Abstract
Verbs have two separate levels of meaning. One level reflects the uniqueness of every verb and is called the “root.” The other level consists of a more austere representation that is shared by all the verbs in a given class and is called the “event structure template.” We explore the following hypotheses about how, with specific reference to the motor features of action verbs, these two distinct levels of semantic representation might correspond to two distinct levels of the mirror neuron system. Hypothesis 1: Root-level motor features of verb meaning are partially subserved by somatotopically mapped mirror neurons in the left primary motor and/or premotor cortices. Hypothesis 2: Template-level motor features of verb meaning are partially subserved by representationally more schematic mirror neurons in Brodmann area 44 of the left inferior frontal gyrus. Evidence has been accumulating in support of the general neuroanatomical claims made by these two hypotheses—namely, that each level of verb meaning is associated with the designated cortical areas. However, as yet no studies have satisfied all the criteria necessary to support the more specific neurobiological claims made by the two hypotheses—namely, that each level of verb meaning is associated with mirror neurons in the pertinent brain regions. This would require demonstrating that within those regions the same neuronal populations are engaged during (a) the linguistic processing of particular motor features of verb meaning, (b) the execution of actions with the corresponding motor features, and (c) the observation of actions with the corresponding motor features.
1. INTRODUCTION
Many traditional approaches to the human conceptual system assume that semantic knowledge is represented separately from, and is qualitatively different than, modality-specific systems for perception, action, and emotion (e.g., Fodor, 1975; Smith, 1978; Pylyshyn, 1984; Barsalou & Hale, 1993; Landauer & Dumais, 1997). According to this classic “disembodied cognition hypothesis” (Mahon and Caramazza, 2008), sensorimotor and affective representations are transduced into amodal structures such as feature lists, semantic networks, frames, etc., and cognitive processes operate on those structures, not on memories of the original experiences. Moreover, the content of all types of concepts, including those encoded by words, is believed to consist entirely of combinations of these abstract symbols.
A very different line of thinking is currently being pursued by a growing number of researchers in linguistics (e.g., Hampe, 2005; Evans & Green, 2006; Bergen, 2007), philosophy (e.g., Prinz, 2005; Gallagher, 2006; Johnson, 2007), psychology (e.g., Pecher & Zwaan, 2005; Gibbs, 2006; Barsalou, 2008b; Klatzky et al., 2008), and neuroscience (e.g., Jeannerod, 2006; Keysers & Gazzola, 2006; Martin, 2007; Haggard et al., 2007; Barsalou, 2008a,c; Kemmerer, in press), all of whom endorse one form or another of what is often called the Embodied Cognition Framework (also known as the Grounded Cognition Framework or the Simulation Framework). The central tenet of this approach is that semantic knowledge is not purely amodal, but is instead anchored in modality-specific input/output systems, such that many forms of conceptual processing involve the transient recapitulation of diverse aspects of sensorimotor and affective experiences. As emphasized recently by Hoenig et al. (in press) and Kemmerer (in press), the notion of modality-specific semantic maps does not rule out the possibility of higher-order integrative memory systems that contain systematically organized “conjunctive units” for binding cross-modal feature correlations; indeed, there is accumulating evidence that, at least for certain kinds of object concepts, integrative systems of this nature may reside in the temporal poles (e.g., Bright et al., 2007; Patterson et al., 2007; Lambon Ralph et al., in press). The most important, and most controversial, claim of the Embodied Cognition Framework, however, is that these integrative systems are not by themselves sufficient for full-fledged conceptual processing; rather, such processing requires that the abstract conjunctive units within the integrative systems activate, in top-down fashion, modality-specific representations that “flesh out,” to varying degrees, the contextually most appropriate concrete content of the relevant ideas (Damasio, 1989a,b,c; Simmons & Barsalou, 2003).
In recent years a great deal of research within the Embodied Cognition Framework has focused on the nature of action concepts, and this is due in large part to the seminal—some would even say “paradigm-shattering” (Ramachandran, 2008)—discovery of mirror neurons roughly 20 years ago (di Pellegrino et al., 1992). These are cells that discharge not only when certain kinds of actions are executed by the self, but also when they are seen or heard being performed by someone else. Thus, mirror neurons appear to represent behavioral patterns per se, and because they neutralize the self-other distinction,1 they may turn out to have profound implications for intersubjective understanding (Hurley, 2008; Iacoboni, 2008). Owing to their remarkable response properties, these cells seem to confirm a prescient statement made by an early advocate of the Embodied Cognition Framework, namely William James (1890, p. 526): “Every representation of a movement awakens in some degree the actual movement which is its object.”
Mirror neurons have been found in a variety of brain regions, but before briefly reviewing those results we would first like to clarify our terminology. There is currently some disagreement over the definition of “mirror neurons.” Cells that fire during both action execution and action observation were first discovered in area F5 of the macaque ventral premotor cortex, and this region has continued to received a great deal of attention over the years. Apparently for this purely historical reason, however, some researchers seem to think that only F5 cells deserve to be called “mirror neurons,” and that cells in other cortical areas that also fire during both action execution and action observation do not qualify. For example, after providing compelling evidence that cells with mirror-like properties—i.e., cells that achieve action observation-execution matching—are broadly distributed across many sectors of the macaque frontal cortex, Raos et al. (2007, p. 12682) conclude that their results “undermine the ‘mirror neuron system’ concept,” and that the more general notion of “mental simulation” is explanatorily superior because it, rather than the former concept, “assigns the role of understanding others’ actions to the entire distributed neural network, which is responsible for the execution of actions.” The same research team recently expressed essentially the same view after extending their work to multiple sectors of the macaque parietal cortex (Evangeliou et al., in press). We believe, however, that “mirror neurons” should be defined by functional rather than anatomical criteria. Indeed, this perspective is adopted in several prominent reviews of the mirror neuron system which indicate that the system is not necessarily limited to F5 (Rizzolatti et al., 2001; Rizzolatti & Craighero, 2004).
Having said that, we consider it noteworthy that in the macaque brain mirror neurons have already been found in an impressively large number of areas:
ventral premotor cortex (di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996; Kohler et al., 2002; Ferrari et al., 2003; Keysers et al., 2003; Nelissen et al., 2005; Raos et al., 2007);
dorsal premotor cortex (Cisek & Kalaska, 2004; Raos et al., 2007);
primary motor cortex (Raos et al., 2004, 2007; Tkach et al., 2007);
several medial frontal regions (Raos et al., 2007);
inferior parietal cortex (Gallese et al., 2002; Fogassi et al., 2005; Evangeliou et al., in press);
superior parietal cortex (Evangeliou et al., in press);
primary and supplementary somatosensory areas (Evangeliou et al., in press).
There is mounting evidence that mirror neurons also exist in a wide range of human brain areas. Despite some important limitations that we address later (Mahon & Caramazza, 2005, 2008; Negri et al., 2007; Turella et al., 2008), numerous human brain mapping studies suggest that the visual or auditory perception of an action engages many of the same neural networks that are recruited during its execution—a remarkable phenomenon which suggests that understanding other people’s actions may involve, to some degree, simulating them in a completely automatic, unconscious manner (we discuss some of this literature in sections 3.1 and 4.1).
In addition, a growing literature suggests that, as predicted by the Embodied Cognition Framework, when people understand linguistic descriptions of actions, motor-related regions in their frontal lobes are engaged (for reviews see Pulvermüller, 2005, 2008; Willems & Hagoort, 2007; Fischer & Zwaan, 2008). So far, linguistically triggered motor resonance has not been investigated in as much detail as the type of motor resonance that is induced by action observation, but there is increasing interest in the provocative idea that comprehending a linguistic description of an action might involve covertly recapitulating the type of action that it refers to, using some of the same brain systems that underlie the execution and observation of that type of action. As yet, however, this line of research has, for the most part, neglected recent advances in linguistic theory, especially regarding the lexical and grammatical encoding of action. The main purpose of this paper is therefore to take some steps toward filling that gap.
In particular, our aim is to explore some possible connections between, on the one hand, the Embodied Cognition Framework as it has hitherto been applied to action concepts and the mirror neuron system, and on the other hand, the Two-Level Theory of verb meaning, which is an approach to analyzing the linguistic representation of action that has not only been supported and refined for over 20 years (for a review see Levin & Rappaport Hovav, 2005), but has also arguably led to deep insights about the fabric of human thought (Pinker, 2007). Basically, the Two-Level Theory holds that verb meanings have two separate levels of structure—one for the “root” or “constant” semantic features that characterize individual verbs, and another for the “event structure templates” or “thematic cores” that are shared by all the verbs in a given class. In section 2 we elaborate this central claim of the theory in greater detail. Then in sections 3 and 4 we explore the following hypotheses about how, with specific reference to the motor features of action verbs, the two distinct levels of semantic representation might correspond to two distinct levels of the mirror neuron system:
Hypothesis 1: Root-level motor features of verb meaning are partially subserved by somatotopically mapped mirror neurons in the left primary motor and/or premotor cortices.
Hypothesis 2. Template-level motor features of verb meaning are partially subserved by representationally more schematic mirror neurons in Brodmann area (BA) 44 of the left inferior frontal gyrus.
We have deliberately shaped these hypotheses in the form of rather bold proposals about how the semantics of action might relate to the mirror system, because our intent is to provide some intriguing theoretical ideas around which both past and future research can be organized. We show that evidence has been accumulating in support of the general neuroanatomical claims made by both hypotheses—namely, that each level of verb meaning is associated with the designated cortical areas. However, we also point out a number of problems, the most important of which is that, to the best of our knowledge, as yet no studies have satisfied all the criteria necessary to support the more specific neurobiological claims made by the two hypotheses—namely, that each level of verb meaning is associated with mirror neurons in the pertinent brain regions. Strictly speaking, such studies would need to demonstrate that within those brain regions overlapping neuronal populations, and ultimately the very same cells (Dinstein et al., 2008), are functionally engaged during all three of the following conditions: (a) the linguistic processing of particular motor features of verb meaning, (b) the execution of actions with the corresponding motor features, and (c) the observation of actions with the corresponding motor features. We suggest several ways in which the Two-Level Theory could help guide future research aimed at evaluating and refining Hypotheses 1 and 2.
Before proceeding, a caveat is in order: Our hypotheses focus rather narrowly on how certain aspects of verb meaning might be linked with mirror neurons in certain regions of the left frontal lobe. The main reason we restricted the hypotheses in these ways is because we felt it necessary to constrain the scope and length of the paper. We would like to point out, however, that even though the hypotheses do not directly address parietal and temporal brain regions, we consider it likely that some of those regions also contribute, in various ways, to the linguistic representation of action. In fact, several recent studies point to the presence of mirror neurons in the left intraparietal sulcus and inferior parietal lobule (e.g., Hamilton & Grafton, 2006, 2007, 2008; Shmuelof & Zohary, 2006; Dinstein et al., 2007), and there is growing evidence that these same regions also support some aspects of verb meaning (e.g., Noppeney et al., 2005; Saccuman et al., 2006; Kemmerer et al., 2008; Tranel et al., 2008). While investigating possible relations between mirror neurons and verb meanings in the left parietal cortex is beyond the purview of this paper, it is clearly an important direction for future research (e.g., see Glenberg & Gallese, submitted, for a new theoretical proposal about the role of action-related frontoparietal circuits in sentence processing). In addition, our hypotheses do not encompass the posterolateral temporal cortex, despite the fact that this region plays a major role, albeit predominantly in the right hemisphere, in biological motion perception (for a review see Blake & Shiffrar, 2007) and has also been implicated, albeit predominantly in the left hemisphere, in the semantic processing of action verbs (e.g., Kable et al., 2002, 2005; Noppeney et al., 2005; Kemmerer et al., 2008; Tranel et al., 2008; Pirog Revill et al., 2008; see also relevant data on thematic roles and event structure provided by, e.g., Wu et al., 2007; Grewe et al., 2007; Bedny et al., in press). We would like to emphasize, however, that even though we do not discuss the posterolateral temporal cortex in detail, we nevertheless refer, at several points in our presentation, to findings about this region that are especially pertinent to our arguments (see in particular sections 3.2.2.1 and 4.2.2.2).
2. THE TWO-LEVEL THEORY OF VERB MEANING
2.1. The Theory in a Nutshell
The Two-Level Theory subsumes a number of complex, well-developed proposals about the linguistic representation of action. These proposals differ in non-trivial ways, but as noted above, all of them share the fundamental assumption that the meanings of verbs have two separate levels of semantic structure (for a brief overview of this research see Levin & Rappaport Hovav, in press; for a broader survey see Levin & Rappaport Hovav, 2005; see also Pinker, 1989, 2007; Levin, 1993; Rappaport Hovav & Levin, 1998; Croft, 1991, 1998; Davis, 2001; Iwata, 2002, 2005, 2008; Van Valin, 2005, 2006; Wunderlich, 2006; Bornkessel et al., 2006; Koenig et al., 2008). One level of meaning reflects the uniqueness of every verb and has been dubbed the “root” or “constant” because it captures idiosyncratic semantic features that (a) distinguish each verb in a given class from all the others, (b) are often concrete and modality-specific in format, and (c) do not interface with grammar. The other level of meaning consists of a more austere representation, referred to variously as the “event structure template” (Rappaport Hovav & Levin, 1998), the “thematic core” (Pinker, 1989), or the “logical structure” (Van Valin, 2005, 2006), that is (a) common to all the verbs in a given class, (b) composed primarily of schematic predicates and variables for arguments, and (c) relevant to the grammatical properties of all the verbs in a given class.
According to the Two-Level Theory, the composite meaning of a verb involves the association of a particular root with a particular template.2 Consider, for example, Change of State verbs—e.g., melt, dry, shatter. All of the verbs in this large class specify that an object undergoes some kind of physical transformation. The verbs share the same skeletal template—roughly, [BECOME [X <STATE>]]—but they vary with respect to the unique roots that flesh out the “state” component of that template (Levin, 1993). Thus, as Pinker (2007, p. 83) puts it, “basic conceptual distinctions assemble themselves into a scaffolding of meaning [at the template level], which has hooks here and there on which to hang images, sounds, emotions, mental movies, and the other contents of consciousness [at the root level].” Below we describe each level of verb meaning in greater depth.
2.2. The Root Level
In her book English verb classes and alternations,Levin (1993) sorted over 3,000 English verbs into well over 200 classes, based on commonalities in both semantic and syntactic properties. The verbs in each class collectively provide a detailed semantic map of a particular conceptual space by making contrasts, often of a remarkably fine-grained nature, along a number of different dimensions. These contrasts reflect the idiosyncratic, lexically discriminative, root meanings of verbs, as illustrated by the semantic distinctions found among the verbs comprising each of the following three classes, all of which fall within the more general category of “putting” verbs—i.e., verbs that denote various ways in which people put things in places (note that the examples provided are only representative, not exhaustive, of each class):
Verbs that specify actions in which an agent applies force to a substance, causing it to move onto or into an object: inject, shower, spatter, splash, splatter, spray, sprinkle, spritz, squirt.
Verbs that specify actions in which an agent enables something, typically a liquid or semisolid substance, to move along a downward path by virtue of gravity: dribble, drip, drop, dump, funnel, ladle, pour, siphon, slosh, spill, spoon.
Verbs that specify actions in which an agent causes a mass to become coextensive with a medium: drench, infuse, saturate, soak, stain, suffuse, interlace, interleave, interweave, intersperse.
The semantic diversity manifested by the verbs comprising each of these classes exemplifies the richness and subtlety of the root level of verb meaning. Indeed, some of the concepts expressed by the verbs above are so specialized that they may be unique to English, as suggested by recent research in semantic typology that has revealed widespread cross-linguistic variation in verb inventories (e.g., McGregor, 2002; Slobin, 2004; Aikhenvald & Dixon, 2006; Levinson & Wilkins, 2006; Majid et al., 2007).
2.3. The Template Level
As indicated above, the verb classes in Levin’s (1993) taxonomy are defined as classes according to commonalities in both semantic composition and syntactic distribution. In fact, one of the central tenets of the Two-Level Theory is that the range of clausal constructions in which the verbs belonging to a particular class can occur is partly determined by a shared semantic “skeleton” that is often called the event structure template. In what follows, we summarize some of the most basic aspects of this level of verb meaning. We focus on Steven Pinker’s (1989) influential approach to this realm of action representation, but we do so mostly for the sake of simplicity, because in actuality many different approaches have been offered and are currently being debated (for an extensive but still only partial review, see Levin & Rappaport Hovav, 2005).
This level captures event structure schemas that are common to all the verbs in a given class. As described by Pinker (1989; see also Pinker, 2007), these representations are formulated in terms of a restricted, perhaps universal, set of semantic devices that include basic conceptual units (e.g., events, states, things, substances, places, goals, intentions), primitive predicates (e.g., acting, going, being, changing, having), force-dynamic relations between entities (e.g., causing, enabling, preventing), physical properties differentiating entities (e.g., animate/inanimate, human/nonhuman, count/mass, substance/aggregate, 0/1/2/3-dimensional4.2.2 extendedness, rigid/flexible, liquid/semisolid), temporal notions (e.g., points vs. regions on a time-line), and various other kinds of cognitive machinery. Later on, in section 4.3, we will attempt to relate the template level of verb meaning to the mirror neuron system, and we will focus specifically on certain higher-order motor representations that capture the ways in which certain classes of verbs encode the goals and intentions of actions.
As already noted, the types of semantic elements described above not only constitute the basic building blocks of the template level of verb meaning, but also influence the range of clausal constructions in which the verbs belonging to a given class can occur. This is because most constructions consist of syntactic patterns that are directly associated with schematic meanings, and in order for a particular verb to occur in a particular construction, its template must be compatible with the inherent meaning of the construction. In the remainder of this section, we elaborate these points by working through a specific example involving interactions between the three classes of verbs described in section 2.2 and the English locative alternation.
The locative alternation involves the syntactic and semantic relations between the two constructions shown in (4):3
-
(4)
- Content-Locative Construction
Syntax: NP1 V NP2 on/in NP3 Semantics: X1 causes Y2 to go to Z3 Verb class 1: Carol1 sprayed/splashed/squirted water2 on the flowers3 Verb class 2: Carol1 dripped/poured/spilled water2 on the flowers3 Verb class 3: *Carol1 drenched/soaked/saturated water2 on the flowers3 - Container-Locative Construction
Syntax: NP1 V NP3 with NP2 Semantics: X1 causes Z3 to change state by means of adding Y2 Verb class 1: Carol1 sprayed/splashed/squirted the flowers3 with water2 Verb class 2: *Carol1 dripped/poured/spilled the flowers3 with water2 Verb class 3: Carol1 drenched/soaked/saturated the flowers3 with water2
Although these two constructions are very similar, it is well-established that they express different ways of subjectively construing the same objective type of event (for a detailed investigation see Iwata, 2008). At the most general level of conceptual analysis, the content-locative means “X causes Y to go to Z,” whereas the container-locative means “X causes Z to change state by means of adding Y.”4 From a cognitive point of view, alternating between these two schematic representations involves a “gestalt shift” (Pinker, 1989, 2007) or a “perspective change” (Tomasello, 1999; Foley, 2007). This is due in part to a general semiotic (essentially iconic) principle regarding the syntax-semantics interface, namely the “affectedness principle,” which maintains that the entity that is syntactically expressed as the direct object NP corresponds semantically to the entity that is most affected by the action (for a review see Levin & Rappaport, 2005; see also Gropen et al., 1991; Kako, 2006a, 2006b; Foley, 2007; Naess, 2007). As a consequence of this principle, constructional alternations that change which entity is mapped onto the direct object position also change which entity is treated as the main “target” or “focus” of the action. In the case of the content-locative, this entity is the one that is caused to move along a trajectory, whereas in the case of the container-locative, it is the one that is caused to change state.
Now, as shown by the example sentences in (4), verbs belonging to class (1) can occur in both locative constructions. Pinker (1989) argues that this is because they are associated with two closely related templates, one corresponding to the schematic meaning of the content-locative, and the other corresponding to the schematic meaning of the container-locative. In contrast, verb classes (2) and (3) are in complementary distribution, with the former only being acceptable in the content-locative, and the latter only being acceptable in the container-locative. These syntactic restrictions reflect language-specific semantic stipulations regarding the templates that are encoded by the verbs in the respective classes. As Pinker (1989, p. 102) puts it: “Language guards its verbs’ grammatically relevant semantic structures vigilantly. In ordinary natural speech, speakers cannot construe the meaning of a verb however they see fit before mapping it onto syntax, even if such a construal is consistent with the referent event.” Note, for example, that one can put water on flowers by spraying the water, dripping the water, or dumping the water so that the flowers become completely drenched. No matter which method is used, our cognitive flexibility allows us to treat the most affected entity as being either the water (because it changes location) or the flowers (because they become wet). That flexibility, however, is independent of language, and if we decide to refer to the actions using the verbs spray, drip, and drench, we immediately find that the range of allowable construals, and hence the range of allowable constructions, is constrained in different ways. Specifically, although spray can mean either “cause to move” (as in spray water on the flowers) or “cause to change state” (as in spray the flowers with water), drip can only have the former meaning (cf. drip water on the flowers vs. *drip the flowers with water), and drench can only have the latter (cf. *drench water on the flowers vs. drench the flowers with water). More precisely, Pinker (1989) argues that drip shares with the other verbs comprising class (2) a rather specialized template that can be glossed roughly as “X enables a mass Y to go to Z via the force of gravity”; conversely, drench shares with the other verbs comprising class (3) a narrowly defined template that can be glossed roughly as “X causes a medium Z to have a mass Y distributed throughout it.”
As our brief description of the locative alternation illustrates, the template level of verb meaning is more remote from consciousness than the root level. That is why laypeople usually have difficulty explaining their own intuitions about the differential acceptability of the sentences shown in (4), and it is why even professional linguists have spent decades engaged in vigorous debate over how best to analyze not only the locative alternation but also a vast array of other phenemena involving the interface between the semantic and syntactic properties of verbs, in English as well as in the 6000+ other languages in the world (e.g., Michaelis and Ruppenhofer, 2001; Van Valin, 2005, 2006; Wunderlich, 2006; Foley, 2007; Naess, 2007; Bowerman & Brown, 2008; Iwata, 2008). It is essential to realize, however, that the controversy is not about whether the template level of meaning exist, but rather about how it should be characterized.
2.4. Hypotheses
The major functional differences between the root and template levels of verb meaning are summarized in Table 1. The root level represents semantic features that distinguish between verbs within the same class; it is pitched at a relatively low level of schematicity; and it is irrelevant to grammar. The template level, on the other hand, captures semantic generalizations that distinguish between entire classes of verbs; it occupies a relatively high level of schematicity; and it is relevant to grammar. In addition, Table 1 indicates what we hypothesize to be the neuroanatomical correlates of some of the motor specifications made at each level of verb meaning. These hypotheses, first set forth in the Introduction, point to possible connections between the semantics of action and the mirror neuron system. To reiterate: Hypothesis 1 holds that root-level motor features of verb meaning are partially subserved by somatotopically mapped mirror neurons in the left primary motor and/or premotor cortices. Hypothesis 2 holds that template-level motor features of verb meaning are partially subserved by representationally more schematic mirror neurons in BA44 of the left inferior frontal gyrus.
Table 1.
The first three rows indicate functional differences between root and template levels of verb meaning. The bottom row indicates the hypothesized neuroanatomical correlates of the motor specifications made at each level of verb meaning.
| Roots | Templates | |
|---|---|---|
| Semantic contrasts | Distinguish between verbs within the same class | Distinguish between entire verb classes |
| Degree of schematicity | Low | High |
| Grammatical relevance | Low | High |
| Hypothesized neural correlates of motor specifications | Somatotopically organized primary motor and/or premotor cortices | Brodmann area 44 |
3. HYPOTHESIS 1: ROOT-LEVEL MOTOR FEATURES OF VERB MEANING ARE PARTIALLY SUBSERVED BY SOMATOTOPICALLY MAPPED MIRROR NEURONS IN THE LEFT PRIMARY MOTOR AND/OR PREMOTOR CORTICES
In this section we approach Hypothesis 1 in the following way. First, we describe the somatotopic organization of the primary motor and premotor cortices, and we briefly review evidence that these regions contain mirror neurons that mediate action observation-execution matching in an effector-congruent fashion. Second, we consider the question of whether these mirror neurons also contribute to representing root-level motor features of the meanings of action verbs, such as the kinematic contrasts that distinguish between bite, throw, and kick. In this context, we introduce Pulvermüller’s (2005, 2008) Semantic Somatotopy Model of verb meaning, which is closely linked to Hypothesis 1, and we summarize pertinent experimental findings from studies employing a wide range of brain mapping techniques. Finally, we conclude by discussing some problems and prospects in this line of research.
3.1. Mirror Neurons
The motor cortex has a heterogeneous architecture that includes the primary motor cortex and at least six premotor areas (e.g., Matelli et al., 1985; Luppino et al., 1991; Matsuzaka et al., 1992; Preuss et al., 1996; Rizzolatti & Luppino, 2001; Dum & Strick, 2002; Hoshi & Tanji, 2007). The primary motor cortex is traditionally thought of as containing a relatively simple map of the body’s muscles, with the tongue and lips represented close to the sylvian fissure, the hand and arm represented at lateral and dorsolateral sites, and the leg and foot represented at the vertex and in the interhemispheric sulcus. Additional somatotopic maps are known to exist in the various premotor areas, but their structure and function are controversial. Interestingly, recent studies with macaque monkeys have generated new findings suggesting that the primary motor cortex as well as some of the caudal premotor areas are topographically parcellated according to different categories of ethologically important behaviors that require the coordination of multiple joints—e.g., climbing/leaping behaviors, reach-to-grasp behaviors, central-space manipulation behaviors, defensive behaviors, hand-to-mouth behaviors, and licking/chewing behaviors (for reviews see Graziano, 2006; Graziano & Aflalo, 2007). These discoveries are broadly consistent with the classic view of an overarching somatotopic organization in which lip/tongue actions are controlled predominantly by ventral regions, arm/hand actions by lateral and dorsolateral regions, and leg/foot actions by dorsal and dorsomedial regions; however, the new findings reveal a cortical design that is not only more complex than previously suspected, but also more functionally adaptive, including for instance at least three separate hand representations that contribute to the animal’s “behavioral repertoire” in different ways.
During the past few years, numerous studies have provided evidence that the macaque motor cortex contains somatotopically organized mirror neurons—i.e., cells that discharge during both the production and the perception of certain types of actions involving particular body parts (for reviews see Rizzolatti et al., 2001; Rizzolatti & Craighero, 2004; Rizzolatti & Sinigaglia, 2008). As noted in the Introduction, research with monkeys has identified mirror neurons in the ventral premotor cortex (di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996a; Kohler et al., 2002; Ferrari et al., 2003; Keysers et al., 2003; Nelissen et al., 2005; Raos et al., 2007), the dorsal premotor cortex (Cisek & Kalaska, 2004; Raos et al., 2007), and the primary motor cortex (Raos et al., 2004, 2007; Tkach et al., 2007). Rizzolatti and Sinigaglia (2008, p. 46) describe mirror neurons as forming a “vocabulary of motor acts, in which the words are represented by populations of neurons [emphasis in original]. Some of these indicate the general goal of the act (holding, grasping, breaking, etc.), others the manner in which a specific motor act can be performed (precision grip, finger prehension, etc.), and lastly, there is a group that designates the temporal segmentation of the motor act in its elementary movements (opening and closing of the hand).” Mirror neurons represent actions independently of agency, which is to say, regardless of the self-other distinction (but cf. footnote 1). So far, the research on mirror neurons in the motor cortex of monkeys has not yet interfaced with the new studies by Graziano and colleagues mentioned above; however, it is clear that Rizzolatti’s “vocabulary” metaphor is similar to Graziano’s notion of a “behavioral repertoire,” and an important direction of future inquiry will be to determine whether the behaviorally category-specific neuronal populations uncovered by Graziano have mirror-like properties.
Turning to the human motor cortex, evidence for somatotopically organized mirror neurons comes from studies employing diverse brain mapping methods, including fMRI (e.g., Filimon et al., 2007), magnetoencephalography (MEG; e.g., Nishitani & Hari, 2000), transcranial magnetic stimulation (TMS; for a review see Fadiga et al., 2005), and direct recordings of cortical activity from implanted subdural electrodes (Tremblay et al., 2004). Remarkably, motor resonance or simulation in primary motor and premotor cortices has been shown to be triggered, sometimes in an effector-congruent form, not just by the observation of veridical dynamic actions, but also by the following types of stimuli:
actions that are perceived only as point-light displays (Saygin et al., 2004, Saygin, 2007);
actions that are merely implied by static pictures (Nishitani & Hari, 2002; Longcamp et al., 2006; Urgesi et al., 2006b; see also Pierno et al., in press);
actions that are performed by robots (Gazzola et al., 2007a; Oberman et al., 2007);
actions that are heard but not seen (Aziz-Zadeh et al., 2004; Lewis et al., 2005; Gazzola et al., 2006; Hauk et al., 2006; Caetano et al., 2007; Kaplan & Iacoboni, 2007; Lahav et al., 2007);
and even, somewhat surprisingly, actions that are biomechanically impossible (Constantini et al., 2005; Romani et al., 2005).
Moreover, this motor resonance is modulated by myriad factors, including the following:
the time course of the observed action (Borroni et al., 2005);
the observer’s attention (Chong et al., 2008);
the observer’s motivation (Cheng et al., 2007);
whether the observer’s posture is similar to the actor’s (Urgesi et al., 2006a);
whether the observer is skilled at performing the action (Calvo-Merino et al., 2006; Cross et al. 2006, in press; Aglioti et al., 2008; van Elk et al., in press);
whether the observer has been trained to simultaneously perform an action that differs from the one that is seen (Catmur et al., 2007);
whether the action is part of the behavioral repertoire of the observer’s cultural “in group” (Molnar- Szakacs et al., 2007);
whether the observer’s skin color is similar to the actor’s (Désy & Théoret, 2007);
whether the observer’s gender is the same as the actor’s (Cheng et al., 2008).
It has been argued, most forcefully by Rizzolatti and colleagues (Rizzolatti et al., 2001; Rizzolatti & Craighero, 2004; Rizzolatti & Sinigaglia, 2008), that the mirror mechanism—that is, the automatic, unconscious process by which perceived actions evoke the corresponding motor programs—plays an essential rather than an ancillary role in understanding other people’s behaviors. This view has been seriously challenged, however, by neuropsychological studies of apraxic patients who evince dissociations between, on the one hand, impaired execution and imitation of certain types of actions, and on the other, preserved recognition of the very same types of actions when they are seen being performed by other individuals (Mahon & Caramazza, 2005, 2008; Negri et al., 2007). These findings suggest that, contrary to the view advocated by Rizzolatti and colleagues, comprehending what someone else is doing may not necessarily require mapping the visually perceived action onto the matching motor program in one’s own behavioral repertoire. This controversy is clearly important and hence warrants greater attention, but we will not discuss it further here, since our main concern involves Hypothesis 1, which focuses on the issue of whether somatotopically organized mirror neurons in the primary motor and/or premotor cortices contribute to representing root-level motor features of the meanings of action verbs. We return to the significance of lesion data, however, in section 3.2.2.3.
3.2. Verb Meanings
3.2.1. The Semantic Somatotopy Model
In a series of influential studies, Pulvermüller has elaborated and supported what he calls the Semantic Somatotopy Model of verb meanings (for reviews see Pulvermüller, 2005, 2008). This model falls squarely under the rubric of the Embodied Cognition Framework, and it incorporates one of the main ideas of Hypothesis 1, namely that root-level semantic distinctions involving the motor aspects of verb meaning are represented in modality-specific format in somatotopically mapped primary motor and/or premotor cortices. To take some of Pulvermüller’s own favorite examples, the model maintains that (a) verbs designating lip/tongue actions, like lick, depend on ventral areas that control those types of actions; (b) verbs designating arm/hand actions, like pick, depend on lateral and dorsolateral areas that control those types of actions; and (c) verbs designating leg/foot actions, like kick, depend on dorsal and dorsomedial areas that control those types of actions (Figure 1). This approach also assumes that relatively subtle kinematic contrasts among the root-level meanings of verbs within the same class—contrasts that may be specified in terms of parameters for the direction, speed, force, and form of movement—are captured by neuronal populations in adjacent or even overlapping cortical regions. For instance, the fine-grained motor distinctions between march, strut, sashay, lurch, trudge, shuffle, and limp (distinctions that are not even made in some languages; see Slobin, 2004) may rely on intertwined cell assemblies in dorsal and dorsomedial primary motor and/or premotor regions that are engaged when such actions are performed.5
Figure 1.
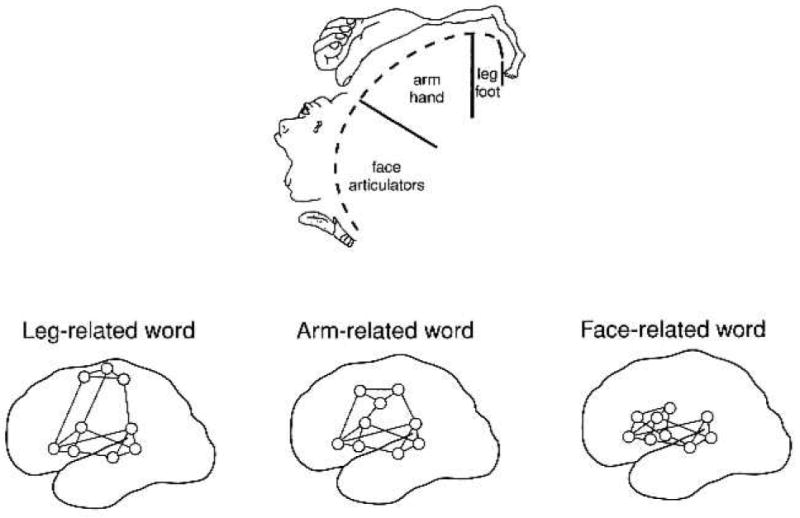
F. Pulvermüller’s (2005, 2008) Semantic Somatotopy Model of action word processing. Top: Somatotopy of the primary motor cortex as revealed by Penfield and Boldrey (1937). Bottom: Distributed neuronal ensembles bind information about word forms and the actions they encode. Because action words can relate to different parts of the body (e.g., kick, pick, lick), the cortical distributions of their root-level motor features differ from each other. (Figure adapted from Pulvermüller, 2008. [NB: Need to get permission.])
Pulvermüller’s Semantic Somatotopy Model maintains that the linguistic representation of action and the execution of action have partially overlapping neural substrates, acquired through Hebbian learning during language development. Hypothesis 1 goes further, however, by proposing a more direct link between the semantics of action and the mirror neuron system. More precisely, this hypothesis maintains that comprehending the unique root-level motor features of verb meanings involves covertly simulating the types of actions that the verbs describe, using some of the same effector-specific brain systems—or, to frame the claim most strongly, mirror neurons—that underlie not only the execution but also the observation of those types of actions. (For a similar proposal see Gallese and Lakoff [2005], and for responses to that paper see Mahon and Caramazza [2005] and Arbib [2008].) This hypothesis predicts a substantial amount of commonality among the somatotopically organized primary motor and/or premotor areas that are recruited when subjects (a) execute particular kinds of actions, (b) observe the same kinds of actions, and (c) process verbs that denote those kinds of actions. (See Turella et al. [2008] for a discussion of the need for studies that putatively focus on mirror neurons to demonstrate that the brain areas under investigation actually contribute to both the execution and the observation of actions, and see Dinstein et al. [2008] for an argument to the effect that execution-observation matching must ultimately be identified at the cellular level.) To date, however, we are only aware of two studies that have used all three sorts of experimental conditions with the same group of subjects—specifically, Postle et al. (in press), which we summarize below, and de Zubicaray et al. (2008, this issue). Needless to say, this is somewhat surprising, given all the excitement that has surrounded mirror neurons in recent years. Nevertheless, a number of studies, drawing on diverse brain mapping methods, have found intriguing primary motor and/or premotor responses to root-level motor aspects of verb meaning either (a) in combination with just action execution, (b) in combination with just action observation, or (c) in isolation. Below we review these experimental findings and discuss some of the empirical and theoretical questions that they raise.
3.2.2. Experimental Findings
3.2.2.1. Activation Patterns
We begin by considering the activation patterns reported by six fMRI studies (see also Aziz-Zadeh & Damasio, 2008; we exclude from our review a recent study by Esopenko et al., in press, that investigates semantic somatotopy linked with language production rather than language comprehension). First, Hauk et al. (2004) compared the cortical activation patterns elicited by the execution of simple bodily actions and the comprehension of action verbs. In a localizer experiment they identified somatotopically organized motor areas for producing elementary movements of the tongue, hands, and feet. In another experiment with the same subjects, they identified somatotopically organized motor areas that were engaged during passive reading of verbs denoting lip/tongue actions (e.g., lick), arm/hand actions (e.g., pick), and leg/foot actions (e.g., kick), relative to a baseline condition involving the perception of strings of meaningless hash marks. The activation patterns elicited by two of the three broad categories of verbs—namely, those denoting arm/hand actions and those denoting leg/foot actions—significantly overlapped the activation patterns associated with the corresponding categories of movements, thereby providing partial support for the notion that, as two commentators put it, “the mere reading of action-related words activates the motor homunculus” (de Lafuente & Romeo, 2004, p. 178).
Second, Aziz-Zadeh et al. (2006) compared the cortical activation patterns triggered by the observation of complex bodily actions and the comprehension of linguistic phrases describing the very same types of actions. In a localizer experiment they identified, on a subject-by-subject basis, somatotopically organized motor areas that responded to the observation of mouth-controlled biting actions, arm/hand-controlled grasping actions, and leg/foot-controlled pressing actions. In another experiment they measured signal changes in each subject’s observationally-defined effector-specific regions of interest (ROIs) while the subjects passively read linguistic phrases about the kinds of bodily actions that were used as stimuli in the localizer experiment (e.g., biting the peach, grasping the pen, pressing the car brake). The results revealed that each ROI responded most strongly to phrases involving the effector for which the ROI was defined on the basis of action observation. A shortcoming is that, of the three pairwise interactions between observation-based ROIs and phrase-based effectors, two were described by the authors as only “marginally significant” (foot vs. hand: p=0.013; foot vs. mouth: p=0.081; hand vs. mouth: p=0.061; Aziz-Zadeh et al., 2006, p. 1818). Nevertheless, the study does provide some evidence that, as a commentator put it, “reading the phrase biting the peach generates activation in the same motor areas as observing someone biting a peach” (Glenberg, 2006, p. R803).
Third, Tettamanti et al. (2005) explored the cortical activation patterns evoked during passive listening to sentences describing lip/tongue actions (e.g., I bite the apple), arm/hand actions (e.g., I grasp the knife), and leg/foot actions (e.g., I kick the ball), relative to sentences describing mental states (e.g., I appreciate sincerity).6 As expected, they found that the three different kinds of sentences generated three different patches of activation in the premotor cortex, aligned in a manner that is mostly consistent with the somatotopic organization of this region.
Fourth, Rüschemeyer et al. (2007) conducted an fMRI study that concerned, in part, the neural substrates of verbs encoding hand actions. In the current context, the most relevant experimental contrast was between a condition in which subjects made lexical decisions about morphologically simple verbs for arm/hand actions (e.g., grasp, throw, stab) and a condition in which subjects made lexical decisions about morphologically simple verbs for abstract events (e.g., think, hope, trust).7 This contrast revealed activations in the lateral precentral gyrus, specifically in regions of the premotor/primary motor cortex frequently associated with the execution and observation of arm/hand actions.
Fifth, in an fMRI study that was motivated by both the Embodied Cognition Framework and the Two-Level Theory, we and our colleagues tested several predictions about the neural correlates of root-level semantic distinctions between verbs belonging to five different classes: Running (e.g., run, jog, walk), Speaking (e.g., mumble, whisper, yell), Hitting (e.g., hit, poke, jab), Cutting (e.g., cut, slice, hack), and Change of State (e.g., shatter, crack, smash) (Kemmerer et al., 2008). The main task involved making fine-grained discriminations among triads of verbs within each class, and the baseline task involved making comparable judgments about strings of characters in Wingdings font. To our surprise, and contrary to Hypothesis 1, Speaking verbs did not engage lip/tongue-related primary motor or premotor cortex, including a region defined as the average between the peak coordinates for mouth-related verbs/sentences reported by Hauk et al. (2004) and Tettamanti et al. (2005). However, in accord with our expectations, Running verbs engaged a leg/foot-related primary motor region, Cutting verbs engaged an arm/hand-related (and tool-related; see Lewis, 2006) premotor region, and Hitting verbs engaged an arm/hand-related primary motor region. Importantly, the localization of primary and premotor regions was based on probabilistic maps derived from meta-analyses of functional neuroimaging studies (Mayka et al., 2006). Finally, Change of State verbs were not expected to activate primary motor or premotor motor cortex, and they did not do so.
Sixth, Postle et al. (in press) recently reported a study with the following design: first, they delineated left-hemisphere primary motor and premotor cortices according to cytoarchitectonically defined probabilistic maps (Eickhoff et al., 2006); second, within those regions they identified somatotopically organized mirror areas—i.e., areas that were activated during both the execution and the observation of simple lip/tongue, arm/hand, and leg/foot movements; and third, they investigated whether those effector-specific ROIs responded significantly more when subjects read correspondingly effector-specific categories of verbs, relative to when they saw several different kinds of control stimuli (specifically, non-body-part-related words, pseudowords, and strings of hash marks). Remarkably, the only significant effect was that verbs for leg/foot actions were associated with the leg/foot observation ROI in premotor cortex; beyond that, the only noteworthy findings were non-significant trends for verbs encoding lip/tongue actions to be linked with the lip/tongue execution and observation ROIs. These results appear to challenge Hypothesis 1. However, it is possible that the outcomes would have been more in agreement with Hypothesis 1 if the authors had used probabilistic maps for the primary motor and premotor cortices that are based not on cytoarchitecture but instead on functional neuroimaging studies (Mayka et al., 2006).
This conjecture gains some plausibility from the following considerations. On the one hand, Postle et al. (in press) used Eickhoff et al.’s (2006) cytoarchitectonically-derived maximum probability maps for primary motor and premotor areas to plot not only their own new data, but also the peak coordinates from three of the fMRI studies reviewed above (specifically, Hauk et al., 2004, Tettamanti et al., 2005, and Aziz-Zadeh et al., 2006). They found, contrary to Hypothesis 1, that many of the peak coordinates fell outside the primary motor and premotor areas. On the other hand, when we checked the same peak coordinates, as well as those reported in the other studies reviewed above (specifically, Rüschemeyer et al., 2007, and Kemmerer et al. 2008), against the probabilistic maps derived by Mayka et al. (2006) from functional neuroimaging studies, we found that all them fell inside the primary motor and/or premotor cortices. These activation foci are shown in Figure 2, plotted on top of the primary motor and premotor sectors of Mayka et al.’s (2006) Human Motor Area Template.
Figure 2.
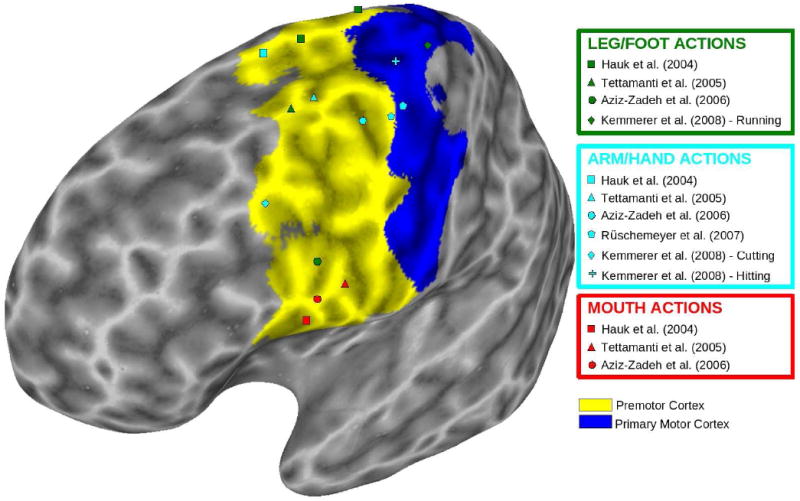
Peak MNI coordinates of left-hemisphere primary motor and premotor activations reported by fMRI studies that have probed the neural substrates of root-level motor features of verbs and sentences encoding and leg/foot actions, arm/hand actions, and mouth actions. Activations are plotted on a color-coded inflated 3D brain with definitions for the primary motor cortex (dark blue) and premotor cortex (yellow) from Mayka et al.’s (2006) Human Motor Area Template (HMAT). In particular, the primary motor cortex ROI corresponds to M1 in the HMAT, and the premotor cortex ROI is defined as the combination of ventral (PMv) and dorsal (PMd) premotor ROIs in the HMAT.
As can be seen, the foci are clustered, at least roughly, in the familiar somatotopic pattern, lending at least modest support to the notion that effector-congruent motor resonance or simulation may be integral to understanding root-level motor properties of the meanings of action verbs. Two aspects of Figure 2 raise serious concerns, however. First, as already noted, Kemmerer et al. (2008) found that Speaking verbs did not significantly activate the ventral lip/tongue portions of either the premotor cortex or the primary motor cortex, despite the fact that these verbs clearly encode various kinds of vocal communicative behaviors that are executed by the articulators. Hence, Figure 2 does not include a symbol for the Speaking verbs condition in that study. Second, although the cluster of activation foci for verbs denoting arm/hand actions is, as expected, concentrated in lateral and dorsolateral regions, it is broadly distributed; likewise, although the cluster of activation foci for verbs denoting leg/foot actions is, as expected, concentrated in dorsal regions, it too is broadly distributed. To some extent, these findings might reflect normal inter-individual variability in cortical maps (e.g., Elbert et al., 1995). Alternatively, they could reflect differences in the particular types of effector-specific actions (and corresponding behavioral repertoires; cf. Graziano, 2006; Graziano & Aflalo, 2007) encoded by the verbs that were used as stimuli across the studies. An obvious outlier, however, is the surprisingly ventral activation peak for leg/foot actions reported by Aziz-Zadeh et al. (2006). It is important to bear in mind that this peak represents the average across all of the subjects, and that the individually determined ROIs (which, as noted above, were used for the key data analyses) were widely scattered, with some residing in dorsal sites, as predicted (see Aziz-Zadeh et al.’s Figure 3). The activation foci for the other studies shown in Figure 2 also represent averages, however, so the unusually ventral average peak found in this study remains peculiar (for further discussion see Aziz-Zadeh & Damasio, 2008). More generally, the question remains: What accounts for the widely scattered distribution of putatively effector-specific peak activations shown in Figure 2? And relatedly: Which types of probabilistic maps of the primary motor and premotor cortices are more appropriate for investigating the neural substrates of root-level motor features of verb meaning—those based on cytoarchitectonics (Eickhoff et al., 2006) or those based on functional neuroimaging studies (Mayka et al., 2006)? Both of these issues warrant greater attention, since they have serious consequences for Hypothesis 1.
At this juncture, we would like to briefly discuss two additional, closely related fMRI studies (Kable et al., 2002, 2005) that are relevant in the current context because they might also be taken as challenging Hypothesis 1. In both studies, subjects made similarity judgments about the meanings of verbs (e.g., determining that digging is more like shoveling than listening, or that skipping is more like bouncing than rolling). When the investigators contrasted the verb conditions with baseline conditions, they found significant activation in the left posterolateral temporal cortex (as did many of the other fMRI studies reviewed above), but not, or at least not consistently, in the left primary motor or premotor cortices (unlike many of the other fMRI studies reviewed above). One possible reason for this outcome is that, because the investigators did not originally set out to test the kind of proposal captured by Hypothesis 1, they did not organize their stimuli into separate, equal-sized classes of verbs defined according to which body parts are predominantly used to perform the designated actions. Thus, specific somatotopically mapped motor areas may not have been engaged above threshold. Support for this interpretation comes from the fact that in our own fMRI study (Kemmerer et al., 2008), we employed a semantic similarity judgment paradigm almost exactly like the one used by Kable et al. (2002, 2005), but with distinct verb classes that were chosen in part because they encode actions that are typically executed with particular body parts. It is notable that in discussing their results, Kable et al. (2005, p. 1864) state that “motor attributes may play a less central role than sensory ones in the representation of action concepts generally, or at least for the specific stimuli we used” (emphasis added).
Finally, a serious issue concerning the fMRI studies reviewed earlier is that they allow for the possibility that the verb-induced activation patterns found in primary motor and/or premotor cortices might not reflect semantic processing per se, but might instead reflect post-comprehension motor imagery of the sort reported by Johnson et al. (2002), Stippich et al. (2002), Ehrsson et al. (2003), Michelon et al. (2006), and Bakker et al. (in press). Some investigators (e.g., Aziz-Zadeh et al., 2006; Tettamanti et al., 2005) argue that the activation patterns in their verb studies are most likely not due to voluntary motor imagery, whereas others (e.g., Kemmerer et al., 2008) are more open to this alternative possibility. A few researchers have recently begun to tackle the imagery question head-on (Tomasino et al., 2007, 2008). For instance, Tomasino et al. (2007) conducted an fMRI study in which subjects silently read action phrases and either deliberately imagined the action or performed a letter detection task. They discovered that the primary motor cortex was only engaged during the imagery condition. This outcome appears to increase the likelihood that the motor activations found in some of the fMRI studies reviewed above may have resulted from subjects adopting a strategy of imagining the bodily movements described by the linguistic stimuli. It is not clear, however, if such an account is correct, and further work is needed to resolve this issue.
3.2.2.2. Processing Speed
One way to shed more light on the question of whether the motor resonance associated with action verb processing reflects automatic semantic retrieval or voluntary post-comprehension imagery is to investigate the time course of meaning access (Hauk et al., 2008b). If neuronal populations in somatotopically mapped primary motor and/or premotor cortices represent root-level motor aspects of verb meaning, they should be activated quite rapidly after verbs are perceived. There is evidence from studies measuring event-related potentials (ERPs) that lexical-semantic information is accessed as early as 150 ms post-word-onset (Penolazzi et al., 2007; Hoenig et al., in press). In a series of ERP experiments that used source localization techniques to identify the neural generators of signals elicited by verbs encoding different body-part-specific categories of actions, Pulvermüller and colleagues found that within the time window of 150-250 ms, verbs for face actions engaged ventral motor-related areas, verbs for arm/hand actions engaged lateral motor-related areas, and verbs for leg/foot actions engaged dorsal motor-related areas (Pulvermüller et al., 2000, 2001; Hauk & Pulvermüller, 2004). Moreover, similar results were obtained in a MEG study in which subjects passively heard action verbs while their attention was focused on a silent video film, thereby supporting the view that the activation of somatotopically mapped motor regions is an automatic rather than a strategic process (Pulvermüller et al., 2005b; see also Shtyrov et al., 2004). Finally, a number of behavioral studies bolster the idea that root-level motor aspects of verb meaning are retrieved extremely fast (e.g., Borreggine & Kaschak, 2006; Boulenger et al., 2006; Kaschak & Borreggine, 2008; Nazir et al., 2008; Sato et al., 2008). Especially noteworthy in this context is a series of reaction time experiments by Zwaan and colleagues. By using the “reading by rotation” paradigm, in which subjects advance incrementally through passages of printed text by turning a knob 5°, these researchers have been revealing many nuances of rapid motor resonance induced not only by verbs, but also by other types of words, during online sentence comprehension (Zwaan & Taylor, 2006; Taylor & Zwaan, 2008; Taylor et al., 2008; Zwaan et al., in press).
These ERP, MEG, and behavioral findings strongly suggest that motor resonance occurs very quickly during action verb comprehension. It is still not clear, though, whether this resonance reflects genuine semantic access or post-semantic processing. First of all, it is noteworthy that the ERP and MEG studies conducted by Pulvermüller and colleagues did not distinguish neuroanatomically between the primary motor and premotor cortices. This is important because, as mentioned above, Tomasino et al.’s (2007) fMRI study suggests that these two sectors of the frontal lobe may play different roles in action verb processing, with the primary motor cortex only being engaged when subjects deliberately imagine the described movements (see also Tomasino et al., 2008, which we discuss below). For the sake of argument, let’s suppose that the rapidly triggered activations that were found in the ERP and MEG studies included the premotor cortex but not the primary motor cortex. Given that the premotor cortex is somatotopically organized, one could argue that such an outcome is still consistent with Hypothesis 1, because the fast premotor activation might reflect an automatic process of motor simulation that is both embodied and legitimately semantic in content. This is not the only available interpretation, however, because as Mahon and Caramazza (2008) point out, an alternative possibility is that root-level motor features of verb meaning might be represented amodally in a non-somatotopically mapped brain region that is engaged prior to, or even simultaneously with, the premotor cortex, which itself might contribute only to post-semantic processing (like the primary motor cortex, according to Tomasino et al., 2007, 2008). Mahon and Caramazza (2008) elaborate this idea more fully as follows: “In order for the speed of motor system activation to be relevant for distinguishing between an embodied and disembodied interpretation, it would have to be known, independently, what types of cognitive processes are interposed between the perception of the action word and the activation of the motor system. In other words, it would have to be known that the activation of the motor system was not mediated by the retrieval of ‘abstract’ conceptual content. Of course, this is precisely the ‘unknown’ that is at issue.” Moreover, it is important to avoid conflating automaticity with necessity because, strictly speaking, even if it were definitively demonstrated that primary motor and/or premotor areas are rapidly engaged during the comprehension of action verbs, this would not entail that those areas are essential for understanding the motor content encoded by the verbs. We turn now to a more detailed consideration of this question of “functional relevance.”
3.2.2.3. Functional Relevance
The most powerful way to argue that somatotopically mapped primary motor and/or premotor cortices represent root-level motor features of verb meaning would be to show that altering the functional state of those brain regions directly influences the processing of the pertinent semantic properties of verbs. Below we summarize several studies that have reported such effects (see also Pulvermüller’s [2005, 2008] treatment of this topic as well as Fischer & Zwaan’s [2008] discussion of “the necessity question”).
A number of studies have used TMS to explore functional interactions between motor areas and action verbs. All of the results are generally consistent with Hypothesis 1, but it must also be acknowledged that the literature contains some puzzling contradictions. Pulvermüller et al. (2005a) found that stimulation of the hand region of the left primary motor cortex led to faster lexical decisions for verbs encoding arm/hand actions compared to verbs encoding leg/foot actions, and that stimulation of the leg region had the opposite effect. However, Buccino et al. (2005) found that stimulation of primary hand and leg regions led to slower reaction times when subjects listened to sentences describing actions performed with the corresponding effectors and also responded to the stimuli with those effectors. In addition, Buccino et al. (2005) discovered that the amplitude of motor evoked potentials (MEPs) recorded from hand and foot muscles was reduced when subjects processed effector-congruent action sentences. But when Glenberg et al. (2008b) conducted a similar study that contrasted sentences describing “transfer events” (e.g., You give the pizza to Andrea) with sentences describing “non-transfer events” (e.g., You smell the pizza with Andrea), they discovered that the amplitude of MEPs recorded from hand muscles was enhanced for the former sentences relative to the latter ones.8 Glenberg et al. (2008b) suggested that the discrepancy between their MEP results and those of Buccino et al. (2005) might be due to Buccino et al.’s (2005) use of sentences with exclusively third person participants. Yet Oliveri et al. (2004) also employed third person stimuli in a study involving verb production and found enhanced MEPs, like Glenberg et al. (2008b), rather than reduced MEPs, like Buccino et al. (2005). Finally, in an especially interesting new study, Gerfo et al. (2008) used repetitive TMS to temporarily suppress the excitability of the hand area of the left primary motor cortex while subjects performed morphological transformations of both verbs and nouns, and found that this kind of neurophysiological interference significantly lengthened reaction times for both action verbs and action nouns, but did not affect reaction times for either state verbs or state nouns.
Although not all of these studies are consistent with each other, overall they support the idea that root-level motor properties of verb meaning may be processed, at least in part, in the primary motor cortex. Nevertheless, they do not exclude the alternative possibility that the observed neurophysiological effects reflect post-semantic processing. In fact, Tomasino et al. (2008) recently investigated this issue and obtained results that, according to their interpretation, favor the alternative view. Specifically, they used TMS to stimulate the hand area of the left primary motor cortex while subjects performed three different kinds of tasks after reading action verbs: (a) they simply indicated when they had finished reading; (b) they estimated the frequency of the verbs; and (c) they imagined performing the action and judged whether it would involve a hand rotation. TMS facilitated performance for just the final task, and this effect was found for all stimulation delays, from 150 to 750 ms post-verb-onset. Tomasino et al. (2008, p. 1924) treat these results as evidence that “motor resonance is intimately related to subjects (explicitly or implicitly) performing mental simulation but not to action meaning encoding per se. Our view is that the presence of M1 [i.e., primary motor] activation during action-related word understanding depends on whether or not, during reading, subjects simulate the movement the words are referring to. In this view, mental simulation (and thus M1 activation) would be a side effect of or a corollary phenomenon to understanding motor-related words, rather than a requirement for language comprehension” (emphasis in original). If it is true that, as Tomasino et al. (2008) maintain, action verbs can lead to post-comprehension imagery mediated by the primary motor cortex within 150 ms, this raises a question as to whether the early-onset ERP and MEG findings discussed in the previous section can be taken as reliable evidence against a post-comprehension imagery explanation. However, as mentioned earlier, it remains possible that root-level motor properties of verb meaning depend on somatotopically mapped premotor areas, and that the retrieval of those properties during online comprehension involves automatically running embodied simulations in those areas, rather than in primary motor areas. Importantly, such a scenario would still be consistent with Hypothesis 1.
Evidence for this view comes from lesion studies. Kemmerer et al. (2001a) administered a battery of six tests, each of which probes knowledge of action verbs/concepts in a unique way, to 89 brain-injured patients with focal, stable, unilateral damage in either the left or right hemisphere. From the perspective of the Two-Level Theory, the tests that were employed cover a wide range of verb classes; however, they have a preponderance of items that focus on hand-action concepts. Of the 89 patients who were studied, 30 were impaired on at least one test. Together, these 30 patients manifested a total of 22 different performance profiles (i.e., combinations of “passes” and “failures”) across the six tests, and each test dissociated from all the others, suggesting that each one may have distinctive processing requirements that can be independently disrupted (see also Kemmerer et al., 2001b). One of the few patients who failed all six tests, and who can therefore be regarded as having severely impaired knowledge of the meanings of action verbs, was 1172JP. His lesion, which is depicted in a separate paper (Kemmerer & Tranel, 2003), is centered in the left posterior inferior frontal gyrus, but it extends superiorly into the lateral portion of the precentral sulcus and the adjacent posterior portion of the middle frontal gyrus—an area that is generally considered to be a hand-related sector of the premotor cortex. Moreover, it is noteworthy that this same premotor region was among the areas of maximal lesion overlap in a related study that focused on 26 patients who failed one or both of two tests that were drawn from the same battery used by Kemmerer et al. (2001a) and that assess knowledge of action concepts independently of the input/output processing of verb forms (Tranel et al., 2003).
These lesion studies strengthen the view that the premotor cortex is functionally relevant to the “semantics of kinematics.” The studies are not without limitations, however, and additional research using the lesion method is urgently needed in order to test Hypothesis 1 more rigorously. In particular, what is necessary at this point are neuropsychological studies that carefully explore whether—and, if so, how—focal lesions to leg/foot-related, arm/hand-related, and lip/tongue-related regions of the primary motor and/or premotor cortices affect the processing of the corresponding body-part-specific root-level motor features of verb meaning. The need for such studies cannot be overestimated, since they will play a key role in evaluating Hypothesis 1. Furthermore, this field of research could benefit greatly from more studies that use repetitive TMS to transiently disrupt, in healthy subjects, the operations of precisely targetted motor areas.
3.3. Problems and Prospects
Stepping back now from the details, we would like to consider some general issues regarding the current status of Hypothesis 1, bearing in mind the broader contexts of the Embodied Cognition Framework and the Two-Level Theory of verb meaning. To reiterate: Hypothesis 1 holds that root-level motor features of verb meaning are partially subserved by somatotopically mapped mirror neurons in the primary motor and/or premotor cortices. While some aspects of this hypothesis receive modest support from the literature reviewed above, many important questions remain unanswered.
First, as noted in section 3.2.2.1, a problem requiring further research is how to demarcate the boundaries of the primary motor and premotor cortices. Different probabilistic maps, derived from cytoarchitectonics (Eickhoff et al., 2006) and functional neuroimaging studies (Mayka et al., 2006), provide different parcellations of these regions. Both types of maps have strengths and weaknesses, but as yet there is no “gold standard” for determining which is more appropriate for purposes of exploring proposals like Hypothesis 1. In addition, as intimated in section 3.1, the somatotopic organization of human motor areas, including both primary motor and premotor cortices, is not nearly as straightforward as is sometimes assumed in the literature on action verbs (e.g., Sanes & Schieber, 2001; Schubotz & von Cramon, 2003). This clearly increases the level of difficulty of investigating the relations between, on the one hand, effector-specific semantic features of action verbs, and on the other, effector-specific regions of the motor system.
Another major problem is that so far only one study (Postle et al., in press) has addressed the central claim of Hypothesis 1—namely, that root-level motor features of verb meaning are linked specifically with mirror neurons in the pertinent frontal regions. This claim predicts that accessing those features requires engaging some of the same neuronal populations that are engaged during both the execution and the observation of the corresponding types of actions. Hence an important direction of future research will be to search for such overlapping mechanisms, taking full advantage of all the available brain mapping methods. For example, understanding verbs expressing different types of arm/hand actions (e.g., pinch vs. poke vs. slap) should ignite certain primary motor and/or premotor cell assemblies that are also ignited when one performs the designated kinds of actions and when one sees them being performed by someone else. Moreover, damage to those cell assemblies should impair not only the capacity to comprehend the idiosyncratic kinematic specifications of the verbs, but also the capacity to correctly execute the associated actions as well as the capacity to fully appreciate them when they are produced by other people. Findings like these are needed in order to substantiate Hypothesis 1, but they have not yet been reported.
Yet another significant issue is one that we have already raised at several points in our review, namely the question of how to determine whether the engagement of the primary motor and/or premotor cortices during a verb comprehension task reflects genuine semantic processing of the root-level motor features of verb meaning, or instead post-comprehension cognitive operations such as the voluntary generation of motor imagery. This issue is very important, both methodologically and theoretically, because it bears on the broader question of how the motor areas of the left hemisphere might contribute to the conceptual knowledge encoded by action verbs. Below we discuss several facets of this complex issue.
At the very outset, it is worthwhile to remind ourselves that embodied and disembodied theories of cognition make different predictions about the involvement of modality-specific cortical areas in semantic processing, and, in this particular case, about the involvement of motor areas in verb comprehension:
Embodied theories claim that root-level motor features of verb meaning are modality-specific in content, and that they depend on somatotopically organized motor areas of the brain. So these theories predict that when those aspects of verb meaning are processed, those areas of the frontal lobe should be engaged.
Disembodied theories claim that root-level motor features of verb meaning are amodal in content, and that they do not depend on somatotopically organized motor areas of the brain. So these theories predict that when those aspects of verb meaning are processed, those areas of the frontal lobe should not be engaged.
Of course, the main concern is that the participants in various verb processing experiments might voluntarily evoke motor images (and perhaps also other kinds of modality-specific images) after understanding the verb stimuli. So any significant engagement of motor areas might index imagery rather than comprehension. Our point, however, is this: According to the basic assumptions of disembodied theories, there is no functional reason why subjects should deliberately generate imagery, unless of course they are overtly instructed to do so. After all, if the semantic content that must be accessed in order to perform a verb comprehension task is abstract, as disembodied theories maintain, why would subjects bother to consistently activate modality-specific representations? In pursuing a similar line of argumentation, Simmons et al. (2007, pp. 2807-8) observe that “it would seem extremely odd for a proponent of amodal accounts to argue that the task cannot be performed using the amodal representations central to amodal theories, but must be performed using additional, ancillary, effortful processes….”
To see how these contrasting theoretical predictions play out in practice, consider once again Tomasino et al.’s (2008) study in which, for one of the conditions, subjects were shown verbs and were instructed to first imagine themselves performing the action and then decide whether it involves a hand rotation. The results suggested that this condition activated hand-related primary motor cortex, and the authors interpreted this activation as the neural signature of motor imagery. However, the question arises as to whether a similar pattern of activity, if not in primary motor cortex then perhaps in premotor cortex, would have emerged if the subjects had simply been instructed to make hand rotation judgments about verb meanings, without first being told to conjure up a mental image of each action. The task would then have reduced to pure property verification, just like in several other studies that have used such a paradigm to test proposals about modality-specific aspects of semantic structure (e.g., Chao et al., 1999; Kellenbach et al., 2001; Kan et al., 2003; Goldberg et al., 2006; Simmons et al., 2007; Hoenig et al., in press). Embodied theories predict that, in such a situation, making hand rotation judgments about verb meanings would still engage hand-related motor areas of the brain. Disembodied theories, however, do not make this prediction.
Nevertheless, it remains possible that when subjects perform various verb comprehension tasks that involve so-called “controlled,” as opposed to “automatic,” processing, they sometimes do deliberately evoke, for whatever reason, explicit motor images immediately after understanding the verbs. Hence an important aim of future research should be to delineate the similarities and differences between, on the one hand, the neural correlates of accessing root-level motor features of verb meaning, and on the other, the neural correlates of evoking rich motor images that instantiate those features. A potentially profitable way to tackle this challenge would be to design studies that allow the investigators to compare, for the very same sets of action verbs, the neural responses of motor areas during (1) explicit imagery conditions and (2) a wide range of semantic conditions involving both controlled and automatic processing. Explicit imagery conditions might consist of presenting subjects with subtly contrasting verbs from the same class (e.g., trudge, limp, stroll) and instructing them to vividly imagine executing the designated bodily movements. Semantic conditions involving controlled processing might include tasks like property verification (e.g., “Does trudge describe a manner of locomotion?”) and semantic similarity judgment (e.g., “Is trudge more like limp or stroll?”). Finally, semantic conditions involving automatic processing might draw upon paradigms like lexical decision (e.g., “Is trudge a real word of English?”) and masked repetition priming (i.e., a technique that probes semantic access in a way that is completely subliminal yet also detectable with both fMRI and ERPs; see Naccache & Dehaene [2001]). For other novel approaches to distinguishing between automatic semantic processing and strategic imagery generation, see Hauk et al. (2008a) and Pirog Revill et al. (2008).
Ideally, a multi-pronged methodology like this would enable researchers to determine whether, independently of voluntary motor imagery, certain cell assemblies in the primary motor and/or premotor cortices reliably “track” certain root-level motor features of verb meaning across multiple experimental conditions. A positive outcome would, of course, support the Embodied Cognition Framework. On the other hand, a negative outcome—that is, one in which neural responses vary across tasks without exhibiting much, if any, consistency for the same sets of verbs—would have more complicated consequences. Of course, one possibility is that it might be taken as evidence against the Embodied Cognition Framework. An alternative possibility, however, is that it might be taken as a sign that we, as a scientific community, should re-think our theoretical views about the degree to which concepts can be regarded as representationally “stable” neurocognitive constructs (for a recent exploration of this topic, see Hoenig et al., in press). Given that, as noted in section 3.1, motor resonance during action observation is modulated by myriad properties of both the actor and the perceiver, it is not inconceivable—in fact, it might even be expected—that motor resonance during verb comprehension is similarly labile, reflecting both the contingencies of situational factors and the vagaries of individual experience (for relevant data see Glenberg et al., 2008a).
To summarize our main points: In order for Hypothesis 1 to be either confirmed or disconfirmed, research must progress along several fronts. First, the neurobiological boundaries of the primary motor and premotor cortices, as well as the somatotopic patterns within those regions, must be demarcated more sharply. Second, more neuroscientific studies must compare the processing of root-level motor features of verb meaning with both the execution and the observation of actions with the corresponding features. Finally, greater efforts must be made to distinguish between authentic semantic processing and post-comprehension cognitive processing, for example by using a wide range of methods to investigate the same classes of verbs.
4. HYPOTHESIS 2: TEMPLATE-LEVEL MOTOR FEATURES OF VERB MEANING ARE PARTIALLY SUBSERVED BY REPRESENTATIONALLY SCHEMATIC MIRROR NEURONS IN BRODMANN AREA 44 OF THE LEFT INFERIOR FRONTAL GYRUS
We turn now to Hypothesis 2. First, we describe a special category of mirror neurons that mediate action observation-execution matching in a way that focuses on the goals and intentions, rather than the manners, of object-directed movements. Cells with these relatively abstract tunings have been identified in area F5 of the macaque ventral premotor cortex, and there is mounting evidence that they also exist in the putative human homologue of that region, namely BA44 of the inferior frontal gyrus. Second, we address the question of whether these mirror neurons play a role in representing the template-level motor aspects of verb meanings—i.e., those aspects of the semantics of action that involve austere event structure schemas which are essentially conceptual generalizations over entire classes of verbs. Finally, we conclude by suggesting several directions for further research on this topic.
4.1. Mirror Neurons
Although some of the mirror neurons that have been found in area F5 of the macaque ventral premotor cortex represent very detailed properties of actions, the majority of them represent actions at a rather schematic level of coding (for reviews see Rizzolatti et al., 2001; Rizzolatti & Craighero, 2004; Rizzolatti & Sinigaglia, 2008). For example, many F5 cells classified as “broadly congruent” mirror neurons have response properties that are selective for a particular kind of goal, regardless of which body part is used to achieve it. As Gallese et al. (1996) have shown, cells of this type may discharge during both execution and observation of hand-controlled grasping actions (same effector, same goal), as well as during observation of mouth-controlled grasping actions (different effector, same goal), but not during observation of hand-controlled placing actions (same effector, different goal). In addition, Umilta et al. (2001) discovered that the firing rate of many mirror neurons in area F5 increases significantly not only when the monkey sees a complete action in which an experimenter reaches for and grasps an object, but also when the monkey sees just the reaching phase of such an action (due to the occlusion of the object) and hence must infer the final grasping phase. These mirror neurons may play an important role in predicting or anticipating the actions of other individuals.
It has been argued that the human homologue of macaque F5 is BA44 (Petrides & Pandya, 1994; Pandya & Yetarian, 1996; Geyer et al., 2000; Arbib & Bota, 2003; for a summary see Arbib & Bota, 2006, p. 153). However, this view has not gone unchallenged (Petrides, 2005; Toni et al., 2008). While acknowledging that this issue remains unresolved, we will adopt here, mainly for exploratory purposes, the assumption that BA44 does correspond roughly to F5.
In keeping with the monkey literature, there is evidence suggesting that BA44 contributes to both the production and the recognition of actions. For example, data from diverse brain mapping methods implicate BA44 in motor resonance for the fine-grained kinematic features of arm/hand actions (MEG: Nishitani & Hari, 2000; fMRI adaptation: Hamilton & Grafton, 2007; Lestou et al., 2008; repetitive TMS: Pobric & Hamilton, 2006; Urgesi et al., 2007; Candidi et al., in press). More importantly for present purposes, a number of recent studies suggest that, like the F5 mirror neurons mentioned above, BA44 also exhibits motor resonance for the goals of actions. First of all, several fMRI studies suggest that BA44 mediates observation-execution matching for the goals of arm/hand actions (e.g., Hamzei et al., 2003; Johnson-Frey et al., 2003; Gazzola et al., 2007a,b; Engel et al., in press; Schubotz & von Cramon, in press).9 In addition, a recent neuropsychological study with a group of patients manifesting limb apraxia found that the left BA44 in particular was the site of greatest lesion overlap for those patients who were impaired on a gesture recognition task that “assessed the ability to judge whether the ultimate goals of transitive gestures are attained or whether the symbolic meaning of intransitive gestures is maintained” (Pazzaglia et al., 2008, p. 3039). Finally, a few fMRI studies have linked BA44 with the ability to understand the covert intentions guiding other people’s actions, particularly when those actions are “stereotypic” (Iacoboni et al., 2005; Brass et al., 2007; Liepelt et al., in press).
Given that this field of inquiry is still quite new, it is not surprising that much of the research described above is controversial. For instance, some of the fMRI studies have been criticized on methodological grounds (Turella et al., 2008), and although the deficit-lesion correlation discovered by Pazzaglia et al. (2008) is impressive, it is also noteworthy that, as the authors acknowledge, non-trivial exceptions to the pattern have been previously reported—specifically, some patients with left BA44 damage have impaired action production but preserved action recognition (e.g., case DR reported by Negri et al., 2007; see also Mahon & Caramazza, 2005, 2008). Despite these limitations, we feel that the idea that BA44 may be involved in higher-order aspects of motor resonance has received enough support to justify an exploration of how template-level motor features of verb meaning might fit into the picture.
4.2. Verb Meanings
4.2.1. How Might Templates be Related to Mirror Neurons?
As indicated in section 2.3, templates are event structure schemas or, in Pinker’s (1989) terms, “thematic cores” that integrate the semantic features shared by all the verbs comprising particular classes. They abstract away from the root-level biomechanical specifications of individual verbs so as to capture overarching patterns of human behavior, patterns that typically consist of agents performing various kinds of goal-directed volitional actions involving one or more other entities. One of the most interesting and important properties of templates is that many of them represent different ways of subjectively construing the same objective sorts of scenarios. For example, as we noted in our discussion of the locative alternation, verbs in class (1), like spray, are associated with two closely related templates: “X causes Y to go to Z,” as in Carol sprayed water on the flowers, and “X causes Z to change state by adding Y,” as in Carol sprayed the flowers with water. These two templates vary with respect to which entity is cognitively treated as the main “target” of the action—the thing that changes location, or the thing that changes state. And as illustrated by the two sentences involving spray, these semantic differences regarding the relative prominence of the participants in the hierarchical goal structures of the templates correspond isomorphically to syntactic differences regarding the relative prominence of the NPs in the hierarchical grammatical structures of the constructions (see the coindexed subscripts indicating linking patterns shown in (4) in section 2.3). However, there are also language-specific restrictions on which templates are encoded by which verbs, or, more accurately, by which verb classes. Recall, for instance, that verbs in class (3), like drip, stipulate that the main affected entity can only be the thing that changes location, and that, conversely, verbs in class (4), like drench, stipulate that it can only be the thing that changes state. In the current context, the key point is this: Template-level motor features of verb meaning capture high-level, differentially construable, aspects of action representation that involve the goals and intentions of agents.10
Now, according to the Embodied Cognition Framework, words and larger linguistic expressions are effectively conventionalized cues for running modality-specific simulations. If this proposal is applied to the kinds of templates that are encoded by verbs and that serve as the semantic interface between verbs and constructions, it predicts that comprehending sentences like Carol sprayed water on the flowers and Carol sprayed the flowers with water involves simulating not only how the described actions are performed in terms of relatively low-level, effector-specific kinematics, but also how they are construed in terms of relatively high-level, effector-neutral goals and intentions—that is, in terms of whether the ultimate purpose of the agent’s action is to affect the water by causing it to move onto the flowers, or to affect the flowers by causing them to become wet. (See Bergen and Chang [2005] for a computational model of a simulation approach to constructional meaning.) On the one hand, as we discussed in section 3, Hypothesis 1 maintains that the first type of simulation depends on somatotopically mapped mirror neurons in the primary motor and/or premotor cortices. On the other hand, as we discuss in the rest of this section, Hypothesis 2 maintains that the second type of simulation depends on representationally more schematic mirror neurons in left BA44. Just like with Hypothesis 1, we are not aware of any studies that have directly addressed Hypothesis 2. That is to say, to our knowledge no studies have attempted to determine whether the same neuronal populations in BA44 are engaged during all three of the following conditions: (a) the linguistic processing of certain template-level motor features of verb meaning, (b) the execution of actions with the corresponding properties, and (c) the observation of actions with the corresponding properties. Nevertheless, the literature does contain some experimental findings that provide preliminary support for Hypothesis 2. Below we review a number of studies that consider BA44 in the context of both sentence processing (section 4.2.2) and single verb processing (section 4.2.3).
4.2.2. Studies Involving Sentence Processing
4.2.2.1. Functional Neuroimaging Studies
Many fMRI studies are pertinent to Hypothesis 2, but we will focus on two in particular. First, we noted in section 3.2.2.1 that Tettamanti et al. (2005) found a somatotopic pattern of activation in the premotor cortex when subjects heard sentences describing actions performed with different body parts (e.g., I bite the apple, I grasp the knife, I kick the ball), relative to when they heard sentences describing psychological states (e.g., I appreciate sincerity). Another fascinating discovery was that when all three types of action sentences were contrasted with the psychological sentences, the only brain region that was significantly engaged was left BA44 (peak MNI coordinates: -52, 10, 16). Crucially, the sentences in each condition had the same syntactic structure, so the only distinguishing factor was semantics; however, because the various kinds of action sentences were averaged together, the engagement of left BA44 must have reflected aspects of meaning that are effector-neutral. In fact, the authors concluded that this activation was “related to the semantics of the presented linguistic material, at an abstract, body-part-independent level” (Tettamanti et al., 2005, p. 277). This interpretation is not only consistent with, but can be enriched by, the Two-Level Theory. Although the authors did not provide a comprehensive list of the stimuli used in each experimental condition, they did give some examples (mentioned above) which suggest that the action sentences instantiated the prototypical transitive construction, shown in (5) (see Naess, 2007, for an in-depth analysis). This construction licenses two-argument verbs that encode a template specifying that a volitional agent acts forcefully on another entity. In contrast, the psychological sentences apparently instantiated the construction shown in (6), which licenses two-argument verbs that encode a template with no motor content whatsoever, specifying instead that a person has a certain mental attitude about something (see Jackendoff, 2007, for a recent analysis that combines linguistic-semantic and social-cognitive perspectives).
-
(5)
Prototypical Transitive Construction
Syntax: NP1 V NP2 Semantics: X1 acts on Y2 in some manner -
(6)
Experiencer-Subject Psych Verb Construction
Syntax: NP1 V NP2 Semantics: X1 has some mental attitude about Y2
Thus, the finding that the action sentences engaged left BA44 significantly more than the psychological sentences supports the idea that this brain region contributes to representing especially the motor aspects of the event structure templates encoded by verbs. Nevertheless, a lingering concern is that, as pointed out by Rüschemeyer et al. (2007), Tettamanti et al.’s (2005) action sentences differed from their psychological sentences not only in having concrete verbs but also in having concrete nouns, making it impossible to be certain that the differential activation found in left BA44 reflected the verbs rather than the nouns.
In another fMRI study that bears on Hypothesis 2, subjects made sensibility judgments about object-directed arm/hand actions that were presented in two different ways—linguistically as spoken sentences,11 and visually as dynamic video clips (Baumgaertner et al., 2007). Two additional conditions involving sentences and videos about inanimate motion events were included to control for action specificity. The investigators found that left BA44 was activated in all of the key contrasts: action sentences vs. non-action sentences (peak MNI coordinates: -57, 12, 24); action videos vs. non-action videos (peak MNI coordinates: -51, 6, 27); and [action sentences + action videos] vs. [non-action sentences + non-action videos] (peak MNI coordinates: -51, 9, 24). It is noteworthy that, as in Tettamanti et al.’s (2005) study, the action and non-action sentences had comparable syntactic structures and hence differed primarily in semantic content. More specifically, all of the action sentences expressed scenarios in which an agent causes something to change location or state by means of operating on it with a tool (e.g., He is sweeping with a broom), whereas all of the non-action sentences expressed scenarios in which an inanimate entity moves as a result of natural forces (e.g., The leaves are swirling through the air). Because the former sentences, but not the latter ones, contained verbs associated with templates involving goal-oriented instrumental actions (Koenig et al., 2008), the imaging results strengthen the notion that those templates rely on left BA44 (although it must also be acknowledged that the sentences in the two conditions also varied in other ways that could conceivably have influenced the imaging results). Moreover, the finding that left BA44 was engaged by object-directed arm/hand actions not only when they were presented as spoken sentences, but also when they were presented as video clips, suggests that this cortical area “is endowed with polymodal capabilities, allowing the processing of higher-level conceptual aspects of action understanding” (Baumgaertner et al., 2007, p. 881).
4.2.2.2. Lesion Studies
If template-level motor features of verb meaning depend on left BA44, as Hypothesis 2 maintains, then damage to that region should impair those features. A few neuropsychological studies that were directly motivated by the Two-Level Theory have obtained results that are consistent with this prediction (Kemmerer, 2000, 2003; Kemmerer & Wright, 2002; for a review see Kemmerer, 2006). One study focused specifically on the locative alternation and discovered the following pattern of performance in two patients—1962RR and 1978JB—who had left inferior frontoparietal lesions that encompassed left BA44 (Kemmerer, 2000; note that the lesion site of one of these patients, 1962RR, is depicted in Kemmerer & Tranel, 2003). Both patients performed well on a verb-picture matching test requiring discrimination between subtle root-level semantic properties of verbs in particular classes, including classes (1), (2), and (3) in section 2.2. However, both patients failed a grammaticality judgment test involving sentences like those shown in (4) in section 2.3. This test assessed the patients’ ability to determine the compatibility between, on the one hand, the templates encoded by the same verbs used in the matching test, and on the other, the schematic meanings of the content-locative and container-locative constructions. As already noted, verbs in class (1), like spray, are associated with two templates, one of which fits with the schematic meaning of the content-locative, as in Carol sprayed water on the flowers, and the other of which fits with the schematic meaning of the container-locative, as in Carol sprayed the flowers with water. In contrast, verbs in class (2), like drip, only encode a single template that licenses occurrence in just the content-locative (cf. Carol dripped water on the flowers vs. *Carol dripped the flowers with water), and verbs in class (3), like drench, only encode a single template that licenses occurrence in just the container-locative (cf. *Carol drenched water on the flowers vs. Carol drenched the flowers with water). Strikingly, the patients judged many ungrammatical sentences as sounding fine, and judged many grammatical sentences as sounding bad. Their errors could not be attributed, however, to a disturbance of either purely syntactic processing or metalinguistic judgment ability, because both patients passed another test that evaluated the integrity of those capacities. Thus, it is possible that the patients’ impairments selectively disrupted their appreciation of the template-level motor features of verb meaning. In other words, using the examples above for purposes of illustration, the patients may have defective understanding of the lexical-semantic stipulations that (a) spray can refer to either of two types of goal-directed action—causing something to move, or causing something to change state; (b) drip can only refer to the first type of action; and (c) drench can only refer to the second type of action. Importantly, the fact that both patients suffered damage to left BA44 supports the notion that this region may be esssential for representing and/or processing those aspects of verb meaning. Furthermore, both patients manifested similar dissociations—namely, good performance on tests probing root-level semantic features, but poor performance on tests probing template-level semantic features—in two other studies that focused on different verb classes and constructions (Kemmerer, 2003; Kemmerer & Wright, 2002). Those studies also obtained comparable behavioral results for two additional patients—1726RO and 1760KS—who had lesions that included left BA44 and/or the underlying white matter. It must be acknowledged, however, that all of these studies have limitations. Most importantly, it remains possible that the patients’ deficits did not affect verb templates per se, but rather the closely related schematic meanings of the pertinent constructions and/or the complex linking patterns that must be established between syntactic and semantic structures during sentence comprehension (e.g., O’Grady & Lee, 2005).
In this context, it is also worth mentioning the well-documented finding that patients with agrammatism—a disorder that is often (but not always) associated with left BA44 lesions (Vanier & Caplan, 1990)—are typically impaired at retrieving action verbs in sentence production tasks as well as in isolation. There is substantial evidence that the degree to which a given verb is difficult for an agrammatic patient to access depends in part on its argument structure(s) (Kegl, 1995; Thompson et al., 1997; Kim & Thompson, 2000, 2004; Kiss, 2000; Luzzatti et al., 2002; De Bleser & Kauschke, 2003). Two distinct kinds of influence have been reported. First, verb retrieval difficulty often increases in proportion to the number of syntactic argument positions that are strictly subcategorized by the verb, such that one-place verbs (e.g., snore) are easier to produce than two-place verbs (e.g., devour),12 which in turn are easier to produce than three-place verbs (e.g., give). This hierarchy is manifested in natural discourse contexts like conversation and storytelling, in experimentally constrained sentence generation and completion tasks, and even in the paradigmatic single word production task—confrontation picture naming. Second, verb retrieval difficulty is also affected by the number of alternative argument structures that are available, such that verbs with just one (e.g., snore, which is intransitive) are easier to produce than verbs with two or more (e.g., eat, which is ambitransitive, i.e., it can be either intransitive or transitive).13 Both of these factors—i.e., the number of strictly subcategorized argument positions, and the number of alternative argument structures—also influence verb retrieval in normal subjects, but the effects are far more dramatic in agrammatic patients.
To be sure, this line of research has yielded many valuable insights. However, it has concentrated mainly on the syntactic aspects of argument structure and has not devoted as much attention to the corresponding semantic aspects, which, according to the Two-Level Theory, include the event structure templates that are encoded by verbs, as well as the schematic meanings that are associated directly with clausal constructions.14 As verbs increase in valence from one to two to three core participants, and as they increase in the range of constructions they can occur in, so too they increase in the intentional and causal complexity of their templates. Indeed, exploring the intricasies of such phenomena within and across languages is a major focus of research in semantic typology (e.g., Croft, 1991; Dixon & Aikhenvald, 2000; Van Valin, 2005; Wunderlich, 2006; Foley, 2007). The upshot: It is conceivable that the verb retrieval deficits exhibited by agrammatic patients with left BA44 lesions are due in part to impaired representation and/or processing of these template-level aspects of verb meaning.
Although we think this interpretation is plausible enough to warrant further investigation, we are well aware that there are many complications, one of which stems from evidence that major participant roles, like actor and undergoer, may be mediated in part by posterior temporoparietal regions that interact closely with areas concerned with biological motion patterns, spatial relations, and perspective-taking (Bornkessel et al., 2005; Grewe et al., 2007; Shetreet et al., 2007; Thompson et al., 2007; Wu et al., 2007; see also Shapiro et al., 1993). Still, BA44 may be essential for computing the hierarchical rankings or event structure configurations of different participant roles and their corresponding syntactic positions during on-line language processing, guided in part by principles of motor cognition that may also be employed during both the execution and observation of goal-directed bodily actions. We return to this issue in section 4.3.
4.2.3. Studies Involving Single Verb Processing
We pointed out in section 2.3 that the template level of verb meaning differs from the root level not only in being more relevant to grammar but also in being less accessible to consciousness. For both of these reasons, it can be difficult to investigate the template level with experimental paradigms that involve single verb processing. Nevertheless, several studies that draw upon such paradigms have generated results bearing on Hypothesis 2.
Studies of confrontation naming using both functional neuroimaging (e.g., Damasio et al., 2001) and the lesion method (e.g., Tranel et al., 2001, 2008) suggest that left BA44 may be more important for the production of action verbs than object nouns. Given the complexity of the naming process, this connection between left BA44 and verbs could reflect any of several different aspects of lexical access, including the retrieval of syntactic and/or morphological and/or phonological information (for reviews see Druks, 2002; Shapiro & Caramazza, 2004; Berlingeri et al., 2008). However, it could also reflect the activation of template-level motor features of verb meaning, especially features that are relatively salient, like those that distinguish between one-, two-, and three-argument templates. This possibility is consistent with the lesion studies discussed in the previous section. Moreover, it dovetails nicely with two recent naming studies—one employing fMRI (Saccuman et al., 2006) and the other using the lesion method (Arévalo et al., 2007)—which found that dependence on left BA44 increases not only for verbs, but also for nouns, as the status of the words increases along the semantic parameter of “manipulability,” which involves the degree to which the words are associated with goal-directed arm/hand movements.
Furthermore, an interesting prediction, based largely on previous studies demonstrating significant task effects in semantic processing (e.g., Kemmerer et al., 2001a; Tomasino et al., 2007, 2008; Berlingeri et al., 2008; Hoenig et al., in press), is as follows: left BA44 should not be significantly engaged when subjects perform verb comprehension tasks that require them to attend specifically to the root level, as opposed to the template level, of meaning. Support for this prediction comes from several fMRI studies in which subjects made root-level semantic similarity judgments among triads of verbs (Kable et al., 2002, 2005; Noppeney et al., 2005; Kemmerer et al., 2008). In Kable et al.’s (2002, 2005) studies, which we discussed in section 3.2.2.1, left BA44 was not activated significantly more for verbs than for baseline conditions. In another study by Noppeney et al. (2005), no significant left BA44 activation was reported, relative to a baseline condition, when subjects made judgments about which of two verbs was most similar in meaning to a third; moreover, this outcome was the same regardless of whether the verbs involved arm/hand actions (e.g., determining that throw is more like toss than hit) or lip/tongue actions (e.g., determining that shout is more like bellow than murmur). Furthermore, in our own fMRI study (Kemmerer et al., 2008), which we discussed in section 3.2.2.1, subjects performed a task very much like the one used by Kable et al. (2002, 2005) and Noppeney et al. (2005), and no significant left BA44 activation was found, relative to a baseline condition, for verbs of Speaking, Hitting, and Change of State. In addition, only 2 voxels at the border of left BA44 and BA45 were above threshold for verbs of Running; however, contrary to the prediction described above, 85 voxels in left BA44 (comprising 7.3% of this area) were above threshold for verbs of Cutting. Now, it must be noted that the null findings just summarized could be due to many factors, including experimental design, statistical thresholding, intersubject variability, and uncertainties regarding the precise boundaries of certain neuroanatomical structures. It is also conceivable, however, that the reason why left BA44 was not engaged—or, in the case of Cutting verbs, was only partially engaged—in the studies by Kable et al., (2002, 2005), Noppeney et al. (2005), and Kemmerer et al. (2008) is because the tasks forced the subjects to focus on the root level, rather than the template level, of verb meaning. Still, for this interpretation to be persuasive, it would be necessary to demonstrate in a new study that when subjects attend to template-level motor features of verb meaning, left BA44 is significantly engaged, but when subjects attend to various root-level semantic properties of the very same verbs, left BA44 is not significantly engaged.
4.3. Problems and Prospects
As we stated at the outset, Hypothesis 2 maintains that template-level motor features of verb meaning are partially subserved by representationally schematic mirror neurons in left BA44. Although this hypothesis is admittedly more speculative than Hypothesis 1, we have shown that it nevertheless receives modest support from a variety of sources. Most importantly, studies involving both sentence processing and single verb processing suggest that left BA44 may indeed contribute to the sorts of event structure schemas that are captured by entire classes of verbs and that strongly influence the range of constructions in which they can occur. On the other hand, as we indicated in section 4.2.1, so far no studies, to our knowledge, have investigated the most intriguing part of Hypothesis 2, which is the proposal that template-level motor features of verb meaning are associated specifically with mirror neurons in left BA44. The status of Hypothesis 2 is therefore similar to that of Hypothesis 1: The kinds of studies that are necessary to either confirm or disconfirm the central claim have not yet been conducted. In particular, the adjudication of Hypothesis 2 will depend on studies that attempt to determine whether the same neuronal populations within left BA44 are engaged not only when subjects access certain well-defined template-level motor features of verb meaning during language processing, but also when they execute and observe bodily actions that involve the very same semantic features. Designing such studies may turn out to be even more challenging than designing studies to address Hypothesis 1, because in this case the pertinent conceptual knowledge is in some respects even more difficult to isolate. We are encouraged by research like that reported by Baumgaertner et al. (2007), which found overlapping activation patterns in left BA44 when subjects made sensibility judgments about goal-directed arm/hand actions (relative to inanimate motion events) that were presented both linguistically as spoken sentences and visually as dynamic video clips. However, this field of inquiry is still very much in its infancy, and the provocative notion that processing the template level of verb meaning might involve a form of motor resonance or simulation clearly requires much more empirical and theoretical investigation.
A potentially productive direction of future work may be to explore connections between Hypothesis 2 and the following two lines of research, both of which focus on left BA44. First, in a series of theoretical, computational, and experimental studies of construction-based sentence processing, Dominey and colleagues argue that left BA44 represents what they call “Scene Event Arrays” (e.g., Dominey et al., 2006; Dominey & Hoen, 2006; Hoen et al., 2006). These are very similar to both the event structure templates that are encoded by verbs and the schematic meanings that are associated with clausal constructions. The same investigators also maintain that left BA44 plays a key role in “structure mapping,” which is essentially a form-to-meaning transformation that involves sequentially linking the referent meanings of the linearly presented core NPs in a sentence with the appropriate semantic argument variables in the given Scene Event Array, under the guidance of the syntactic and semantic specifications of the applicable verb and construction. Second, according to a theory of sentence processing called the “Extended Argument Dependency Model” (Bornkessel & Schlesewsky, 2006), left BA44 subserves the function of “prominence computation” (for supporting fMRI studies see Bornkessel et al., 2005; Grewe et al., 2006). This is analogous to Dominey et al.’s (2006) structure mapping process, since both operations involve determining the hierarchical syntactic and semantic rankings of the various participants in the described event, based on both grammatical and conceptual factors. For example, the two constructions comprising the locative alternation differ with respect to which entity is syntactically realized as the direct object NP and semantically construed as the main “target” of the action. Computing these different prominence hierarchies may be one of the responsibilities of left BA44.
The closely related notions of “structure mapping” and “prominence computation” both seem to reflect an even more fundamental notion—namely, that goal-directed bodily actions always unfold in both space and time, starting inside the agent and progressing outward toward other entities. We think this basic fact may play a pivotal role in future work aimed at bridging the gap between the template level of the semantics of action and the BA44 component of the mirror neuron system. Actions have a spatiotemporal structure not only when they are performed and perceived in real life, but also when they are described in language, and we find it very interesting that the most common type of linearization of action in language reflects the corresponding type of linearization of action in real life. Imagine, for example, a situation in which a woman reaches out and grasps a cup. In languages worldwide, there is an overwhelming tendency for such an action to be described by a sentence instantiating the prototypical transitive construction, in which the NP encoding the actor usually occurs before the NP encoding the undergoer, as in The woman grasped the cup (see the analysis and map of 1,228 languages provided by Dryer, 2005). In fact, this word order pattern—i.e., the strong tendency for subjects (actors) to precede objects (undergoers)—is arguably one of the most solidly grounded universals of human languages, and it is a major ingredient of many cognitively, functionally, and typologically oriented approaches to clause structure (e.g., Croft, 1998; Van Valin, 2005, 2006; Foley, 2007; Naess, 2007; Langacker, 2008; see also Klein & Perdue, 1997).
Thus, it can safely be assumed that, with respect to predictive or anticipatory spatiotemporal sequencing, the canonical linguistic description of a simple transitive action ordinarily parallels the real-world unfolding of that action. When viewed from the perspective of the Embodied Cognition Framework, this remarkable isomorphism feeds into the notion that, as implied by Hypothesis 2, understanding a sentence like The woman grasped the cup may involve a form of high-level motor resonance that consists of simulating the goal organization of the designated action, drawing on representationally schematic mirror neurons in left BA44 that subserve, in part, the very same goal organization not only when one actually performs the action, but also when one sees someone else perform it. Although this view is still quite speculative, it is consistent with much of the literature summarized above. Moreover, similar ideas have appeared in a number of recent theoretical and experimental papers (e.g., Arbib, 2006; Fiebach & Schubotz, 2006; Hoen et al., 2006; Koechlin & Jubault, 2006; van Schie et al., 2006; van Elk et al., 2007; Bahlmann et al., in press; Glenberg & Gallese, submitted).
We believe the Two-Level Theory has the potential to contribute a great deal to this growing literature, since it provides a well-developed framework for characterizing the main topic of linguistic interest—namely, template-level motor features of verb meaning. Perhaps most interestingly, this theory shows in considerable detail how, as illustrated by the locative alternation, the very same action can sometimes be conceptualized, for purposes of linguistic communication, as having multiple, subjectively distinct, causal frames or profiles. Indeed, this is a large part of what makes “information packaging in the clause” (Foley, 2007) such a fascinating and cognitively significant phenomenon, and it is one of the many ways in which research on the semantics of action can enrich research on the mirror neuron system.
Clearly, however, future efforts to test Hypothesis 2 must confront numerous challenges. For example, an important aim should be to design experiments that allow the investigators to distinguish between, on the one hand, left BA44 responses that might plausibly reflect template-level motor features of verb meaning, and on the other, left BA44 responses that might plausibly reflect purely syntactic aspects of the kinds of linearization principles described by Bornkessel & Schlesewsky (2006). It will also be necessary to take into account, and attempt to rule out, alternative explanations that draw upon research linking the left posterior inferior frontal gyrus with relatively general “cognitive control” operations involving the detection and resolution of conflicts between multiply active representations, especially in the linguistic domain (e.g., Novick et al., 2005; Thompson-Schill et al., 2005). These are only some of the many concerns that would need to be addressed by future attempts to test Hypothesis 2.
5. CONCLUSION
We have explored the possibility that two separate levels of verb meaning map onto two separate levels of the mirror neuron system. Hypothesis 1 holds that root-level motor features of verb meaning depend on somatotopically organized mirror neurons in the left primary and/or premotor cortices. Hypothesis 2 holds that template-level motor features of verb meaning depend on representationally more schematic mirror neurons in BA44 or the left inferior frontal gyrus. Evidence has been accumulating in support of the associations between semantic levels and brain regions postulated by these two hypotheses. However, to our knowledge, as yet no studies have shown that the relevant aspects of verb meaning are linked specifically with mirror neurons in the relevant cortical areas. This would require demonstrating that the same neuronal populations, and ultimately the same cells, are essential for (a) processing certain motor features of verb meaning, (b) executing actions with the corresponding motor features, and (c) observing actions with the corresponding motor features. Although some studies have met two of these criteria, we are not aware of any that have met all three. Another important direction of future research will be to explore possible connections between the semantics of action and the various components of the mirror neuron system that lie outside the frontal regions considered by Hypotheses 1 and 2. Overall, investigating in detail the complex relations between verb meanings and mirror neurons promises to significantly deepen our understanding of the shared representations that underlie human communication and intersubjectivity.
Acknowledgments
For statistical advice we thank Tom Talavage, and for generous comments on previous versions of the paper we thank Arthur Glenberg, Natalya Kaganovich, Gina Kuperberg, Nathan Lautz, Brad Mahon, Evie Malaia, Megan MacPherson, Luke Miller, Steve Pinker, Mikkel Wallentin, Ronnie Wilbur, and Rolf Zwaan. We are especially grateful to Greig de Zubicaray, Anjan Chatterjee, and Eileen Cardillo for providing very detailed and constructive feedback. Needless to say, we alone are responsible for all remaining flaws.
Footnotes
See Jeannerod (2007) and Tsakiris et al. (2007) for two among several current theories of how the self-other distinction is ultimately achieved with respect to the production and perception of actions.
As described in section 2.3, sometimes a single root can be associated with multiple templates, yielding systematic verbal polysemy. The extent of such polysemy is, however, a matter of debate.
NP = noun phrase; V = verb; X, Y, and Z = semantic arguments. Coindexed subscripts indicate linking patterns between syntax, semantics, and phonology/orthography, following the formalism of Jackendoff (2002); the numbering is arbitrary.
This gloss of the schematic meaning of the container-locative is not a full decomposition, because the second sub-event, labeled simply “adding Y,” is actually identical to the schematic meaning of the content-locative. Thus, the core sense of the container-locative is, in greater detail, something like “X acts on Z, causing Z to change state, by means of acting on Y, causing Y to go to Z.” For further elucidation of these semantic issues, see Pinker’s (1989) geometric representations and Davis’s (2001) more formal representations; see also Iwata’s (2008) in-depth analysis.
Although many English action verbs elaborate the motor component of meaning in specific ways, it is important to note that some do not. For example, after surveying roughly 1,900 English action verbs involving the thematic role commonly known in the linguistics literature as “instrument,” Koenig et al. (2008, p. 175) conclude that “verbs that require or allow instruments constrain the end states of the situations they describe more than they constrain the agent’s initial activity.” Pulvermüller’s model and our Hypothesis 1 focus on verbs that do encode relatively specific motor information.
The sentences were presented in Italian.
The verbs were presented in German.
The stimuli were presented in Italian. It is noteworthy that MEPs were enhanced not only by sentences describing concrete object transfer, but also by sentences describing abstract information transfer (e.g., Arthur presents the argument to you)—a finding that, according to the authors, supports the notion of “a relatively general, transfer action schema in motor cortex, probably in the mirror neuron system” (Glenberg et al., 2008b, p. 916). See Glenberg et al. (2008a) for a related behavioral study, and see footnote 10 for more information about transfer constructions.
The intraparietal sulcus and inferior parietal lobule may also contribute to goal representation during both the production and the recognition of arm/hand actions (e.g., Hamilton & Grafton, 2006, 2007, 2008).
Another instructive example is the dative alternation: Bill threw the ball to Bob has the schematic meaning “X causes Y to go to Z” (analogous to the content-locative), whereas Bill threw Bob the ball has the schematic meaning “X causes Z to receive Y.” Note that only the second construction entails successful transfer of possession. As Pinker (2007, p. 60) points out, “Señor Jones taught Spanish to the students is compatible with his fruitlessly lecturing to dullards who don’t remember a word. But Señor Jones taught the students Spanish carries more of an implication that the students now know Spanish—that they metaphorically possess it.” See Rappaport Hovav and Levin (2008) for one of the most recent linguistic analyses. See Kaschak and Glenberg (2000) and Glenberg et al. (2008a, 2008b) for relevant psycholinguistic and neurolinguistic studies.
The sentences were presented in German.
For exceptions to this tendency see Jonkers and Bastiaanse (1996, 1997, 1998).
A problem, however, is that some verbs can occur in a surprisingly wide range of constructions, making it difficult to determine which associations between verbs and constructions are stored in long-term memory and which are computed “on the fly,” so to speak. Consider, for example, the following sentences based on the verb kick: Bill kicked the ball (transitive); Bill kicked the ball into the lake (caused motion); Bill kicked at the ball (conative); Bill kicked Bob the ball (ditransitive); Bill kicked Bob black and blue (resultative); Bill kicked Bob in the knee (body-part possessor ascension); Bill kicked his foot against the chair (contact against); Bill kicked his way through the crowd (X’s way); Horses kick (habitual). Even the verb snore, which was classified above as being intransitive, can occur in additional constructions—e.g., the sentence Bill snored his way to fame and fortune would be quite felicitous in the context of a snoring contest. For discussion see, e.g., Jackendoff (2002), Goldberg (1995), and Levin & Rappaport Hovav (2005).
Some of the studies cited above (e.g., Kim & Thompson, 2000, 2004) provide evidence that agrammatic patients have intact verb comprehension. However, the tasks only assessed knowledge of grammatically irrelevant root-level properties of verb meaning.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nature Neuroscience. 2008;11:1109–1116. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Aikhenvald AY, Dixon RMW, editors. Serial verb constructions: A cross-linguistic typology. Oxford: UK: Oxford University Press; 2006. [Google Scholar]
- Arbib MA. The Mirror System Hypothesis on the linkage of action and languages. In: Arbib M, editor. Action to language via the mirror neuron system. Cambridge, UK: Cambridge University Press; 2006. pp. 3–47. [Google Scholar]
- Arbib MA. From grasp to language: Embodied concepts and the challenge of abstraction. Journal of Physiology, Paris. 2008;102:4–20. doi: 10.1016/j.jphysparis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Arbib MA, Bota M. Language evolution: Neural homologies and neuroinformatics. Neural Networks. 2003;16:1237–1260. doi: 10.1016/j.neunet.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Arbib MA, Bota M. Neural homologies and the grounding of neurolinguistics. In: Arbib MA, editor. Action to language via the mirror neuron system. Cambridge, UK: Cambridge University Press; 2006. pp. 136–174. [Google Scholar]
- Arévalo A, Perani D, Cappa SF, Butler A, Bates E, Dronker N. Action and object processing in aphasia: From nouns and verbs to the effect of manipulability. Brain and Language. 2007;100:79–94. doi: 10.1016/j.bandl.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Damasio AR. Embodied semantics for actions: Findings from functional brain imaging. Journal of Physiology, Paris. 2008;102:35–39. doi: 10.1016/j.jphysparis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J. Left hemisphere motor facilitation in response to manual action sounds. European Journal of Neuroscience. 2004;19:2609–2612. doi: 10.1111/j.0953-816X.2004.03348.x. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Current Biology. 2006;16:1818–23. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Bahlmann J, Schubotz RI, Friederici AD. Hierarchical sequencing engages Broca’s area. NeuroImage. doi: 10.1016/j.neuroimage.2008.04.249. in press. [DOI] [PubMed] [Google Scholar]
- Bakker M, De Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I. Cerebral correlates of motor imagery of normal and precision gait. NeuroImage. doi: 10.1016/j.neuroimage.2008.03.020. in press. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Cognitive and neural contributions to understanding the conceptual system. Current Directions in Psychological Science. 2008a;17:91–95. [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008b;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounding symbolic operations in the brains modal systems. In: Semin GR, Smith ER, editors. Embodied grounding: Social, cognitive, affective, and neuroscientific approaches. Cambridge, UK: Cambridge University Press; 2008c. pp. 9–42. [Google Scholar]
- Barsalou LW, Hale CR. Components of conceptual representation: From feature lists to recursive frames. In: Van Mechelen I, Hampton J, Michalski R, Theuns P, editors. Categories and concepts. San Diego, CA: Academic Press; 1993. pp. 97–144. [Google Scholar]
- Baumgaertner A, Buccino G, Lange R, McNamara A, Binkofski F. Polymodal conceptual processing of human biological actions in the left inferior frontal lobe. European Journal of Neuroscience. 2007;25:881–889. doi: 10.1111/j.1460-9568.2007.05346.x. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts are more than percepts: The case of action verbs. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3039-08.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen B. Experimental methods for simulation semantics. In: Gonzalez-Marquez M, Mittelberg I, Coulson S, Spivey MJ, editors. Methods in cognitive linguistics. Amsterdam: John Benjamins; 2007. pp. 277–301. [Google Scholar]
- Bergen B, Chang N. Embodied Construction Grammar in simulation-based language understanding. In: Östman & J-O, Fried M, editors. Construction grammars: Cognitive grounding and theoretical extensions. Amsterdam: John Benjamins; 2005. pp. 147–190. [Google Scholar]
- Berlingeri M, Crepaldi D, Roberti R, Scialfa G, Luzzatti C, Paulesu E. Nouns and verbs in the brain: Grammatical class and task specific effects as revealed by fMRI. Cognitive Neuropsychology. 2008;25:528–558. doi: 10.1080/02643290701674943. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ. A parieto-premotor network for object manipulation: Evidence from neuroimaging. European Journal of Neuroscience. 1999;11:3276–3286. [Google Scholar]
- Binkofski F, Buccino G. The role of ventral premotor cortex in action execution and action understanding. Journal of Physiology, Paris. 2006;99:396–405. doi: 10.1016/j.jphysparis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Schlesewsky M. The Extended Argument Dependency Model: A neurocognitive approach to sentence comprehension across languages. Psychological Review. 2006;113:787–821. doi: 10.1037/0033-295X.113.4.787. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Schlesewsky M, Comrie B, Friederici AD, editors. Semantic role universals and argument linking. Berlin: Mouton de Gruyter; 2006. [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, von Cramon DY, Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. NeuroImage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Borreggine KL, Kaschak MP. The action-sentence compatibility effect: It’s all in the timing. Cognitive Science. 2006;30:1097–1112. doi: 10.1207/s15516709cog0000_91. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Research. 2005;1065:115–124. doi: 10.1016/j.brainres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA. Cross-talk between language processes and overt motor behavior in the first 200 ms of processing. Journal of Cognitive Neuroscience. 2006;18:1607–1615. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Bowerman M, Brown P, editors. Crosslinguistic perspectives on argument structure: Implications for learnability. New York: Lawrence Erlbaum; 2008. [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergeley G. Investigating action understanding: Inferential processes versus action understanding. Current Biology. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss HE, Longe O, Stamatakis EA, Tyler LK. Conceptual structure modulates anteromedial temporal involvement in processing verbally presented object properties. Cerebral Cortex. 2007;17:1066–1073. doi: 10.1093/cercor/bhl016. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain and Language. 2004;89:370–376. doi: 10.1016/S0093-934X(03)00356-0. [DOI] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action- related sentences modulates the activity of the motor system: A combined TMS and behavioral study. Cognitive Brain Research. 2005;24:355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Caetano G, Jousmäki V, Hari R. Actor’s and observer’s primary motor cortices stabilize similarly after seen or heard motor actions. Proceedings of the National Academy of Sciences. 2007;104:9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Candidi M, Urgesi C, Ionta S, Aglioti SM. Virtual lesion of ventral premotor cortex impairs visual perception of biomechanically possible but not impossible actions. Social Neuroscience. doi: 10.1080/17470910701676269. in press. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Current Biology. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lee P-L, Yang C-Y, Lin C-P, Hung D, Decety J. Gender differences in the mu rhythm of the human mirror-neuron system. PloS ONE. 2008;3:e2113. doi: 10.1371/journal.pone.0002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Meltzoff AN, Decety J. Motivation modulates the activity of the human mirror- neuron system. Cerebral Cortex. 2007;17:1979–1986. doi: 10.1093/cercor/bhl107. [DOI] [PubMed] [Google Scholar]
- Chong TTJ, Williams MA, Cunnington R, Mattingley JB. Selective attention modulates inferior frontal gyrus activity during action observation. NeuroImage. 2008;40:298–307. doi: 10.1016/j.neuroimage.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Constantini M, Galati G, Ferretti A, Caulo M, Tartaro A, Romani GL, Aglioti SM. Neural systems underlying observation of humanly impossible movements: An fMRI study. Cerebral Cortex. 2005;15:1761–1767. doi: 10.1093/cercor/bhi053. [DOI] [PubMed] [Google Scholar]
- Croft W. Syntactic categories and grammatical relations: The cognitive organization of information. Chicago: University of Chicago Press; 1991. [Google Scholar]
- Croft W. Event structure in argument linking. In: Butt M, Geuder W, editors. The projection of arguments. Stanford: CSLI Publications; 1998. pp. 21–63. [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: Observation of dance by dancers. NeuroImage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Kraemer DJM, Hamilton AF, Kelley WM, Grafton ST. Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex. doi: 10.1093/cercor/bhn083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems level proposal for the neural substrates of recall and recognition. Cognition. 1989a;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Computation. 1989b;1:123–132. [Google Scholar]
- Damasio AR. Concepts in the brain. Mind and Language. 1989c;4:24–28. [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. Neural correlates of naming actions and of naming spatial relations. NeuroImage. 2001;13:1053–1064. doi: 10.1006/nimg.2001.0775. [DOI] [PubMed] [Google Scholar]
- Davis AR. Linking by types in the hierarchical lexicon. Stanford: CSLI Publications; 2001. [Google Scholar]
- De Bleser R, Kauschke C. Acquisition and loss of nouns and verbs: Parallel or divergent patterns? Journal of Neurolinguistics. 2003;16:213–229. [Google Scholar]
- de Lafuente V, Romo R. Language abilities of motor cortex. Neuron. 2004;41:178–180. doi: 10.1016/s0896-6273(04)00004-2. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Current Biology. 2008;18:454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Désy M-C, Théoret H. Modulation of motor cortex excitability by physical similarity with an observed hand action. PLoS ONE. 2007;2:e971. doi: 10.1371/journal.pone.0000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray GI, Postle N, McMahon K, Meredith M, Ashton R. Mirror neurons, the representation of word meaning, and the foot of the left frontal convolution: An fMRI study. Brain and Language. 2008 doi: 10.1016/j.bandl.2008.09.011. this issue. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–182. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. Journal of Neurophysiology. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Current Biology. 2008;18:R13–R18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RMW, Aikhenvald AY, editors. Changing valency: Case studies in transitivity. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Dominey PF, Hoen M. Structure mapping and semantic integration in a construction-based neurolinguistic model of sentence processing. Cortex. 2006;42:476–479. doi: 10.1016/s0010-9452(08)70381-2. [DOI] [PubMed] [Google Scholar]
- Dominey PF, Hoen M, Inui T. A neurolinguistic model of grammatical construction processing. Journal of Cognitive Neuroscience. 2006;18:2088–2107. doi: 10.1162/jocn.2006.18.12.2088. [DOI] [PubMed] [Google Scholar]
- Druks J. Verbs and nouns: A review of the literature. Journal of Neurolinguistics. 2002;15:289–315. [Google Scholar]
- Dryer MS. Order of subject, object, and verb. In: Haspelmath M, Dryer MS, Gil D, Comrie B, editors. The world atlas of language structures. Oxford, UK: Oxford University Press; 2005. pp. 30330–333. [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiology and Behavior. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. Journal of Neurophysiology. 2003;90:3304–3316. doi: 10.1152/jn.01113.2002. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Engel A, Burke M, Fiehler K, Bien S, Rösler F. What activates the human mirror neuron system during observation of artificial movements: Bottom-up visual features or top-down intentions? Neuropsychologia. doi: 10.1016/j.neuropsychologia.2008.01.025. in press. [DOI] [PubMed] [Google Scholar]
- Esopenko C, Borowsky R, Cummine J, Sarty G. Mapping the semantic homunculus: A functional and behavioral analysis of overt semantic generation. Brain Topography. doi: 10.1007/s10548-008-0043-8. in press. [DOI] [PubMed] [Google Scholar]
- Evans V, Green M. Cognitive linguistics. Mahwah: Lawrence Erlbaum; 2006. [Google Scholar]
- Fadiga L, Craighero L, Olivier E. Human motor cortex excitability during the perception of others’ action. Current Opinion in Neurobiology. 2005;15:213–218. doi: 10.1016/j.conb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. European Journal of Neuroscience. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schubotz RI. Dynamic anticipatory processing of hiearchical sequential events: A common role for Broca’s area and ventral premotor cortex across domains? Cortex. 2006;42:499–502. doi: 10.1016/s0010-9452(08)70386-1. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. NeuroImage. 2007;37:1315–1328. doi: 10.1016/j.neuroimage.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Zwaan RA. Embodied language: A review of the role of the motor system in language comprehension. Quarterly Journal of Experimental Psychology. 2008;61:825–850. doi: 10.1080/17470210701623605. [DOI] [PubMed] [Google Scholar]
- Fodor JA. The language of thought. Cambridge, MA: Harvard University Press; 1975. [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersim F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Foley WA. A typology of information packaging in the clause. In: Shopen T, editor. Language typology and syntactic description, Vol 1: Clause structure. Cambridge, UK: Cambridge University Press; 2007. pp. 362–446. [Google Scholar]
- Gallagher S. How the body shapes the mind. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolatti G. Action representation and the inferior parietal lobule. In: Prinz W, Hommel B, editors. Attention and Performance XIX Common mechanisms in perception and action. Oxford, UK: Oxford University Press; 2002. pp. 247–266. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22:455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic mirror system in humans. Current Biology. 2006;16:1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gazzola V, van der Worp H, Mulder T, Wicker B, Rizzolatti G, Keysers C. Aplasics born without hands mirror the goal of hand actions with their feet. Current Biology. 2007a;17:1235–1240. doi: 10.1016/j.cub.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. NeuroImage. 2007b;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gerfo EL, Oliveri M, Torriero S, Salerno S, Koch G, Caltagirone C. The influence of rTMS over prefrontal and motor areas in a morphological task: Grammatical vs. semantic effects. Neuropsychologia. 2008;46:764–770. doi: 10.1016/j.neuropsychologia.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anatomy and Embryology. 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Gibbs RW. Embodiment and cognitive science. Cambridge, UK: University of Cambridge Press; 2006. [Google Scholar]
- Glenberg AM. Naturalizing cognition: The integration of cognitive science and biology. Current Biology. 2006;16:R802–R804. doi: 10.1016/j.cub.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Gallese V. Action-based language: A theory of language acquisition, production, and comprehension. doi: 10.1016/j.cortex.2011.04.010. submitted. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L. Use-induced motor plasticity affects the processing of abstract and concrete language. Current Biology. 2008a;18:R290–R291. doi: 10.1016/j.cub.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L, Riggio L, Palumbo D, Buccino G. Processing abstract language modulates motor system activity. Quarterly Journal of Experimental Psychology. 2008b;61:905–919. doi: 10.1080/17470210701625550. [DOI] [PubMed] [Google Scholar]
- Goldberg AE. Constructions. Chicago: University of Chicago Press; 1995. [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. Journal of Neuroscience. 2006a;26:4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by PET: 2. Observation compared with imagination. Experimental Brain Research. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Graziano M. The organization of behavioral repertoire in motor cortex. Annual Review of Neuroscience. 2006;29:104–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56:239–251. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M. Linguistic prominence and Broca’s area: The influence of animacy as a linearization principle. NeuroImage. 2006;32:1395–1402. doi: 10.1016/j.neuroimage.2006.04.213. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel-Schlesewsky I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M. The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. NeuroImage. 2007;35:343–352. doi: 10.1016/j.neuroimage.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Gropen J, Pinker S, Hollander M, Goldberg R. Affectedness and direct objects: The role of lexical semantics in the acquisition of verb argument structure. Cognition. 1991;41:153–195. doi: 10.1016/0010-0277(91)90035-3. [DOI] [PubMed] [Google Scholar]
- Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. Journal of Neuroscience. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. The motor hierarchy: From kinematics to goals and intentions. In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition. Oxford, UK: Oxford University Press; 2007. pp. 381–408. [Google Scholar]
- Hamilton AF, Grafton ST. Action outcomes are represented in human inferior frontoparietal cortex. Cerebral Cortex. 2008;18:1160–1168. doi: 10.1093/cercor/bhm150. [DOI] [PubMed] [Google Scholar]
- Hampe B. From perception to meaning. Berlin: Mouton de Gruyter; 2005. [Google Scholar]
- Hamzei F, Rijntjes M, Dettmers C, Glauche V, Weiller C, Buchel C. The human action recognition system and its relationship to Broca’s area: An fMRI study. NeuroImage. 2003;19:637–644. doi: 10.1016/s1053-8119(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermüller F. Neurophysiological distinction of action words in the fronto-central cortex. Human Brain Mapping. 2004;21:191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Shtyrov Y, Pulvermüller F. The sound of actions as reflected by mismatch negativity: Rapid activation of cortical sensory-motor networks by sounds associated with finger and tongue movements. European Journal of Neuroscience. 2006;23:811–821. doi: 10.1111/j.1460-9568.2006.04586.x. [DOI] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Kherif F, Pulvermüller F. Imagery or meaning? Evidence for a semantic origin of category-specific brain activity in metabolic imaging. European Journal of Neuroscience. 2008a;27:1856–1866. doi: 10.1111/j.1460-9568.2008.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Shtyrov Y, Pulvermüller F. The time course of action and action-word comprehen- sion in the human brain as revealed by neurophysiology. Journal of Physiology, Paris. 2008b;102:50–58. doi: 10.1016/j.jphysparis.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta J. The essential role of Broca’s area in imitation. European Journal of Neuroscience. 2003;17:1123–1128. doi: 10.1046/j.1460-9568.2003.02530.x. [DOI] [PubMed] [Google Scholar]
- Hoen M, Pachot-Clouard M, Segebarth C, Dominey PF. When Broca experiences the Janus syndrome: An ER-fMRI study comparing sentence comprehension and cognitive sequence processing. Cortex. 2006;42:605–623. doi: 10.1016/s0010-9452(08)70398-8. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Sim EJ, Bochev V, Herrnberger B, Kiefer M. Conceptual flexibility in the human brain: Dynamic recruitment of semantic maps from visual, motor, and motion-related areas. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.20123. in press. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: Anatomical connectivity and functional properties. Current Opinion in Neurobiology. 2007;17:1–9. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hurley S. The Shared Circuits Model: How control, mirroring and simulation can enable imitation, deliberation, and mindreading. Behavioral and Brain Sciences. 2008;31:1–22. doi: 10.1017/S0140525X07003123. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Mirroring people. Farrar, Straus, & Giroux; 2008. [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror system. PloS Biology. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iwata S. Does MANNER count or not? Manner-of-motion verbs revisited. Linguistics. 2002;40:61–110. [Google Scholar]
- Iwata S. Locative alternation and two levels of verb meaning. Cognitive Linguistics. 2005;16:355–407. [Google Scholar]
- Iwata S. Locative alternation: A lexical-constructional approach. Amsterdam: John Benjamins; 2008. [Google Scholar]
- Jackendoff R. Foundations of language. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- Jackendoff R. Language, consciousness, culture. Cambridge, MA: MIT Press; 2007. [Google Scholar]
- James W. Principles of psychology. II. MacMillan; London: 1890. New edition: Dover: New York, 1950. [Google Scholar]
- Jeannerod M. Motor cognition: What actions tell the self. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- Jeannerod M. From my self to other selves: A revised framework for the self/other differentiation. In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition. Oxford, UK: Oxford University Press; 2007. pp. 233–248. [Google Scholar]
- Johnson M. The meaning of the body. Chicago: University of Chicago Press; 2007. [Google Scholar]
- Johnson SH, Grafton ST. From “acting on” to “acting with”: The functional anatomy of object- oriented action schemata. Progress in Brain Research. 2003;142:127–139. doi: 10.1016/S0079-6123(03)42010-4. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ. Selective activation of a parieto-frontal circuit during implicitly imagined prehension. NeuroImage. 2002;17:1693–1704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrar C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39:1053–1058. doi: 10.1016/s0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Jonkers R, Bastiaanse R. The influence of instrumentality and transitivity on action naming in Broca’s and anomic aphasia. Brain and Language. 1996;55:37–39. doi: 10.1016/j.bandl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Jonkers R, Bastiaanse R. Verb retrieval in isolation and sentence context in Broca’s aphasics: The effect of transitivity. Brain and Language. 1997;60:33–36. [Google Scholar]
- Jonkers R, Bastiaanse R. How selective are selective word class deficits? Tw case studies of action and object naming. Aphasiology. 1998;12:245–256. [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. Journal of Cognitive Neuroscience. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual representations of action in the lateral temporal cortex. Journal of Cognitive Neuroscience. 2005;17:1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kako E. The semantics of syntactic frames. Language and Cognitive Processes. 2006a;21:562–575. [Google Scholar]
- Kako E. Thematic role properties of subjects and objects. Cognition. 2006b;101:1–42. doi: 10.1016/j.cognition.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon KO, Minor JK, Thompson-Schill SL. Role of mental imagery in a property verification task: fMRI evidence for perceptual representations. Cognitive Neuropsychology. 2003;20:525–540. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Kaplan J, Iacoboni M. Multimodal action representation in human ventral premotor cortex. Cognitive Processing. 2007;8:103–113. doi: 10.1007/s10339-007-0165-z. [DOI] [PubMed] [Google Scholar]
- Kaschak MP, Borreggine KL. Temporal dynamics of the action-sentence compatibility effect. Quarterly Journal of Experimental Psychology. 2008;61:883–895. doi: 10.1080/17470210701623852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschak MP, Glenberg AM. Constructing meaning: The role of affordances and grammatical constructions in sentence comprehension. Journal of Memory and Language. 2000;43:508–529. [Google Scholar]
- Kegl J. Levels of representation and units of access relevant to agrammatism. Brain and Language. 1995;50:151–200. doi: 10.1006/brln.1995.1044. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Large, colorful, or noisy? Attribute- and modality- specific activations during retrieval of perceptual attribute knowledge. Cognitive, Affective, and Behavioral Neuroscience. 2001;1:207–221. doi: 10.3758/cabn.1.3.207. [DOI] [PubMed] [Google Scholar]
- Kemmerer D. Grammatically relevant and grammatically irrelevant features of verb meaning can be independently impaired. Aphasiology. 2000;14:997–1020. [Google Scholar]
- Kemmerer D. Why can you hit someone on the arm but not break someone on the arm? A neuropsychological investigation of the English body-part possessor ascension construction. Journal of Neurolinguistics. 2003;16:13–36. [Google Scholar]
- Kemmerer D. Action verbs, argument structure constructions, and the mirror neuron system. In: Arbib M, editor. Action to language via the mirror neuron system. Cambridge, UK: Cambridge University Press; 2006. pp. 347–373. [Google Scholar]
- Kemmerer D. How words capture visual experience: The perspective from cognitive neuroscience. In: Malt B, Wolff P, editors. Words and the world: How words capture experience. Oxford, UK: Oxford University Press; in press. [Google Scholar]
- Kemmerer D, Gonzalez Castillo J, Talavage T, Patterson S, Wiley C. Neuroanatomical distribution of five semantic components of verbs: Evidence from fMRI. Brain and Language. 2008 doi: 10.1016/j.bandl.2007.09.003. in press. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Tranel D. A double dissociation between the meanings of action verbs and locative prepositions. NeuroCase. 2003;9:421–435. doi: 10.1076/neur.9.5.421.16551. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Tranel D, Barrash J. Patterns of dissociation in the processing of verb meanings in brain-damaged subjects. Language and Cognitive Processes. 2001a;16:1–34. [Google Scholar]
- Kemmerer D, Tranel D, Barrash J. Addendum to “Patterns of dissociation in the processing of verb meanings in brain-damaged subjects”. Language and Cognitive Processes. 2001b;16:461–463. [Google Scholar]
- Kemmerer D, Wright SK. Selective impairment of knowledge underlying reversative un-prefixation: Further evidence for the autonomy of grammatical semantics. Journal of Neurolinguistics. 2002;15:403–32. [Google Scholar]
- Kessler K, Biermann-Ruben K, Jonas M, Siebner HR, Bäumer T, Münchau A, Schnitzler A. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. NeuroImage. 2006;33:227–238. doi: 10.1016/j.neuroimage.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Towards a unifying neural theory of social cognition. Progress in Brain Research. 2006;156:383–406. doi: 10.1016/S0079-6123(06)56021-2. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kohler E, Umilta MA, Nanetti L, Fogassi L, Gallese V. Audiovisual mirror neurons and action recognition. Experimental Brain Research. 2003;153:628–636. doi: 10.1007/s00221-003-1603-5. [DOI] [PubMed] [Google Scholar]
- Kim M, Thompson CK. Patterns of comprehension and production of nouns and verbs in agrammatism: Implications for lexical organization. Brain and Language. 2000;74:1–25. doi: 10.1006/brln.2000.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Thompson CK. Verb deficits in Alzheimer’s disease and agrammatism: Implications for lexical organization. Brain and Language. 2004;88:1–20. doi: 10.1016/s0093-934x(03)00147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss R. Effect of verb complexity on agrammatic aphasics’ sentence production. In: Bastiaanse R, Grodzinsky Y, editors. Grammatical disorders in aphasia. London: Whurr; 2000. pp. 152–170. [Google Scholar]
- Klatzky RL, MacWhinney B, Behrmann M. Embodiment, ego-space, and action. Philadelphia: Psychology Press; [Google Scholar]
- Klein W, Perdue C. The Basic Variety (or: Couldn’t natural languages be much simpler?) Second Language Research. 1997;13:301–347. [Google Scholar]
- Koechlin E, Jubault T. Broca’s area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Koenig J-P, Mauner G, Bienvenue B, Conklin K. What with? The anatomy of a (proto)-role. Journal of Semantics. 2008;25:175–220. [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Lahav A, Saltzman E, Schlaug G. Action representation of sound: Audiomotor recognition network while listening to newly acquired actions. Journal of Neuroscience. 2007;27:308–314. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer TK, Dumais ST. A solution to Plato’s problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychological Review. 1997;104:211–240. [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge in underpinned by the temporal pole bilaterally. Cerebral Cortex. doi: 10.1093/cercor/bhn131. in press. [DOI] [PubMed] [Google Scholar]
- Langacker RW. Cognitive grammar. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- Lestou V, Pollick FE, Kourtzi Z. Neural substrates of action understanding at different description levels in the human brain. Journal of Cognitive Neuroscience. 2008;20:324–341. doi: 10.1162/jocn.2008.20021. [DOI] [PubMed] [Google Scholar]
- Levin B. English verb classes and alternations. Chicago, IL: University of Chicago Press; 1993. [Google Scholar]
- Levin B, Rappaport Hovav M. Argument realization. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Levin B, Rappaport Hovav M. Lexical conceptual structure. In: von Heusinger K, Maienborn C, Portner P, editors. Semantics: An international handbook of natural language meaning. Berlin: Mouton de Gruyter; in press. [Google Scholar]
- Levinson SC, Wilkins D. Grammars of space: Explorations in cognitive diversity. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. The Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Brefczynski JA, Phinney RE, Janik JJ, DeYoe EA. Distinct cortical pathways for processing tool versus animal sounds. Journal of Neuroscience. 2005;25:5148–5158. doi: 10.1523/JNEUROSCI.0419-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepelt R, von Cramon DY, Brass M. How do we infer others’ goals from non-stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. NeuroImage. doi: 10.1016/j.neuroimage.2008.08.007. in press. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Tanskanen T, Hari R. The imprint of action: Motor cortex involvement in visual perception of handwritten letters. NeuroImage. 2006;33:681–688. doi: 10.1016/j.neuroimage.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Experimental Brain Research. 1991;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Raggi R, Zonca G, Pistarini C, Contardi A, Pinna GD. Verb-noun double dissociation in aphasic lexical impairments: The role of word frequency and imageability. Brain and Language. 2002;81:432–444. doi: 10.1006/brln.2001.2536. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cognitive Neuropsychology. 2005;22:480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology, Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Majid A, Bowerman M, van Staden M, Boster JS. The semantics of “cutting and breaking” events: A crosslinguistic perspective. Cognitive Linguistics. 2007;18:133–152. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behavioral Brain Research. 1985;18:125–136. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: Neuronal activity during a learned motor task. Journal of Neurophysiology. 1992;68:653–662. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. NeuroImage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor WB. Verb classification in Australian languages. Berlin: Mouton de Gruyter; 2002. [Google Scholar]
- Michaelis LA, Ruppenhofer J. Beyond alternations. Stanford, CA: CSLI Publications; 2001. [Google Scholar]
- Michelon P, Vettel JM, Zacks JM. Lateral somatotopic organization during imagined and prepared movements. Journal of Neurophysiology. 2006;95:811–822. doi: 10.1152/jn.00488.2005. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta J. Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action observation. Cerebral Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Wu AD, Robles RJ, Iacoboni M. Do you see what I mean? Cortico-spinal excitability during observation of culture-specific gestures. PLoS ONE. 2007;2:e626. doi: 10.1371/journal.pone.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Dehaene S. The priming method: Imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cerebral Cortex. 2001;11:966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- Naess A. Prototypical transitivity. Amsterdam: John Benjamins; 2007. [Google Scholar]
- Nazir TA, Boulenger V, Roy A, Silber B, Jeannerod M, Paulignan Y. Language-induced motor perturbations during the execution of a reaching movement. Quarterly Journal of Experimental Psychology. 2008;61:933–943. doi: 10.1080/17470210701625667. [DOI] [PubMed] [Google Scholar]
- Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology. 2007;24:795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Observing others: Multiple action representations in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proceedings of the National Academy of Sciences. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Viewing lip forms: Cortical dynamics. Neuron. 2002;36:1211–1220. doi: 10.1016/s0896-6273(02)01089-9. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Kiebel S, Friston KJ, Price CJ. Action selectivity in parietal and temporal cortex. Cognitive Brain Research. 2005;25:641–649. doi: 10.1016/j.cogbrainres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Oberman LM, McCleery JP, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron activity during the observation of human and robotic actions: Toward an analysis of the human qualities of interactive robots. Neurocomputing. 2007;70:2194–2203. [Google Scholar]
- O’Grady W, Lee M. A mapping theory of agrammatic comphrension deficits. Brain and Language. 2005;92:91–100. doi: 10.1016/j.bandl.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Oliveri, et al. All talk and no action: A transcranial magnetic stimulation study of motor cortex activation during action word production. Journal of Cognitive Neuroscience. 2004;16:374–381. doi: 10.1162/089892904322926719. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yetarian EH. Comparison of prefrontal architecture and connections. Philosophical Transactions of the Royal Society of London, B. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Smania N, Corato E, Aglioti SM. Neural underpinnings of gesture discrimination in patients with limb apraxia. Journal of Neuroscience. 2008;28:3030–3041. doi: 10.1523/JNEUROSCI.5748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecher D, Zwaan RA. Grounding cognition: The role of perception and action in memory, language, and thinking. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Penfield W, Boldrey E. Somatic sensory and motor representation in the cerebral cortex as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penolazzi B, Hauk O, Pulvermüller F. Early semantic context integration and lexical access as revealed by event-related potentials. Biological Psychology. 2007;74:374–388. doi: 10.1016/j.biopsycho.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philosophical Transactions of the Royal Society, B. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropychology. IX. New York: Elsevier; 1994. pp. 17–58. [Google Scholar]
- Pierno AC, Tubaldi F, Turella L, Grossi P, Barachino L, Gallo P, Castiello U. Neurofunctional modulation of brain regions by the observation of pointing and grasping actions. Cerebral Cortex. doi: 10.1093/cercor/bhn089. in press. [DOI] [PubMed] [Google Scholar]
- Pinker S. Learnability and cognition. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Pinker S. The stuff of thought. New York: Viking; 2007. [Google Scholar]
- Pirog Revill K, Aslin RA, Tanenhaus MK, Bavelier D. Neural correlates of partial lexical activation. Proceedings of the National Academy of Sciences. 2008;105:13111–13115. doi: 10.1073/pnas.0807054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Hamilton AF. Action understanding requires the left inferior frontal cortex. Current Biology. 2006;16:524–529. doi: 10.1016/j.cub.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI. Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. NeuroImage. doi: 10.1016/j.neuroimage.2008.08.006. in press. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: A microstimulation study. Journal of Comparative Neurology. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Prinz JJ. The return of concept empiricism. In: Cohen H, Lefebvre C, editors. Handbook of categorization in cognitive science. St Louis: Elsevier; 2005. [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain embodiment of category-specific semantic memory circuits. In: Semin GR, Smith ER, editors. Embodied grounding: Social, cognitive, affective, and neuroscientific approaches. Cambridge, UK: Cambridge University Press; 2008. pp. 71–97. [Google Scholar]
- Pulvermüller F, Härle M, Hummel F. Neurophysiological distinction of verb categories. NeuroReport. 2000;11:2789–2793. doi: 10.1097/00001756-200008210-00036. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Härle M, Hummel F. Walking or talking? Behavioral and neurophysiological correlates of action verb processing. Brain and Language. 2001;78:143–168. doi: 10.1006/brln.2000.2390. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikulin V, Ilmoniemi R. Functional links between motor and language systems. European Journal of Neuroscience. 2005a;21:793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y, Ilmoniemi R. Brain signatures of meaning access in action word recognition. Journal of Cognitive Neuroscience. 2005b;17:884–892. doi: 10.1162/0898929054021111. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Computation and cognition. Cambridge, MA: MIT Press; 1984. [Google Scholar]
- Ramachandran VS. Reflecting on the mind. Science. 2008;452:814–815. [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Observation of action: Grasping with the mind’s hand. NeuroImage. 2004;23:193–201. doi: 10.1016/j.neuroimage.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Mental simulation of action in the service of action perception. Journal of Neuroscience. 2007;27:12675–12683. doi: 10.1523/JNEUROSCI.2988-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport Horav M, Levin B. Building verb meanings. In: Butt M, Geuder W, editors. The projection of arguments. Stanford: CSLI Publications; 1998. pp. 97–134. [Google Scholar]
- Rappaport Hovav M, Levin B. The English dative alternation: The case for verb sensitivity. Journal of Linguistics. 2008;44:129–167. [Google Scholar]
- Rizzolatti G, Buccino G. The mirror neuron system and its role in imitation and language. In: Dehaene S, Duhamel J-R, Hauser MD, Rizzolatti G, editors. From monkey brain to human brain. Cambridge, MA: MIT Press; 2005. pp. 213–234. [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. Mirrors in the brain. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- Romani M, Cesari P, Urgesi C, Facchini S, Aglioti SM. Motor faciliation of the human corticospinal system during observation of bio-mechanically impossible movements. NeuroImage. 2005;26:755–763. doi: 10.1016/j.neuroimage.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Rüschemeyer S-A, Brass M, Friederici AD. Comprehending prehending: Neural correlates of processing verbs with motor stems. Journal of Cognitive Neuroscience. 2007;19:855–865. doi: 10.1162/jocn.2007.19.5.855. [DOI] [PubMed] [Google Scholar]
- Saccuman MC, Cappa SF, Bates EA, Arevalo A, Rosa PD, Danna M, Perani D. The impact of semantic reference on word class: An fMRI study of action and object naming. NeuroImage. 2006;32:1865–1878. doi: 10.1016/j.neuroimage.2006.04.179. [DOI] [PubMed] [Google Scholar]
- Sakreida K, Schubotz RI, Wolfensteller U, von Cramon DY. Motion class dependency in observers’ motor areas revealed by functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:1335–1342. doi: 10.1523/JNEUROSCI.4170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Schieber MH. Orderly somatotopy in primary motor cortex: Does it exist? NeuroImage. 2001;13:968–974. doi: 10.1006/nimg.2000.0733. [DOI] [PubMed] [Google Scholar]
- Sato M, Mengarelli M, Riggio L, Gallese V, Buccino G. Task related modulation of the motor system during language processing. Brain and Language. 2008;105:83–90. doi: 10.1016/j.bandl.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Saygin AP. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130:2452–2461. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Hagler DJ, Bates E, Sereno MI. Point-light biological motion perception activates human premotor cortex. Journal of Neuroscience. 2004;24:6181–6188. doi: 10.1523/JNEUROSCI.0504-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Functional-anatomical concepts of human premotor cortex: Evidence from fMRI and PET studies. NeuroImage. 2003;20:S120–S131. doi: 10.1016/j.neuroimage.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. The case of pretense: Observing actions and inferring goals. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21049. in press. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A. The organization of lexical knowledge in the brain. In: Gazzaniga MS, editor. The cognitive neurosciences III. Cambridge, MA: MIT Press; 2004. pp. 803–814. [Google Scholar]
- Shapiro LR, Gordon B, Hack N, Killackey J. Verb-argument structure processing in complex sentences in Broca’s and Wernicke’s aphasia. Brain and Language. 1993;45:423–447. doi: 10.1006/brln.1993.1053. [DOI] [PubMed] [Google Scholar]
- Shetreet E, Palti D, Friedmann N, Hadar U. Cortical representation of verb processing in sentence comprehension: Number of complements, subcategorization, and thematic frames. Cerebral Cortex. 2007;17:1958–1969. doi: 10.1093/cercor/bhl105. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. A mirror representation of others’ actions in the human anterior parietal cortex. Journal of Neuroscience. 2006;26:9736–9742. doi: 10.1523/JNEUROSCI.1836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtyrov Y, Hauk O, Pulvermüller F. Distributed neuronal networks for encoding category-specific semantic information: The mismatch negativity to action words. European Journal of Neuroscience. 2004;19:1083–1092. doi: 10.1111/j.0953-816x.2004.03126.x. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Barsalou LW. The similarity-in-topography principle: Reconciling theories of conceptual deficits. Cognitive Neuropsychology. 2003;20:451–486. doi: 10.1080/02643290342000032. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45:2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin DI. The many ways to search for a frog: Linguistic typology and the expression of motion events. In: Strömqvist S, Verhoeven L, editors. Relating events in narrative: typological and contextual perspectives. Mahwah, NJ: Erlbaum; 2004. pp. 219–258. [Google Scholar]
- Smith EE. Theories of semantic memory. In: Estes WK, editor. Handbook of learning and cognitive processes. Vol. 6. Hillsdale, NJ: Lawrence Erlbaum Associates; 1978. [Google Scholar]
- Stippich C, Ochmann H, Sartor K. Somatotopic mapping of the human primary sensorimotor cortex during motor imagery and motor execution by functional magnetic resonance imaging. Neuroscience Letters. 2002;331:50–54. doi: 10.1016/s0304-3940(02)00826-1. [DOI] [PubMed] [Google Scholar]
- Taylor LJ, Zwaan RA. Motor resonance and linguistic focus. Quarterly Journal of Experimental Psychology. 2008;61:896–904. doi: 10.1080/17470210701625519. [DOI] [PubMed] [Google Scholar]
- Taylor LJ, Lev-Ari S, Zwaan RA. Inferences about action engage action systems. Brain and Language. 2008 doi: 10.1016/j.bandl.2007.08.004. in press. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D. Listening to action-related sentences activates fronto-parietal motor circuits. Journal of Cognitive Neuroscience. 2005;17:273–81. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam MM. Neural correlates of verb argument structure processing. Journal of Cognitive Neuroscience. 2007;19:1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Lange KL, Schneider SL, Shapiro LP. Agrammatic and non-brain-damaged subjects’ verb and verb argument structure production. Aphasiology. 1997;11:473–490. [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. Journal of Neuroscience. 2007;27:13241–13250. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The cultural origins of human cognition. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- Tomasino B, Fink GR, Sparing R, Dafotakis M, Weiss PH. Action verbs and the primary motor cortex: A comparative TMS study of silent reading, frequency judgments, and motor imagery. Neuropsychologia. 2008;46:1915–1926. doi: 10.1016/j.neuropsychologia.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Werner CJ, Weiss PH, Fink GR. Stimulus properties matter more than perspective: An fMRI study of mental imagery and silent reading of action phrases. NeuroImage. 2007;36:T128–T141. doi: 10.1016/j.neuroimage.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Toni I, de Lange FP, Noordzij ML, Hagoort P. Language beyond action. Journal of Physiology, Paris. 2008;102:71–79. doi: 10.1016/j.jphysparis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR. A neural basis for the retrieval of words for actions. Cognitive Neuropsychology. 2001;18:655–670. doi: 10.1080/02643290126377. [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, Damasio A. Neural correlates of conceptual knowledge for actions. Cognitive Neuropsychology. 2003;20:409–32. doi: 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Tranel D, Manzel K, Asp E, Kemmerer D. Naming static and dynamic actions: Neuropsychological evidence. Journal of Physiology, Paris. 2008;102:80–94. doi: 10.1016/j.jphysparis.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G, Robert M, Pascual-Leone A, Lepore F, Nguyen DK, Carmant L, Bouthillierm A, Théoret H. Action observation and execution: Intracranial recordings in a human subject. Neurology. 2004;63:937–938. doi: 10.1212/01.wnl.0000137111.16767.c6. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Schütz-Bosbach S, Gallagher S. On agency and body-ownership: Phenomenological and neurcognitive reflections. Consciousness and Cognition. 2007;16:645–660. doi: 10.1016/j.concog.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Turella L, Pierno AC, Tubaldi F, Castiello U. Mirror neurons in humans: Consisting or confounding evidence? Brain and Language. 2008 doi: 10.1016/j.bandl.2007.11.002. In press. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing: A neurophysiological study. Neuron. 2001;32:91–101. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Fabbro F, Romani M, Aglioti SM. Motor faciliation during action observation: Topographic mapping of the target muscle and influence of the onlooker’s posture. European Journal of Neuroscience. 2006a;23:2522–2530. doi: 10.1111/j.1460-9568.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Ionta S, Aglioti SM. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nature Neuroscience. 2007;10:30–31. doi: 10.1038/nn1815. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Moro V, Candidi M, Aglioti SM. Mapping implied body actions in the human motor system. Journal of Neuroscience. 2006b;26:7942–7949. doi: 10.1523/JNEUROSCI.1289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Lindemann O, Bekkering H. Using conceptual knowledge in action and language. In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition. Oxford, UK: Oxford University Press; 2007. pp. 575–599. [Google Scholar]
- Van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage. doi: 10.1016/j.neuroimage.2008.07.057. in press. [DOI] [PubMed] [Google Scholar]
- van Schie HT, Toni I, Bekkering H. Comparable mechanisms for action and language: Neural systems behind Intentions, goals, and means. Cortex. 2006;42:495–498. doi: 10.1016/s0010-9452(08)70385-x. [DOI] [PubMed] [Google Scholar]
- Van Valin RD. Exploring the syntax-semantic interface. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Van Valin RD. Some universals of verb semantics. In: Mairal R, Gil J, editors. Linguistic universals. Cambridge, UK: Cambridge University Press; 2006. pp. 155–178. [Google Scholar]
- Vanier M, Caplan D. CT-scan correlates of agrammatism. In: Menn L, Obler LK, editors. Agrammatic aphasia. Vol. 1. Amsterdam: John Benjamins; 1990. pp. 37–115. [Google Scholar]
- Wallentin M, Lund TE, Ostergaard S, Ostergaard L, Roepstorff A. Motion verb sentences activate left posterior middle temporal cortex despite static context. NeuroReport. 2005;16:649–652. doi: 10.1097/00001756-200504250-00027. [DOI] [PubMed] [Google Scholar]
- Willems RM, Hagoort P. Neural evidence for the interplay between language, gesture, and action: A review. Brain and Language. 2007;101:278–289. doi: 10.1016/j.bandl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Wu DH, Waller S, Chatterjee A. The functional neuroanatomy of thematic role and locative relational knowledge. Journal of Cognitive Neuroscience. 2007;19:1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]
- Wunderlich D. Towards a structural typology of verb classes. In: Wunderlich D, editor. Advances in the theory of the lexicon. Berlin: Mouton de Gruyter; 2006. pp. 58–166. [Google Scholar]
- Zwaan RA, Taylor LJ. Seeing, acting, understanding: Motor resonance in language comprehension. Journal of Experimental Psychology: General. 2006;135:1–11. doi: 10.1037/0096-3445.135.1.1. [DOI] [PubMed] [Google Scholar]
- Zwaan RA, Taylor LJ, de Boer M. Motor resonance as a function of narrative time: Further tests of the Linguistic Focus Hypothesis. Brain and Language. doi: 10.1016/j.bandl.2008.11.004. in press. [DOI] [PubMed] [Google Scholar]


