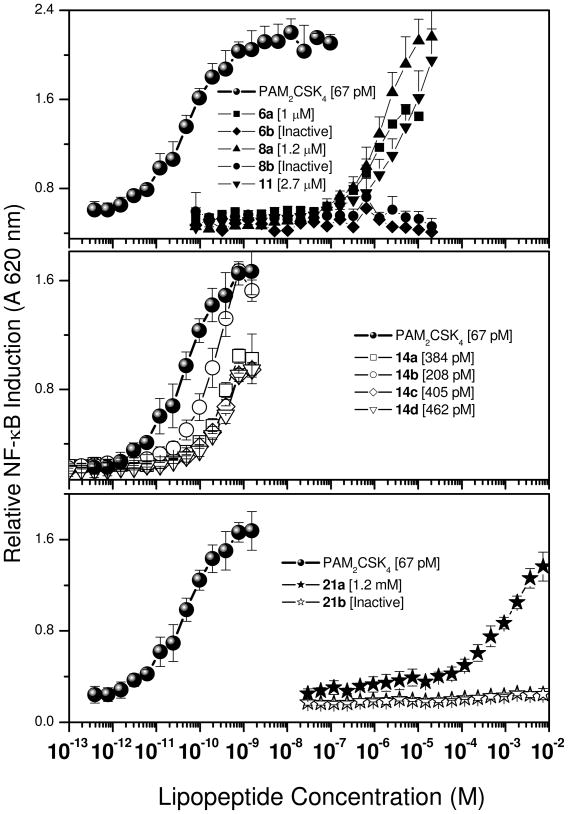
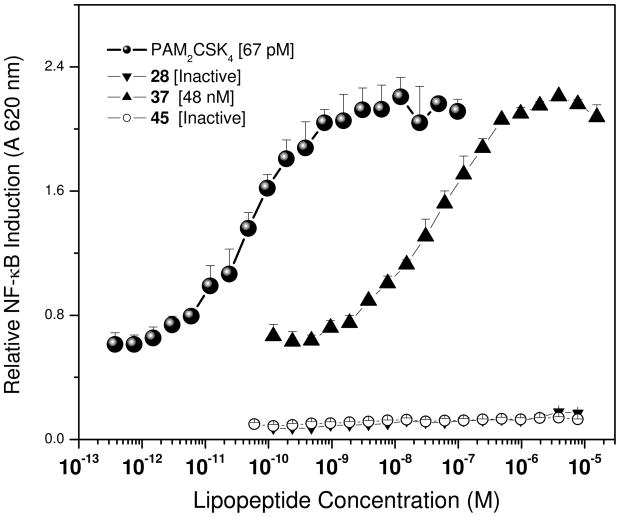
Much of the previous work in the literature concerning the structure-activity relationships in lipopeptides have utilized murine TLR2, and in an effort to confirm the minimal part-structure of the lipopeptide that was TLR-agonistic on human TLR2, we first targeted the stereoselective syntheses of 1,2-dipalmitoyl (R)- and -(S)-3-(2-aminoethylthio)propanediol (6a and 6b, Scheme 1) starting from the corresponding enantiopure 1,2-isopropylideneglycerol. The (R)-cysteamine lipoamino acid 6a was weakly active (IC50: 1 μM, Fig. 1) relative to the commercially available reference lipopeptide PAM2CSK4 (IC50: 67 pM), while the (S)-analogue 6b was completely inactive. Coupling L-serine to the free amine of the cysteamine resulted in the lipodipeptides 8a and 8b; similar to the cysteamine analogues, only 8a was active with an IC50 of 1.2 μM; it is to be noted that in these latter compounds, the dipeptide unit is inverted since, unlike in the naturally occurring as well as in synthetic PAM2CSK4, it is the carboxyl group of the serine that is coupled to the lipopeptide. The L-cysteine-containing lipoamino acid, compound 11 (Scheme 2) was also found to be weakly TLR2-agonistic, which is in contradistinction to the virtually absent activity on murine cells described earlier.47 The results emphasize differences in ligand specificity between murine and human TLR2. The difference in activity between the R- and S-isomers which was consistent with previous SAR results,30;51 served to focus our subsequent SAR in which we elected to examine only the R-diacylthioglycerol analogues.
Scheme 1.
Reagents and conditions: a: p-TsCl, pyr, DCM, 0 °C-r.t., 8 h;
b: N-Boc-2-aminoethanethiol, NaH, DMF, 0 °C-r.t., 8 h;
c: 70% AcOH, r.t., 12 h;
d: C15H31COCl, pyr, DMAP, DCM, 0 °C-r.t., 10 h;
e: TFA, r.t., 30 min;
f: Boc-L-Serine-OH, EDCI, EDIPA, DMAP, DCM, 0 °C-r.t., 8 h;
g: TFA, r.t., 30 min.
Figure 1.
TLR2 agonistic activities of the lipopeptide analogues. HEK293 cells stably transfected with a TLR2-NF-κB-SEAP reporter gene was used in a 384 well format. Data points represent means and sd determined on triplicate samples.
Scheme 2.
Reagents and conditions: a: PPh3, I2, imidazole, toluene, 90 °C, 2 h;
b: Boc-L- or -D-Cys-OMe, TEA, DMF, 85°C, 4 h;
c: i) 70% AcOH, r.t., 24 h; ii) C15H31COCl, pyr, DMAP, DCM, 0 °C- r.t., 8 h; iii)TFA, r.t., 30 min;
d: i) LiOH, THF/H2O, r.t., 10 h; ii) H-L-Ser(OtBu)-OtBu•HCl or H-L-Ser(OtBu)-OMe•HCl or H-D-Ser(OtBu)-OMe•HCl, EDCI, EDIPA, DMAP, DCM, 0 °C-r.t., 8 h;
e: i) 70% AcOH, r.t.,12 h; ii) C15H31COCl, pyr, DMAP, DCM, 0 °C-r.t., 10 h;
f: TFA, r.t., 30 min.
The PAM2Cys-Ser compounds of the 14 series comprising of combination of L- or D- amino acids at either position, and with the carboxyl terminus as either the carboxylic acid or the methyl ester (Scheme 2) were all highly active with the IC50 values in the mid-picomolar range (Fig. 1), signifying that the stereochemistry of the dipeptide unit is non-critical as long as the orientation of the amide bond is in the correct configuration as discussed earlier. These results are consonant with the crystal structure of PAM2CSK4 complexed with TLR2, in which the carbonyl Cys-Ser amide bond forms H-bonding with Pro315 and the NH of the amide interacts with Y326, but the side-chains of the dipeptide unit show no significant van der Waals interactions within the binding pocket.45 The relative unimportance of the side-chains of the dipeptide core are also in agreement with observations that in naturally-occurring lipopeptides, the second amino acid is far less conserved, and can be replaced by other residues with non-bulky side-chains such as Gly,46;48–50 presumably as long as steric interactions within the narrow neck region of the binding pocket45 are not compromised. While the PAM2CS methylester compounds are indeed highly active, they are about a fifth as potent as the reference PAM2CSK4 lipopeptide. We have found that, as with other amphipathic compounds that we have recently characterized,52 the apparent differences in potencies are related, at least in part, to the substantially higher binding of the more hydrophobic PAM2CS analogues to albumin present in cell culture media, details of which will be published elsewhere.
It was of particular interest to examine the role of the thioether bridge between the diacyl and dipeptide units. O-alkylation of R-1,2-isopropylideneglycerol with racemic ethyl 2,3-ethoxypropanoate yielded the key intermediate 15, from which the oxoether-bridged compounds were obtained (21a and 21b, Scheme 3) in which two of the three stereocenters were held fixed. The activity of 21a (lipopeptide terminating with L-Ser) was about eight orders of magnitude lower (IC50: 1.2 mM) than that of PAM2CSK4 while 21b (D-Ser) was inactive at the highest concentration tested (10 mM; Fig. 1). While these results are indicative of the absolute requirement of the thioether linkage, we do not yet understand why the stereochemistry of the terminal Ser residue manifests in differential activity only in the ether-linked (21a, 21b), but not in the thioether-linked analogues (14a-d).
Scheme 3.
Reagents and conditions: a: Ethyl 2,3-epoxypropanoate, BF3•OEt2, DCM, r.t., 5 h;
b: p-TsCl, TEA, DCM, 0°C-r.t., 8 h;
c: NaN3, DMF, r.t., 12 h;
d: i) PPh3, H2O, THF, reflux, 6 h; ii) Boc2O, DCM, r.t., 8 h;
e: i) LiOH, THF/H2O, r.t., 10 h; ii) H-L-Ser(tBu)-OMe•HCl or H-D-Ser(t-Bu)-OMe•HCl, EDCI, EDIPA, DMAP, DCM, 0°C-r.t., 8 h;
f: i) 70% AcOH, r.t., 12 h; ii) C15H31COCl, pyr, DMAP, DCM, 0°C-r.t., 10 h;
g: TFA, r.t., 30 min.
We next tested whether the ester-linked palmitoyl groups could be replaced with potentially hydrolytically more stable ether and amide linkages without compromising activity. Synthesis of analogue 28 with the ether-linked C16 hydrocarbon appendages could not be obtained by direct O-alkylation of an advanced intermediate such as the deprotected species from series 12 under a variety of conditions. This necessitated the installation of the ether-linked C16 groups on the glycerol backbone (23) first, followed by transformation of the free hydroxyl to the iodo intermediate (25), and subsequent S-alkylation with cysteine (Scheme 4). We found that the ether analogue 28 was entirely inactive (Fig. 2).
Scheme 4.
Reagents and conditions: a: BnBr, NaH, DMF, 0 °C- r.t., 8 h;
b: i) 70% AcOH, r.t., 12 h; ii) C16H33I, NaH, DMF, 0 °C- r.t., 8 h;
c: i) Pd/C, H2, 8 h; ii) TsCl, pyr, DMAP, CH3CN, 70 °C, 12 h;
d: I2, KI, DMF, 80 °C, 24 h;
e: Boc-L-Cys-OMe, TEA, DMF, 85 °C, 2 h;
f: i) LiOH, THF/H2O, r.t., 10 h; ii) H-L-Ser(tBu)-OMe •HCl, EDCI, EDIPA, DMAP, DCM, 0 °C-r.t., 8 h;
g: TFA, r.t., 30 min.
Figure 2.
TLR2 agonistic activities of the ether, monoamide and diamide lipopeptide analogues. HEK293 cells stably transfected with a TLR2-NF-κB-SEAP reporter gene was used in a 384 well format. Data points represent means and sd determined on triplicate samples.
We next investigated the bioisoteric replacement of one (internal, secondary alcohol-derived; Scheme 5) ester as well as both the esters (Scheme 6) with amide-linked hydrocarbon chains. Synthesis of the monoamide analogue 37 proceeded smoothly starting from D-Ser-OMe (29). The diamide analogue, 45, however, was more problematic to synthesize. Acetonide deprotection of the monoamide precursor 35, followed by attempts at converting the free hydroxyl group to the amine via an azide intermediate was unsuccessful. The strategy of first N-acylating (R) -methyl 2,3-diaminopropanoate with palmitoyl chloride, and then converting the ester to the iodo via the alcohol as described in Scheme 5 was also not fruitful because of unexpected difficulty in the conversion of the alcohol to the iodo group. This is probably due either to steric bulk of the long-chain amides or to an internal H-bond between the free alcohol and one of the amide groups. We therefore resorted to first installing the iodo on a N, N′-di-Boc-protected 2,3-diaminopropane-1-ol, followed by S-alkylation with N-Troc-L-Cys-OMe (Scheme 6), affording the required orthogonality of the protecting groups The amines on the diaminopropane-thiol fragment of compound 41 were then acylated with hexadecanoic acid. Next, the base-labile N-Troc protecting group on the cysteine unit had to be converted to the N-Boc derivative in order to carry out subsequent base-catalyzed de-esterfication step. Coupling of the terminal Ser residue proceeded smoothly.
Scheme 5.
Reagents and conditions: a: C15H31COCl, aq.NaHCO3, EtOAc, r.t.,1 h;
b: 2,2-DMP, PPTS, toluene, 90 °C, 22 h;
c: LiBH4, THF, 0 °C(3h) - r.t.(6h);
d: PPh3, I2, toluene, 90 °C, 2 h;
e: Boc-L-Cys-OMe, TEA, DMF, 85 °C, 2 h;
f: i) Ba(OH)2, AcCN/H2O, 60 °C, 1 h; ii) H-L-Ser(tBu)-OMe·HCl, EDCI, EDIPA, DMAP, DCM, 0°C- r.t., 8 h;
g: i) 70% AcOH, r.t.,12 h; ii) C15H31COCl, pyr, DMAP, DCM, 0 °C-r.t., 10 h;
h: TFA, r.t., 30 min.
Scheme 6.
Reagents and conditions: a: i) (Boc)2O, TEA, DCM, r.t., 2 h; ii) MeOH, EDCI, HOBt, TEA, DMAP, DCM, 10 h;
b: NaBH4, MeOH, THF, reflux, 4 h;
c: I2, PPh3, imidazole, DCM, 0 °C-rt, 2 h;
d: Troc-L-Cys-OMe, TEA, DMF, 85 °C, 2 h;
e: i) TFA, r.t., 30 min; ii) C15H31COOH, EDCI, HOBt, TEA, DMAP, DMF, 60 °C, 10 h;
f: i) Zn dust, AcOH, H2O, THF, r.t., 1 h; ii) (Boc)2O, TEA, DCM, r.t., 1 h;
g: i) Ba(OH)2, THF/H2O, 60 °C, 1 h; ii) H-L-Ser(tBu)-OMe•HCl, EDCI, HOBt, EDIPA, DMAP, DMF, 60 °C, 10 h;
h: TFA, r.t., 30 min.
The monoamide derivative 37 was found to be partially active (IC50: 48 nM, Fig. 2), although weaker by about three orders of magnitude compared to the reference lipopeptide PAM2CSK4, while the diamide derivative 45 was completely inactive. The progressive loss of activity from the monoamide to the diamide analogue suggests that both ester groups are important for maximal TLR2-stimulatory activity, although this is yet to be formally tested by synthesizing the regioisomer of 37 with the amide group replacing the external (primary alcohol-derived) ester group.
We were also keen to test the possibility that some of the inactive compounds could perhaps behave as TLR2 antagonists, particularly with 8a and 8b, since we had designed these compounds with inverted cysteamine-serine amide bonds in the hope that this would be the case. It could be noted in this context that TLR2-mediated immunopathology has been implicated in a number of inflammatory bowel diseases,53–56 and the only reported TLR2 receptor antagonists are rather weak lipolanthionine derivatives.57 Although we were disappointed that 6a, 6b, 21a-b, 28, 37 and 45 were all inactive as TLR2 receptor antagonists, the negative results add to the SAR data in that they demonstrate that these compounds do not engage TLR2.
In addition to the lipopeptides being potentially useful as immunotherapeutic agents for cancer, they also display potent adjuvant properties. Lipopeptides greatly enhance MHC Class I-restricted CTL proliferation to an immunodominant influenza peptide Mx58-66 in a human autologous DC/CD8+ T cell co-culture model.58 Lipopeptide-primed dendritic cells also stimulate the proliferation of allogeneic naïve CD8+ CTLs,58 and immunization of mice with influenza-specific peptide results in greatly enhanced numbers of interferon-γ-secreting CD8+ CTLs when lipopeptides were used as adjuvants.59 Similarly, MALP-2 induces robust, long-lived CTL responses against HIV-1 Tat protein.60;61 The synthetic methods that we have developed are easily scalable, and the availability of a free amino group in PAM2CS should allow the facile introduction of electrophilic labels such as isothiocyanate for covalent coupling to vaccine antigens. Differential protein binding should also allow considerable control of pharmacokinetic properties. These are currently being investigated.
Experimental Section
Chemistry
All of the solvents and reagents used were obtained commercially and used as such unless noted otherwise. Moisture or air-sensitive reactions were conducted under argon atmosphere in oven-dried (120°C) glass apparatus. Solvents were removed under reduced pressure using standard rotary evaporators. Flash column chromatography was carried out using silica gel 635 (60–100 mesh), while thin-layer chromatography was carried out on silica gel CCM pre-coated aluminum sheets. All yields reported refer to isolated material characterized by 1H and 13C NMR, and mass spectrometry. Purity for all final compounds was confirmed to be at least 97% by LC-MS using a Zorbax Eclipse Plus 4.6 × 150 mm, 5μ analytical reverse phase C18 column with H2O-isopropanol and H2O-CH3CN gradients, and an Agilent ESI-TOF mass spectrometer (mass accuracy: 3 ppm) operating in the positive ion acquisition mode.
Syntheses of Compounds 2a ((R)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 4-methylbenzenesulfonate and 2b ((S)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 4-methylbenzenesulfonate)
O-tosylation of (S)-(+)- [1a] and (R)-(-)-2,2-dimethyl-1,3-dioxolane-4-methanol [1b]. To enantiopure (S)-(+)- or (R)-(−)-2,2-dimethyl-1,3-dioxolane-4-methano1 (compounds 1a and 1b, respectively, 1.0 g, 7.57 mmol, Sigma-Aldrich, Inc., St. Louis, MO) dissolved in anhydrous DCM (30 mL) and cooled to 0 °C was added pyridine (1.22 mL, 15.14 mmol), followed by p-toluenesulfonyl chloride (p-TsCl; 2.89 g, 22.70 mmol). The reaction solution was brought to r.t. and stirred for 8 h. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 49:1) to afford the literature compounds 2a (R) and 2b (S) in 96–98% yields.62;63
Syntheses of Compounds 3a ((R)-tert-butyl 2-((2,2-dimethyl-1,3-dioxolan-4-yl)methylthio) ethylcarbamate) and 3b ((S)-tert-butyl 2-((2,2-dimethyl-1,3-dioxolan-4-yl)methylthio) ethylcarbamate)
To a solution of 2a or 2b (800 mg, 2.80 mmol) in anhydrous DMF (15 mL) cooled to 0 °C was added sodium hydride (NaH; 101 mg, 4.19 mmol). After stirring for 30 min at 0 °C, N-Boc-cysteamine (N-Boc-2-aminoethanethiol; 595 mg, 3.36 mmol) was added dropwise. The reaction solution was then brought to r.t. and stirred for 8 h. After quenching unreacted NaH with 1 M HCl, the solvent was removed under reduced pressure, and the residue was dissolved in EtOAc and washed with water. The water layer was extracted thrice with EtOAc. The combined organic layers were washed with brine and dried over Na2SO4. After removal of solvent under reduced pressure, the residue was purified by flash column chromatography (hexane:EtOAc = 5:1) to afford the title compounds 3a and 3b as colorless oils (90–94%).
3a
1H NMR (400 MHz, CDCl3) δ 4.98 (d, J = 27.4, 1H), 4.22 (p, J = 6.2, 1H), 4.07 (dd, J = 6.1, 8.3, 1H), 3.67 (dd, J = 6.4, 8.3, 1H), 3.35 (m, 2H), 2.78–2.58 (m, 4H), 1.41 (s, 9H), 1.40 (s, 3H), 1.32 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 155.78, 109.67, 79.48, 75.58, 68.82, 39.79, 33.01, 28.40, 26.90, 25.55. MS (ESI) calculated for C13H25NO4S, m/z 291.15, found 292.16 (M+H)+ and 314.14 (M+Na) +.
3b
1H NMR (400 MHz, CDCl3) δ 4.92 (s, 1H), 4.23 (p, J = 6.2, 1H), 4.12–4.04 (m, 1H), 3.68 (dd, J = 6.4, 8.3, 1H), 3.31 (m, 2H), 2.79–2.58 (m, 4H), 1.43 (s, 9H), 1.41 (d, J = 0.4, 3H), 1.34 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 155.78, 109.65, 79.45, 75.57, 68.81, 39.80, 24.95, 32.98, 28.40, 26.89, 25.55. MS (ESI) calculated for C13H25NO4S, m/z 291.15, found 292.15 (M+H)+ and 314.14 (M+Na+).
Syntheses of Compounds 4a ((R)-tert-butyl 2-(2,3-dihydroxypropylthio)ethylcarbamate) and 4b ((S)-tert-butyl 2-(2,3-dihydroxypropylthio)ethylcarbamate)
Acetonide deprotection of compounds 3a and 3b (900 mg, 3.09 mmol) was achieved by adding 70% acetic acid (AcOH:H2O = 7:3) and stirring at r.t. for 12 h. After removal of solvent under vacuum, the reaction residue was dissolved in EtOAc, and washed with sat. NaHCO3 solution. The aqueous layer was extracted thrice with EtOAc. The combined EtOAc layers were washed with brine and dried over Na2SO4. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 3:1) to afford the title compounds 4a and 4b (88–91%).
4a
1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 8.3, 1H), 7.34 (d, J = 8.0, 1H), 4.93 (s, 1H), 3.85–3.75 (m, 1H), 3.70 (dt, J = 5.2, 10.3, 1H), 3.55 (dd, J = 5.9, 11.3, 1H), 3.31 (d, J = 5.3, 2H), 2.77–2.57 (m, 4H), 1.42 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 156.20, 79.80, 70.40, 65.26, 39.85, 35.53, 32.96, 28.40. MS (ESI) calculated for C10H21NO4S, m/z 251.12, found 252.13 (M+H)+ and 274.11 (M+Na)+.
4b
1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H), 6.98 (s, 1H), 4.87 (s, 1H), 3.79 (ddd, J = 4.2, 6.9, 12.0, 1H), 3.75–3.68 (m, 1H), 3.55 (dt, J = 5.6, 11.3, 1H), 3.32 (d, J = 3.9, 2H), 2.78–2.55 (m, 4H), 1.43 (s, 9H). MS (ESI) calculated for C10H21NO4S, m/z 251.34, found 252.13 (M+H) + and 274.12 (M+Na) +.
Syntheses of Compounds 5a ((R)-3-(2-(tert-butoxycarbonylamino)ethylthio)propane-1,2-diyl dipalmitate) and 5b ((S)-3-(2-(tert-butoxycarbonylamino)ethylthio)propane-1,2-diyl dipalmitate)
O-palmitoylation of compounds 5a and 5b. To a solution of compound 4a and 4b (200 mg, 0.80 mmol) in anhydrous DCM (8 mL) at 0 °C were added pyridine (385 μL, 4.78 mmol) and a catalytic amount of 4-(dimethylamino)pyridine (DMAP). Palmitoyl chloride (876 mg, 3.19 mmol) was then added dropwise and the reaction was brought to r.t., after which the reaction solution was stirred for10 h. The reaction mixture was sequentially washed with water, sat. NaHCO3, brine, and then dried over Na2SO4. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 8:1) to afford the title compounds 5a and 5b as white solids (90–92%).
5a
1H NMR (400 MHz, CDCl3) δ 5.12 (m, 1H), 4.88 (s, 1H), 4.34 (dd, J = 3.5, 11.9, 1H), 4.15 (dd, J = 6.0, 11.9, 1H), 3.30 (d, J = 6.1, 2H), 2.68 (dd, J = 6.5, 11.5, 4H), 2.38–2.24 (m, 4H), 1.62–1.54 (m, 4H), 1.42 (s, 9H), 1.25 (d, J = 10.6, 48H), 0.86 (t, J = 6.9, 6H). 13C NMR (126 MHz, CDCl3) δ 173.42, 173.16, 155.77, 79.50, 70.30, 63.56, 39.59, 34.31, 34.12, 43.14, 31.94, 29.72, 29.69, 29.66, 29.61, 29.52, 29.46, 29.38, 29.31, 29.26, 29.15, 29.13, 29.09, 28.39, 24.91, 24.74, 22.71, 14.15. MS (ESI) calculated for C42H81NO6S, m/z 727.58, found 728.59 (M+H) + and 750.57 (M+Na) +.
5b
1H NMR (400 MHz, CDCl3) δ 5.12 (m, 1H), 4.88 (s, 1H), 4.34 (dd, J = 3.5, 11.9, 1H), 4.15 (dd, J = 6.0, 11.9, 1H), 3.30 (d, J = 6.1, 2H), 2.68 (dd, J = 6.4, 11.5, 4H), 2.34–2.25 (m, 4H), 1.64–1.56 (m, 4H), 1.42 (s, 9H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). MS (ESI) calculated for C42H81NO6S, m/z 727.58, found 728.59 (M+H) + and 750.57 (M+Na) +.
General procedure for N-Boc deprotection: Syntheses of Compounds 6a ((R)-3-(2-aminoethylthio)propane-1,2-diyl dipalmitate) and 6b ((S)-3-(2-aminoethylthio)propane-1,2-diyl dipalmitate)
To 5a and 5b was added excess dry trifluoroacetic acid (TFA) and stirred at r.t. for 30 min. TFA was removed by purging nitrogen and the residue was thoroughly washed with diethyl ether to obtain the title compounds as flaky white solids(98–100%).
6a
1H NMR (400 MHz, DMSO) δ 7.83 (s, 1H), 5.18–5.06 (m, 1H), 4.30 (dd, J = 2.7, 11.9, 1H), 4.10 (dd, J = 7.2, 12.0, 1H), 2.99 (t, J = 7.2, 2H), 2.90–2.65 (m, 4H), 2.32–2.14 (m, 4H), 1.58–1.42 (m, 4H), 1.23 (s, 48H), 0.84 (t, J = 6.8, 6H). 13C NMR (126 MHz, DMSO) δ 172.47, 172.29, 69.64, 63.47, 33.62, 33.53, 33.37, 31.29, 30.87, 29.06, 29.03, 29.01, 28.93, 28.75, 28.71, 28.50, 28.42, 28.38, 24.47, 24.38, 22.08, 13.91. MS (ESI) calculated for C37H73NO4S, m/z 627.53, found 628.51 (M+H)+.
6b
1H NMR (400 MHz, DMSO) δ 7.80 (s, 1H), 5.16–5.05 (m, 1H), 4.30 (dd, J = 2.8, 11.9, 1H), 4.10 (dd, J = 7.1, 12.0, 1H), 2.99 (t, J = 7.1, 2H), 2.87–2.65 (m, 4H), 2.33–2.20 (m, 4H), 1.50 (d, J = 6.2, 4H), 1.23 (s, 48H), 0.84 (t, J = 6.8, 6H). MS (ESI) calculated for C37H73NO4S, m/z 627.53, found 628.51 (M+H)+.
Syntheses of Compounds 7a ((6 R,13R)-6-(hydroxymethyl)-2,2-dimethyl-4,7-dioxo-3-oxa-11-thia-5,8-diazatetradecane-13,14-diyl dipalmitate) and 7b ((6S,13R)-6-(hydroxymethyl)-2,2-dimethyl-4,7-dioxo-3-oxa-11-thia-5,8-diazatetradecane-13,14-diyl dipalmitate)
The terminal L-serine of the dipeptide unit was appended to 6a and 6b by conventional solution phase coupling procedures. To 6a and 6b (200 mg, 0.27 mmol) in anhydrous DCM (8 mL) at 0 °C were added Boc-L-Ser-OH (Bachem Americas, Inc., Torrance, CA, 83 mg, 0.40 mmol), (3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI; 103 mg, 0.54 mmol), N, N-diisopropylethylamine (EDIPA; 53.4 μL, 0.32 mmol), and a catalytic amount of DMAP. The reaction mixture was stirred at 0 °C for 30 min, and then brought to r.t., and stirred for an additional 8 h. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 3:2) to afford the title compounds 7a or 7b as white solids (75–80%).
7a
1H NMR (400 MHz, CDCl3) δ 6.92 (s, 1H), 5.54 (s, 1H), 5.11 (dt, J = 5.0, 9.8, 1H), 4.36 (dd, J = 3.3, 11.9, 1H), 4.16–4.04 (m, 3H), 3.64 (s, 1H), 3.56–3.37 (m, 2H), 2.90 (s, 1H), 2.77–2.61 (m, 4H), 2.29 (td, J = 4.5, 7.5, 4H), 1.58 (s, 4H), 1.44 (s, 9H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 172.30, 172.13, 170.33, 154.96, 79.32, 69.11, 62.38, 61.73, 53.57, 37.04, 33.11, 32.90, 31.01, 30.72, 28.50, 28.46, 28.44, 28.29, 28.16, 28.09, 27.92, 27.10, 23.68, 21.49, 12.92. MS (ESI) calculated for C45H86N2O8S, m/z 814.61, found 837.56 (M+Na) +.
7b
1H NMR (400 MHz, CDCl3) δ 6.91 (s, 1H), 5.53 (s, 1H), 5.12 (qd, J = 3.4, 6.5, 1H), 4.35 (dd, J = 3.4, 11.9, 1H), 4.16–4.03 (m, 3H), 3.64 (s, 1H), 3.57–3.34 (m, 2H), 2.91 (s, 1H), 2.78–2.61 (m, 4H), 2.29 (td, J = 4.1, 7.6, 4H), 1.60 (s, 4H), 1.44 (s, 9H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). MS (ESI) calculated for C45H86N2O8S, m/z 814.61, found 837.56 (M+Na) +.
Syntheses of Compounds 8a ((R)-3-(2-((R)-2-amino-3-hydroxypropanamido)ethylthio)propane-1,2-diyl dipalmitate) and 8b ((S)-3-(2-((R)-2-amino-3-hydroxypropanamido)ethylthio)propane-1,2-diyl dipalmitate)
7a and 7b were deprotected with TFA as described earlier in the general procedure for N-Boc deprotection (see syntheses of 6a, 6b). The title compounds were obtained as flaky white solids(99–100%).
8a
1H NMR (400 MHz, DMSO) δ 8.51 (t, J = 5.7, 1H), 8.08 (s, 1H), 5.47 (s, 1H), 5.08 (dd, J = 4.5, 11.4, 1H), 4.31 (dd, J = 2.7, 11.9, 1H), 4.10 (dd, J = 7.1, 12.0, 1H), 3.69 (dd, J = 9.1, 33.4, 3H), 3.28 (dd, J = 6.5, 13.3, 2H), 2.74 (ddd, J = 6.6, 14.1, 21.4, 2H), 2.62 (t, J = 6.9, 2H), 2.32–2.19 (m, 4H), 1.56–1.45 (m, 4H), 1.23 (s, 48H), 0.85 (t, J = 6.8, 6H). 13C NMR (126 MHz, DMSO) δ 172.50, 172.27, 166.69, 69.88, 63.45, 60.27, 54.35, 38.52, 33.55, 33.37, 31.28, 31.04, 30.80, 29.05, 29.02, 29.00, 28.92, 28.91, 28.74, 28.71, 28.70, 28.41, 28.36, 24.47, 24.39, 22.08, 13.92. MS (ESI) calculated for C40H78N2O6S, m/z 714.56, found 715.54 (M+H) +.
8b
1H NMR (400 MHz, DMSO) δ 8.51 (t, J = 5.4, 1H), 8.08 (s, 1H), 5.48 (s, 1H), 5.09 (d, J = 5.8, 1H), 4.30 (dd, J = 2.6, 11.9, 1H), 4.10 (dd, J = 7.1, 11.9, 1H), 3.85–3.57 (m, 3H), 3.32–3.26 (m, 2H), 2.80 (dd, J = 5.8, 14.1, 1H), 2.65 (ddd, J = 7.0, 13.9, 20.3, 3H), 2.31–2.22 (m, 4H), 1.50 (d, J = 6.8, 4H), 1.23 (s, 48H), 0.84 (t, J = 6.7, 6H). 13C NMR (126 MHz, DMSO) δ 172.85, 172.63, 167.10, 70.25, 63.84, 60.65, 54.71, 38.92, 33.92, 33.75, 31.66, 31.42, 31.17, 29.44, 29.41, 29.39, 29.30, 29.13, 29.10, 29.09, 28.80, 28.75, 24.80, 22.46, 14.18. MS (ESI) calculated for C40H78N2O6S, m/z 714.56, found 715.54 (M+H) +.
Synthesis of Compound 9 ((R)-4-(iodomethyl)-2,2-dimethyl-1,3-dioxolane)
To a solution of (S)-(+)-2,2-dimethyl-1,3-dioxolane-4-methanol (compound 1a, 3.0 g, 22.70 mmol) in toluene (50 mL) was added triphenylphosphine (PPh3; 37.15 g, 27.38 mmol), imidazole (4.64 g, 68.10 mmol) and iodine (7.0 g, 29.51 mmol).64 The reaction mixture was stirred at 90 °C for 2 h. After removal of toluene under reduced pressure, the residue was dissolved in DCM, washed with sat. Na2S2O3 (to quench the unreacted iodine), brine, and dried over Na2SO4. The solvent was then removed under vacuum and the residue was purified by flash column chromatography (hexane:EtOAc = 49:1) to afford the literature compound 965 as a colorless liquid (5.44 g, 99%).
9
1H NMR (400 MHz, CDCl3) δ 4.30–4.21 (m, 1H), 4.12 (dd, J = 6.1, 8.6, 1H), 3.76 (dd, J = 5.4, 8.6, 1H), 3.23 (dd, J = 4.6, 9.8, 1H), 3.12 (dd, J = 8.5, 9.8, 1H), 1.43 (s, 1H), 1.32 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 109.47, 74.63, 68.57, 26.11, 24.57, 5.67.
Syntheses of Compounds 10a ((R)-methyl 2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-1,3-dioxolan-4-yl)methylthio)propanoate) and 10b ((S)-methyl 2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-1,3-dioxolan-4-yl)methylthio)propanoate)
N-Boc protected L- and D- cysteine methyl esters were first obtained by treating H-L- and D-Cys-OMe3HCl (Sigma-Aldrich) with di-tert-butyl dicarbonate (Boc2O; 2.0 eq.) in DCM, followed by slow addition of triethylamine (TEA; 1.0 eq.) at 0 °C. DCM was removed under vacuum after the reaction was stirred at 0 °C for 2 h and the residue was then purified by flash column chromatography with 10% EtOAc/hexane.) S-alkylation of Boc-L-Cys-OMe and Boc-D-Cys-OMe with 9 were then carried out. Compound 10 was synthesized as follows: to a solution of 9 (900 mg, 3.72 mmol) in anhydrous DMF (8 mL) was added TEA (1.55 mL, 11.15 mmol), followed by Boc-L- or D-Cys-OMe (673 mg, 2.86 mmol). The reaction solution was stirred at 85 °C for 4 h. After removal of DMF under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 9:1) to afford 10a or 10b, respectively as viscous oils (60–63%).
10a
1H NMR (400 MHz, CDCl3) δ 5.39 (d, J = 7.1, 1H), 4.52 (d, J = 7.3, 1H), 4.21 (p, J = 6.2, 1H), 4.06 (dd, J = 6.1, 8.3, 1H), 3.74 (s, 3H), 3.66 (dd, J = 6.4, 8.3, 1H), 3.02 (d, J = 3.2, 2H), 2.73 (dd, J = 6.0, 13.4, 1H), 2.62 (dd, J = 6.5, 13.4, 1H), 1.43 (s, 9H), 1.40 (s, 3H), 1.33 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 171.49, 155.23, 109.73, 80.18, 75.64, 68.68, 53.45, 52.58, 38.78, 35.22, 28.31, 26.84, 25.55. MS (ESI) calculated for C15H27NO6S, m/z 349.16, found 372.10 (M+Na) +.
10b
1H NMR (400 MHz, CDCl3) δ 5.43 (d, J = 7.0, 1H), 4.52 (s, 1H), 4.26–4.18 (m, 1H), 4.06 (dd, J = 6.1, 8.3, 1H), 3.76 (d, J = 11.0, 3H), 3.66 (dd, J = 6.5, 8.3, 1H), 3.02 (ddd, J = 5.2, 13.8, 19.4, 2H), 2.75 (dd, J = 5.9, 13.5, 1H), 2.63 (dd, J = 6.3, 13.5, 1H), 1.43 (s, 9H), 1.41 (s, 3H), 1.34 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 171.49, 155.23, 109.73, 80.18, 75.64, 68.68, 53.45, 52.58, 35.78, 35.22, 28.31, 26.84, 25.55. MS (ESI) calculated for C15H27NO6S, m/z 349.16, found 372.10 (M+Na) +.
General procedure for acetonide deprotection and palmitoylation of the 1,2-isopropylideneglycerol unit: Synthesis of Compound11 ((R)-3-((R)-2-amino-3-methoxy-3-oxopropylthio)propane-1,2-diyl dipalmitate)
To 10a (200 mg, 0.57 mmol) was added 15 mL of 70% acetic acid (AcOH:H2O = 7:3) and the reaction was stirred at r.t. for 24h. After complete removal of AcOH and water under reduced pressure, O-palmitoylation of the diol was carried out by sequential addition of pyridine (278 μL, 3.44 mmol), a catalytic amount of DMAP, followed by palmitoyl chloride (709 μL, 2.29 mmol) to the diol dissolved in anhydrous DCM (15 mL) and precooled to 0 °C. After stirring at 0 °C for 30 min, the reaction solution was brought to r.t. and stirred for 8 h. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 19:1) to afford the N-Boc-protected intermediate as a colorless oil. Finally, N-Boc deprotection was carried out as described earlier (see syntheses of 6a, 6b) to obtain the title compound 11 as a white glassy solid (435 mg, 95%). 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 2H), 5.13 (s, 1H), 4.41–4.06 (m, 3H), 3.81 (s, 3H), 3.20 (d, J = 41.1, 2H), 2.74 (s, 2H), 2.29 (dd, J = 7.9, 15.5, 4H), 1.58 (d, J = 4.7, 4H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 173.66, 173.63, 168.19, 69.95, 63.47, 53.73, 52.50, 34.24, 34.02, 32.91, 32.46, 31.94, 29.72, 29.70, 29.68, 29.52, 29.38, 29.30, 29.13, 24.85, 22.70, 14.13. MS (ESI) calculated for C39H75NO6S, m/z 685.53, found 686.59 (M+H) +.
General Procedure for de-esterification of 10a and 10b, and subsequent coupling of serine: Syntheses of Compounds 12a-d
10a and 10b were de-esterified with aqueous LiOH (165 mg, 6.87 mmol dissolved in 6mL water/15 mL THF) at r.t. for 10h. 1 M HCl solution was then added (to quench unreacted LiOH and to convert the lithium salt to the free-acid form) until a pH of 4 was reached. After removal of THF under vacuum, the residue was extracted thrice with DCM. The combined DCM layers were washed with brine and dried over Na2SO4. After removal of DCM under vacuum, H-L-Ser(tBu)-OtBu·HCl, or H-L-Ser(tBu)- OMe·HCl, or H-D-Ser(tBu)-OMe·HCl (all from Bachem Americas, Inc., Torrance, CA, 437 mg, 2.06 mmol) were coupled to the de-esterified intermediates as described earlier for the syntheses of 7a and 7b. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 4:1) to afford the corresponding compounds 12a-d as viscous oils (32–38%).
12a ((S)-tert-butyl 3-tert-butoxy-2-((R)-2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-1,3-dioxolan-4-yl)methylthio)propanamido)propanoate)
1H NMR (400 MHz, CDCl3) δ 7.31 (s, 1H), 5.48 (s, 1H), 4.70–4.47 (m, 2H), 4.38–4.22 (m, 2H), 4.07 (dd, J = 6.1, 8.2, 1H), 3.79–3.71 (m, 1H), 3.67 (dd, J = 6.6, 8.2, 1H), 3.59–3.45 (m, 1H), 3.10 (d, J = 6.5, 1H), 2.96 (ddd, J = 6.1, 13.9, 43.9, 1H), 2.74 (ddd, J = 6.1, 13.5, 44.1, 1H), 1.43 (s, 18H), 1.33 (s, 3H), 1.22 (s, 3H), 1.11 (d, J = 4.0, 9H). MS (ESI) calculated for C25H46N2O8S, m/z 534.30, found 557.29 (M+Na) +.
12b ((S)-methyl 3-tert-butoxy-2-((R)-2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-1,3-dioxolan-4-yl)methylthio)propanamido)propanoate)
1H NMR (400 MHz, CDCl3) δ 7.07 (d, J = 8.3, 1H), 5.48 (s, 1H), 4.71–4.59 (m, 1H), 4.41–4.22 (m, 2H), 4.15–4.02 (m, 1H), 3.80 (dd, J = 2.9, 9.1, 1H), 3.72 (s, 3H), 3.68 (dd, J = 6.5, 8.3, 1H), 3.55 (dd, J = 3.2, 9.1, 1H), 3.06 (ddd, J = 5.9, 12.2, 19.8, 1H), 2.98–2.87 (m, 1H), 2.85–2.76 (m, 1H), 2.71 (dt, J = 6.8, 13.5, 1H), 1.44 (s, 9H), 1.42 (s, 3H), 1.34 (s, 3H), 1.12 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 170.47, 170.41, 155.28, 109.70, 75.70, 74.81, 73.52, 68.73, 61.69, 54.10, 53.12, 52.43, 35.61, 35.22, 28.31, 27.30, 26.87, 25.56. MS (ESI) calculated for C22H40N2O8S, m/z 492.25, found 515.18 (M+Na) +.
12c ((R)-methyl 3-tert-butoxy-2-((R)-2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-1,3-dioxolan-4-yl)methylthio)propanamido)propanoate)
1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1H), 5.55 (s, 1H), 4.71 (d, J = 8.2, 1H), 4.39 (s, 1H), 4.35–4.25 (m, 1H), 4.16–4.08 (m, 1H), 3.85 (d, J = 9.0, 1H), 3.76 (s, 3H), 3.70 (t, J = 7.3, 1H), 3.57 (d, J = 9.0, 1H), 3.13 (d, J = 15.0, 1H), 2.93 (dd, J = 6.1, 13.9, 1H), 2.86–2.71 (m, 2H), 1.48 (s, 9H), 1.45 (s, 3H), 1.38 (s, 3H), 1.15 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 170.60, 170.34, 155.39, 109.70, 75.52, 74.79, 73.50, 68.68, 61.75, 53.99, 52.86, 52.41, 35.51, 30.95, 28.31, 27.30, 26.88, 25.52. MS (ESI) calculated for C22H40N2O8S m/z 492.25, found 515.18 (M+Na) +.
12d ((S)-methyl 3-tert-butoxy-2-((S)-2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-1,3-dioxolan-4-yl)methylthio)propanamido)propanoate)
1H NMR (400 MHz, CDCl3) δ 7.07 (d, J = 8.3, 1H), 5.48 (s, 1H), 4.71–4.59 (m, 1H), 4.41–4.22 (m, 2H), 4.15–4.02 (m, 1H), 3.80 (dd, J = 2.9, 9.1, 1H), 3.72 (s, 3H), 3.68 (dd, J = 6.5, 8.3, 1H), 3.55 (dd, J = 3.2, 9.1, 1H), 3.06 (ddd, J = 5.9, 12.2, 19.8, 1H), 2.98–2.87 (m, 1H), 2.85–2.76 (m, 1H), 2.71 (dt, J = 6.8, 13.5, 1H), 1.44 (s, 9H), 1.42 (s, 3H), 1.34 (s, 3H), 1.12 (s, 9H). MS (ESI) calculated for C22H40N2O8S, m/z 492.25, found 515.18 (M+Na) +.
Syntheses of Compounds 13a-d
Acetonide deprotection followed by O-palmitoylation of 12a-d were performed as per the general procedure outlined earlier (synthesis of compound 11) to afford the corresponding compounds 13a-d in 90–95% yields.
13a ((R)-3-((R)-2-(tert-butoxycarbonylamino)-3-((S)-1,3-di-tert-butoxy-1-oxopropan-2-ylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (400 MHz, CDCl3) δ 7.02 (d, J = 7.9, 1H), 5.37 (s, 1H), 5.19–5.09 (m, 1H), 4.51 (dt, J = 2.9, 8.0, 1H), 4.31 (dd, J = 3.5, 11.9, 2H), 4.14 (dd, J = 5.9, 11.9, 1H), 3.76 (dd, J = 2.9, 8.8, 1H), 3.51 (dd, J = 3.0, 8.8, 1H), 2.93 (d, J = 6.2, 2H), 2.79 (d, J = 6.1, 2H), 2.30 (ddd, J = 5.8, 10.8, 12.0, 4H), 1.59 (dd, J = 6.6, 12.8, 5H), 1.43 (d, J = 2.6, 17H), 1.23 (s, 48H), 1.12 (d, J = 2.6, 9H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 173.40, 173.13, 170.13, 168.92, 81.97, 73.17, 70.32, 63.52, 61.97, 53.51, 35.61, 34.31, 34.11, 33.71, 33.13, 31.94, 29.72, 29.68, 29.66, 29.53, 29.38, 29.33, 29.32, 29.16, 29.15, 28.30, 28.02, 27.34, 24.90, 24.74, 22.71, 14.14. MS (ESI) calculated for C54H102N2O10S, m/z 970.73, found 993.82 (M+Na) +.
13b ((R)-3-((R)-3-((S)-3-tert-butoxy-1-methoxy-1-oxopropan-2-ylamino)-2-(tert-butoxycarbonylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (400 MHz, CDCl3) δ 7.06 (d, J = 8.1, 1H), 5.17 (s, 1H), 4.69–4.58 (m, 1H), 4.31 (dd, J = 3.5, 11.9, 2H), 4.15 (dd, J = 5.9, 11.9, 1H), 3.80 (dd, J = 3.0, 9.1, 1H), 3.72 (s, 3H), 3.55 (dd, J = 3.2, 9.1, 1H), 2.94 (d, J = 6.1, 2H), 2.79 (d, J = 5.9, 2H), 2.29 (td, J = 4.9, 7.5, 4H), 1.59 (s, 4H), 1.43 (s, 9H), 1.23 (s, 48H), 1.12 (s, 9H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 173.37, 173.14, 170.44, 170.30, 155.22, 73.51, 70.29, 63.49, 61.67, 53.14, 52.41, 35.52, 34.30, 34.09, 33.20, 31.93, 29.71, 29.51, 29.37, 29.32, 29.31, 29.15, 29.14, 28.29, 27.29, 24.90, 24.88, 22.70, 14.13. MS (ESI) calculated for C51H96N2O10S, m/z 928.68, found 951.57 (M+Na) +.
13c ((R)-3-((R)-3-((R)-3-tert-butoxy-1-methoxy-1-oxopropan-2-ylamino)-2-(tert-butoxycarbonylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (400 MHz, CDCl3) δ 7.15–7.06 (m, 1H), 5.19–5.11 (m, 1H), 4.65 (d, J = 8.4, 1H), 4.31 (dd, J = 3.6, 11.9, 2H), 4.14 (dd, J = 5.9, 11.9, 1H), 3.81 (dd, J = 2.9, 9.0, 1H), 3.72 (s, 3H), 3.53 (dd, J = 3.3, 9.0, 1H), 2.94 (dd, J = 14.0, 20.2, 2H), 2.78 (s, 2H), 2.29 (dd, J = 7.5, 13.4, 4H), 1.59 (d, J = 6.7, 4H), 1.44 (s, 9H), 1.23 (s, 47H), 1.12 (d, J = 1.9, 9H), 0.86 (t, J = 6.9, 6H). 13C NMR (126 MHz, CDCl3) δ 173.76, 172.63, 171.22, 170.29, 73.10, 70.42, 63.59, 55.56, 52.96, 34.36, 34.05, 31.93, 30.96, 29.73, 29.68, 29.61, 29.55, 29.38, 29.32, 29.15, 19.12, 24.84, 22.70, 14.13. MS (ESI) calculated for C51H96N2O10S, m/z 928.68, found 951.57 (M+Na) +.
13d ((R)-3-((S)-3-((S)-3-tert-butoxy-1-methoxy-1-oxopropan-2-ylamino)-2-(tert-butoxycarbonylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (400 MHz, CDCl3) δ 7.06 (d, J = 8.1, 1H), 5.17 (s, 1H), 4.69–4.58 (m, 1H), 4.31 (dd, J = 3.5, 11.9, 2H), 4.15 (dd, J = 5.9, 11.9, 1H), 3.80 (dd, J = 3.0, 9.1, 1H), 3.72 (s, 3H), 3.55 (dd, J = 3.2, 9.1, 1H), 2.94 (d, J = 6.1, 2H), 2.79 (d, J = 5.9, 2H), 2.29 (td, J = 4.9, 7.5, 4H), 1.59 (s, 4H), 1.43 (s, 9H), 1.23 (s, 48H), 1.12 (s, 9H), 0.86 (t, J = 6.8, 6H). MS (ESI) calculated for C51H96N2O10S, m/z 928.68, found 951.57 (M+Na) +.
General Procedure for one-step deprotection of N-Boc and O-tBu: Syntheses of Compounds 14a-d
13a-d were deprotected with dry TFA as described earlier (general procedure for N-Boc deprotection) which allowed for the simultaneous deprotection of the N-Boc and O-tBu groups. Consequently, 14a was obtained as the –Ser-OH (free acid), while 14b-d were the –Ser-OMe esters. All title compounds (14a-d) were obtained as white glassy solids in near-quantitative yields.
14a ((S)-2-((R)-2-amino-3-((R)-2,3-bis(palmitoyloxy)propylthio)propanamido)-3-hydroxypropanoic acid)
1H NMR (400 MHz, DMSO) δ 9.10 (d, J = 7.4, 1H), 8.82 (d, J = 7.9, 1H), 8.23 (s, 1H), 5.16 (d, J = 7.5, 1H), 4.81–4.58 (m, 1H), 4.42–4.23 (m, 2H), 4.18–4.07 (m, 1H), 3.79 (dd, J = 4.8, 11.0, 1H), 3.70–3.58 (m, 1H), 3.07 (dt, J = 6.7, 13.0, 1H), 2.94–2.65 (m, 3H), 2.31–2.13 (m, 4H), 1.55–1.44 (m, 4H), 1.22 (s, 48H), 0.84 (t, J = 6.7, 6H). 13C NMR (126 MHz, DMSO) δ 179.71, 177.74, 177.51, 176.39, 74.88, 68.75, 66.29, 60.04, 56.60, 38.87, 38.78, 38.61, 38.23, 36.53, 34.30, 34.25, 34.20, 34.17, 34.13, 34.03, 33.98, 33.95, 33.76, 33.68, 33.63, 29.71, 29.62, 27.32, 19.16. MS (ESI) calculated for C41H78N2O8S, m/z 758.55, found 759.66 (M+H) +.
14b ((R)-3-((R)-2-amino-3-((S)-3-hydroxy-1-methoxy-1-oxopropan-2-ylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (500 MHz, CDCl3) δ 8.21 (s, 1H), 5.28 (s, 1H), 5.20–5.07 (m, 1H), 4.79–4.60 (m, 2H), 4.47–4.26 (m, 2H), 4.14–4.04 (m, 1H), 3.99–3.90 (m, 1H), 3.74 (s, 3H), 3.24–2.97 (m, 2H), 2.75 (s, 2H), 2.36–2.23 (m, 4H), 1.57 (s, 4H), 1.23 (s, 48H), 0.86 (t, J = 7.0, 6H). 13C NMR (126 MHz, CDCl3) δ 174.39, 173.97, 169.90, 70.27, 70.08, 63.65, 63.52, 61.90, 52.92, 34.32, 34.05, 31.93, 29.73, 29.68, 29.55, 59.53, 29.38, 29.0, 29.14, 29.11, 24.89, 24.86, 24.82, 22.70, 14.12. MS (ESI) calculated for C42H80N2O8S, m/z 772.56, found 773.53 (M+H)+.
14c ((R)-3-((R)-2-amino-3-((R)-3-hydroxy-1-methoxy-1-oxopropan-2-ylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (400 MHz, CDCl3) δ 8.86–8.37 (m, 1H), 5.40–5.22 (m, 1H), 5.21–5.01 (m, 1H), 4.72–4.42 (m, 2H), 4.42–4.16 (m, 2H), 4.08 (s, 1H), 4.01–3.88 (m, 1H), 3.73 (s, 3H), 3.39–2.98 (m, 2H), 2.73 (s, 2H), 2.29 (s, 4H), 1.56 (s, 4H), 1.23 (s, 48H), 0.85 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 174.22, 173.92, 70.02, 69.83, 63.66, 63.60, 53.02, 34.30, 34.05, 31.94, 29.73, 29.69, 29.56, 29.55, 29.39, 29.32, 29.16, 29.13, 24.88, 24.83, 22.70, 14.13. MS (ESI) calculated for C42H80N2O8S, m/z 772.56, found 773.53 (M+H)+.
14d ((R)-3-((S)-2-amino-3-((S)-3-hydroxy-1-methoxy-1-oxopropan-2-ylamino)-3-oxopropylthio)propane-1,2-diyl dipalmitate)
1H NMR (400 MHz, CDCl3) δ 8.74 (s, 1H), 7.87 (s, 1H), 5.32 (s, 1H), 5.13 (s, 1H), 4.46 (d, J = 80.5, 3H), 4.13 (s, 1H), 3.99 (s, 2H), 3.78 (s, 3H), 3.23 (s, 2H), 2.78 (s, 2H), 2.33 (s, 4H), 1.61 (s, 4H), 1.61 (s, 3H), 1.27 (s, 48H), 0.89 (t, J = 6.7, 6H). 13C NMR (126 MHz, CDCl3) δ 174.49, 173.76, 70.42, 63.59, 55.56, 53.70, 52.96, 48.56, 34.36, 34.05, 31.93, 29.73, 29.68, 29.55, 29.38, 29.32, 29.15, 29.12, 24.84, 22.70, 14.13. MS (ESI) calculated for C42H80N2O8S, m/z 772.56, found 773.53 (M+H)+.
Synthesis of compound 15 (ethyl 3-(((S)-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)-2-hydroxypropanoate)
To a solution of ethyl-2,3-epoxypropanoate (Sigma-Aldrich, Inc., 375 mg, 2.91 mmol) in anhydrous DCM (10 mL) was added boron trifluoride diethyl etherate (BF3•OEt2; 83 mg, 0.58 mmol), followed by (S)-(+)- 2,2-dimethyl-1,3-dioxolane-4-methanol (1a, 1.20 g, 8.72 mmol).66 The reaction mixture was stirred at r.t. for 5 h. After removal of solvent under reduced pressure, a mixture of ethyl 3-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)-2-hydroxypropanoate (compound 15) and (S)-(+)- 2,2-dimethyl-1,3-dioxolane-4-methanol (unreacted starting material, 1a) were obtained. The Rf values of both the required product as well as the starting material were identical which presented difficulty in chromatographic isolation. The mixture was therefore subjected to selective protection of the primary hydroxyl group of the acetonide-protected glycerol 1a with TBDMSCl (tert-butyldimethylsilyl chloride), which allowed the facile isolation of compound 15 by flash column chromatography using 20% EtOAc/hexane (433 mg, 60%). 1H NMR (400 MHz, CDCl3) δ 4.34–4.22 (m, 4H), 4.05 (dd, J = 6.4, 8.3, 1H), 3.88–3.80 (m, 2H), 3.75 (ddd, J = 6.3, 7.5, 8.2, 1H), 3.66–3.52 (m, 2H), 3.15 (dd, J = 6.6, 15.6, 1H), 1.44–1.27 (m, 9H). 13C NMR (126 MHz, CDCl3) δ 171.49, 171.44, 108.42, 73.59, 73.55, 72.11, 72.04, 71.56, 69.95, 69.85, 65.57, 65.54, 60.85, 28.68, 25.68, 25.67, 24.37, 24.33, 13.17. MS (ESI) calculated for C11H10O6, m/z 248.13, found 271.12 (M+Na+) and 519.245(M+M+Na) +.
Synthesis of compound 16 (ethyl 3-(((S)-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)-2-tosyloxy)propanoate)
O-tosylation was performed by sequentially adding TEA (590 μL, 4.23 mmol) and p-TsCl (806 mg, 4.23 mmol) to a solution of 15 (350 mg, 1.41 mmol) in anhydrous DCM (10 mL) cooled to 0 °C. The reaction mixture was brought to r.t. and stirred for 8 h. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 9:1) to afford the title compound 16 (443 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.4, 2H), 7.31 (d, J = 8.0, 2H), 5.02–4.96 (m, 1H), 4.18–4.06 (m, 3H), 3.96–3.77 (m, 3H), 3.62 (ddd, J = 3.4, 6.3, 8.4, 1H), 3.54–3.38 (m, 2H), 2.42 (s, 3H), 1.35 (s, 3H), 1.31 (s, 3H), 1.19 (t, J = 7.1, 3H). 13C NMR (126 MHz, CDCl3) δ 165.34, 143.94, 132.12, 132.09, 128.55, 126.92, 108.23, 108.20, 75.73, 73.22, 71.20, 69.59, 65.31, 60.90, 25.48, 24.18, 20.49, 12.77. MS (ESI) calculated for C18H26O8, m/z 402.13, found 425.13 (M+Na) +.
Synthesis of compound 17 (ethyl 2-azido-3-(((S)-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)propanoate)
To a solution of compound 16 (380 mg, 0.94 mmol) in anhydrous DMF (10 mL) was added sodium azide (184 mg, 2.83 mmol). The reaction mixture was stirred at r.t. for 12 h. After removal of DMF under vacuum, the reaction residue was dissolved in DCM, washed with water, brine, and dried over Na2SO4. After removal of solvent under reduced pressure, the residue was purified by flash column chromatography (hexane:EtOAc = 9:1) to afford the title compound 17 (245 mg, 95%). 1H NMR (400 MHz, CDCl3) δ 4.29–4.18 (m, 3H), 3.74 (ddd, J = 6.2, 8.4, 11.2, 1H), 3.61–3.48 (m, 2H), 1.39 (d, J = 0.9, 3H), 1.32 (d, J = 5.0, 3H), 1.29 (t, J = 7.1, 3H). 13C NMR (126 MHz, CDCl3) δ 166.54, 107.70, 107.67, 72.67, 70.55, 69.89, 64.82, 60.53, 59.86, 24.94, 23.58, 12.37. MS (ESI) calculated for C11H19N3O5, m/z 273.13, found 296.14 (M+Na) +.
Synthesis of compound 18 (ethyl 2-(tert-butoxycarbonylamino)-3-(((S)2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)propanoate)
Reduction of the azide to the amine under Staudinger conditions.67 To 17 (230 mg, 0.84 mmol) dissolved in THF (8 mL) and H2O (30.3 mg, 1.68 mmol) was added PPh3 (265 mg, 1.01 mmol). The reaction mixture was refluxed for 6 h. After removal of solvent, the reaction residue was dissolved in anhydrous DCM (5 mL) and di-tert-butyl dicarbonate (Boc2O; 551 mg, 2.52 mmol) and TEA (351 μL, 2.52 mmol) was added and stirred at r.t. for 8h. The solvent was removed under vacuum and the residue was purified by flash column chromatography (hexane:EtOAc = 17:3) to afford the title compound 18 (272 mg, 93%). 1H NMR (400 MHz, CDCl3) δ 5.37 (t, J = 8.2, 1H), 4.42–4.32 (m, 1H), 4.26–4.13 (m, 3H), 3.99 (dd, J = 6.4, 8.3, 1H), 3.94–3.86 (m, 1H), 3.75–3.63 (m, 2H), 3.55–3.40 (m, 2H), 1.43 (s, 9H), 1.38 (d, J = 1.9, 3H), 1.33 (d, J = 0.6, 3H), 1.25 (t, J = 7.1, 3H). 13C NMR (126 MHz, CDCl3) δ 168.21, 153.15, 107.09, 77.59, 70.07, 69.60, 69.36, 64.18, 59.17, 51.70, 25.95, 24.32, 23.02, 11.81. MS (ESI) calculated for C16H29NO7, m/z 347.19, found 370.28 (M+Na) +.
Syntheses of compounds 19a ((S)-methyl 3-tert-butoxy-2-(2-(tert-butoxycarbonylamino)-3-(((S)-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)propanamido)propanoate) and 19b ((R)-methyl 3-tert-butoxy-2-(2-(tert-butoxycarbonylamino)-3-(((S)-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)propanamido)propanoate)
De-esterification of the ethyl ester of 18 and subsequent coupling to H-L- and -D-Ser(tBu)-OMe3HCl were performed as described for the syntheses of compounds 12a-d to yield 19a and 19b, respectively (70–75%).
19a
1H NMR (400 MHz, CDCl3) δ 7.21 (s, 1H), 5.48 (s, 1H), 4.71–4.62 (m, 1H), 4.25 (dt, J = 5.9, 11.7, 2H), 4.06–3.98 (m, 1H), 3.89 (ddd, J = 2.4, 4.0, 9.5, 1H), 3.82–3.68 (m, 5H), 3.66–3.50 (m, 4H), 1.44 (s, 9H), 1.40 (d, J = 2.4, 3H), 1.33 (d, J = 2.2, 3H), 1.15–1.08 (m, 9H). 13C NMR (126 MHz, CDCl3) δ 168.88, 168.87, 168.81, 108.18, 73.14, 62.15, 71.24, 71.00, 69.90, 65.30, 65.06, 60.56, 51.78, 51.03, 28.38, 26.98, 25.97, 25.43, 24.10. MS (ESI) calculated for C22H40N2O9, m/z 476.27, found 499.19 (M+Na) +.
19b
1H NMR (400 MHz, CDCl3) δ 7.21 (s, 1H), 5.49 (s, 1H), 4.66 (ddd, J = 3.2, 7.2, 11.3, 1H), 4.25 (ddd, J = 5.7, 11.6, 17.0, 2H), 4.06–3.98 (m, 1H), 3.89 (ddd, J = 2.4, 4.0, 9.5, 1H), 3.84–3.76 (m, 1H), 3.75–3.67 (m, 4H), 3.64–3.50 (m, 4H), 1.44 (s, 9H), 1.42–1.38 (m, 3H), 1.36–1.31 (m, 3H), 1.13–1.08 (m, 9H). 13C NMR (126 MHz, CDCl3) δ 167.90, 167.83, 107.19, 72.16, 71.16, 70.27, 70.03, 68.94, 64.32, 64.09, 59.58, 50.80, 50.05, 27.40, 26.00, 24.99, 24.46, 23.12. MS (ESI) calculated for C22H40N2O9, m/z 476.27, found 499.19 (M+Na) +.
Syntheses of compounds 20a ((S)-3-(3-((S)-3-tert-butoxy-1-methoxy-1-oxopropan-2-ylamino)-2-(tert-butoxycarbonylamino)-3-oxopropoxy)propane-1,2-diyl dipalmitate), and 20b ((S)-3-(3-((R)-3-tert-butoxy-1-methoxy-1-oxopropan-2-ylamino)-2-(tert-butoxycarbonylamino)-3-oxopropoxy)propane-1,2-diyl dipalmitate)
Acetonide deprotection and O-palmitoylation were performed as described earlier (see syntheses of 13a-d). 20a or 20b were obtained in 80–83% yield.
20a
1H NMR (400 MHz, CDCl3) δ 7.11 (t, J = 8.3, 1H), 5.40 (s, 1H), 5.16 (dd, J = 3.3, 5.5, 1H), 4.66 (tt, J = 3.0, 8.8, 1H), 4.38–4.22 (m, 2H), 4.18–4.06 (m, 1H), 3.96–3.76 (m, 2H), 3.71 (d, J = 1.5, 3H), 3.66–3.49 (m, 4H), 2.28 (dd, J = 7.7, 15.5, 4H), 1.58 (d, J = 5.7, 4H), 1.44 (s, 9H), 1.23 (s, 48H), 1.13–1.08 (m, 9H), 0.85 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 172.18, 171.88, 169.40, 168.79, 154.25, 79.11, 72.29, 69.96, 69.69, 68.70, 68.63, 68.58, 68.55, 68.48, 68.44, 68.41, 61.19, 60.94, 51.90, 51.66, 51.17, 33.06, 32.91, 30.74, 28.52, 28.48, 28.32, 28.18, 18.12, 27.94, 27.11, 26.09, 23.70, 21.51, 12.94. MS (ESI) calculated for C51H96N2O11, m/z 912.70, found 935.54 (M+Na) +.
20b
1H NMR (400 MHz, CDCl3) δ 7.11 (t, J = 9.0, 1H), 5.40 (s, 1H), 5.23–5.11 (m, 1H), 4.66 (tt, J = 3.0, 8.6, 1H), 4.30 (ddd, J = 4.3, 10.2, 15.3, 2H), 4.11 (ddt, J = 5.0, 6.0, 10.3, 1H), 3.91–3.76 (m, 2H), 3.71 (d, J = 1.3, 3H), 3.68–3.49 (m, 4H), 2.28 (dd, J = 7.8, 15.5, 4H), 1.58 (dd, J = 6.9, 12.8, 4H), 1.44 (s, 9H), 1.23 (s, 48H), 1.14–1.07 (m, 9H), 0.85 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 171.84, 171.56, 168.96, 168.46, 153.92, 78.72, 71.96, 69.62, 69.40, 68.37, 68.30, 68.25, 68.22, 68.15, 68.08, 60.86, 60.31, 51.57, 51.32, 50.84, 32.74, 32.58, 30.41, 28.19, 28.15, 27.99, 27.85, 27.79, 27.62, 26.78, 25.72, 23.38, 21.18, 12.61. MS (ESI) calculated for C51H96N2O11, m/z 912.70, found 935.54 (M+Na) +.
Syntheses of compound 21a ((S)-3-(2-amino-3-((S)-3-hydroxy-1-methoxy-1-oxopropan-2-ylamino)- 3-oxopropoxy)propane-1,2-diyl dipalmitate; trifluoroacetate) and 21b ((S)-3-(2-amino-3-((R)-3-hydroxy-1-methoxy-1-oxopropan-2-ylamino)-3-oxopropoxy)propane-1,2-diyl dipalmitate; trifluoroacetate)
One-step deprotection of the N-Boc and O-tBu groups utilizing TFA was carried out as described earlier (syntheses of 14a-d). Title compounds were obtained as white glassy solids 21a or 21b (99–100%).
21a
1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H), 8.03 (d, J = 28.1, 1H), 5.28 (s, 1H), 5.15 (s, 1H), 4.71 (dd, J = 69.3, 103.2, 2H), 4.17 (ddd, J = 7.3, 17.4, 18.6, 2H), 4.06–3.81 (m, 3H), 3.76 (d, J = 14.3, 3H), 3.58 (dd, J = 10.2, 15.7, 2H), 2.28 (dd, J = 7.3, 11.9, 4H), 1.56 (s, 4H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 172.76, 172.47, 172.41, 172.34, 168.73, 68.50, 68.31, 68.22, 67.59, 61.22, 61.10, 60.49, 54.08, 53.97, 51.92, 51.63, 51.47. 32.90, 32.78, 30.63, 28.42, 28.37, 28.24, 28.08, 28.02, 27.85, 27.80, 23.55, 21.40, 12.82. MS (ESI) calculated for C42H80N2O9, m/z 756.59, found 757.45 (M+H) +.
21b
1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 8.04 (s, 1H), 5.28 (s, 1H), 5.15 (s, 1H), 4.58 (d, J = 51.7, 2H), 4.18 (d, J = 42.7, 2H), 3.90 (s, 3H), 3.76 (d, J = 14.5, 3H), 3.59 (s, 2H), 2.28 (dd, J = 7.4, 11.7, 4H), 1.56 (s, 4H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 172.11, 171.82, 171.76, 171.69, 168.06, 67.84, 67.64, 67.55, 66.89, 60.55, 60.41, 59.84, 53.42, 53.32, 51.27, 50.98, 50.82, 32.24, 32.14, 29.97, 27.76, 27.72, 27.58, 27.42, 27.35, 27.19, 27.14, 22.89, 20.74, 12.16. MS (ESI) calculated for C42H80N2O9, m/z 756.59, found 757.45 (M+H) +.
Synthesis of compound 22 ((S)-4-(benzyloxymethyl)-2,2-dimethyl-1,3-dioxolane)
O-benzylation of 1a was performed by first adding sodium hydride (NaH; 60% in mineral oil, 363 mg) to a solution of (S)-(+)-2,2-dimethyl-1,3-dioxolane-4-methanol (1a, 200 mg, 1.51 mmol) in anhydrous DMF (5 mL) on ice. After stirring for 10 min, benzyl bromide (BnBr; 360 μL, 3.03 mmol) was added slowly to the reaction mixture. The reaction was brought to r.t. after 30 min and stirred for an additional 8 h. NaH was quenched by slowly adding methanol to the reaction vessel at 0 °C. After removal of solvent, the residue was dissolved in DCM and washed with water (1X) and the resulting water layer was extracted with DCM (3X). The combined DCM layers were washed with brine and dried over Na2SO4. After removal of solvent, the residue was purified by flash column chromatography (hexane:EtOAc = 19:1) to yield the title compound 22 (330 mg, 98%). 1H NMR (400 MHz, CDCl3) δ 7.36–7.30 (m, 4H), 7.29–7.24 (m, 1H), 4.62–4.51 (m, 2H), 4.33–4.25 (m, 1H), 4.04 (dd, J = 6.4, 8.3, 1H), 3.73 (dd, J = 6.3, 8.3, 1H), 3.54 (dd, J = 5.7, 9.8, 1H), 3.46 (dd, J = 5.5, 9.8, 1H), 1.40 (s, 3H), 1.35 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 136.87, 127.39, 126.73, 126.71, 108.39, 73.68, 72.47, 70.00, 65.81, 25.74, 24.35. MS (ESI) calculated for C13H18O3, m/z 222.13, found 245.13 (M+Na)+.
Synthesis of compound 23 ((R)-((2,3-bis(hexadecyloxy)propoxy)methyl)benzene)
Deprotection of the acetonide protecting group of 22 was achieved by stirring the compound (180 mg, 0.81 mmol) in 70% acetic acid (AcOH:H2O = 7:3) as described for the syntheses of 4a and 4b. After complete removal of acetic acid and water under reduced pressure, the residue was dissolved in DMF (5 mL) and cooled to 0 °C. The resulting diol was O-alkylated with 1-iodohexadecane (1.14g, 3.24 mmol) in the presence of NaH (60 % in mineral oil, 770 mg) in anhydrous DMF (8 mL) at 0 °C. The reaction mixture was stirred for 30 min at 0 °C and the temperature was then raised to r.t. for 8 h. The excess NaH was quenched by slowly adding methanol to the reaction at 0 °C. After removal of solvent, the residue was dissolved in DCM and washed with water (1X). And the resulting water layer was extracted with DCM (3X). The combined DCM layers were washed with brine and dried over Na2SO4. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 49:1) to afford a white solid (470 mg, 92%). 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J = 4.3, 4H), 7.28–7.24 (m, 1H), 4.54 (s, 2H), 3.62–3.44 (m, 7H), 3.41 (t, J = 6.7, 2H), 1.60–1.47 (m, 4H), 1.26 (d, 52H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 138.42, 128.31, 127.58, 77.88, 73.34, 71.66, 70.79, 70.62, 70.24, 31.93, 30.09, 29.72, 29.66, 29.52, 29.38, 26.10, 22.70, 14.14. MS (ESI) calculated for C42H78O3, m/z 630.60, found 653.61 (M+Na)+.
Synthesis of compound 24 ((R)-2,3-bis(hexadecyloxy)propyl 4-methylbenzenesulfonate)
O-debenzylation followed by O-tosylation of 23 en route to the iodo intermediate 25 was obtained as follows: Hydrogenolysis of 23 (500 mg, 0.79 mmol) dissolved in a mixture of 20 mL MeOH/DCM (1:1) was carried out using Pd(OH)2/C (500 mg) and H2 at 55 psi in a Parr apparatus for 8 h. The catalyst was removed by filtration over celite, and the solvent was removed to afford the O-debenzylated intermediate as a white solid (425 mg, ~100%). After drying completely, the solid was dissolved in anhydrous CH3CN (15 mL), followed by addition of pyridine (320 μL, 3.96 mmol) and a catalytic amount of DMAP. Then p-TsCl (755 mg, 3.96 mmol) was added and the reaction mixture was heated at 70 °C for 12 h. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 17:1) to afford the title compound 24 (527 mg, 96%). 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.2, 2H), 7.31 (d, J = 8.5, 2H), 4.13 (dd, J = 4.1, 10.3, 1H), 4.00 (dd, J = 5.8, 10.3, 1H), 3.62–3.54 (m, 1H), 3.47–3.41 (m, 2H), 3.38 (t, 2H), 3.33 (t, J = 6.7, 2H), 2.42 (s, 3H), 1.45 (s, 4H), 1.23 (s, 52H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 143.66, 131.93, 128.75, 126.98, 75.16, 70.75, 69.80, 68.64, 68.30, 30.91, 28.85, 28.69, 28.65, 28.63, 28.60, 28.50, 28.46, 28.44, 28.35, 25.01, 24.94, 21.68, 20.62, 13.11. MS (ESI) calculated for C42H78O5S, m/z 694.56, found 717.52 (M+Na)+.
Synthesis of compound 25 ((R)-1-(1-(hexadecyloxy)-3-iodopropan-2-yloxy)hexadecane)
The O-tosyl derivative 24 was converted to the iodo derivative 25. Iodine (310 mg, 12.23 mmol) and potassium iodide (KI; 2.03 g, 12.23 mmol) were added to a solution of 25 (850 mg, 1.22 mmol) in anhydrous DMF (15 mL) and the reaction was stirred at 80 °C for 24 h. After removal of DMF under vacuum, the residue was diluted with DCM and washed with water (1X), brine (1X) and dried over Na2SO4. The solvent was removed and the residue was purified by flash column chromatography (hexane:EtOAc = 49:1) to yield the title compound 25 (700 mg, 88%). 1H NMR (400 MHz, CDCl3) δ 3.51 (m, 3H), 3.45–3.39 (m, 3H), 3.37–3.24 (m, 3H), 1.60–1.50 (m, 4H), 1.23 (s, 52H), 0.86 (t, J = 6.9, 6H). 13C NMR (126 MHz, CDCl3) δ 76.40, 70.86, 70.73, 69.25, 30.91, 28.91, 28.69, 28.66, 28.65, 28.61, 28.45, 28.43, 28.35, 25.09, 25.07, 21.68, 13.11, 6.39. MS (ESI) calculated for C35H71IO2, m/z 650.45, found 673.42 (M+Na)+.
Synthesis of compound 26 ((6R,10R)-methyl 10-(hexadecyloxy)-2,2-dimethyl-4-oxo-3,12-dioxa-8-thia-5-azaoctacosane-6-carboxylate)
S-alkylation ofBoc- L-Cys-OMe with 25 was carried out as follows: Boc-L-Cys-OMe (752 mg, 3.20 mmol) and TEA (445 μL, 3.20 mmol) were added to the solution of 25 (260 mg, 0.40 mmol) in anhydrous DMF (8 mL). The reaction was stirred at 85 °C for 2h. After removal of DMF under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 19:1) to afford the title compound 26 (291 mg, 96%). 1H NMR (500 MHz, CDCl3) δ 5.53 (d, J = 8.0, 1H), 4.51 (s, 1H), 3.73 (s, 3H), 3.48 (m, 5H), 3.40 (m, 6.7, 2H), 3.07–2.93 (m, 2H), 2.75 (dd, J = 4.9, 13.7, 1H), 2.63 (dd, J = 6.0, 13.7, 1H), 1.57–1.49 (m, 4H), 1.42 (s, 9H), 1.31–1.20 (m, 52H), 0.85 (t, J = 7.0, 6H). 13C NMR (126 MHz, CDCl3) δ 170.63, 154.23, 28.97, 77.55, 70.68, 70.28, 69.55, 52.47, 51.47, 34.44, 33.51, 30.90, 28.97, 28.69, 28.64, 28.35, 27.28, 25.08, 25.05, 21.68, 13.13. MS (ESI) calculated for C44H87O6S, m/z 757.63, found 780.61 (M+Na)+.
Synthesis of compound 27 ((5S,8R,12R)-methyl 8-(tert-butoxycarbonylamino)-12-(hexadecyloxy)- 2,2-dimethyl-7-oxo-3,14-dioxa-10-thia-6-azatriacontane-5-carboxylate)
Compound 26 was obtained as a viscous oil (228 mg, 64%) via procedures similar to those described for the syntheses of compounds 12a-d. 1H NMR (500 MHz, CDCl3) δ 5.60 (s, 1H), 4.64 (d, J = 8.1, 1H), 4.30 (s, 1H), 3.79 (dd, J = 3.0, 9.1, 1H), 3.71 (s, 3H), 3.60–3.47 (m, 7H), 3.45–3.35 (m, 2H), 2.95 (dd, J = 5.8, 13.9, 1H), 2.87 (dd, J = 6.9, 13.8, 1H), 2.78 (s, 2H), 1.58–1.49 (m, 4H), 1.43 (s, 6H), 1.30–1.20 (m, 52H), 1.11 (s, 8H), 0.85 (t, J = 7.0, 6H). 13C NMR (126 MHz, CDCl3) δ 169.58. 169.46, 154.24, 77.23, 72.40, 70.68, 70.27, 69.54, 60.72, 52.13, 51.33, 34.57 33.34, 30.90, 28.95, 28.69, 28.64, 28.62, 28.50, 28.35, 27.28, 26.26, 25.08, 25.03, 21.68, 13.13. MS (ESI) calculated for C51H100N2O8S, m/z 900.72 found 923.69 (M+Na)+.
Synthesis of compound 28 ((S)-methyl 2-((R)-2-amino-3-((R)-2,3-bis(hexadecyloxy)propylthio)propanamido)-3-hydroxypropanoate; trifluoroacetate)
N-Boc deprotection with neat TFA was performed as described for the synthesis of 6a and 6b. 1H NMR (400 MHz, CDCl3) δ 8.30 (s, 1H), 4.64 (s, 1H), 4.32 (s, 1H), 4.00–3.82 (m, 1H), 3.74 (s, 3H), 3.61–3.48 (m, 3H), 3.47–3.37 (m, 3H), 3.18–3.07 (m, 1H), 3.01–2.89 (m, 1H), 2.86–2.66 (m, 1H), 1.59–1.47 (m, 4H), 1.23 (s, 48H), 0.86 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 170.05, 77.85, 71.80, 71.12, 70.91, 61.75, 55.32, 53.06, 52.74, 34.98, 34.32, 31.93, 29.73, 29.68, 29.55, 29.51, 29.42, 29.38, 26.06, 25.87, 22.69, 14.12. MS (ESI) calculated for C42H84N2O6S, m/z 744.61 found 645.61 (M+H)+.
Synthesis of compound 30 ((R)-methyl 3-hydroxy-2-palmitamidopropanoate)
N-palmitoylation of 29 was carried out as follows: H-D-Ser-OMe•HCl (29) was N-acylated by first generating the free-base by the addition of 10 mL of sat. aqueous NaHCO3 solution to a solution of 29 (200 mg, 1.04 mmol, Bachem) in EtOAc (10 mL), and then adding palmitoyl chloride (377 μL, 1.24 mmol) dropwise to the reaction. The reaction mixture, after stirring for 1 h, was extracted with EtOAc (3X). The combined EtOAc layers were washed with brine and dried over Na2SO4. After removal of solvent under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 7:3) to afford the title compound as a white solid (286 mg, 77%). 1H NMR (500 MHz, CDCl3) δ 6.61 (s, 1H), 4.65–4.58 (m, 1H), 3.92 (dd, J = 3.6, 11.1, 1H), 3.83 (d, J = 11.1, 1H), 3.73 (s, 3H), 2.25–2.18 (m, 3H), 1.64–1.54 (m, 2H), 1.27–1.18 (m, 24H), 0.83 (t, J = 6.9, 3H). 13C NMR (126 MHz, CDCl3) d173.98, 171.65, 63.23, 54.59, 52.68, 36.48, 31.82, 29.70, 29.67, 29.66, 29.64, 29.52, 29.37, 29.36, 29.26, 25.59, 22.69, 14.12. MS (ESI) calculated for C20H39NO4, m/z 357.29, found 358.30 (M+H)+ and 380.28 (M+Na)+.
Synthesis of compound 31 ((R)-methyl 2,2-dimethyl-3-palmitoyloxazolidine-4-carboxylate)
Compound 30 was acetonide protected with 2,2-dimethyoxypropane (2,2-DMP; 24 mL, 195.8 mmol) and pyridinium p-toluenesulfonate (PPTS; 281 mg, 1.12 mmol) by adding the above reagents to compound 30 (2.0 g, 5.59 mmol) in toluene (25 mL), and the reaction refluxed at 90 °C for 22 h. After concentrating under reduced pressure, the residue was purified by flash column chromatography (hexane:EtOAc = 9:1) to afford the title compound as a white solid (2.0 g, 90%). 1H NMR (500 MHz, CDCl3) δ 4.42 (dd, J = 1.3, 6.3, 1H), 4.20 (dd, J = 1.4, 9.3, 1H), 4.14 (dd, J = 6.3, 9.3, 1H), 3.78 (s, 3H), 2.18–2.03 (m, 2H), 1.67 (s, 2H), 1.63–1.57 (m, 2H), 1.54 (s, 3H), 1.28–1.20 (m, 24H), 0.85 (t, J = 7.0, 3H). 13C NMR (126 MHz, CDCl3) δ 171.15, 170.17, 96.62, 66.99, 59.49, 52.89, 35.64, 31.93, 29.70, 29.66, 29.64, 29.56, 29.48, 29.37, 29.25, 25.15, 24.57, 23.57, 22.70, 14.13. MS (ESI) calculated for C23H43NO4, m/z 397.59, found 398.33 (M+H)+ and 420.31 (M+Na)+.
Synthesis of compound 32 ((S)-1-(4-(hydroxymethyl)-2,2-dimethyloxazolidin-3-yl)hexadecan-1-one)
The ester functional group of 31 was reduced to the primary alcohol by first diluting lithium borohydride (LiBH4; 2.0 M in THF, 1.89 mL, 3.77 mmol) in 5 mL of THF (5 mL) cooled to 0°C for 30 min. Comound 31 (500 mg, 1.26 mmol), dissolved in THF (5 mL) was then added dropwise, after which the reaction was stirred at 0 °C for 3 h and then maintained at r.t. for an additional 6 h. After quenching the unreacted LiBH4 with water, the reaction mixture was extracted with EtOAc (3X) and the combined EtOAc layers were washed by brine and dried over Na2SO4. The solvent was removed under vacuum and the resulting residue was purified by flash column chromatography (hexane:EtOAc = 4:1) to yield a white solid (489 mg, 81%). 1H NMR (500 MHz, CDCl3) δ 4.40 (s, 1H), 4.04 (d, J = 9.0, 1H), 4.01–3.88 (m, 2H), 3.64 (d, J = 10.5, 2H), 2.39–2.26 (m, 2H), 1.62 (s, 2H), 1.59 (d, J = 17.8, 4H), 1.52 (s, 2H), 1.25 (d, J = 22.7, 24H), 0.86 (t, J = 6.9, 3H). 13C NMR (126 MHz, CDCl3) δ 170.41, 95.27, 65.43, 62.93, 58.55, 35.49, 31.94, 29.71, 29.68, 29.65, 29.54, 29.38, 26.83, 25.20, 22.93, 22.71, 14.15. MS (ESI) calculated for C23H43NO4, m/z 369.58, found 370.33 (M+H)+, 392.31 (M+Na)+.
Synthesis of compound 33 ((R)-1-(4-(iodomethyl)-2,2-dimethyloxazolidin-3-yl)hexadecan-1-one)
Compound 33 (151 mg, 97%) was obtained following the procedure described for the synthesis of 9. 1H NMR (400 MHz, CDCl3) δ 4.17–4.10 (m, 2H), 4.03–3.96 (m, 1H), 3.26 (dd, J = 9.9, 11.4, 1H), 3.16 (dt, J = 2.5, 9.9, 1H), 2.34–2.16 (m, 2H), 1.67–1.62 (m, 3H), 1.53–1.46 (m, 3H), 1.39–1.16 (m, 26H), 0.86 (t, J = 6.9, 3H). MS (ESI) calculated for C22H42INO2m/z 479.23, found 502.21 (M+Na)+.
General procedure for S-alkylation: Synthesis of compound 34 (methyl 2-(tert-butoxycarbonylamino)-3-(((R)-2,2-dimethyl-3-palmitoyloxazolidin-4-yl)methylthio)propanoate)
To a solution of 33 (300 mg, 0.63 mmol) in anhydrous DMF (8 mL) was added TEA (872 μL, 6.26mmol), followed by Boc-L-Cys-OMe (1.03 g, 4.38 mmol). The reaction solution was stirred at 85°C for 2 h. After removal of DMF under vacuum, the residue was purified by flash column chromatography (hexane:EtOAc = 9:1) to afford 34 as a viscous oil (356 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 5.32–5.24 (m, 1H), 4.61–4.48 (m, 1H), 4.03 (d, J = 9.2, 1H), 3.95–3.89 (m, 1H), 3.87–3.81 (m, 1H), 3.76 (s, 3H), 3.07–2.92 (m, 2H), 2.79–2.63 (m, 2H), 2.36–2.17 (m, 2H), 1.70–1.58 (m, 6H), 1.43 (s, 9H), 1.36–1.17 (m, 27H), 0.86 (t, J = 6.9, 3H). 13C NMR (126 MHz, CDCl3) δ 171.10, 169.73, 155.02, 95.54, 80.37, 66.36, 57.42, 53.39, 52.79, 35.86, 35.49, 35.46, 31.92, 29.70, 29.69, 29.68, 29.66, 29.59, 29.37, 28.27, 26.96, 25.08, 22.90, 22.70, 14.15. MS (ESI) calculated for C31H158N2O6S, m/z 586.40, found 609.39 (M+Na)+.
Synthesis of compound 35 ((S)-methyl 3-tert-butoxy-2-((R)-2-(tert-butoxycarbonylamino)-3-(((R)- 2,2-dimethyl-3-palmitoyloxazolidin-4-yl)methylthio)propanamido)propanoate)
Compound 34 (400 mg, 0.68 mmol) in 15 mL THF was de-esterified using procedures as described for the syntheses of 12a-d with the exception that barium hydroxide octahydrate (645 mg, 2.04 mmol, in 6 mL H2O at 60 °C, 1h), and not LiOH was used; we elected to use the much milder Ba(OH)2 conditions in view of potential lability of the amide bond in 34. Subsequent coupling to H-L-Ser(tBu)-OMe•HCl was carried out as described earlier for 12a-d. Compound 35 was obtained as a viscous oil (274 mg, 55%). 1H NMR (400 MHz, CDCl3) δ 7.03 (d, J = 8.2, 1H), 5.40 (s, 1H), 4.63 (d, J = 8.2, 1H), 4.35–4.24 (m, 1H), 4.11–4.02 (m, 1H), 3.96–3,87 (m, 2H), 3.81 (dd, J = 2.8, 9.1, 1H), 3.74–3.69 (m, 3H), 3.54 (dd, J = 3.2, 9.1, 1H), 3.05–2.94 (m, 1H), 2.93–2.79 (m, 2H), 2.77–2.67 (m, 1H), 2.38–2.23 (m, 2H), 1.61 (s, 5H), 1.53–1.48 (m, 3H), 1.31–1.20 (m, 24H), 1.12 (s, 9H), 0.85 (t, J = 6.8, 3H). 13C NMR (126 MHz, CDCl3) δ 173.80, 170.63, 170.51, 170.02, 95.57, 80.38, 73.69, 73.58, 66.43, 63.58, 61.68, 61.61, 57.45, 53.15, 52.50, 52.48, 51.49, 36.71, 35.40, 35.13, 34.94, 32.92, 31.93, 30.95, 29.69, 29.66, 29.65, 29.54, 29.52, 29.38, 29.37, 29.31, 28.32, 28.28, 27.30, 27.28, 26.96, 25.71, 25.16, 22.96, 22.70. MS (ESI) calculated for C38H71N3O8S, m/z 729.50, found 752.48 (M+Na)+.
Synthesis of compound 36 ((5S,8R,12R)-methyl 8-(tert-butoxycarbonylamino)-2,2-dimethyl-7,14-dioxo-12-(palmitoyloxymethyl)-3-oxa-10-thia-6,13-diazanonacosane-5-carboxylate)
The general procedure of acetonide deprotection and subsequent O-palmitoylation described earlier for the synthesis of 11 was utilized. The reaction residue was purified by flash column chromatography (hexane:EtOAc = 3:1) to afford the intermediate as a colorless oil. (203 mg, 80%). 1H NMR (500 MHz, CDCl3) δ 6.21 (d, J = 7.9, 1H), 5.56 (d, J = 6.7, 1H), 4.68–4.58 (m, 1H), 4.43–4.31 (m, 2H), 4.21 (dd, J = 5.0, 11.3, 1H), 4.08 (dd, J = 4.9, 11.3, 1H), 3.81 (dd, J = 3.0, 9.1, 1H), 3.72 (s, 3H), 3.55 (dd, J = 3.3, 9.1, 1H), 3.01 (dd, J = 5.5, 13.9, 1H), 2.91–2.79 (m, 2H), 2.75–2.64 (m, J = 5.4, 14.0, 1H), 2.35–2.26 (m, 2H), 2.23–2.10 (m, 2H), 1.63–1.55 (br s, 4H), 1.43 (s, 6H), 1.30–1.19 (m, 52H), 1.14–1.10 (m, 8H), 0.85 (t, J = 7.0, 6H). 13C NMR (126 MHz, CDCl3) δ 173.90, 173.29, 170.59, 170.47, 155.40, 80.21, 73.57, 64.46, 61.65, 53.72, 53.14, 52.47, 48.83, 36.70, 34.12, 31.93, 29.71, 29.67, 29.55, 29.50, 29.46, 29.42, 29.37, 29.30, 29.27, 29.18, 28.31, 27.27, 25.65, 24.88, 22.70, 14.15. MS (ESI) calculated for C51H97N3O9S, m/z 927.69, found 950.68 (M+Na)+.
Synthesis of compound 37 ((R)-3-((R)-2-amino-3-((S)-3-hydroxy-1-methoxy-1-oxopropan-2-ylamino)-3-oxopropylthio)-2-palmitamidopropyl palmitate; trifluoroacetate)
The previously described N-Boc/O-tBu deprotection procedure (see syntheses of 14a-d) was used. The title compound was obtained as a flaky yellow solid (99%). 1H NMR (500 MHz, CDCl3) δ 8.46 (s, 1H), 6.57 (s, 1H), 4.73–4.59 (m, 1H), 4.53–4.34 (m, 1H), 4.34–4.23 (m, 1H), 4.23–4.15 (m, 1H), 4.15–4.06 (br s, 1H), 4.03–3.84 (m, 2H), 3.79–3.67 (m, 3H), 3.21–2.93 (m, 2H), 2.90–2.63 (m, 2H), 2.37–2.10 (m, 4H), 1.64–1.49 (m, 4H), 1.32–1.16 (m, 48H), 0.85 (t, J = 6.9, 6H). 13C NMR (126 MHz, CDCl3) δ 174.32, 170.36, 170.21, 64.40, 53.13, 52.76, 52.64, 50.29, 49.20, 36.61, 34.02, 31.94, 29.75, 29.73, 29.72, 29.70, 29.68, 29.60, 29.55, 29.38, 29.34, 29.28, 29.23, 29.20, 29.12, 25.70, 24.85, 24.78, 22.70, 14.13. MS (ESI) calculated for C42H81N3O7S, m/z 771.58, found 772.59 (M+H)+ and 794.57 (M+Na)+.
Synthesis of compound 38 ((R)-methyl 2,2,11,11-tetramethyl-4,9-dioxo-3,10-dioxa-5,8-diazadodecane-6-carboxylate)
D-2,3-diaminopropionic acid monohydrochloride (Sigma-Aldrich, 500 mg, 3.56 mmol) was N-boc protected by Boc2O (2.33 g, 10.67 mmol) in the presence of TEA (1.49 mL, 10.67 mmol) in DCM (10 mL). After stirring at r.t. for 2 h, the solvent was removed under vacuum and the residue was purified by silica flash chromatography (DCM:MeOH = 17:3). The free carboxyl group of the intermediate was converted to methyl ester by dissolving it in anhydrous DCM (10 mL), followed by sequential addition of EDCI (1.11 g, 5.81 mmol), 1-hydroxybenzotriazole hydrate (HOBt; 785 mg, 5.81 mmol), TEA (810 μL, 5.81 mmol), anhydrous MeOH (353 μL, 8.71 mmol) and a catalytic amount of DMAP. After stirring at r.t. for 10 h, solvent was removed from the reaction under vacuum and the reaction residue was dissolved in DCM, washed with water (1X), brine (1X) and dried over Na2SO4. The residue was purified by silica flash chromatography (hexane:EtOAc = 22: 3) to afford the title compound 38 (702 mg, 62%). 1H NMR (400 MHz, CDCl3) δ 5.40 (s, 1H), 4.82 (s, 1H), 4.32 (s, 1H), 3.73 (s, 3H), 3.56–3.40 (m, 2H), 1.44–1.39 (m, 18H). 13C NMR (126 MHz, CDCl3) δ 171.30, 156.13, 155.41, 80.14, 79.85, 54.17, 52.65, 42.40, 30.95, 28.29, 28.28. MS (ESI) calculated for C14H26N2O6, m/z 318.18, found 341.17 (M+Na)+ and 659.35 (M+M+Na)+.
Synthesis of compound 39 ((R)-tert-butyl 3-hydroxypropane-1,2-diyldicarbamate)
Sodium borohydride (NaBH4; 285 mg, 7.54 mmol) was added to a solution of 39 (600mg, 2.20mmol) for reducing the ester to the corresponding primary alcohol. We used NaBH4 as an alternative to LiBH4 described in the synthesis of 32 with significantly better yields. The reaction mixture was refluxed at 75 °C and then anhydrous methanol (3 mL) was added dropwise over a period of 1 h. After refluxing for an additional 3 h, the reaction was acidified to pH 2.0 with 1 M HCl and THF was removed under vacuum. The residue was extracted with DCM (3X) and the combined DCM layers were washed with brine and dried over Na2SO4. The residue was purified by silica flash chromatography (hexane:EtOAc = 3:1) to yield 39 as a colorless oil (525 mg, 96%). 1H NMR (400 MHz, CDCl3) δ 5.10 (s, 1H), 4.97 (s, 1H), 3.76–3.45 (m, 4H), 3.41–3.16 (m, 2H), 1.58–1.36 (m, 18H). 13C NMR (126 MHz, CDCl3) δ 157.75, 155.74, 80.41, 79.62, 61.56, 52.27, 40.12, 30.95, 28.36, 28.29. MS (ESI) calculated for C13H16N2O5, m/z 290.18, found 313.18 (M+Na)+ and 603.37 (M+M+Na)+.
Synthesis of compound 40 ((R)-tert-butyl 3-iodopropane-1,2-diyldicarbamate)
A procedure essentially identical to that described for the synthesis of 9 was followed; however the reaction was carried out at 0 °C and then warming to r.t. instead of at 90 °C in an effort to minimize side-reactions and improve yield. No significant improvements in yield, unfortunately, were obtained. Compound 40 was obtained as a white solid (489 mg, 71%). 1H NMR (400 MHz, CDCl3) δ 5.30 (s, 1H), 4.83 (s, 1H), 3.62 (s, 1H), 3.44–3.21 (m, 4H), 1.46 (s, 18H). 13C NMR (126 MHz, CDCl3) δ 155.76, 154.44, 79.00, 50.54, 43.16 29.92, 27.31, 27.19, 7.69. MS (ESI) calculated for C13H25N2O4, m/z 400.09, found 423.09 (M+Na)+ and 823.18 (M+M+Na)+.
Synthesis of compound 41 ((6R,10R)-methyl 10-(tert-butoxycarbonylamino)-1,1,1-trichloro-15,15-dimethyl-4,13-dioxo-3,14-dioxa-8-thia-5,12-diazahexadecane-6-carboxylate)
Orthogonal protection of the amines of the 2,3-diaminopropionic acid fragment, and of the amine of the cysteine unit was necessary. We elected to utilize Troc-L-Cys-OMe, which was obtained from L-cystine dimethylester (Sigma-Aldrich) as follows. 2,2,2-trichloroethyl chloroformate (805 μL, 5.86 mmol) was slowly added to a stirring solution of L-cystine dimethylester dihydrochloride (500 mg, 1.47 mmol) in anhydrous DCM (10 mL) and pyridine (10 mL) cooled to 0 °C. The reaction was brought to r.t. and stirred for 8 h. After removal of solvent under vacuum, the residue was purified by flash chromatography (hexane:EtOAc = 4:1) to afford N-Troc cystine dimethylester as a white solid. The disulfide bond of the N-Troc-protected cystine ester was reduced by dissolving the solid in THF (10 mL), and adding tributylphosphine (Bu3P; 542 μL, 2.20 mmol) and H2O (132 μL, 7.33 mmol). The reaction was stirred at r.t. for 2 h. After removal of solvent, the residue was purified by flash column chromatography (hexane:EtOAc = 9:1) to afford the title compound as a oil (410 mg, 90%). The characterization data for Troc-L-Cys-OMe (Scheme 6, step d) is provided in the Supporting Information. S- alkylation of Troc L-Cys-OMe with 40 was performed as described earlier for 26. Compound 41 was obtained as a mixture with the oxidized N-Troc cystine dimethylester as ascertained by LC-MS and 1H NMR. The Rfs of both components were virtually identical, rendering the isolation of 41 very difficult by flash chromatography. Reverse-phase HPLC employing several solvent combinations also did not yield good separations. We reasoned, however, that N-Troc cystine dimethylester would be inert to both N-Boc deprotection and N-acylation conditions that were to follow (see synthesis of 42 below). We therefore proceeded without further purification.
Synthesis of compound 42 ((6R,10R)-methyl 1,1,1-trichloro-4,13-dioxo-10-palmitamido-3-oxa-8-thia-5,12-diazaoctacosane-6-carboxylate)
A crude sample of 41 (200 mg, 0.34 mmol) was dissolved in excess dry TFA and stirred at r.t. for 30 min to effect N-Boc deprotection on the 2,3-diaminopropionic acid fragment. Excess TFA was removed by purging nitrogen. The resulting diamino intermediate was coupled with palmitic acid (264 mg, 1.03 mmol) using EDCI (197 mg, 1.03 mmol), HOBt (139 mg, 1.03 mmol) and TEA (287 mL, 2.06 mmol), and a catalytic amount of DMAP in anhydrous DMF (8 mL). The reaction was stirred at 60 °C for 10 h. After DMF was removed under reduced pressure, the residue was dissolved with DCM and washed with water (1X), brine (1X) and dried over Na2SO4. The residue was purified by silica flash chromatography (hexane:EtOAc = 39:11) to afford the title compound 42 (185 mg, 64%). 1H NMR (500 MHz, CDCl3) δ 6.90 (d, J = 6.5, 1H), 6.24–6.14 (m, 1H), 4.82–4.73 (m, 1H), 4.73–4.63 (m, 1H), 4.62–4.54 (m, 1H), 3.98 (br s, 1H), 3.76 (d, J = 4.3, 3H), 3.53–3.43 (m, 1H), 3.41–3.30 (m, 1H), 3.12–2.97 (m, 2H), 2.92–2.78 (m, 2H), 2.57–2.43 (m, 1H), 2.23–2.09 (m, 4H), 1.63–1.50 (m, 4H), 1.22 (s, 48H), 0.85 (t, J = 6.9, 6H). 13C NMR (126 MHz, CDCl3) δ 173.07, 171.90, 168.27, 151.86, 92.87 72.24, 52.01, 50.44, 48.60, 34.42, 34.16, 32.08, 31.71, 29.48, 27.27, 27.24, 27.22, 27.10, 27.07, 26.92, 26.85, 23.17, 20.25, 11.69. MS (ESI) calculated for C42H78Cl3N3O6S, m/z 857.47, found 880.45, 881.45, 882.45 (M+Na+ with expected chlorine isotopic mass-spectral envelope).
Synthesis of compound 43 ((6R,10R)-methyl 2,2-dimethyl-4,13-dioxo-10-palmitamido-3-oxa-8-thia-5,12-diazaoctacosane-6-carboxylate)
The base-labile N-Troc protecting group on the cysteine unit had to be converted to the N-Boc derivative in order to carry out subsequent base-catalyzed de-esterfication step (see synthesis of 44 below). Accordingly, 42 (350mg, 0.41 mmol) was dissolved in THF (3 mL) and zinc dust (266 mg, 4.07 mmol), AcOH (15 mL) and H2O (1 mL) was added to the reaction. After stirring at r.t. for 1 h, zinc was filtered on celite and the filtrate was concentrated under reduced pressure. Reprotection with Boc2O was achieved by dissolving the completely dried intermediate in DCM and the addition of Boc2O (444 mg, 2.04 mmol) in the presence of TEA (284 μL, 2.04 mmol), and stirring the reaction mixture at r.t. for 1h. After the solvent was removed, the residue was purified by silica flash chromatography (hexane:EtOAc = 7:3) to yield the desired product 43 as a white solid (217 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 6.81 (s, 1H), 5.43 (s, 1H), 4.55 (s, 1H), 4.03 (s, 1H), 3.82–3.77 (m, 3H), 3.53 (s, 1H), 3.42 (s, 1H), 3.11–2.86 (m, 3H), 2.30–2.13 (m, 4H), 1.69–1.56 (m, 4H), 1.54–1.44 (m,7H), 1.27 (s, 48H). 13C NMR (126 MHz, CDCl3) δ 173.14, 141.89, 169.32, 169.22, 78.11, 72.52, 51.39, 50.52, 48.69, 48.31, 40.02, 39.64, 34.60, 34.46, 33.77, 33.19, 32.16, 29.76, 27.55, 27.52, 27.50, 27.38, 27.36, 27.21, 27.17, 27.14, 27.12, 26.13, 23.57, 23.50, 23.48, 23.37, 20.53. MS (ESI) calculated for C44H85N3O6S, m/z 783.62, found 806.61 (M+Na)+.
Synthesis of compound 44 ((5S,8R,12R)-methyl 8-(tert-butoxycarbonylamino)-2,2-dimethyl-7,15-dioxo-12-palmitamido-3-oxa-10-thia-6,14-diazatriacontane-5-carboxylate)
De-esterification followed by serine coupling was carried out as described for the synthesis of 35. (75%) 1H NMR (400 MHz, CDCl3) δ 6.91 (s, 1H), 6.52 (s, 1H), 5.52 (s, 1H), 4.68–4.61 (m, 1H), 3.83–3.77 (m, 1H), 3.74–3.69 (m, 3H), 3.59–3.51 (m, 1H), 3.50–3.32 (m, 2H), 2.98–2.83 (m, 2H), 2.23–2.09 (m, 4H), 1.65–1.51 (m, 4H), 1.46–1.40 (m, 8H), 1.22 (s, 48H), 1.14–1.09 (m, 9H), 0.85 (t, J = 6.8, 6H). 13C NMR (126 MHz, CDCl3) δ 174.15, 173.17, 169.65, 169.56, 154.54, 79.12, 72.61, 60.69, 52.18, 51.40, 50.55, 41.27, 35.75, 35.61, 30.91, 29.92, 28.69, 28.66, 28.65, 28.54, 28.52, 28.39, 28.38, 28.35, 28.32, 28.30, 27.32, 27.29, 26.26, 24.76, 24.67, 21.67, 13.11. MS (ESI) calculated for C51H98N4O8S, m/z 926.71, found 949.70 (M+Na)+.
Synthesis of compound 45 ((S)-methyl 2-((R)-2-amino-3-((R)-2,3-dipalmitamidopropylthio)- propanamido)-3-hydroxypropanoate; trifluoroacetate)
Compound 45 was deprotected with TFA as described earlier. The title compounds were obtained as a flaky yellow solid (99%). 1H NMR (500 MHz, CDCl3) δ 7.21–7.11 (m, 1H), 7.20–7.11 (m, 1H), 4.70–4.54 (m, 1H), 4.51–4.37 (m, 1H), 3.90 (s, 2H), 3.71 (s, 3H), 3.49–3.35 (m, 1H), 3.33–3.22 (m, 1H), 3.21–3.01 (m, 1H), 2.80–2.53 (m, 1H), 2.15 (s, 3H), 1.53 (s, 3H), 1.25 (d, J = 21.5, 49H), 0.85 (t, J = 6.9, 6H). 13C NMR (126 MHz, CDCl3) δ 132.65, 131.11, 129.02, 68.37, 52.91, 38.92, 36.93, 36.56, 36.55, 32.15, 30.56, 30.37, 29.97, 29.88, 29.83, 29.76, 29.68, 29.60, 29.56, 29.54, 29.51, 29.47, 29.46, 29.13, 25.98, 25.86, 25.77, 23.94, 23.20, 22.92, 14.41, 14.34, 14.28, 11.17. MS (ESI) calculated for C42H82N4O6S, m/z 770.60, found 771.60 (M+H)+.
NF-κB induction
The induction of NF-κB was quantified using HEK-Blue-2™ cells as previously described by us.42 HEK-Blue™-2 cells (HEK293 cells stably transfected with TLR2, MD2, CD14, and sAP) were from InvivoGen (San Diego, CA), and were maintained in HEK-Blue™ Selection medium containing zeocin and normocin. Stable expression of secreted alkaline phosphatase (sAP) under control of NF-κB/AP-1 promoters is inducible by the TLR2 agonists, and extracellular sAP in the supernatant is proportional to NF-κB induction. HEK-Blue-2 cells were incubated at a density of ~105 cells/ml in a volume of 80 μl/well, in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluency was achieved, and subsequently graded concentrations of stimuli. sAP was assayed spectrophotometrically using an alkaline phosphatase-specific chromogen (present in HEK-detection medium as supplied by the vendor) at 620 nm.
Supplementary Material
Acknowledgments
This work was supported in part by NIH/NIAID contract HHSN272200900033C, and the University of Kansas Start-up Funds.
Abbreviations
- EDIPA
N, N-diisopropylethylamine
- IC50
concentration inducing half-maximal response
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-12
interleukin-12
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- PAM2CSK4
S-[2,3-bis-(palmitoyloxy)-(2RS)-propyl]-R-cysteinyl-S-seryl-S-lysyl-S-lysyl-S-lysyl-S-lysine
- PAMP
pathogen-associated molecular pattern
- PE
phycoerythrin
- PGN
peptidoglycan
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Supporting Information: 1H, 13C, and mass spectral data of all intermediates and final target compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Haux J. Infection and cancer. Lancet. 2001;358:155–156. doi: 10.1016/S0140-6736(01)05369-7. [DOI] [PubMed] [Google Scholar]
- 2.Bauduer F. Medicine in ancient Egypt, a fascinating level of modernity. Presse Med. 2001;30:1236–1239. [PubMed] [Google Scholar]
- 3.SPRUNT WH. Imhotep. N Engl J Med. 1955;253:778–780. doi: 10.1056/NEJM195511032531808. [DOI] [PubMed] [Google Scholar]
- 4.Coley WB. A Preliminary Note on the Treatment of Inoperable Sarcoma by the Toxic Product of Erysipelas. Post-graduate. 1893;8:278–286. [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway CA., Jr An ancient system of host defense. Curr Opin Immunol. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA, Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K. Functions of toll-like receptors: lessons from KO mice. C R Biol. 2004;327:581–589. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Kirschning CJ, Schumann RR. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr Top Microbiol Immunol. 2002;270:121–144. doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- 11.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007 doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi O, Akira S. Signaling pathways activated by microorganisms. Curr Opin Cell Biol. 2007;19:185–191. doi: 10.1016/j.ceb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 15.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–68. 357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 16.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;19:358, 2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A controlled field trial of the effectiveness of cholera and cholera El Tor vaccines in the Philippines. Preliminary report. Philippines Cholera Committee. Bull World Health Organ. 1965;32:603–625. [PMC free article] [PubMed] [Google Scholar]
- 18.Vollmer W, Holtje JV. The architecture of the murein (peptidoglycan) in gram-negative bacteria: vertical scaffold or horizontal layer(s)? J Bacteriol. 2004;186:5978–5987. doi: 10.1128/JB.186.18.5978-5987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dmitriev BA, Toukach FV, Holst O, Rietschel ET, Ehlers S. Tertiary structure of Staphylococcus aureus cell wall murein. J Bacteriol. 2004;186:7141–7148. doi: 10.1128/JB.186.21.7141-7148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burge RE, Fowler AG, Reaveley DA. Structure of the peptidogylcan of bacterial cell walls. I. J Mol Biol. 1977;117:927–953. doi: 10.1016/s0022-2836(77)80006-5. [DOI] [PubMed] [Google Scholar]
- 21.Baddiley J. Bacterial cell walls and membranes. Discovery of the teichoic acids. Bioessays. 1989;10:207–210. doi: 10.1002/bies.950100607. [DOI] [PubMed] [Google Scholar]
- 22.Coley J, Duckworth M, Baddiley J. The occurrence of lipoteichoic acids in the membranes of gram-positive bacteria. J Gen Microbiol. 1972;73:587–591. doi: 10.1099/00221287-73-3-587. [DOI] [PubMed] [Google Scholar]
- 23.Stadelmaier A, Morath S, Hartung T, Schmidt RR. Synthesis of the first fully active lipoteichoic acid. Angew Chem Int Ed Engl. 2003;42:916–920. doi: 10.1002/anie.200390243. [DOI] [PubMed] [Google Scholar]
- 24.Deininger S, Stadelmaier A, von Aulock S, Morath S, Schmidt RR, Hartung T. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J Immunol. 2003;170:4134–4138. doi: 10.4049/jimmunol.170.8.4134. [DOI] [PubMed] [Google Scholar]
- 25.Fischer W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol. 1994;183:61–76. doi: 10.1007/BF00277157. [DOI] [PubMed] [Google Scholar]
- 26.Kostyal DA, Butler GH, Beezhold DH. A 48-kilodalton Mycoplasma fermentans membrane protein induces cytokine secretion by human monocytes. Infect Immun. 1994;62:3793–3800. doi: 10.1128/iai.62.9.3793-3800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlradt PF, Meyer H, Jansen R. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry. 1996;35:7781–7786. doi: 10.1021/bi9602831. [DOI] [PubMed] [Google Scholar]
- 30.Metzger J, Jung G, Bessler WG, Hoffmann P, Strecker M, Lieberknecht A, Schmidt U. Lipopeptides containing 2-(palmitoylamino)-6,7-bis(palmitoyloxy) heptanoic acid: synthesis, stereospecific stimulation of B-lymphocytes and macrophages, and adjuvanticity in vivo and in vitro. J Med Chem. 1991;34:1969–1974. doi: 10.1021/jm00111a008. [DOI] [PubMed] [Google Scholar]
- 31.Lex A, Wiesmuller KH, Jung G, Bessler WG. A synthetic analogue of Escherichia coli lipoprotein, tripalmitoyl pentapeptide, constitutes a potent immune adjuvant. J Immunol. 1986;137:2676–2681. [PubMed] [Google Scholar]
- 32.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect Immun. 2005;73:5212–5216. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 35.Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem. 2001;276:22041–22047. doi: 10.1074/jbc.M010481200. [DOI] [PubMed] [Google Scholar]
- 36.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 2005;272:6354–6364. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 37.Spohn R, Buwitt-Beckmann U, Brock R, Jung G, Ulmer AJ, Wiesmuller KH. Synthetic lipopeptide adjuvants and Toll-like receptor 2--structure-activity relationships. Vaccine. 2004;22:2494–2499. doi: 10.1016/j.vaccine.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 38.Nahori MA, Fournie-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CR, Saint GI, Werts C. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol. 2005;175:6022–6031. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 39.Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. Lipopolysaccharides of Bacteroides fragilis, Chlamydia trachomatis and Pseudomonas aeruginosa signal via toll-like receptor 2. J Med Microbiol. 2004;53:735–740. doi: 10.1099/jmm.0.45598-0. [DOI] [PubMed] [Google Scholar]
- 40.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2008;83:692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 41.Heine H, Ulmer AJ. Recognition of bacterial products by toll-like receptors. Chem Immunol Allergy. 2005;86:99–119. doi: 10.1159/000086654. [DOI] [PubMed] [Google Scholar]
- 42.Kimbrell MR, Warshakoon H, Cromer JR, Malladi S, Hood JD, Balakrishna R, Scholdberg TA, David SA. Comparison of the immunostimulatory and proinflammatory activities of candidate Gram-positive endotoxins, lipoteichoic acid, peptidoglycan, and lipopeptides, in murine and human cells. Immunol Lett. 2008;118:132–141. doi: 10.1016/j.imlet.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt J, Welsch T, Jager D, Muhlradt PF, Buchler MW, Marten A. Intratumoural injection of the toll-like receptor-2/6 agonist ‘macrophage-activating lipopeptide-2’ in patients with pancreatic carcinoma: a phase I/II trial. Br J Cancer. 2007;97:598–604. doi: 10.1038/sj.bjc.6603903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grabiec A, Meng G, Fichte S, Bessler W, Wagner H, Kirschning CJ. Human but not murine toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J Biol Chem. 2004;279:48004–48012. doi: 10.1074/jbc.M405311200. [DOI] [PubMed] [Google Scholar]
- 45.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Prass W, Ringsdorf H, Bessler W, Wiesmuller KH, Jung G. Lipopeptides of the N-terminus of Escherichia coli lipoprotein: synthesis, mitogenicity and properties in monolayer experiments. Biochim Biophys Acta. 1987;900:116–128. doi: 10.1016/0005-2736(87)90283-5. [DOI] [PubMed] [Google Scholar]
- 47.Bessler WG, Cox M, Lex A, Suhr B, Wiesmuller KH, Jung G. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol. 1985;135:1900–1905. [PubMed] [Google Scholar]
- 48.Metzger J, Wiesmuller KH, Schaude R, Bessler WG, Jung G. Synthesis of novel immunologically active tripalmitoyl-S-glycerylcysteinyl lipopeptides as useful intermediates for immunogen preparations. Int J Pept Protein Res. 1991;37:46–57. doi: 10.1111/j.1399-3011.1991.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 49.Metzger J, Jung G. Mycoloylpeptides and other lipopeptide adjuvants from higher aldoketene dimers. Angew Chem Int Ed Engl. 1987;26:336–338. [Google Scholar]
- 50.Metzger J, Olma A, Leplawy MT, Jung G. Synthesis of a B-lymphocyte activating ?-methylserine containing lipopentapeptide. Z Naturforsch B. 1987;42:1197–1201. [Google Scholar]
- 51.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen TB, Suresh Kumar EV, Sil D, Wood SJ, Miller KA, Warshakoon HJ, Datta A, David SA. Controlling Plasma Protein Binding: Structural Correlates of Interactions of Hydrophobic Polyamine Endotoxin Sequestrants with Human Serum Albumin. Mol Pharm. 2008 doi: 10.1021/mp8001123. [DOI] [PubMed] [Google Scholar]
- 53.Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Vermeire S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- 54.Canto E, Ricart E, Monfort D, Gonzalez-Juan D, Balanzo J, Rodriguez-Sanchez JL, Vidal S. TNF alpha production to TLR2 ligands in active IBD patients. Clin Immunol. 2006;119:156–165. doi: 10.1016/j.clim.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Nara K, Kurokawa MS, Chiba S, Yoshikawa H, Tsukikawa S, Matsuda T, Suzuki N. Involvement of innate immunity in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2008;152:245–251. doi: 10.1111/j.1365-2249.2008.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albert EJ, Marshall JS. Aging in the absence of TLR2 is associated with reduced IFN-gamma responses in the large intestine and increased severity of induced colitis. J Leukoc Biol. 2008;83:833–842. doi: 10.1189/jlb.0807557. [DOI] [PubMed] [Google Scholar]
- 57.Seyberth T, Voss S, Brock R, Wiesmuller KH, Jung G. Lipolanthionine peptides act as inhibitors of TLR2-mediated IL-8 secretion. Synthesis and structure-activity relationships. J Med Chem. 2006;49:1754–1765. doi: 10.1021/jm050585d. [DOI] [PubMed] [Google Scholar]
- 58.Reschner A, Moretta A, Landmann R, Heberer M, Spagnoli GC, Padovan E. The ester-bonded palmitoyl side chains of Pam3CysSerLys4 lipopeptide account for its powerful adjuvanticity to HLA class I-restricted CD8+ T lymphocytes. Eur J Immunol. 2003;33:2044–2052. doi: 10.1002/eji.200323776. [DOI] [PubMed] [Google Scholar]
- 59.Lau YF, Deliyannis G, Zeng W, Mansell A, Jackson DC, Brown LE. Lipid-containing mimetics of natural triggers of innate immunity as CTL-inducing influenza vaccines. Int Immunol. 2006;18:1801–1813. doi: 10.1093/intimm/dxl114. [DOI] [PubMed] [Google Scholar]
- 60.Borsutzky S, Fiorelli V, Ebensen T, Tripiciano A, Rharbaoui F, Scoglio A, Link C, Nappi F, Morr M, Butto S, Cafaro A, Muhlradt PF, Ensoli B, Guzman CA. Efficient mucosal delivery of the HIV-1 Tat protein using the synthetic lipopeptide MALP-2 as adjuvant. Eur J Immunol. 2003;33:1548–1556. doi: 10.1002/eji.200323954. [DOI] [PubMed] [Google Scholar]
- 61.Borsutzky S, Ebensen T, Link C, Becker PD, Fiorelli V, Cafaro A, Ensoli B, Guzman CA. Efficient systemic and mucosal responses against the HIV-1 Tat protein by prime/boost vaccination using the lipopeptide MALP-2 as adjuvant. Vaccine. 2006;24:2049–2056. doi: 10.1016/j.vaccine.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 62.Zarbin PH, Arrigoni EB, Reckziegel A, Moreira JA, Baraldi PT, Vieira PC. Identification of male-specific chiral compound from the sugarcane weevil Sphenophorus levis. J Chem Ecol. 2003;29:377–386. doi: 10.1023/a:1022634012212. [DOI] [PubMed] [Google Scholar]
- 63.Zhou D, Lagoja IM, Rozenski J, Busson R, Van Aerschot A, Herdewijn P. Synthesis and properties of aminopropyl nucleic acids. Chembiochem. 2005;6:2298–2304. doi: 10.1002/cbic.200500170. [DOI] [PubMed] [Google Scholar]
- 64.Enders D, Lenzen A, Backes M, Janeck C, Catlin K, Lannou MI, Runsink J, Raabe G. Asymmetric total synthesis of the 1-epi-aglycon of the cripowellins A and B. J Org Chem. 2005;70:10538–10551. doi: 10.1021/jo0518093. [DOI] [PubMed] [Google Scholar]
- 65.Andre C, Bolte J, Demuynck C. Syntheses of 4-deoxy-D-fructose and enzymic affinity study. Tetrahedron Asymmetry. 1998;9:3737–3739. [Google Scholar]
- 66.Prestat G, Baylon C, Heck MP, Grasa GA, Nolan SP, Mioskowski C. New strategy for the construction of a monotetrahydrofuran ring in Annonaceous acetogenin based on a ruthenium ring-closing metathesis: application to the synthesis of Solamin. J Org Chem. 2004;69:5770–5773. doi: 10.1021/jo049505o. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez R, Ros A, Magriz A, Dietrich H, Lassaletta JM. Enantioselective synthesis of cis-α-substituted cycloalkanols and trans-cycloalkyl amines thereof. Tetrahedron. 2007;63:6755–6763. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.