Abstract
The theory of mixtures is applied to the analysis of the passive response of cells to osmotic loading with neutrally charged solutes. The formulation, which is derived for multiple solute species, incorporates partition coefficients for the solutes in the cytoplasm relative to the external solution, and accounts for cell membrane tension. The mixture formulation provides an explicit dependence of the hydraulic conductivity of the cell membrane on the concentration of permeating solutes. The resulting equations are shown to reduce to the classical equations of Kedem and Katchalsky (Kedem and Katchalsky, 1958, 1961) in the limit when the membrane tension is equal to zero and the solute partition coefficient in the cytoplasm is equal to unity. Numerical simulations demonstrate that the concentration-dependence of the hydraulic conductivity is not negligible; the volume response to osmotic loading is very sensitive to the partition coefficient of the solute in the cytoplasm, which controls the magnitude of cell volume recovery; and the volume response is sensitive to the magnitude of cell membrane tension. Deviations of the Boyle-van't Hoff response from a straight line under hypo-osmotic loading may be indicative of cell membrane tension.
Introduction
The fundamental physical mechanisms of solute and water transport across the cell membrane have long been studied in the field of cell membrane biophysics, and there exist a number of formalisms aiming to characterize transport through membrane channels and/or lipid bilayers. These formalisms include a one-parameter (solute permeability) model (Mazur et al., 1974), a classic two-parameter (water and solute permeability) model (Jacobs and Stewart, 1932) and a commonly used three-parameter model (water and solute permeability and a solute-solvent interaction term) developed by Kedem and Katchalsky (Kedem and Katchalsky, 1958). The parameters of interest (permeabilities) can be extracted from the formulation when the cell volume change is measured in the experiment, assuming that the cell volume change is due purely to the volume of the water (and solute) that enters or exudes from the cell. These formulations have been derived from the general theory of irreversible thermodynamics (Onsager, 1931a, b; Staverman, 1952).
In more recent decades, the field of rational mechanics has addressed problems in the mixture of fluids (solvent and solutes) (Mills, 1966) as well as the mixture of fluids and solids (deformable porous media) (Atkin and Craine, 1976; Bowen, 1980; Mow et al., 1980; Frank and Grodzinsky, 1987; Lai et al., 1991; Huyghe and Janssen, 1997; Gu et al., 1998). In this study we propose to apply the theory of mixtures to the analysis of the passive response of cells to osmotic loading. For ease of comparison with the more classical treatment of Kedem and Katchalsky (Kedem and Katchalsky, 1958, 1961), the analysis is limited to non-electrolytes. However, the formulation is generalized to multiple solute species; it also incorporates partition coefficients for the solutes in the cytoplasm relative to the external solution, and accounts for cell membrane tension.
Methods
In this analysis the cell is modeled as a fluid-filled membrane, where the membrane is described by the mixture theory equations presented below. These equations are generally valid for neutral solute transport in uncharged porous media. In the following sections, the general equations of mixture theory are reduced to the case of a thin membrane, specialized to the case of one permeating and one non-permeating solute, and then compared to the classical Kedem-Katchalsky model.
Governing Equations of Mixture Theory
In the presentation of the mixture theory equations we use the notation of Gu et al. (Gu et al., 1998), along with the generalizations to the solvent and solute momentum equations and solute constitutive equation as described by Mauck et al. (Mauck et al., 2003). The mixture consists of a solid matrix (α = s), solvent (α = w) and solutes (α ≠ s, w), with each constituent assumed to be intrinsically incompressible and neutrally charged. The momentum equations for the mixture as a whole and for the solvent and solutes are given respectively by
| (1) |
| (2) |
where p is the fluid (solution) pressure, σe is the effective (or elastic) stress in the solid matrix, μ̃α the chemical potential of the solvent or solute, vα is the velocity of constituent α, fαβ is the diffusive drag coefficient between constituents α and β (with fβα = fαβ), and ρα is the apparent density of constituent α. In this treatment, the solute-solvent mixture is assumed to be dilute and ideal (solute activity coefficients and osmotic coefficients of unity) so that the constitutive relations for the solvent and solute chemical potentials are given by
| (3) |
| (5) |
where cα is the solute concentration on a solvent volume basis, Mα is the molecular weight of solute α, R is the universal gas constant and θ is the absolute temperature. Since the pore size distribution within the solid matrix may vary, solutes may not have access to all of the solvent available within the matrix due to steric volume exclusion effects. Mauck et al. (Mauck et al., 2003) extended the formulation of the solute chemical potential to account for this effect by incorporating the partition coefficient κα which, for an ideal solution, is the solubility of solute α inside the mixture relative to free solution. Thus cα/κα is the concentration of solute per volume of accessible solvent; κα is assumed to be constant in this analysis. is the true density of the solvent and is the chemical potential of constituent α in the standard state (when in the case of solutes, and when all cα = 0 and p = 0 in the case of the solvent). The solute standard state is a constant, usually taken to be (Tinoco, 2002). Substituting Eqs. (3)–(4) into Eq.(2) and recognizing that ρα = Mαφwcα (α ≠ s, w), and where φw is the volume fraction of the solvent in the mixture, yields
| (5) |
| (6) |
This formulation is simplified to account for friction between solutes and the solvent and between solutes and the mixture while neglecting the solute-against-solute diffusive drag. The non-zero diffusive drag coefficients are given by (Lai et al., 1991; Gu et al., 1998; Mauck et al., 2003; Meerveld et al., 2003)
| (7) |
where k is the hydraulic permeability of the solvent in the solid matrix, Dα is the solute diffusivity in the mixture and is its diffusivity in free solution. In general, due to steric exclusion effects and tortuosity, Dα is smaller than . From these equations we can interpret the diffusion coefficient of a solute in free solution to result only from the frictional drag of the solute with the solvent (fwα); in the presence of a solid matrix however, the diffusion coefficient results from the frictional drag between solute and solvent and between solute and solid matrix (fwα + fws). Substituting Eq.(7) into Eqs.(5)–(6) yields
| (8) |
| (9) |
Equation (9) can be solved for vα − vs in terms of vw − vs; substituting this result into Eq.(8) and rewriting vβ − vw as (vβ − vs)+(vs−vw), these equations can be solved simultaneously to yield
| (10) |
| (11) |
where
| (12) |
φw(vw − vs) is the volumetric flux of solvent relative to the solid matrix and φwcα(vα − vs) is the molar flux of solute α relative to the solid matrix. The material properties of the mixture include the hydraulic permeability of the solid matrix to pure solvent, k; k̃ is the hydraulic permeability of the matrix to the solution (solvent + solutes).
In addition to these momentum equations the equations of conservation of mass for the mixture as a whole and for the individual solutes are given by
| (13) |
| (14) |
where, for dilute solutions, we have taken into account that the volume fraction of solutes is much smaller than that of the solvent, φα ≪ φw.
The equations presented above are generalized relations for diffusion-convection problems in deformable porous media, where the solution is dilute. In these expressions, φw depends on the matrix dilatation according to
| (15) |
where is the porosity in the reference configuration of zero deformation and F is the deformation gradient of the solid matrix.
The general form of boundary or interface conditions applicable to mixture problem of this type are given by
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
where [[·]] denotes the difference, across the interface, of the quantity in the brackets, and n is the unit outward normal to the interface. These equations assume that the interface is defined somewhere on the solid matrix, which is the most common case (e.g., the cell membrane surface). Note that if the boundary or interface is not permeable to solute α, then Eq.(18) is equivalent to φwcα (vα − vs)·n = 0 and Eq.(21) is not applicable. Eqs.(16)–(18) are kinematic conditions that stipulate continuity of solid matrix velocity, relative solvent flux, and relative solute molar flux across the interface, respectively. Eqs.(19)–(21) represent continuity of the total traction, solvent chemical potential, and solute chemical potentials across the interface, respectively.
Mixture Model for Thin Semi-Permeable Membrane
For transport across a thin membrane of thickness h, the gradients in pressure and concentration across the membrane may be represented in scalar form by grad p · n ≈ −Δp/h and grad cα · n ≈ −Δcα/h, where n is the unit normal to the membrane surface. In this notation, Δp = p(r)− p(r + h), and similarly for Δcα, where r is the coordinate direction normal to the membrane. The flux vector components normal to the membrane are given by
| (22) |
| (23) |
Using Eqs.(10)–(11), these expressions reduce to
| (24) |
| (25) |
where c̅α in the last term now represents the average solute concentration in the membrane.
Evaluating the projection of the vector equation in Eq.(1) along n, and reducing the resulting expression to the case of a thin spherical membrane under symmetric conditions yields
| (26) |
where T is the surface tension in the membrane (in units of force per unit length), a is the spherical membrane radius, and is the normal effective (elastic) stress in the radial direction.
According to the mixture formulation the concentrations cα appearing in the above expressions refer to quantities inside the membrane. To express these equations using concentrations in the cytoplasm and in the external solution, boundary conditions of the form given in Eq.(21) are needed. For the interface between the membrane and cytoplasm,
| (27) |
where pi is the fluid pressure in the cytoplasm; is the concentration of solute α in the cytoplasm; is the partition coefficient of the solute between the cytoplasm and the external solution and κα is the corresponding partition coefficient between the membrane and external solution. Similarly, for the interface between the membrane and external solution,
| (28) |
where pe is the fluid pressure and is the concentrations of solute α in the external solution. Using these relations Δp, and Δcα can be evaluated from the difference, and c̅α from the mean, of the respective expressions in Eqs.(27) and (28) to yield
| (29) |
| (30) |
Substituting these expressions, along with Eq.(26), into Eqs.(24)–(25) produces
| (31) |
| (32) |
where
| (33) |
Lp is the membrane hydraulic conductivity (or conductance), Pα is the membrane permeability of solute α and σα is Staverman's reflection coefficient. These expressions are consistent with the physical meaning attributed to the material parameters of the Kedem-Katchalsky model (Kedem and Katchalsky, 1961). For example, Staverman's coefficient is equal to zero when the solute can diffuse through the membrane as easily as through pure solvent (no membrane resistance), . Conversely, if the solute cannot diffuse through the membrane, καDα = 0, which leads to σα = 1 and Pα = 0. Substituting Eqs.(30)2 and (33) into Eq.(12) provides an explicit dependence of Lp on the external concentrations,
| (34) |
where Lp0 = k/h is the value of Lp in the absence of solutes.
Integrating the mixture continuity of mass equation in Eq.(13) and recognizing that all velocities are equal to zero at the cell center yields Jw = φw (vw − vs) · n = −vs · n where vs is the velocity of the cell membrane. It follows from this result that Jw A = −dV/dt where A is the cell surface area (A = 4πa2) and V is its volume (V = 4πa3/3). By integrating the solute continuity of mass equation in Eq.(14) over the cell volume, we find that , where is the number of moles of solute inside the cell. Substituting all these results into Eqs.(31)–(32) yields
| (35) |
| (36) |
In these expressions the internal concentrations of solutes are related to the corresponding number of moles via
| (37) |
where is the average water content (porosity) of the cell. Furthermore, from kinematic considerations under finite deformation as given in Eq.(15),
| (38) |
where and Vr are the cell water content and volume in the reference configuration (in the initial state prior to osmotic loading), respectively. Note that for all solute species are prescribed as boundary conditions. For a non-permeating solute we have κα = 0, which leads to σα = 1 and Pα = 0 according to Eq.(33), so that Eq.(36) yields . Thus, for a non-permeating solute, is constant (i.e., the number of moles of non-permeating solute inside the cell remains constant). Substituting Eqs.(37)–(38) into Eqs.(35)–(36) produces a set of coupled nonlinear ordinary differential equations in the unknowns and V(t) which can be solved subject to appropriate initial conditions, given a constitutive relation between the membrane tension T and surface area A.
Permeating and Non-Permeating Solutes
The analysis can now proceed to consider solutes which can permeate across the membrane, and solutes which cannot. For the case of one permeating (α = p) and one non-permeating (α = n) solute species, it follows that Jn = 0, so that the only remaining relations are
| (39) |
| (40) |
with
| (41) |
To evaluate the equilibrium response for the system of equations in Eqs.(39)–(40), let dnp/dt = 0 and dV/dt = 0, which yields
| (42) |
This equilibrium solution is equally valid in the reference state (assuming that the reference state is at equilibrium), so that
| (43) |
where the subscript r refers to quantities evaluated in the reference state. Substituting Eqs.(37)–(38) into the above equilibrium solutions and recognizing that the number of moles of non-permeating solute inside the cell remains constant, , produces an expression for the equilibrium values of and V (t),
| (44) |
Since the cell membrane tension T will generally be a function of its areal strain, the expression for V/Vr in Eq.(44) would need to be solved nonlinearly for the radius a of the cell, except in the special case when T = 0. Clearly the equilibrium response to osmotic loading is independent of solvent and solute transport parameters (LP, Pp, σp). In addition to being a function of the initial and final concentrations of permeating and non-permeating solutes, it is dependent on the cell membrane tension, the permeating solute partition coefficient in the cytoplasm, and the water content of the cell.
Note that under hyper-osmotic loading with very high concentrations of a non-permeating solute, , the relative volume change can be used to assess the water content (or equivalently, the osmotically inactive solid fraction, ) inside the cell under the reference configuration,
| (45) |
Three-Parameter Kedem-Katchalsky Model
It can be noted that the above equations reduce to the classical three-parameter (LP, Pp, σp) Kedem-Katchalsky model (Kedem and Katchalsky, 1958, 1961; Kleinhans, 1998) if the membrane surface tension is neglected (T = 0) and the permeating solute partition coefficient is assumed to be unity within the cytoplasm (). Thus from Eqs.(31)–(32),
| (46) |
| (47) |
where we have replaced 2T/a with pi − pe as per Eq.(29)2 for ease of comparison. However, even in this special reduced form the hydraulic conductivity is still found to be concentration-dependent according to Eq.(41),
| (48) |
This result suggests that the mixture formulation is more general than the classical phenomenological equations.
Results
To analyze the influence of the various material parameters on the response of a cell to osmotic loading, we propose to investigate each parameter independently of others. In contrast to the classical three-parameter K-K model, the general set of equations in (39)–(41) has five parameters, Lp0, Pp, σp, κp and , in addition to the need for a constitutive relation between the membrane tension T and area A. Since the governing differential equations are nonlinear specific numerical examples are given to illustrate the influence of the parameters that are not traditionally included in the K-K model. In all cases the temperature is given by θ = 25°C = 298K and the cell radius and water content in the reference state are ar = 10 µm, . The hydraulic conductivity of the membrane at zero solute concentration is taken as Lp0 = 5 × 10−14 m3/N · s; the solute permeability in the limit when κp = 1 is taken as φwDα/h = 5 × 10−8 m/s; and for simplicity it is assumed that so that σα = 1 − κα/2 according to Eq.(33). Integration of the nonlinear ordinary differential equations was performed numerically using the Runge-Kutta method of order 4 (Conte and De Boor, 1980) in a custom-written program; numerical convergence was achieved in all cases.
Concentration-Dependence of Membrane Hydraulic conductivity
When examining Eqs.(39)–(41), it can be noted that the parameter κp appears explicitly only in the expression for Lp in Eq.(41). If we find that the concentration-dependence of Lp has a negligible effect on the overall response of Eqs.(39)–(41), then the five-parameter model would effectively be reduced to a four-parameter model (Lp0, Pp, σp and ). This is investigated numerically using the following representative choice of parameters:
| (49) |
The response to hyper-osmotic loading with a permeable solute is analyzed, with
| (50) |
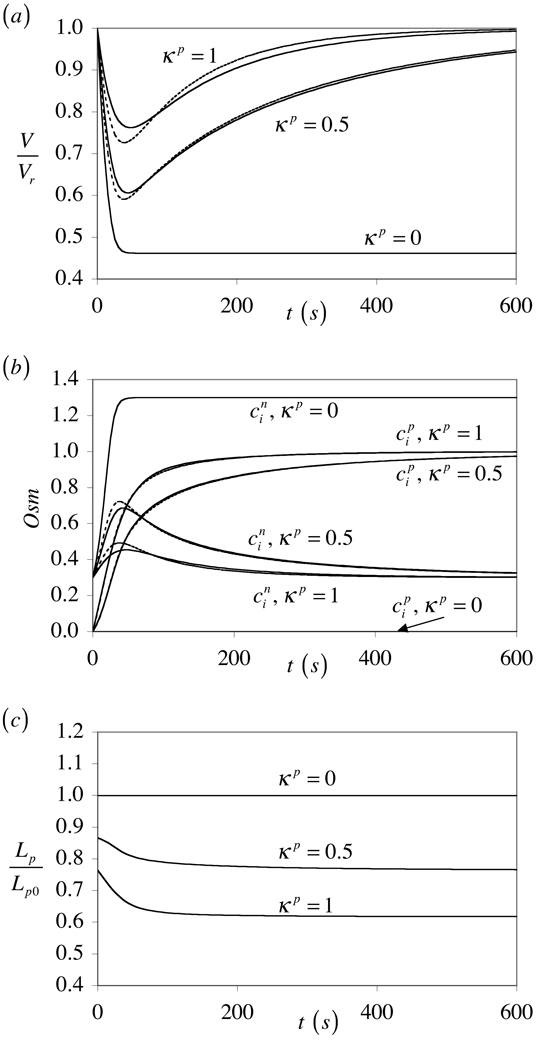
For this analysis the membrane tension is taken as T = 0. The resulting cell volume response, V/Vr, is presented in Figure 1a. For κp = 1 and 0.5, the volume initially decreases before recovering to its initial value at equilibrium (as also predicted from the closed-form equilibrium solution in Eq.(44)). This is the classical response to hyper-osmotic loading, whereby water initially exudes from the cell due to the higher external solute concentration; as the external permeating solute slowly diffuses into the cell however, the concentrations of permeating and non-permeating solutes inside and outside eventually balance themselves (Figure 1b) and water flows back into the cell until there is complete volume recovery. In the limiting case of κp = 0 however, the solute is completely excluded from the membrane and thus cannot permeate across it. Consequently, water will exude from the cell as the volume decreases, until the decrease in volume produces a balance between the concentration of non-permeating solute inside () and the concentrations of permeating and non-permeating solutes outside (Figure 1b). In this case there is no volume recovery achieved at equilibrium. The variation of the concentration-dependent Lp as a function of time is shown in Figure 1c. When the solute can permeate across the cell membrane (κp > 0), the value of Lp drops significantly below that of Lp0, particularly when κp = 1. The greatest difference observed in V/Vr between concentration-dependent and constant values of Lp is thus observed at κp = 1 (Figure 1a).
Figure 1.
Comparison of cell response to hyper-osmotic loading, when the hydraulic conductivity Lp is taken to be concentration-dependent (solid lines) [Eq.(41)] or assumed constant (dashed lines) [Lp = Lp0], for various values of the solute partition coefficient in the membrane, κp. The case κp = 0 is equivalent to hyper-osmotic loading with a non-permeating solute. (a) Relative cell volume response, V/Vr. (b) Internal concentration of permeating solute, , and non-permeating solute, . (c) Concentration-dependent normalized membrane hydraulic conductivity, Lp/Lp0.
Effect of Permeating Solute Partition Coefficient In Cytoplasm
According to the equations for the equilibrium solution, Eq.(44), the partition coefficient for the permeating solute between the cytoplasm and external solution, , influences the equilibrium value of V/Vr. The complete time-dependent response of a cell to hyper-osmotic loading is investigated for various values of , using the material parameters
| (51) |
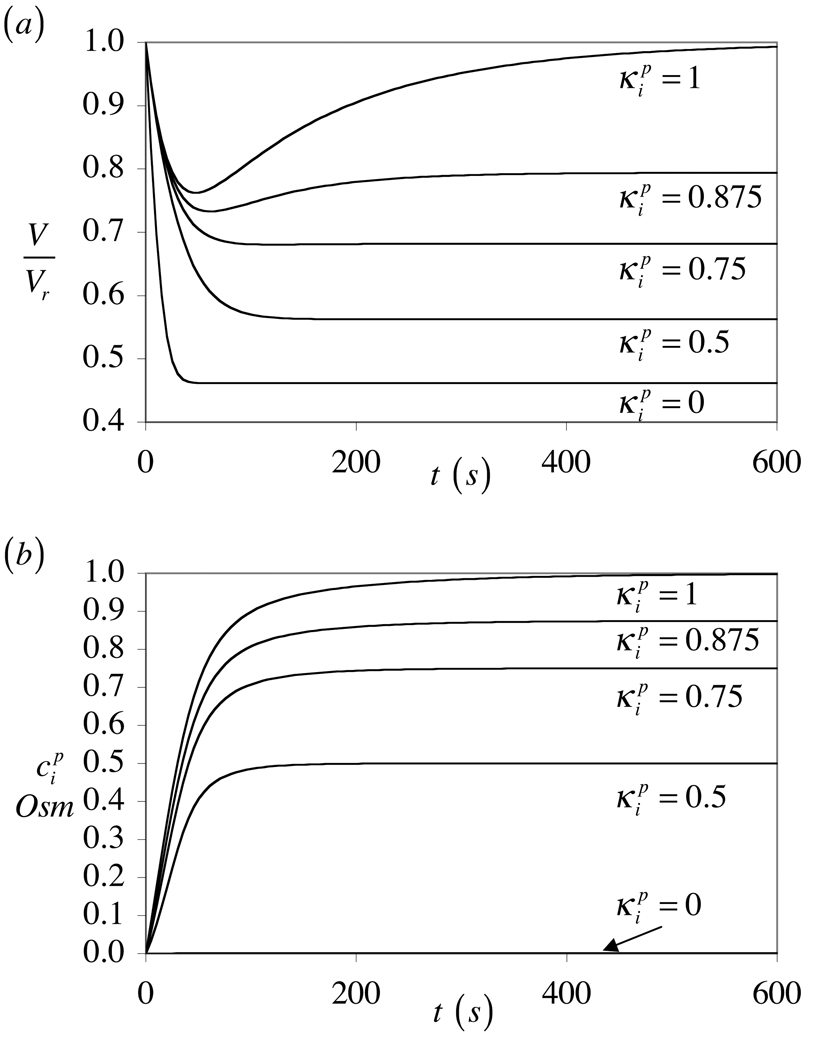
In this analysis, Lp is concentration-dependent, T = 0, and the external solute concentrations are given by Eq.(50). Note that the limiting case requires special treatment in the numerical solution since the permeating solute concentration inside the cell must reduce to zero, which is equivalent to osmotic loading with a non-permeating solute. Results show that has a very significant influence on the relative change in volume of the cell (Figure 2a). Only partial volume recovery is achieved when . The reason for this outcome is apparent from Figure 2b, which shows that the internal concentration of permeating solute decreases with decreasing values of . Interestingly, as becomes smaller, the volume response to osmotic loading with a permeating solute becomes qualitatively similar to the response to loading with a non-permeating solute, as can be construed from the responses at and in Figure 2a. This serves as a cautionary note in the interpretation of experimental data, namely that the lack of volume recovery following osmotic loading does not necessarily imply that the solute is non-permeating. The response shown in Figure 2a is remarkably similar to the recent experimental study of Lucio et al. (Lucio et al., 2003).
Figure 2.
Response to hyper-osmotic loading for various values of the solute partition coefficient in the cytoplasm, : (a) Relative cell volume V/Vr, and (b) internal concentration of permeating solute, .
Effect of Membrane Tension
To investigate the influence of membrane tension on the volumetric response, we now provide a constitutive relation between T and the areal strain. In this formulation it is assumed that the membrane is slack below a threshold value of the areal strain and supports tensile stresses above that threshold (Guilak et al., 2002),
| (52) |
where A0 is the cell surface area above which tension develops in the membrane. For simplicity in the current analysis, it is assumed that this threshold transition point coincides with the reference configuration of the cell, A0 = Ar. Thus tension will exist in the membrane under hypo-osmotic loading (swelling) relative to the reference state, but not under hyper-osmotic loading (shrinking). Furthermore, the power law of Eq.(52) is implemented in its simplest form, with β = 1. This leaves the area expansion modulus K as a parameter that can be varied in the analysis. The numerical example uses the following choice of parameters,
| (53) |
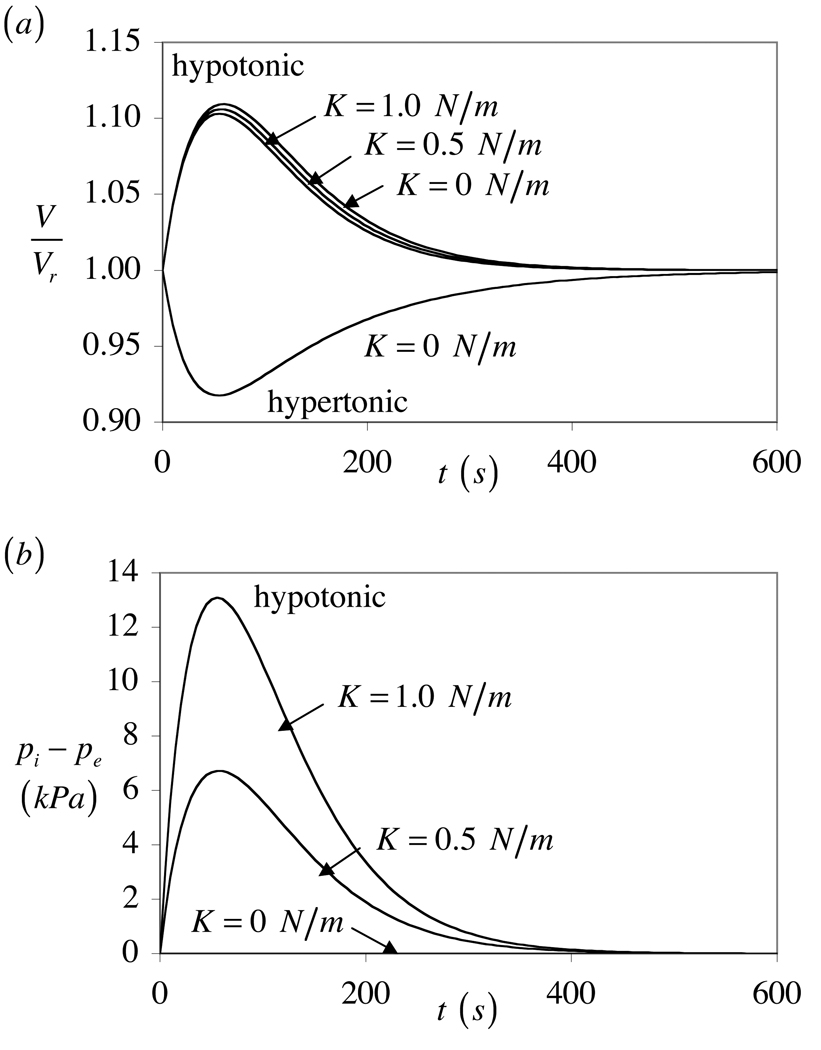
This range of K is based on values reported for red blood cells (Evans and Waugh, 1977; Waugh and Evans, 1979) and lipid membranes (Needham and Nunn, 1990). A representative case of hypo-osmotic loading with a permeating solute is obtained with
| (54) |
whereas hyper-osmotic loading is obtained with
| (55) |
The resulting relative volume change of the cell is presented in Figure 3a, showing an asymmetric response between hyper- and hypo-osmotic loading even in the absence of membrane tension, as supported from experiments (Curry et al., 2000). When membrane tension does develop (K > 0), the peak volumetric change decreases and pressure rises inside the cell as the membrane expands under hypo-osmotic loading. The pressure then returns to zero as the cell recovers its initial volume (Figure 3b).
Figure 3.
Response to hyper-osmotic and hypo-osmotic loading with a permeating solute, for various values of the area expansion modulus K. Membrane tension occurs only under hypo-osmotic loading in this example. (a) Relative cell volume V/Vr, and (b) pressure difference between the inside and outside of the cell, pi − pe.
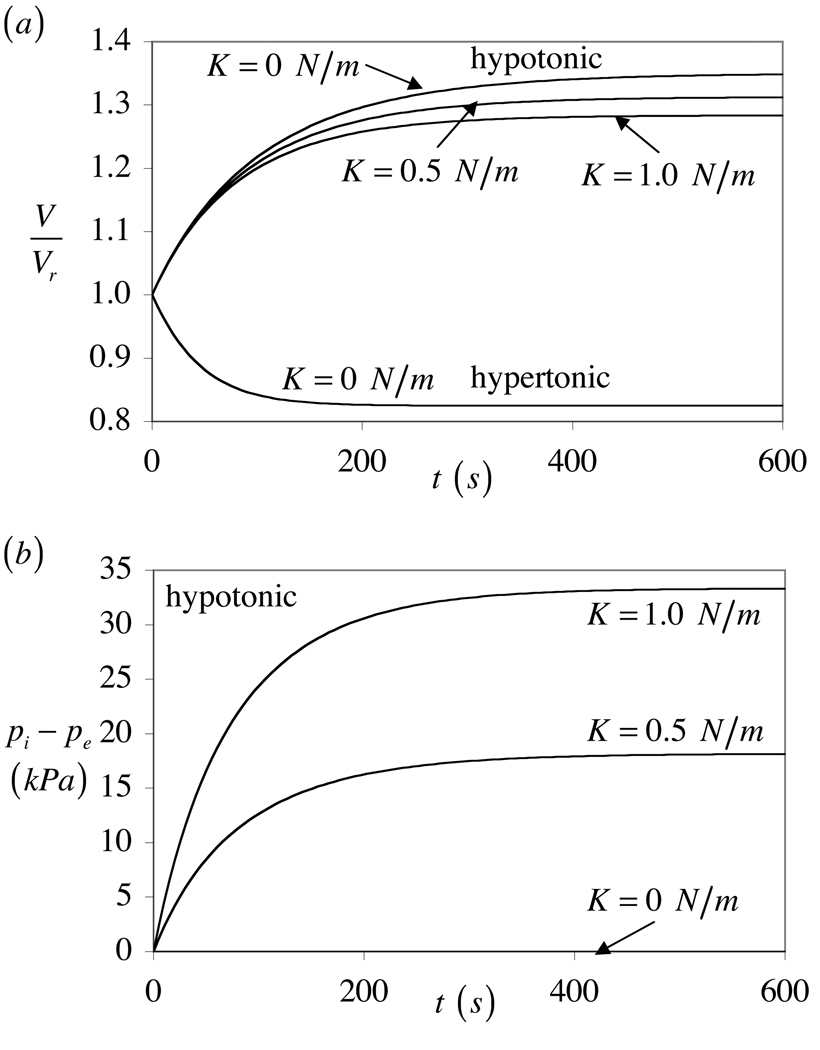
The response to loading with a non-permeating solute can similarly be simulated with the representative conditions
| (56) |
for hypo-osmotic loading and
| (57) |
for hyper-osmotic loading. The asymmetric volume response between hyper- and hypo-osmotic loading is even more apparent with non-permeating solutes (Figure 4a). There is no volume recovery under loading with a non-permeating solute, and for K > 0 the internal fluid pressure remains elevated at steady-state, to balance the membrane tension (Figure 4b). The equilibrium volume increase under hypo-osmotic loading is smaller with increasing membrane stiffness.
Figure 4.
Response to hyper-osmotic and hypo-osmotic loading with a non-permeating solute, for various values of the area expansion modulus K. (a) Relative cell volume V/Vr, and (b) pressure difference between the inside and outside of the cell, pi − pe.
In addition to analyzing the transient response to osmotic loading, we can also plot the equilibrium volume change in response to osmotic loading with a non-permeating solute, as V/Vr versus , for various values of the membrane area expansion modulus K. This is achieved by substituting the constitutive relation of Eq.(52) into the equilibrium solution of Eq.(44), for the special case A0 = Ar and β = 1,
| (58) |
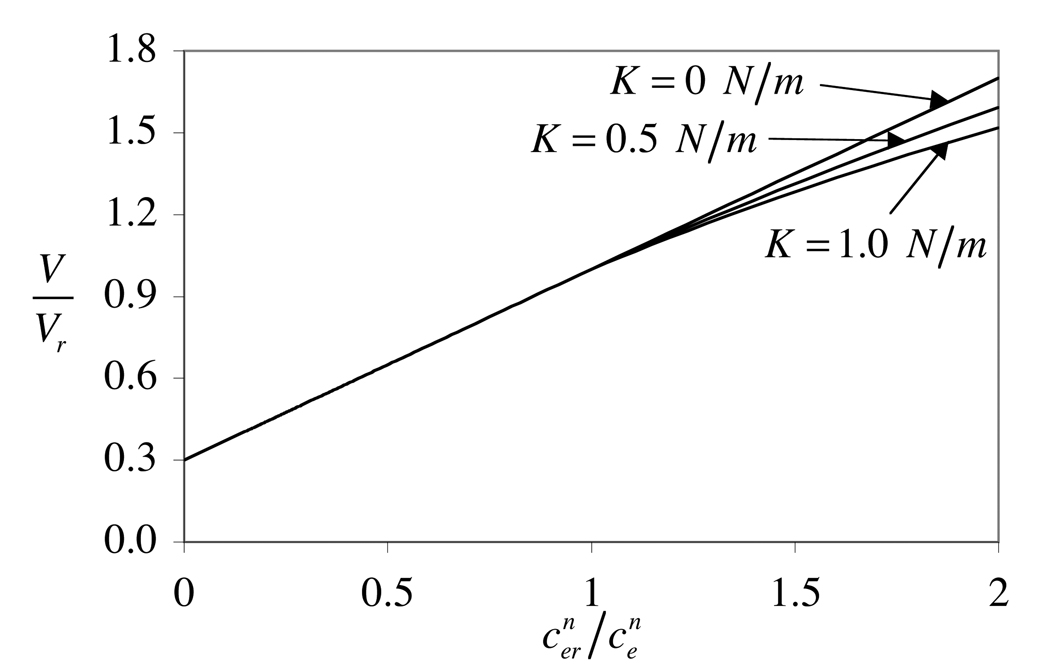
where V/Vr = (a/ar)3 and A/Ar = (a/ar)2. Under hypo-osmotic loading (), this is a non-linear equation in the unknown cell radius a. For a reference configuration , the equilibrium response V/Vr is plotted against in Figure 5. When there is no membrane tension, the response is linear and corresponds to the classical Boyle-van't Hoff relation or Ponder plot, which has been confirmed experimentally (Guilak et al., 2002; Xu et al., 2003). According to Eq.(58) the slope of the line is and the intercept at the origin is . In the presence of membrane tension however, the response becomes nonlinear, with tapering off of the volume increase under hypo-osmotic loading.
Figure 5.
Equilibrium response of V/Vr for various values of the area expansion modulus K. When K > 0, a transition occurs from a linear to a non-linear response at .
Discussion
Mixture theory has been used to formulate the equations for osmotic loading of a fluid-filled spherical membrane representative of a cell. The fundamental principles of mixture theory represent a generalization of the phenomenological equations that are based on irreversible thermodynamics (Onsager, 1931a, b; Staverman, 1952). The mixture theory equations, Eqs. (10)–(12), are not limited to thin membranes but are applicable to any continuum consisting of a mixture of a neutral solid, solvent, and solute constituents. The material parameters appearing in these equations are the familiar properties of transport theory, including the permeability of the solvent through the porous solid matrix, and the diffusivities of the solutes in the mixture and in free solution. By reducing these governing equations to the special case of a spherically-shaped thin membrane a formulation was obtained which is a generalized form of the classical Kedem-Katchalsky equations. Equivalences were established between the material parameters of mixture theory and the classical phenomenological parameters, as presented in Eq.(33). These equivalences are intuitive and consistent with the physical interpretations attributed to the phenomenological parameters (Kedem and Katchalsky, 1961). They are also comparable to the relations presented by Gu et al. (Gu et al., 1993), though it should be noted that these authors included charge effects, which allows them to model the membrane potential, while neglecting the friction between solutes and the solid matrix (fsα = 0). One noteworthy generalization over the Kedem-Katchalsky model is the formulation of a concentration-dependent membrane hydraulic conductivity as shown in the general expression of Eq.(34) and in the special case of Eq.(48). The dependence of Lp on concentration has been alluded to in previous studies (Williams and Comper, 1990), though we believe that the explicit dependence derived from mixture theory in this study has not been previously reported. Another generalization relative to the Kedem-Katchalsky model is the incorporation of surface tension in the reduction to a thin spherical membrane (Evans et al., 1976).
The requisite interface boundary conditions of Eqs.(16)–(21) were applied to the mixture formulation to investigate the cell response to osmotic loading. One particular feature which bears note is the incorporation of a partition coefficient for solutes in the mixture, relative to free solution. The resulting governing equations for osmotic loading of a fluid-filled membrane, Eqs.(31)–(32), are more general than the three-parameter Kedem-Katchalsky model, Eq.(46)–(47), though they can be reduced to that model in the limit when the cytoplasm partition coefficient for permeating solutes is equal to unity and the membrane tension is neglected.
An assumption which is implicit in the current model as well as the K-K model is that the diffusion of solutes within the cytoplasm occur at time scales significantly shorter than the transport of solutes and solvents across the membrane. Therefore homogeneous distributions of solute concentration are assumed inside the cell. The mixture formulation assumes the existence of a solid matrix in the cell (the osmotically inactive volume) since the intracellular (osmotically active) water content () can be less than 100% and the solute partition coefficient () can be less than unity. However the stiffness of this matrix, and resistance to intracellular flow through the matrix, are neglected in the current formulation, under the assumption that the magnitude of osmotic pressure developing inside the cell (e.g., Figure 3b and Figure 4b) is considerably higher than the stress magnitudes resulting from matrix strains, and resistance to water and solute transport across the cell membrane is significantly greater than for intracellular transport.
The numerical examples investigated in this study suggest that the concentration-dependence of the membrane hydraulic conductivity, Lp, can be significant, particularly when the membrane partition coefficient for the permeating solute, κp, is close to unity (Figure 1). However, when osmotic loading is performed with a non-permeating solute, Lp remains constant and equal to Lp0 (Figure 1c), as is apparent from Eqs.(41) and (48) with . This observation suggests that, experimentally, Lp0 can be determined by osmotic loading with a non-permeating solute.
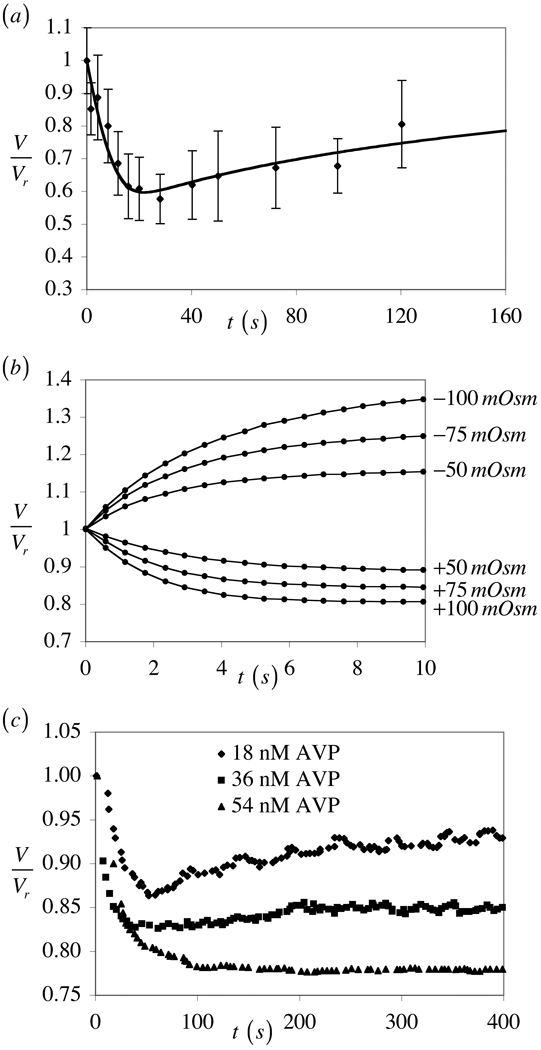
The theoretical predictions of this model are in very good agreement with experimental results available in the literature. For example, the response to hyperosmotic loading with a permeating solute, as shown in Figure 1 for the case κp ≠ 0 agrees very well with the experiments of Xu et al. (Xu et al., 2003) where bovine chondrocytes were osmotically loaded with glycerol (Figure 6a). The asymmetric response to hyper- and hypo-osmotic loading with a non-permeating solute predicted in Figure 4 is very similar to the experiments of Curry et al. (Curry et al., 2000) who performed osmotic loading of rabbit spermatozoa (Figure 6b). It is also interesting that varying the partition coefficient of the permeating solute in the cytoplasm can produce partial volume recoveries (Figure 2) which are remarkably similar to experimental measurements by Lucio et al. (Lucio et al., 2003) in kidney cells treated with various doses of vasopressin hormone (Figure 6c). This agreement suggests that the role of the partition coefficient in osmotic loading of cells may be quite significant and should be investigated in greater detail. Quantitative differences in the time scales and magnitude of volume changes between these experimental studies and the theoretical predictions of the current study are simply due to differences in cell sizes (radius a) and choices of material coefficients (Lp and Pp) and boundary conditions (values of and ).
Figure 6.
(a) Hyper-osmotic loading of bovine chondrocytes with a permeating solute (1.4 M glycerol) at 21°C, with solid curve representing prediction from K-K model (Reprinted from Med Eng Phys, Vol. 25, Xu, X., Cui, Z., Urban, J. P., Measurement of the chondrocyte membrane permeability to Me2SO, glycerol and 1,2-propanediol, pp. 573–579, Copyright (2003), with permission from The Institute of Engineering and Physics in Medicine); (b) Osmotic loading of rabbit spermatozoa at 25°C with non-permeating solutes (sucrose for hyper-osmotic loading and dilution of cell culture media with distilled water for hypo-osmotic loading) (Reprinted from Cryobiology, Vol. 41, Curry, M. R., Kleinhans, F. W., Watson, P. F., Measurement of the water permeability of the membranes of boar, ram, and rabbit spermatozoa using concentration-dependent self-quenching of an entrapped fluorophore, pp. 167–173, Copyright (2000), with permission from Elsevier); (c) Hyper-osmotic loading of Madin Darby canine kidney cell with sodium chloride in the presence of different concentrations of the hormone vasopressin, at 25°C (Reprinted figure with permission from Lucio, A. D., Santos, R. A., Mesquita, O. N., Phys Rev E Stat Nonlin Soft Matter Phys 68, 041906, 2003. Copyright 2003 by the American Physical Society).
Finally, the numerical simulations incorporating cell membrane elasticity show that surface tension can have a non-negligible influence on the volumetric response to osmotic loading (Figure 3, Figure 4). Experimental measurements of the equilibrium response to loading with a non-permeating solute at various concentrations may be used to infer the membrane's area expansion modulus if the Boyle-van't Hoff relationship is found to significantly deviate from a straight line under hypo-osmotic loading (Figure 5). For bovine chondrocytes, measurements to date demonstrate a linear relationship (Guilak et al., 2002; Xu et al., 2003) more akin to the case K = 0 N/m (“perfect osmometer”) in Figure 5, suggesting that membrane tension may be negligible for those cells.
Acknowledgments
This study was supported with funds from the National Institutes of Health (R21 AR48791, R01 AR46532).
Nomenclature
- a
cell radius
- A
cell surface area
- cα
concentration of solute α inside the mixture, on a solvent volume basis
concentration of solute α in the standard state
- c̅α
average concentration of solute α inside the membrane
concentrations of solute α in external solution
concentration of solute α in cytoplasm
- Dα
solute diffusivity in mixture of solid and fluid
solute diffusivity in free solution
- F
deformation gradient of the solid matrix
- fαβ
diffusive drag coefficient between constituents α and β
- Jw
volume flux of solvent across membrane
- Jα
molar flux of solute α across membrane
- k
hydraulic permeability of solid matrix to pure solvent
- k̃
hydraulic permeability of solid matrix to solution (solvent + solutes)
- Lp
membrane hydraulic conductivity (or conductance)
- Mα
molecular weight of solute α
number of moles of solute α inside the cell
- p
fluid pressure inside the mixture
- pe
fluid pressure in external solution
- pi
fluid pressure in cytoplasm
- Pα
membrane permeability of solute α
- R
universal gas constant
- T
surface tension in membrane
- V
cell volume
- vα
velocity of constituent α
- α = n
refers to non-permeating solute
- α = p
refers to permeating solute
- α = s
refers to solid matrix
- α = w
refers to solvent
- φα
volume fraction of constituent α in the mixture
volume fraction of osmotically active water in the cell
- κα
partition coefficient of solute α inside mixture relative to free solution
partition coefficient of solute α between cytoplasm and external solution
- μ̃α
chemical potential of solvent (α = w) or solute
chemical potential of constituent α in the standard state
- ρα
apparent density of constituent α
true density of solvent
- θ
absolute temperature
- σe
effective stress tensor in solid matrix
normal effective stress in radial direction
- σα
Staverman's reflection coefficient for solute α
References
- Atkin RJ, Craine RE. Continuum Theories of Mixtures: Basic Theory and Historical Development. Q J Mech Appl Math. 1976;29:209–244. [Google Scholar]
- Bowen RM. Incompressible porous media models by use of the theory of mixtures. International Journal of Engineering Science. 1980;18:1129–1148. [Google Scholar]
- Conte SD, De Boor C. Elementary numerical analysis: an algorithmic approach. New York: McGraw-Hill; 1980. [Google Scholar]
- Curry MR, Kleinhans FW, Watson PF. Measurement of the water permeability of the membranes of boar, ram, and rabbit spermatozoa using concentration-dependent self-quenching of an entrapped fluorophore. Cryobiology. 2000;41:167–173. doi: 10.1006/cryo.2000.2277. [DOI] [PubMed] [Google Scholar]
- Evans EA, Waugh R, Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976;16:585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Waugh R. Osmotic correction to elastic area compressibility measurements on red cell membrane. Biophys J. 1977;20:307–313. doi: 10.1016/S0006-3495(77)85551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank EH, Grodzinsky AJ. Cartilage electromechanics--II. A continuum model of cartilage electrokinetics and correlation with experiments. J Biomech. 1987;20:629–639. doi: 10.1016/0021-9290(87)90283-1. [DOI] [PubMed] [Google Scholar]
- Gu WY, Lai WM, Mow VC. Transport of fluid and ions through a porouspermeable charged-hydrated tissue, and streaming potential data on normal bovine articular cartilage. J Biomech. 1993;26:709–723. doi: 10.1016/0021-9290(93)90034-c. [DOI] [PubMed] [Google Scholar]
- Gu WY, Lai WM, Mow VC. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. J Biomech Eng. 1998;120:169–180. doi: 10.1115/1.2798299. [DOI] [PubMed] [Google Scholar]
- Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82:720–727. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe JM, Janssen JD. Quadriphasic mechanics of swelling incompressible porous media. International Journal of Engineering Science. 1997;35:793–802. [Google Scholar]
- Jacobs MH, Stewart DR. A simple method for the quantitative measurement of cell permeability. Journal of Cell Computational Physiology. 1932;1:71–82. [Google Scholar]
- Kedem O, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958;27:229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Kedem O, Katchalsky A. A physical interpretation of the phenomenological coefficients of membrane permeability. J Gen Physiol. 1961;45:143–179. doi: 10.1085/jgp.45.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans FW. Membrane permeability modeling: Kedem-Katchalsky vs a two-parameter formalism. Cryobiology. 1998;37:271–289. doi: 10.1006/cryo.1998.2135. [DOI] [PubMed] [Google Scholar]
- Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- Lucio AD, Santos RA, Mesquita ON. Measurements and modeling of water transport and osmoregulation in a single kidney cell using optical tweezers and videomicroscopy. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:041906. doi: 10.1103/PhysRevE.68.041906. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P, Leibo SP, Miller RH. Permeability of the bovine red cell to glycerol in hyperosmotic solutions at various temperatures. J Membr Biol. 1974;15:107–136. doi: 10.1007/BF01870084. [DOI] [PubMed] [Google Scholar]
- Meerveld Jv, Molenaar MM, Huyghe JM, Baaijens FPT. Analytical solution of compression, free swelling and electrical loading of saturated charged porous media. Transport in Porous Media. 2003;50:111–126. [Google Scholar]
- Mills N. Incompressible mixture of newtonian fluids. International Journal of Engineering Science. 1966;4:97–112. [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Needham D, Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onsager L. Reciprocal relations in irreversible processes. I. Phys Rev. 1931a;37:405–426. [Google Scholar]
- Onsager L. Reciprocal relations in irreversible processes. II. Phys Rev. 1931b;38:2265–2279. [Google Scholar]
- Staverman AJ. Non-equilibrium thermodynamics of membrane processes. Tr Faraday Soc. 1952;48:176–185. [Google Scholar]
- Tinoco I. Physical Chemistry: Principles and applications in biological sciences. Upper Saddle River, NJ: Prentice-Hall; 2002. [Google Scholar]
- Waugh R, Evans EA. Thermoelasticity of red blood cell membrane. Biophys J. 1979;26:115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RPW, Comper WD. Osmotic flow caused by polyelectrolytes. Biophys Chem. 1990;36:223–234. doi: 10.1016/0301-4622(90)80028-6. [DOI] [PubMed] [Google Scholar]
- Xu X, Cui Z, Urban JP. Measurement of the chondrocyte membrane permeability to Me2SO, glycerol and 1,2-propanediol. Med Eng Phys. 2003;25:573–579. doi: 10.1016/s1350-4533(03)00073-0. [DOI] [PubMed] [Google Scholar]