Summary
Fingolimod (FTY720) is a first-in-class orally bioavailable compound that has shown efficacy in advanced clinical trials for the treatment of multiple sclerosis (MS). In vivo, fingolimod is phosphorylated to form fingolimod-phosphate, which resembles naturally occurring sphingosine 1-phosphate (S1P), an extracellular lipid mediator whose major effects are mediated by cognate G protein-coupled receptors. There are at least five S1P receptor subtypes, known as S1P1–5, four of which bind fingolimod-phosphate. These receptors are expressed on a wide range of cells that are involved in many biological processes relevant to MS. S1P1 plays a key role in the immune system, regulating lymphocyte egress from lymphoid tissues into the circulation. Fingolimod-phosphate initially activates lymphocyte S1P1 via high-affinity receptor binding, yet subsequently induces S1P1 down-regulation that prevents lymphocyte egress from lymphoid tissues, thereby reducing autoaggressive lymphocyte infiltration into the central nervous system (CNS). S1P receptors are also expressed by many CNS cell types and have been shown to influence cell proliferation, morphology and migration. Fingolimod crosses the blood–brain barrier and may therefore have direct CNS effects, distinguishing it from immunologically targeted MS therapies. Prophylactic administration of fingolimod to animals with experimental autoimmune encephalitis (EAE), a model of MS, completely prevents development of EAE features, while therapeutic administration significantly reduces clinical severity of EAE. Therapeutic efficacy observed in animal studies has been substantiated in phase 2 and phase 3 trials involving patients with relapsing or relapsing-remitting MS.
Keywords: fingolimod, multiple sclerosis, sphingosine 1-phosphate receptor modulator, sphingosine 1-phosphate
INTRODUCTION
Multiple sclerosis (MS) is a chronic autoimmune and neurodegenerative disease of the central nervous system (CNS) associated with irreversible progression of disability; it affects up to 2.5 million people worldwide (1–3). Disability in MS reflects myriad neurological sequelae and includes physical and cognitive impairment, as well as fatigue, pain, depression and bladder dysfunction. Untreated, the disease may progress, significantly interfering with lifestyle and career plans and shortening lifespan. The primary cause is unknown; however, a key aspect of the pathology of MS is generally believed to be the activation of autoimmune lymphocytes in the periphery. These cells proliferate and mature within lymphoid tissues. They then egress into the blood and cross the blood–brain barrier (BBB), entering the CNS, where their autoaggressive nature produces inflammation, demyelination, axonal damage, gliosis and, ultimately, neurodegeneration (4–9).
Inflammatory tissue damage early in the course of the disease can be overcome to some extent by endogenous CNS repair mechanisms (including remyelination, regeneration and restoration of adequate nerve conduction). Ultimately, however, these mechanisms provide only partial recovery and as MS progresses CNS repair increasingly fails, due in part to recurring inflammatory attacks (2, 5, 8, 9). Disability in patients with MS is caused by incomplete recovery from inflammatory relapses, gliotic changes and progressive irreversible neurodegeneration (5, 10). Accordingly, the degree of disability in MS reflects a balance between damage to the CNS and the extent to which this is countered by endogenous repair processes (9–11). Novel treatments for MS that beneficially affect not only the immune system to reduce inflammation but also the CNS to promote neuroprotection and repair are therefore desirable (12, 13).
Most current disease-modifying therapies (DMTs) for MS primarily target the immunological inflammatory component of the disease without acting directly on the CNS; such DMTs have been shown to be only partially effective (12). First-line DMTs – interferon β-1a or −1b (IFNβ-1a, 1b) and glatiramer acetate – provide a reduction in relapse rate of 29–34% compared with placebo over a 2-year period and show modest effectiveness at slowing disability progression (14, 15), whereas no current DMT is effective in patients with primary progressive MS (16). Another DMT, natalizumab, appears to be more effective than current first-line DMTs, as suggested by results of a phase 3 study which demonstrated a reduction in relapse rate of approximately 65% compared with placebo, and a reduction in risk of sustained disability progression over 2 years of approximately 42% (17). However, use of natalizumab is restricted to a subgroup of patients (those who have rapidly evolving, severe relapsing–remitting MS (RRMS) or who have high disease activity despite treatment with first-line DMT), because of safety concerns – most notably the increased risk of progressive multifocal leukoencephalopathy, a rare but debilitating demyelinating disease of the brain which can be fatal (18–22). A chemotherapeutic agent, mitoxantrone, has also been approved; however, cumulative cardiac toxicity and drug-induced acute myelogenous leukemia limits its use to patients with rapidly worsening or progressive relapsing forms of MS (23). These two drugs are therefore generally not used as first-line therapies for MS.
All currently approved MS treatments are injected (subcutaneously or intramuscularly) or are given by intravenous infusion which can be associated with reduced convenience, compliance and with injection- or infusion-related adverse effects (24–28). Given the limitations of current interventions, management of MS could be significantly improved by new treatments that influence not only the immune system but also the pathological changes in the CNS, while also being amenable to oral administration, thus avoiding the drawbacks of parenteral administration. Several oral agents are currently in development, including cladribine, laquinimod, teriflunomide, BG-12 and fingolimod (FTY720) – the subject of this review (12, 28). As the lead compound in a new class of agents – sphingosine 1-phosphate (S1P) receptor modulators (4, 29) – fingolimod is currently being assessed in one of the largest phase 3 MS study programs ever undertaken (30), having shown promise in a phase 2, 6-month placebo-controlled study in patients with relapsing MS in which oral fingolimod significantly reduced annualized relapse rate and inflammatory activity according to MRI scans compared with placebo (31). This paper reviews current understanding of the role of receptor-mediated S1P signaling within the body and its relevance to MS pathology, along with data for fingolimod-induced modulation of S1P receptors. Evidence for beneficial effects of fingolimod on MS obtained from studies in experimental autoimmune encephalitis (EAE) animal models of MS and clinical data from patients with relapsing MS are also discussed.
SPHINGOSINE 1-PHOSPHATE (S1P) BIOLOGY AND ITS RELEVANCE TO MULTIPLE SCLEROSIS PATHOLOGY
Sphingolipids were first identified in ethanolic brain extracts in the 1870s and were named after the Greek mythological creature, the Sphinx, because of their enigmatic nature (32). S1P represents a minor constituent of total sphingolipids. However, in the last decade the structural relationship of S1P to lysophospholipids suggested that it might have a related signaling function, which was initially believed to be as an intracellular second messenger (32). The discovery that the actions of S1P are mediated by cell surface G protein-coupled receptors (GPCRs) (33–36), now known as S1P receptors, opened the way for studies which showed that S1P has important roles as an extracellular lipid mediator in higher organisms (37).
S1P is produced by phosphorylation of sphingosine by ubiquitously expressed sphingosine kinases (38–41). It is present at concentrations of 100–1000 nM in blood (42–44) and, as with most small lipids, is preferentially bound to albumin and other plasma proteins. S1P signaling through its cognate GPCRs has key roles in processes relevant to MS, including inflammation and repair (4, 29, 45, 46). Indeed, recent studies suggest an association between S1P biology and MS pathology (41). S1P receptors have been implicated in disease progression on the basis of studies in animal models of MS (47, 48), while data from a human study suggest that there is a disturbance in sphingolipid metabolism in MS patients (49).
There are five known S1P receptor subtypes, S1P1–5, and these are expressed on a wide range of cell types, including lymphocytes and neural cells (50–57). S1P1–3 are widely distributed in the immune and cardiovascular systems and the CNS; S1P1 is highly expressed on T and B lymphocytes (35, 38). In adults, S1P4 is generally confined to lymphoid and hematopoietic tissues, and S1P5 is predominantly located in the CNS white matter (Table 1) (51, 58). Patterns of expression of S1P receptors can change with the activation and functional status of cells (59, 60).
TABLE 1.
Distribution and key functions of sphingosine 1-phosphate (S1P) receptor subtypes (29, 34–36, 47, 57, 61, 62, 127).
| Receptor | Distribution (mRNA) | Key functions |
|---|---|---|
| S1P1 | Ubiquitous (high expression on lymphocytes, neural cells, vasculature) |
|
| S1P2 | Ubiquitous |
|
| S1P3 | Ubiquitous, including CNS (neural cells/astrocytes), endothelium |
|
| S1P4 | Lymphocytes (low) |
|
| S1P5 | Brain/white matter, Oligodendrocytes |
|
S1P receptors are involved in multiple biological processes, including leukocyte recirculation, neural cell proliferation, morphological changes, migration, endothelial cell function, vasoregulation and cardiovascular development, as summarized in Table 1 (29, 46, 47, 50–57, 61, 62). In particular, S1P1 expressed on lymphocytes regulates the normal egress of lymphocytes from lymphoid tissues (38–40, 60, 63), while S1P receptors expressed in the CNS have been shown to modulate functions relevant to MS neuropathology, including neurogenesis, neural function and migration (as described below) (4, 41, 46, 61, 62, 64, 65). In addition, evidence suggests that S1P1–3 on smooth muscle and endothelial cells plays pivotal roles in regulating vascular homeostasis and vascular permeability, while S1P1 on atrial myocytes is involved in the control of heart rate (39, 66, 67).
EFFECTS OF FINGOLIMOD IN THE IMMUNE SYSTEM
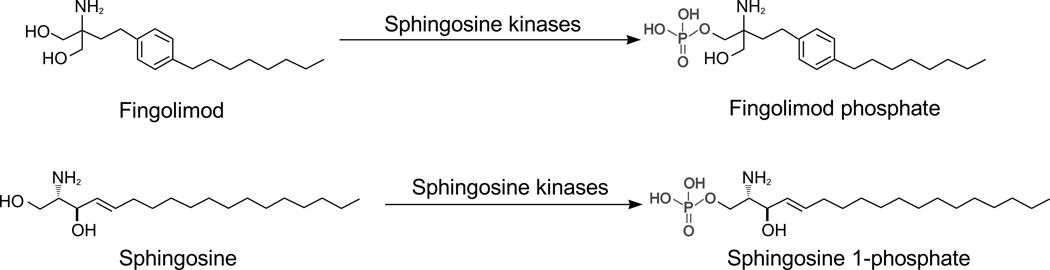
As a structural analog of natural sphingosine (68), fingolimod can undergo rapid phosphorylation in vivo by sphingosine kinase 2 (69) to produce fingolimod-phosphate which binds to four of the five S1P receptors (S1P1 and S1P3–5) with high affinity (0.3–3.1 nM) (29, 35, 38–40, 61, 62, 64, 70) (Fig. 1). Fingolimod exerts its therapeutic effects through modulation of S1P receptors by fingolimod-phosphate, and may well achieve its beneficial effects in patients with MS through receptor-mediated actions both on the immune system and in the CNS (4, 39, 46, 59, 70).
FIG. 1.
Fingolimod is a structural analogue of naturally occurring sphingosine. Fingolimod (molecular weight, 307.47 g/mol), like sphingosine (molecular weight, 299.49 g/mol) is phosphorylated by sphingosine kinases, in particular sphingosine kinase 2, to produced fingolimod phosphate (molecular weight, 387.47 g/mol) while sphingosine undergoes phosphorylation to sphingosine 1-phosphate (molecular weight, 379.47 g/mol).
Role of S1P Receptors in Lymphocyte Recirculation
S1P and S1P receptors play important roles in normal immune function. Adaptive immunity depends on regular circulation of lymphocytes between blood and lymphoid tissue in the search for antigens. When an activating antigen is encountered in the lymph nodes, T cells are retained in the lymph node where naïve T cells become activated and central memory T cells (TCM) are reactivated. Following activation, these T cells return to the blood circulation, allowing them to reach sites of inflammation.
Retention in lymphoid tissues and recirculation of lymphocytes back to the blood circulation is regulated in part by a concentration gradient of S1P between lymphoid tissues and other tissues or body fluids, which is sensed through S1P1 expressed on lymphocytes (Fig. 2a) (39, 63). When a suitable antigen is encountered in the lymph node, expression of S1P1 on T cells is transiently downregulated, allowing the T cells to remain in contact with antigen-presenting cells and to become activated. After clonal expansion of the activated T cells, cell-surface expression of S1P1 is upregulated, allowing cells to respond to the S1P gradient. S1P concentrations are higher in body fluids and tissues than in lymphoid tissues, thus creating a gradient which drives egress of lymphocytes into the circulation (38, 40, 60, 63). The S1P levels in different tissues are maintained by the orchestrated activities of enzymes that regulate sphingolipid metabolism, including kinases, lyases and phosphatases (39, 63).
FIG. 2.
Lymphocyte egress from lymph nodes is driven by a sphingosine 1-phosphate (S1P) gradient (40).
a) S1P concentrations are higher in body fluids than in lymphoid tissues. Lymphoctyes egress from the lymphoid tissues into the circulation along the S1P gradient.
b) Fingolimod downregulates S1P1 on lymphocytes and thereby prevents lymphocyte egress from lymphoid tissues. This in turn reduces the infiltration of autoaggressive cells into the central nervous system.
CNS, central nervous system; S1P, sphingosine 1-phosphate; S1P1, sphingosine 1-phosphate receptor subtype 1
The essential role of expressed S1P1 on lymphocytes and their interaction with S1P in the control of lymphocyte recirculation has been elucidated in elegant studies with knockout mice that lack S1P1 or sphingosine kinases. These studies show that S1P1 and S1P are necessary for the egress of thymocytes from the thymus, and for egress of T and B cells from lymphoid tissue (32, 40, 60, 63). In mice lacking lymphoid S1P1, immature T cell precursors are able to enter the thymus but mature T cells are unable to exit the organ. Other studies have shown that S1P1-deficient lymphocytes transferred into wild-type mice are retained within peripheral lymphoid tissue (60).
Effects of Fingolimod on Lymphocyte Recirculation
Oral fingolimod is thought to provide therapeutic benefit in MS by preventing normal lymphocyte egress from lymphoid tissues, thus reducing the infiltration of autoaggressive lymphocytes into the CNS, where they would cause inflammation and tissue damage (38, 60, 71–73). This action of fingolimod is predominantly mediated by modulation of S1P1 on lymphocytes.
Phosphorylated fingolimod binds with high affinity to S1P1 expressed on lymphocytes. Initial receptor activation is paradoxically followed by S1P1 functional antagonism, whereby receptors are internalized and degraded, thus reducing or eliminating them from the lymphocyte cell surface (40, 60, 70, 74, 75). This downregulation renders lymphocytes unresponsive to the normal S1P gradient and thus deprives them of the obligatory signal that would ordinarily allow them to egress from lymphoid tissues and recirculate to the periphery (Fig. 2b) (40). Oral fingolimod thus prevents normal egress of lymphocytes, including autoaggressive forms, which are retained in lymph organs and away from sites of inflammation (38, 40, 60, 63, 71, 76).
Fingolimod has been shown to selectively retain T cells that regularly traffic through lymph nodes and which express the homing receptor, CCR7. In a recent study involving patients with relapsing MS, fingolimod was found to prevent the egress of CCR7-positive naïve T cells and TCM from the lymph nodes but to spare CCR7-negative effector memory cells (TEM) (Fig. 3) (72). The percentages of naïve T cells and TCM in peripheral blood were significantly reduced in patients treated with fingolimod compared with untreated patients, and consequently the percentages of TEM in peripheral blood increased significantly in fingolimod-treated patients compared with untreated patients. While fingolimod reduced the numbers of both CD4+ and CD8+ T cells, the effect was more pronounced for the CD4+ T-cell subset.
FIG. 3.
Treatment with oral fingolimod prevents the egress of naïve and central memory T cells (TCM) from lymph nodes into the circulation but spares effector memory T cells (TEM). Percentages of naïve T cells, TCM and TEM cells in peripheral blood in patients with relapsing multiple sclerosis treated with fingolimod and in untreated patients. Adapted from Mehling et al. 2008 (72).
MS, multiple sclerosis; naïve, naïve T cells (CCR7+, CD45RA+); TCM cells, central memory T cells (CCR7+, CD45RA−); TEM cells, effector memory T cells (CCR7−, CD45RA−).
Evidence suggests that the autoaggressive lymphocytes important in MS are primarily of the TCM subset (77). These subsets include the pro-inflammatory Th17 cells, which produce the inflammatory cytokine interleukin 17 (IL-17) and have been implicated in MS pathogenesis. Production of IL-17 and enrichment of Th17 has been noted in active MS lesions (78), while data from human in vitro models have shown that Th17 cells migrate across the BBB, disrupt BBB tight junctions and promote CNS inflammation through lymphocyte recruitment (73, 79). In addition, Th17 cells produce pro-apoptotic granzyme B and can kill human neurons in vitro (79). In patients with relapsing MS, oral fingolimod has been shown to reduce the number of Th17 cells in peripheral blood (73). Analysis of T-cell subpopulations showed that levels of IL-17-producing cells were significantly lower in MS patients treated with fingolimod than in untreated patients.
Oral fingolimod does not appear to affect TEM (72), cells that do not express the CD62L and CCR7 lymph-node-homing receptors and so do not recirculate through lymph nodes on a regular basis (77, 80–83). This has significant implications, since peripheral TEM (i.e., in gut epithelial surfaces, small intestine lamina propria, lung, liver, kidney, peritoneum, bone marrow and blood) are thought to be involved in the rapid containment of locally invading pathogens (77), and may be important for immune surveillance and memory immune responses in peripheral tissues (71, 72, 80). Their maintenance during fingolimod exposure could help retain desirable immunological functions.
As a result of lymphocyte retention, overall peripheral blood lymphocyte counts are reduced during treatment with oral fingolimod. This was initially observed in animal models in which fingolimod reversibly reduced the number of circulating peripheral CD4+ and CD8+ lymphocytes (60, 84), as well as in healthy volunteers and in patients with relapsing MS (84, 85). However, the retention of lymphocyte subsets induced by fingolimod does not appear to result in obvious cell accumulation or abnormal lymph node enlargement (lymphadenopathy), reflecting the fact that only about 2% of the total lymphocyte population circulates in the blood at any time (86). Moreover, once treatment is stopped, lymphocyte counts return to normal values within 4–8 weeks (85). Thus, the reduction in peripheral blood lymphocyte counts by oral fingolimod is reversible and reflects redistribution of lymphocytes to the lymphoid tissues rather than lymphocyte destruction, as seen with cytotoxic agents.
Effects of Fingolimod on Lymphocyte Function
Accumulating data indicate that while fingolimod modulates lymphocyte egress, it does not inhibit lymphocyte effector functions; many normal immune response functions are therefore maintained during treatment (87). For example, animal studies have shown that the activation, proliferation and effector functions of T and B cells are not impaired by treatment with fingolimod; in response to antigen challenge, B and T cells can be induced to proliferate, CD8+ T cells develop cytotoxic functions, CD4+ T cells develop B-cell helper functions, and B cells develop antibody responses in models of systemic viral infection (40, 63, 71, 87–89).
Since fingolimod retains recently activated primary T cells and TCM in lymphoid tissues, local immune responses that depend on these cells can be reduced or delayed, which may increase the risk of infections, including common viral infections such as colds and influenza (31, 71, 90). However, memory immune responses throughout the body that depend on local peripheral TEM are unlikely to be inhibited because these cells do not recirculate through lymph nodes and thus are not retained by fingolimod (84, 91).
In addition, oral fingolimod does not appear to inhibit humoral immunity to primary systemic viral or bacterial infections, since it does not suppress the generation of virus-specific or bacterium-specific cytotoxic T cells in the lymph nodes that are responsible for killing cells infected with pathogens (71). In mice infected with lymphocytic choriomeningitis virus or vesicular stomatitis virus, treatment with fingolimod retained more than 95% of circulating lymphocytes in the lymph nodes but did not impair the induction of humoral immunity or specific cytotoxic CD8+ T cells (71). Similarly, no impairment of CD8+ T cell immunity to Listeria monocytogenes was observed (92). Moreover, antigen-primed T cells from fingolimod-treated and control mice produced comparable amounts of IFNγ in response to antigen challenge (92).
Taken together, these data indicate that fingolimod selectively sequesters naïve T cells and TCM, including Th17 cells, within lymphoid tissues. These lymphocyte subsets are believed to be important for inducing the neurological damage associated with MS; their containment in lymphoid tissues is therefore expected to have beneficial effects in patients with MS. As a result of cell retention within lymphoid tissues, peripheral blood lymphocyte counts are reduced during treatment with fingolimod. This effect is readily reversed when fingolimod treatment is stopped, because lymphocytes are redistributed and not destroyed. Intrinsic lymphocyte functions are not affected by treatment with fingolimod and TEMs are spared, whereas local immune responses dependent on naïve T cells and TCM migration to tissues are expected to be reduced or delayed.
S1P SIGNALING IN THE CNS AND EFFECTS OF FINGOLIMOD ON NEURAL CELLS
Preliminary preclinical evidence indicates that fingolimod may also have direct effects within the CNS. Fingolimod is able to cross the BBB (93); following oral administration, fingolimod is found in the CNS where S1P receptors are expressed on most neural lineages and resident CNS cells, particularly neurally derived glia and neurons. By modulating the S1P receptors expressed on CNS cells (45, 46, 52–55, 94–96), fingolimod may have a direct impact on neuropathological processes such as neurodegeneration, gliosis and endogenous repair mechanisms (4, 29, 46, 59). Evidence for the functions of S1P in the CNS and the effects of fingolimod on different neural lineages is reviewed next.
Effects of S1P Signaling in the CNS
Sphingolipids and S1P receptors are found in the CNS where they have been shown to influence neurogenesis, neural cell function and migration (4, 29, 39, 41, 45, 46, 52–56, 59, 62, 64, 94–96). S1P receptors are expressed by virtually all neural cell lineages in the CNS, including oligodendrocytes, neurons, astrocytes and non-neurally derived microglia (Fig. 4) (4, 39, 45, 46, 48, 52–55, 94–96), and S1P signaling has been found to be important for multiple aspects of normal neural function (46, 64, 97). In addition, levels of S1P in the spinal cord are increased following injury (62). S1P has chemo-attractant activity for neural stem/progenitor cells, which have been reported to migrate towards sites of injury in the CNS, and S1P1 has been implicated in this migration process (62). Furthermore, recent studies in EAE, an animal model of MS, have suggested a key role for the S1P1 on neural cells in disease progression (47), and there is also evidence for altered sphingolipid metabolism in MS patients (compared with healthy control subjects), which could contribute to myelin disruption (49). The following sections describe some of the activities of S1P and S1P receptors in different neural lineages and review the evidence suggesting that fingolimod may interact with S1P receptors on neural cells to modulate their function in the treatment of MS.
FIG. 4.
Distribution of sphingosine 1-phosphate (S1P) receptor subtypes on neural cells (44, 39, 46).
OLG, oligodendrocyte; OPC, oligodendrocyte progenitor cell; S1P, sphingosine 1-phosphate; S1P1–5, sphingosine 1-phosphate receptor subtypes 1–5.
Oligodendrocytes
Remyelination has been documented to occur in human MS lesions and animal models of MS. Remyelination is a complex process requiring cellular processes of proliferation, migration, adhesion, process extension/retraction and differentiation (98). S1P signaling has significant effects on these processes in oligodendrocytes (59, 61, 98–100). S1P5 and S1P1 are both expressed on oligodendrocytes and their relative levels of expression appear to depend on developmental stages of the cell (59, 96). Activation of S1P receptor subtypes initiates distinct intracellular signaling pathways that can produce opposing effects (34, 35, 56, 57). The cellular responses mediated by S1P receptors may thus vary with developmental stage and involved receptor subtypes. For example, S1P has been reported to affect process outgrowth in pre-oligodendrocytes but not in mature cells, and to promote survival of mature rat oligodendrocytes but not of precursors cells (61). In addition, it has been reported that cross-talk can occur between S1P receptors and neurotrophin receptors, since downregulation of sphingosine kinase (which regulates S1P levels) abolishes the protective effect of neurotrophin-3 on survival of cultured oligodendrocyte progenitors (99). Fingolimod exposure in vitro has also been reported to increase the number of both progenitor and mature oligodendrocytes, to protect oligodendrocytes from cell death induced by cytokines or the withdrawal of growth factors, and to modulate process outgrowth (both retraction and extension) (59, 101, 102). The effects of fingolimod on human oligodendrocytes and process extension/retraction were found to be time-, dose- and stage-dependent, which may in part reflect the relative levels of expression of the relevant receptors. These effects could formally alter the myelination or remyelination processes (59).
Neurons
S1P receptors are expressed in brain areas showing active neurogenesis (52, 96, 103), while knockout mice with a constitutive genetic deletion of S1P1 showed defective neuronal development (64). Cell culture studies have shown that S1P affects process extension induced by nerve growth factor in dorsal root ganglion neurons. As with oligodendrocytes, the cellular responses appear to depend on the stage of differentiation and the relative expression of S1P receptors (41). In addition, S1P has been shown to promote the migration of neural stem/progenitor cells towards areas of damage in the CNS via S1P1, which are abundantly expressed on neural stem/progenitor cells (62). S1P signaling may also be neuroprotective, as suggested from data obtained from several different in vitro systems (104–106). These observations suggest that receptor-mediated S1P signaling may be involved in promoting some aspects of neuronal injury repair.
Fingolimod has been shown to affect neuronal function in both in vitro and in vivo studies (107, 108). In neuronal cell cultures, fingolimod increased the levels of the endogenous neuroprotectant, brain-derived neurotrophic factor, in a dose-, time- and activation-dependent manner (109), while in a DA rat model of chronic relapsing–remitting EAE, treatment with fingolimod prevented a decrease in axonal density that occurred within the optic nerve of control animals (108) while restoring neuronal function, as measured by normalization of electrophysiological responses (108) and improvements in motor function (110). The receptor mechanisms involved in this response require further clarification, in particular, identification of the target cells involved and the identity and state of the S1P receptors.
Astrocytes
Astrocytes are, like oligodendrocytes, glial cells and are known to play an active role in CNS inflammatory diseases such as MS. Astrocytes have the ability to act like immune cells, enhancing immune responses that can inhibit myelin repair, as occurs during gliosis and glial scar formation. However, they can also be protective and limit inflammation while supporting oligodendrocyte and axonal regeneration (111). Reactive astrocytes appear to play a beneficial role, especially in the acute stage following CNS injury, but may later inhibit CNS regeneration (112). Studies delivering S1P in vivo and in vitro have reported nervous system inflammatory responses that induce morphological changes in neural cells and astrocytes, and increase the expression of glial fibrillary acidic protein (GFAP) (113). GFAP is associated with astrogliosis, which is known to inhibit endogenous repair mechanisms such as remyelination.
Astrocytes have been shown to express S1P1, S1P2, S1P3 and S1P5, with S1P3 and S1P1 being expressed at greater levels than the other two receptor subtypes (114). The results of various studies suggest that S1P can influence astrocyte proliferation, migration and astrogliosis (98, 114–117). In vitro, S1P induced activation and proliferation of astrocytes, while in vivo, injection of S1P into the striata of mouse brains induced astrogliosis (113). In addition, fingolimod has been shown to affect S1P receptor-mediated signaling and migration of astrocytes in vitro (115, 118). These mechanisms can be explained by direct effects of receptor modulation. However, proof-of-concept studies on a related lysophospholipid receptor signaling system in astrocytes – lysophosphatidic acid – indicate that indirect effects may also be important following receptor modulation (65).
POTENTIAL IMPACT OF FINGOLIMOD ON THE TREATMENT OF MULTIPLE SCLEROSIS
The effects of fingolimod on the signs, symptoms and progression of MS have been investigated in multiple animal models of MS and in clinical trials in patients with relapsing or relapsing-remitting MS which are reviewed next (31, 119–121).
Animal Models of MS
The effects of fingolimod on MS animal models (using EAE) at different clinical stages and with different histopathologies have been reported (29, 48, 76, 110, 122, 123). Prophylactic administration of fingolimod prevented development of the clinical features of EAE, while therapeutic treatment with fingolimod at different stages of disease, including late chronic stages, reduced and even reversed the clinical signs of established disease (Fig. 5) (48, 76, 122–124). In particular, in the Biozzi mouse model of relapsing–remitting EAE, treatment with fingolimod at the second inflammatory episode (i.e., when damage was clearly established, with pathological and clinical evidence of late-stage disease) significantly reduced clinical disease features and improved motor function, as measured by the RotaRod system, and reduced axonal loss, compared with vehicle controls (110). Further studies have suggested that fingolimod may provide some form of neuroprotection: treatment with fingolimod in a DA rat EAE model reversed paralysis in animals with established EAE and normalized electrophysiological responses (108). These studies documented improvements in clinical features as well as reductions in inflammatory infiltrates, axonal loss and demyelination, as measured by histology and imaging, although the precise mechanisms underlying these effects remain to be determined (48, 76, 110, 122–124).
FIG. 5.
Oral fingolimod has prophylactic and therapeutic effects in animal models of multiple sclerosis. Fingolimod was administered at a dose of 0.3 mg/kg on days 0–11 (prophylactic administration), days 12–28 (therapeutic administration) or days 40–53 (rescue therapy) after immunization in a rat experimental autoimmune encephalitis model. (123)
Reproduced from with permission from Wiley: Brain Pathology. Foster et al Brain Pathol 2009;19(2):254–66. Copyright 2009.
***p ≤ 0.001.
Clinical score was determined from physical and functional signs as follows: 0 = healthy; 1 = flaccid tail; 2 = hind-limb weakness; 3 = paralysis of one or both hind limbs; 4 = forelimb paralysis; 5 = death.
The effects of fingolimod on myelin protein expression and demyelination have also been investigated in EAE studies. In the SJL/J mouse EAE model of MS that can show a relapsing-remitting disease, fingolimod was found to normalize the expression of myelin proteins (48), while in the DA rat model of chronic relapsing–remitting EAE, fingolimod reduced brain inflammation and demyelination after immunization with syngeneic CNS tissue, with associated improvements in physical and functional measures (123). Late treatment with fingolimod has also been shown to reduce the extent of demyelination (measured by magnetic transfer resonance) in DA rats with EAE induced by myelin oligodendrocyte glycoprotein (124).
Taken together, these MS animal studies suggest that therapeutic administration of fingolimod can have marked ameliorating effects on disease manifestations throughout the course of disease, including the late stages.
Clinical Efficacy and Safety of Fingolimod
The promising therapeutic activity of fingolimod against MS demonstrated in animal models has been substantiated by the results of a placebo-controlled phase 2 study in patients with relapsing MS (31) and a phase 3 study in patients with relapsing-remitting MS (119–121). In the 6-month phase 2 study, which involved 281 patients with relapsing MS (from 32 centers across Europe and Canada), oral fingolimod, administered once daily at a dose of 1.25 mg or 5.0 mg, significantly reduced both the cumulative number of gadolinium (Gd)-enhancing lesions by up to 80% and annualized relapse rates by more than 50% compared with placebo. Results from the open-label extension of this study in which all patients received fingolimod indicated that continuous treatment with oral fingolimod for up to 48 months maintained suppression of clinical and MRI disease activity; at 48 months, 63–70% of patients were relapse-free and 97% of patients were free from Gd-enhancing lesions (125). Initial reports of data from a 12-month phase 3 study indicate that fingolimod reduced relapse rate and MRI lesion activity to a significantly greater degree than a currently approved MS therapy, IFNβ-1a, (119–121) while data from a 2-year placebo-controlled study are awaited with interest.
As with all medications, oral fingolimod is not without risk of adverse events, and a variety of side-effects have been documented in the phase 2 and phase 3 studies (31, 119–121, 125). Two deaths were reported in a phase 3 study that involved 1292 patients, both of which were due to herpes infection, and both occurred in the study arm in which patients received the higher of the two fingolimod doses tested. The extent to which fingolimod contributed to the deaths is unclear, however, since both cases involved confounding factors. Oral fingolimod is generally well tolerated in patients with relapsing forms of MS and is not associated with injection-site reactions or flu-like symptoms (31, 125). Mean blood lymphocyte counts were reduced to approximately 30% from baseline during therapy with fingolimod and remained stable over time (85). There was a slight increase in the incidence of mild infections, mainly nasopharyngitis and herpes zoster reactivation. Transient decreases in heart rate (reported frequently) and the occurrence of conduction blocks (rarely reported) were observed on treatment initiation, but resolved quickly despite continued therapy. These changes reflect the effects of fingolimod on S1P receptors on atrial myocytes, as have been observed in animal studies (126). Mild dose-dependent decreases in pulmonary function were observed on initiation of therapy but pulmonary function remained stable with long-term therapy. These effects on pulmonary function reflect the action of fingolimod on S1P receptors on airway endothelial cells and smooth muscle (67). Macular edema, reversible with drug discontinuation, has been reported in <1% of patients. Seven cases of skin cancer were observed over the course of the phase 2 study; all were successfully excised and no new cases were reported during months 36–48. Oral fingolimod is currently being evaluated further in a large phase 3 clinical study program involving more than 3300 patients with MS. In addition to the 1-year study comparing oral fingolimod with IFNβ-1a administered intramuscularly once weekly in patients with RRMS, this clinical trial program includes two, 2-year placebo-controlled studies of fingolimod, also in patients with RRMS. Because of its mechanism of action that may include direct neural effects, oral fingolimod is also being evaluated in a multicenter phase 3 study in patients with primary progressive MS.
CONCLUSIONS
The discovery of fingolimod and its targeted S1P receptors has raised the possibility of a new generation of medicines for MS. These could have the advantages of oral administration, while beneficially affecting not only the immune system to reduce inflammatory damage but also the CNS to promote neuroprotection and repair. Accumulating data indicate that oral fingolimod exerts beneficial effects in patients with MS by modulating cognate S1P receptors, particularly the S1P1 subtype. The peripheral effects on immune cells prevent the egress of autoaggressive lymphocytes from lymphoid tissues, thus reducing their infiltration into the CNS and decreasing the potential for inflammatory tissue damage. Moreover, fingolimod may also act directly on neural and resident non-neural CNS cells to reduce neurodegenerative processes and to promote endogenous repair processes, and has been shown to prevent or reduce neurological features of MS in animal models, which may be attributable not only to immunological modulation but also to CNS effects. Results from a phase 2 study in patients with relapsing MS have demonstrated a significant and promising therapeutic benefit for oral fingolimod in the treatment of MS; this beneficial response is being investigated further in an extensive phase 3 clinical development program. Further studies are required to elucidate fully the mechanism of action of fingolimod in the treatment of MS and to establish its role in managing this debilitating disease. Both scientific and clinical data indicate that fingolimod represents an important step forwards in the development of better MS treatments, being an oral therapy with a novel mode of action and that is generally well tolerated. Results from the ongoing phase 3 clinical studies will provide further important insights into the clinical utility of fingolimod in the near future.
Acknowledgments
The authors thank Rowena Hughes, PhD (Oxford PharmaGenesis™ Ltd) for editorial assistance, collating the comments of authors, and editing the paper for submission, along with Ms. Danielle Letourneau and Ji Woong Choi, PhD for additional editorial help. This review was supported by the NIH (JC) and Novartis Pharmaceuticals Corporation (JC, H-PH).
Sources of support
JC receives current research support from the National Institutes of Health (NIMH, NINDS, NIDA, NICHD and NIDCD) and Pfizer, Inc. He has received past research support from Novartis Pharma, AG, and honoraria for speaking engagements or scientific advisory panels from Novartis Pharma, AG, GlaxoSmithKline, Biogen Idec, and Amira Pharmaceuticals.
HPH reports having received honoraria for speaking at symposia and serving on steering committees from BayerSchering, BioMS, BiogenIdec, MerckSerono, Novartis, and Teva, with approval by the Rector of Heinrich-Heine-University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Confavreux C, Vukusic S. Accumulation of irreversible disability in multiple sclerosis: from epidemiology to treatment. Clin Neurol Neurosurg. 2006;108:327–332. doi: 10.1016/j.clineuro.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Luchinetti C, Rodriguez M, et al. Multiple Sclerosis. New England Journal Medicine. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Neurology atlas. 2004 http://www.who.int/mental_health/neurology/neurogy_atlas_review_references.pdf.
- 4.Dev KK, Mullershausen F, Mattes H, et al. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117(1):77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 6.Odoardi F, Kawakami N, Klinkert WE, et al. Blood-borne soluble protein antigen intensifies T cell activation in autoimmune CNS lesions and exacerbates clinical disease. Proc Natl Acad Sci USA. 2007;104:18625–18630. doi: 10.1073/pnas.0705033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 8.Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;184:37–44. doi: 10.1016/j.jneuroim.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 10.Frohman EM, Filippi M, Stuve O, et al. Characterizing the mechanisms of progression in multiple sclerosis: evidence and new hypotheses for future directions. Arch Neurol. 2005;62(9):1345–1356. doi: 10.1001/archneur.62.9.1345. [DOI] [PubMed] [Google Scholar]
- 11.Kerschensteiner M, Bareyre FM, Buddeberg BS, et al. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J Exp Med. 2004;200(8):1027–1038. doi: 10.1084/jem.20040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmer B, Hartung HP. Toward the development of rational therapies in multiple sclerosis: what is on the horizon? Ann Neurol. 2007;62:314–326. doi: 10.1002/ana.21289. [DOI] [PubMed] [Google Scholar]
- 13.Virley DJ. Developing therapeutics for the treatment of multiple sclerosis. NeuroRx. 2005;2(4):638–649. doi: 10.1602/neurorx.2.4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association of British Neurologists. ABN guidelines for treatment of multiple sclerosis with β-interferons and glatiramer acetate. 2007 http://www.theabn.org/downloads/ABN-MS-Guidelines-2007.pdf.
- 15.Goodin DS, Frohman EM, Garmany GP, Jr, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58(2):169–178. doi: 10.1212/wnl.58.2.169. [DOI] [PubMed] [Google Scholar]
- 16.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 17.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 18.Natalizumab. Summary of Product Characteristics. 2009 http://emc.medicines.org.uk/medicine/18447/SPC/TYSABRI+300+mg+concentrate+for+solution+for+infusion/
- 19.Natalizumab. Prescribing Information. 2006 http://www.biogenidec.com/site/TYSABRI-PI-MedGuide.pdf.
- 20.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 21.Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 22.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353(4):414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 23.Mitoxantrone. Prescribing Information. 2008 http://www.novantrone.com/assets/pdf/novantrone_prescribing_info.pdf.
- 24.Samuel L, Lowenstein EJ. Recurrent injection site reactions from interferon beta 1-b. J Drugs Dermatol. 2006;5(4):366–367. [PubMed] [Google Scholar]
- 25.Langer-Gould A, Moses HH, Murray TJ. Strategies for managing the side effects of treatments for multiple sclerosis. Neurology. 2004;63(11) Suppl 5:S35–S41. doi: 10.1212/wnl.63.11_suppl_5.s35. [DOI] [PubMed] [Google Scholar]
- 26.Cox D, Stone J. Managing self-injection difficulties in patients with relapsing-remitting multiple sclerosis. J Neurosci Nurs. 2006;38(3):167–171. doi: 10.1097/01376517-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Adherence to long-term therapies: evidence for action. 2003 http://www.who.int/chp/knowledge/publications/adherence_introduction.pdf.
- 28.Cohen BA, Rieckmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract. 2007;61(11):1922–1930. doi: 10.1111/j.1742-1241.2007.01561..x. [DOI] [PubMed] [Google Scholar]
- 29.Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin Investig Drugs. 2007;16(3):283–289. doi: 10.1517/13543784.16.3.283. [DOI] [PubMed] [Google Scholar]
- 30.Adachi K, Chiba K. FTY720 Story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspectives in Medicinal Chemistry. 2007;1:11–23. [PMC free article] [PubMed] [Google Scholar]
- 31.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima N, Ishii I, Contos JJ, et al. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- 34.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279(20):20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 35.Ishii I, Fukushima N, Ye X, et al. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 36.Chun J. How the lysophospholipid got its receptor. The Scientist. 2007;21:48–54. [Google Scholar]
- 37.Chun J. Immunology. The sources of a lipid conundrum. Science. 2007;316(5822):208–210. doi: 10.1126/science.1142239. [DOI] [PubMed] [Google Scholar]
- 38.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 39.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115(1):84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4(7):1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 41.Toman RE, Payne SG, Watterson KR, et al. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166(3):381–392. doi: 10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol Ther. 2000;88(2):115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 43.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349(Pt 2):385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 45.Gardell SE, Dubin AE, Chuns J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12(2):65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8(1):155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- 47.Gardell S, Choi JW, Anliker B, et al. Evidence for Neural S1P Receptor Signaling in EAE and FTY720 Efficacy. Mult Scler. 2007;13:S70. [Google Scholar]
- 48.Webb M, Tham CS, Lin FF, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153(1–2):108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler D, Bandaru VV, Calabresi PA, et al. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131(Pt 11):3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang G, Contos JJ, Weiner JA, et al. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227(1):89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92(5):913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 52.Chun J. Lysophospholipid receptors: implications for neural signaling. Crit Rev Neurobiol. 1999;13(2):151–168. doi: 10.1615/critrevneurobiol.v13.i2.20. [DOI] [PubMed] [Google Scholar]
- 53.Chun J, Contos JJ, Munroe D. A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs) Cell Biochem Biophys. 1999;30(2):213–242. doi: 10.1007/BF02738068. [DOI] [PubMed] [Google Scholar]
- 54.Chun J, Weiner JA, Fukushima N, et al. Neurobiology of receptor-mediated lysophospholipid signaling. From the first lysophospholipid receptor to roles in nervous system function and development. Ann N Y Acad Sci. 2000;905:110–117. doi: 10.1111/j.1749-6632.2000.tb06543.x. [DOI] [PubMed] [Google Scholar]
- 55.Chun J. Lysophospholipids in the nervous system. Prostaglandins Other Lipid Mediat. 2005;77(1–4):46–51. doi: 10.1016/j.prostaglandins.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Mutoh T, Chun J. Lysophospholipid activation of G protein-coupled receptors. Subcell Biochem. 2008;49:269–297. doi: 10.1007/978-1-4020-8831-5_10. [DOI] [PubMed] [Google Scholar]
- 57.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- 58.Watterson K, Sankala H, Milstien S, et al. Pleiotropic actions of sphingosine-1-phosphate. Prog Lipid Res. 2003;42(4):344–357. doi: 10.1016/s0163-7827(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 59.Miron VE, Jung CG, Kim HJ, et al. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63(1):61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 60.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 61.Jaillard C, Harrison S, Stankoff B, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25(6):1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura A, Ohmori T, Ohkawa R, et al. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25(1):115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- 63.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8(12):1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 64.Mizugishi K, Yamashita T, Olivera A, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25(24):11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Sampaio ESTC, Choi JW, Gardell SE, et al. Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem. 2008;283(12):7470–7479. doi: 10.1074/jbc.M707758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gergely P, Wallström E, Nuesslein-Hildesheim B, et al. Phase I study with the selective S1P1/S1P5 receptor modulator BAF312 indicates that S1P1 rather than S1P3 mediates transient heart rate reduction in humans. Mult Scler. 2009;15:S125. [Google Scholar]
- 67.Brinkmann V, Baumruker T. Pulmonary and vascular pharmacology of sphingosine 1-phosphate. Curr Opin Pharmacol. 2006;6(3):244–250. doi: 10.1016/j.coph.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Nofer JR, Bot M, Brodde M, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115(4):501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 69.Billich A, Bornancin F, Devay P, et al. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278(48):47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 70.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 71.Pinschewer DD, Ochsenbein AF, Odermatt B, et al. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164(11):5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 72.Mehling M, Brinkmann V, Antel J, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71(16):1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 73.Mehling M, Lindberg R, Kuhle J, et al. Oral fingolimod (FTY720) treatment reduces peripheral IL-17-producing TH17 cells in patients with multiple sclerosis. Mult Scler. 2008;14:S234. [Google Scholar]
- 74.Oo ML, Thangada S, Wu MT, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282(12):9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 75.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. Faseb J. 2004;18(3):551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 76.Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305(1):70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 77.Brinkmann V, Metzler B, Matloubian M, et al. The mode of action of Fingolimod (FTY720), an oral sphingosine 1-phosphate receptor modulator that is highly effective in human multiple sclerosis (Phase II) Mult Scler. 2006;12:S100. [Google Scholar]
- 78.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172(1):146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masopust D, Vezys V, Marzo AL, et al. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 81.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 82.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 83.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 84.Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6(13–14):1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 85.Schmouder R, Aradhye S, O'Connor P, et al. Pharmacodynamic effects of oral fingolimod (FTY720) Mult Scler. 2006;12 Suppl 1:S101. [Google Scholar]
- 86.Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig. 1992;70(7):539–544. doi: 10.1007/BF00184787. [DOI] [PubMed] [Google Scholar]
- 87.Brinkmann V, Chen S, Feng L, et al. FTY720 alters lymphocyte homing and protects allografts without inducing general immunosuppression. Transplant Proc. 2001;33(1–2):530–531. doi: 10.1016/s0041-1345(00)02126-6. [DOI] [PubMed] [Google Scholar]
- 88.Xie JH, Nomura N, Koprak SL, et al. Sphingosine-1-phosphate receptor agonism impairs the efficiency of the local immune response by altering trafficking of naive and antigen-activated CD4+ T cells. J Immunol. 2003;170(7):3662–3670. doi: 10.4049/jimmunol.170.7.3662. [DOI] [PubMed] [Google Scholar]
- 89.Metzler B, Gfeller P, Wieczorek G, et al. Modulation of T cell homeostasis and alloreactivity under continuous FTY720 exposure. Int Immunol. 2008;20(5):633–644. doi: 10.1093/intimm/dxn023. [DOI] [PubMed] [Google Scholar]
- 90.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) in relapsing ms: 24-month results of the phase II study. Mult Scler. 2006;12:S101. [Google Scholar]
- 91.Yopp AC, Fu S, Honig SM, et al. FTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol. 2004;173(2):855–865. doi: 10.4049/jimmunol.173.2.855. [DOI] [PubMed] [Google Scholar]
- 92.Kursar M, Janner N, Pfeffer K, et al. Requirement of secondary lymphoid tissues for the induction of primary and secondary T cell responses against Listeria monocytogenes. Eur J Immunol. 2008;38(1):127–138. doi: 10.1002/eji.200737142. [DOI] [PubMed] [Google Scholar]
- 93.Foster CA, Howard LM, Schweitzer A, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the CNS during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323(2):469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 94.Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63(23):2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chun J, Rosen H. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr Pharm Des. 2006;12(2):161–171. doi: 10.2174/138161206775193109. [DOI] [PubMed] [Google Scholar]
- 96.McGiffert C, Contos JJ, Friedman B, et al. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1–3) in angiogenesis. FEBS Lett. 2002;531(1):103–108. doi: 10.1016/s0014-5793(02)03404-x. [DOI] [PubMed] [Google Scholar]
- 97.Herr DR, Grillet N, Schwander M, et al. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27(6):1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miron VE, Schubart A, Antel JP. Central nervous system-directed effects of FTY720 (fingolimod) J Neurol Sci. 2008;274(1–2):13–17. doi: 10.1016/j.jns.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 99.Saini HS, Coelho RP, Goparaju SK, et al. Novel role of sphingosine kinase 1 as a mediator of neurotrophin-3 action in oligodendrocyte progenitors. J Neurochem. 2005;95(5):1298–1310. doi: 10.1111/j.1471-4159.2005.03451.x. [DOI] [PubMed] [Google Scholar]
- 100.Novgorodov AS, El-Alwani M, Bielawski J, et al. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. Faseb J. 2007;21(7):1503–1514. doi: 10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]
- 101.Barske C, Osinde M, Mir AK, et al. FTY720 (fingolimod) enhances the number of progenitor and mature oligodendrocytes. Mult Scler. 2007;13:S148. [Google Scholar]
- 102.Coelho RP, Payne SG, Bittman R, et al. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323(2):626–635. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- 103.Harada J, Foley M, Moskowitz MA, et al. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88(4):1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 104.Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17(18):6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chin TY, Hwang HM, Chueh SH. Distinct effects of different calcium-mobilizing agents on cell death in NG108–15 neuroblastoma X glioma cells. Mol Pharmacol. 2002;61(3):486–494. doi: 10.1124/mol.61.3.486. [DOI] [PubMed] [Google Scholar]
- 106.Shinpo K, Kikuchi S, Moriwaka F, et al. Protective effects of the TNF-ceramide pathway against glutamate neurotoxicity on cultured mesencephalic neurons. Brain Res. 1999;819(1–2):170–173. doi: 10.1016/s0006-8993(98)01354-7. [DOI] [PubMed] [Google Scholar]
- 107.Deogracias R, Matsumoto T, Klein C, et al. FTY720 induces BDNF production in neuronal cell cultures. Neurology. 2008;70:A373. [Google Scholar]
- 108.Balatoni B, Storch MK, Swoboda EM, et al. FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain Res Bull. 2007;74(5):307–316. doi: 10.1016/j.brainresbull.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 109.Deogracias R, Klein C, Matsumoto T, et al. Expression of brain-derived neutrophic factor is regulated by FTY720 in cultured neurons. Mult Scler. 2008;14:S243. [Google Scholar]
- 110.Giovannoni G, Al-Izki S, Pryce G, et al. Control of chronic relapsing progressive EAE with fingolimod. AAN: 60th Annual Meeting of the American Academy of Neurology; Poster; 2008 12–17 April; Chicago, USA. 2008. [Google Scholar]
- 111.Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65(17):2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 113.Sorensen SD, Nicole O, Peavy RD, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64(5):1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- 114.Rao TS, Lariosa-Willingham KD, Lin FF, et al. Pharmacological characterization of lysophospholipid receptor signal transduction pathways in rat cerebrocortical astrocytes. Brain Res. 2003;990(1–2):182–194. doi: 10.1016/s0006-8993(03)03527-3. [DOI] [PubMed] [Google Scholar]
- 115.Mullershausen F, Craveiro LM, Shin Y, et al. Phosphorylated FTY720 promotes astrocyte migration through sphingosine-1-phosphate receptors. J Neurochem. 2007;102(4):1151–1161. doi: 10.1111/j.1471-4159.2007.04629.x. [DOI] [PubMed] [Google Scholar]
- 116.Rao TS, Lariosa-Willingham KD, Lin FF, et al. Growth factor pre-treatment differentially regulates phosphoinositide turnover downstream of lysophospholipid receptor and metabotropic glutamate receptors in cultured rat cerebrocortical astrocytes. Int J Dev Neurosci. 2004;22(3):131–135. doi: 10.1016/j.ijdevneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 117.Yamagata K, Tagami M, Torii Y, et al. Sphingosine 1-phosphate induces the production of glial cell line-derived neurotrophic factor and cellular proliferation in astrocytes. Glia. 2003;41(2):199–206. doi: 10.1002/glia.10180. [DOI] [PubMed] [Google Scholar]
- 118.Osinde M, Mullershausen F, Dev KK. Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology. 2007;52(5):1210–1218. doi: 10.1016/j.neuropharm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 119.Kappos L, Cohen J, Pelletier J, et al. Safety findings from a 12-month phase III study (TRANSFORMS) comparing oral fingolimod (FTY720) and intramuscular interferon beta-1a for relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:S245. [Google Scholar]
- 120.Cohen J, Pelletier J, Kappos L, et al. Oral fingolimod (FTY720) significantly reduced relapse rate compared with intramuscular interferon beta-1a in relapsing-remitting multiple sclerosis: clinical results from a 12-month phase III study (TRANSFORMS) Mult Scler. 2009;15:S132. [Google Scholar]
- 121.Barkhof F, Cohen J, Comi G, et al. Oral fingolimod (FTY720) significantly reduced MRI inflammatory activity compared with intramuscular interferon beta-1a in relapsing-remitting multiple sclerosis: MRI findings from a 12-month phase III study (TRANSFORMS) Mult Scler. 2009;15:S21. [Google Scholar]
- 122.Kataoka H, Sugahara K, Shimano K, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2(6):439–448. [PubMed] [Google Scholar]
- 123.Foster CA, Mechtcheriakova D, Storch MK, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2009;19(2):254–266. doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schubart A, Howard LM, Seabrook T, et al. FTY720 suppresses ongoing EAE and promotes a remyelinating environment preventing axonal degeneration within the CNS. Neurology. 2008;70:A339. [Google Scholar]
- 125.Montalban X, O'Connor P, Antel J, et al. Oral fingolimod (FTY720) shows sustained low rates of clinical and MRI disease activity in patients with relapsing multiple sclerosis: 4-year results from a phase II extension. Neurology. 2009;72:A313. [Google Scholar]
- 126.Koyrakh L, Roman MI, Brinkmann V, et al. The heart rate decrease caused by acute FTY720 administration is mediated by the G protein-gated potassium channel I. Am J Transplant. 2005;5(3):529–536. doi: 10.1111/j.1600-6143.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 127.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008;1781(9):531–539. doi: 10.1016/j.bbalip.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]