Abstract
Rationale
p120-catenin (p120) is an armadillo family protein that binds to the cytoplasmic domain of classical cadherins and prevents cadherin endocytosis. The role of p120 in vascular development is unknown.
Objective
The purpose of this study is to examine the role of p120 in mammalian vascular development by generating a conditionally mutant mouse lacking endothelial p120 and determining the effects of the knockout on vasculogenesis, angiogenic remodeling, and the regulation of endothelial cadherin levels.
Methods and Results
A conditional Cre/loxP gene deletion strategy was used to ablate p120 expression, using the Tie2 promoter to drive endothelial Cre recombinase expression. Mice lacking endothelial p120 died embryonically beginning at E11.5. Major blood vessels appeared normal at E9.5. However, both embryonic and extraembryonic vasculature of mutant animals were disorganized and displayed decreased microvascular density by E11.5. Importantly, both vascular endothelial (VE)-cadherin and N-cadherin levels were significantly reduced in vessels lacking p120. This decrease in cadherin expression was accompanied by reduced pericyte recruitment and hemorrhaging. Furthermore, p120-null cultured endothelial cells exhibited proliferation defects that could be rescued by exogenous expression of VE-cadherin.
Conclusions
These findings reveal a fundamental role for p120 in regulating endothelial cadherin levels during vascular development, as well as microvascular patterning, vessel integrity, and endothelial cell proliferation. Loss of endothelial p120 results in lethality due to decreased microvascular density and hemorrhages.
Keywords: Cadherin, endothelial, adhesion
Introduction
Vascular endothelial cells line blood vessels and regulate the movement of solutes, fluids, and cells between the plasma and tissue extracellular space. In addition, endothelial cells are active participants in inflammatory responses and wound healing and undergo dynamic alterations in cell surface adhesive potential, migratory activity, and proliferative capacity. Endothelial cell adhesion molecules have long been appreciated to play key roles in vascular biology and pathophysiology. In particular, vascular endothelial cadherin (VE-cadherin) has been implicated in the regulation of vascular barrier function1-3, inflammatory cell transmigration4, and endothelial cell proliferation and morphogenesis during neovascularization5.
VE-cadherin mediates adhesion through homophilic, calcium-dependent interactions between neighboring endothelial cells and couples this adhesive activity to the actin cytoskeleton at the adherens junction1, 6. Cytoplasmic interactions between the cadherin tail and armadillo family proteins such as β-catenin, plakoglobin, and p120-catenin (p120) are thought to regulate cadherin adhesive function3, 7. β-catenin and plakoglobin have been shown to mediate associations between cadherins and the cytoskeleton8, 9, although the precise molecular interactions that lead to this linkage remain unclear. p120-catenin binds to the juxtamembrane domain of classical cadherins10, 11. First discovered as a Src phosphorylation substrate12, p120 was later shown to be an armadillo family protein13. A central function of p120 is to regulate cadherin stability14. Previous studies have shown that p120 prevents clathrin-dependent endocytosis of VE-cadherin and thus stabilizes VE-cadherin at the plasma membrane15-17. Through this activity, cellular levels of p120 act as a set point, or rheostat, for control of cell surface and steady state cadherin expression levels18-20. Additionally, p120 is an important regulator of members of the Rho family of small GTPases21, and functions in the nucleus to regulate transcription through interactions with Kaiso22.
While many of these activities of p120 have been elucidated using in vitro studies, the functions of p120 in vivo are less clear. In Drosophila23, 24 and C. elegans25, p120 plays a supporting but nonessential role in cadherin stability and cell adhesion. However, global loss of p120 is lethal in vertebrates, resulting in severe morphogenetic defects in Xenopus26, 27, and embryonic lethality in mice28 and zebrafish29. Tissue-specific p120 ablation in the mouse likewise results in a variety of defects. The conditional knockout of p120 in the salivary gland using the Cre/LoxP system resulted in disorganized ducts, reductions in E-cadherin levels, and the formation of epithelial masses that followed a cancer-like growth progression30. Conditional p120 knockout in forebrain neuroepithelia resulted in reduced density of neuronal spines and synapses, an effect owing more to the misregulation of Rho GTPases than changes in N-cadherin levels31. Furthermore, an epidermal conditional p120 knockout mouse displayed a chronic inflammatory response caused by NFκB activation, also likely downstream of altered regulation of Rho family GTPases32. These and other unpublished results demonstrate that tissue-specific ablation of p120 produces a wide range of phenotypes with differing degrees of severity.
The role of p120 in mammalian vascular development has not been addressed. In order to explore the functions of this junctional protein in vivo, a conditional mouse knockout approach was utilized to ablate endothelial p120 expression. The results presented here demonstrate that p120 is essential for vascular development and remodeling and that its conditional endothelial ablation results in embryonic lethality. Mice lacking endothelial p120 exhibit a reduction in VE-cadherin and N-cadherin levels, as well as hemorrhages, decreased microvascular density, reduced pericyte coverage, and disorganized vascular networks in both embryonic and extraembryonic tissues. These findings reveal a fundamental role for p120 in vascular development and endothelial function in vivo.
Methods
Mice with LoxP sites in introns 2 and 8 of the p120 gene were generated as described previously30. Tie2-Cre-expressing C57BL/6 mice were obtained from The Jackson Laboratory #004128. Detailed descriptions of tissue processing, antibodies used for immunofluorescence, microscopy and image quantitation, primary cell isolation, and cultured cell experiments can be found in the supplemental expanded Materials and Methods section.
Results
Loss of endothelial p120-catenin is embryonic lethal
A conditional gene ablation strategy was utilized to define the function of p120 in mouse vascular development. Mice harboring a LoxP-flanked allele of the p120 gene30 were crossed with transgenic mice expressing Cre recombinase driven by the Tie2 promoter (Online Figure I A), resulting in endothelial Cre expression beginning at developmental day E7.533. Fewer than expected pups harboring the p120flox/flox; Cre+ (conditional mutant) genotype were observed in litters (Online Fig. I B), suggesting that deletion of the p120 gene in endothelial cells resulted in an embryonic lethal phenotype. A series of timed mating experiments revealed that the conditional mutant animals began to exhibit lethality at developmental day E12.5, with approximately 40% lethality observed by E14.5 (Online Fig. I C). Additionally, a fraction of the conditional mutant pups died shortly after birth. The remaining genotypic mutants survived into adulthood, with some animals exhibiting small size and failure to thrive and others exhibiting no obvious abnormalities.
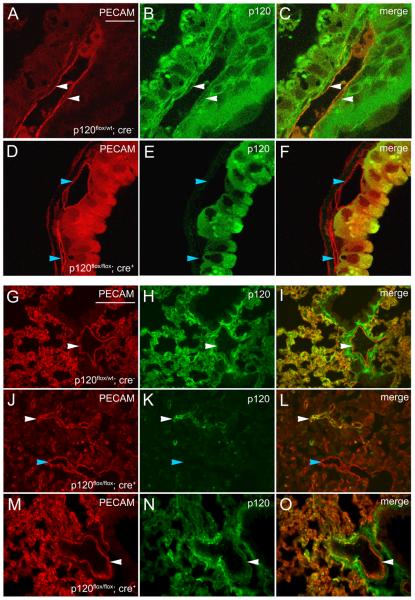
To verify loss of p120 protein expression, yolk sacs were cryosectioned and examined by immunofluorescence microscopy for p120 and the endothelial adhesion molecule PECAM-1 (platelet/endothelial cell adhesion molecule 1)34. p120 colocalized with PECAM-1 in the vasculature of control mice (Fig. 1 A-C) but not in vessels of conditional mutant animals (Fig. 1 D-F). Similar results were observed in embryonic tissue (see Online Fig. II and Fig. 4). p120 expression in endothelial cells was also examined in surviving adult animals harboring the conditional mutant genotype (p120flox/flox; Cre+). Analysis of p120 expression in lung tissue of control mice (Fig. 1 G-I) revealed extensive colocalization between p120 and PECAM-1. p120 expression could also be detected in endothelial cells of surviving mutants. Interestingly, in a mutant animal that was significantly smaller than control littermates, endothelial p120 expression was mosaic and microvascular density was dramatically reduced (Fig. 1 J-L). In contrast, p120 expression appeared normal in genotypically mutant animals that survived into adulthood with no obvious phenotype (Fig. 1 M-O). The inefficient deletion of this p120 allele in some animals was also observed in the mammary gland and prostate of mice expressing Cre driven by the MMTV (mouse mammary tumor virus) promoter and in intestinal epithelium of mice expressing Cre using the villin promoter (Reynolds et al., unpublished). Several other genes have been reported to exhibit mosaic deletion using Cre-loxP systems in the mouse35, 36. In the model system reported here, this mosaic deletion of p120 results in reduced rates of lethality. However, we examined many tens of embryos that exhibited efficient p120 ablation as assessed by immunostaining. In the phenotypic analysis described below, we summarize the phenotype representative of animals with complete endothelial p120 deletion. Importantly, genotypically mutant animals survived because of inefficient deletion of endothelial p120 rather than overcoming loss of p120 expression. From these findings, we conclude that the expression of p120 in mouse vascular endothelial cells is required for survival, and loss of p120 in endothelial cells results in embryonic lethality beginning around E12.5.
Figure 1.
p120 is successfully ablated in conditional mutant mice but deletion is mosaic in some animals. E13.5 yolk sacs were cryosectioned and stained for p120 and PECAM-1 to identify blood vessels. The markers colocalized in control tissues (C), but mutant blood vessels lacked p120 (F), demonstrating that the Cre/LoxP-mediated deletion of p120 was effective. Representative blood vessels expressing p120 are marked by white arrowheads and vessels lacking p120 are indicated by blue arrowheads. Scalebar in A is 20μM. Lung tissues from young adult mice were cryosectioned and stained for p120 and PECAM-1. Representative large vessels expressing p120 are labeled with white arrowheads; blue arrowheads indicate large vessels lacking p120. Control lung tissue exhibited robust p120 expression (H) and dense microvasculature (G). p120 colocalized with the endothelial marker PECAM-1 (I). Genotypically mutant lung tissue from a physically small littermate revealed extensive p120 loss (K) with small patches of p120 expression which colocalized with PECAM-1 (L). Note reduced microvascular density and intact large vessels (J). Another genotypically mutant, surviving littermate displayed normal body size and exhibited normal p120 levels (N) and microvascular density (M) comparable to control animals. Scalebar in G is 100μM
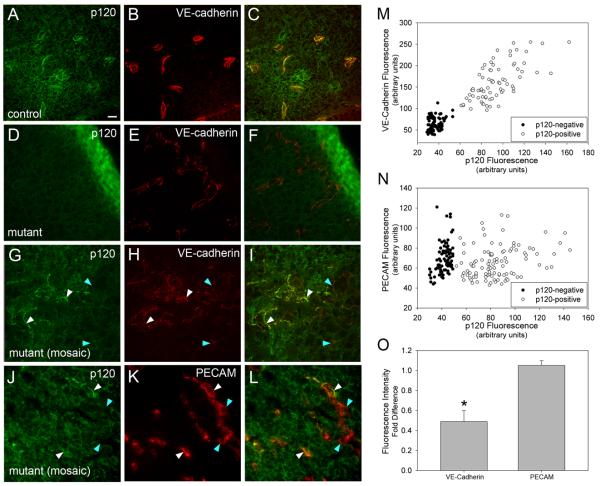
Figure 4.
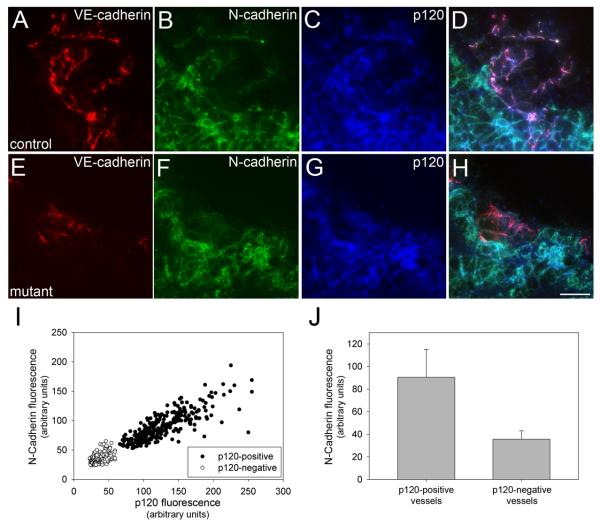
VE-cadherin levels are decreased in embryonic tissues lacking endothelial p120. E10.5 embryos were fixed, cryosectioned, and stained for p120, VE-cadherin, and PECAM-1. In control tissues, p120 (A) and VE-cadherin colocalize in blood vessels (C). In conditionally mutant tissue, VE-cadherin expression is retained (E), but vascular p120 is absent (D). Tissue from genotypically mutant mice which exhibit mosaic p120 expression (G) have reduced levels of VE-cadherin in areas lacking p120 (H - blue arrowheads), compared to areas which have positive staining for vascular p120 (white arrowheads). To verify the specificity of this effect, mosaic mutant tissues were stained for PECAM-1 (J,K,L). Note that PECAM-1 expression is similar in endothelial cells that express p120 (white arrowheads) and those lacking p120 (blue arrowheads). To quantify the reduction in VE-cadherin in p120-negative vessels, peak fluorescence of VE-cadherin (M) or PECAM (N) was plotted against peak fluorescence of p120. VE-cadherin levels were significantly reduced in a manner dependent on p120, while PECAM levels were independent of p120 levels. The fold difference of the average fluorescence intensity of VE-cadherin in p120-negative over p120-positive vessels was 0.489, compared to 1.052 for PECAM (O). (t-test, p ≤ 0.001). Scalebar is 20 μM.
Deletion of endothelial p120 causes defects in microvascular patterning and hemorrhages
To define the vascular defects resulting from the ablation of the p120 gene in endothelial cells, a series of timed mating experiments was conducted and vessel formation analyzed beginning at E9.5. PECAM-1 staining of transverse sections revealed no apparent defect in dorsal aortae lacking p120 (Online Fig. II D-F). These findings suggest that formation of large vessels by vasculogenesis proceeds normally in the absence of endothelial p120. In contrast to the dorsal aortae, intersomitic vessels form by sprouting angiogenesis37. Whole-mounted E9.5 embryos from six litters showed no defects in intersomitic vessel organization or vertebral arteries in mutant animals (Online Fig. II G, H). Thus, major vessels formed by both vasculogenesis and sprouting angiogenesis were indistinguishable in conditional mutant and control embryos at E9.5.
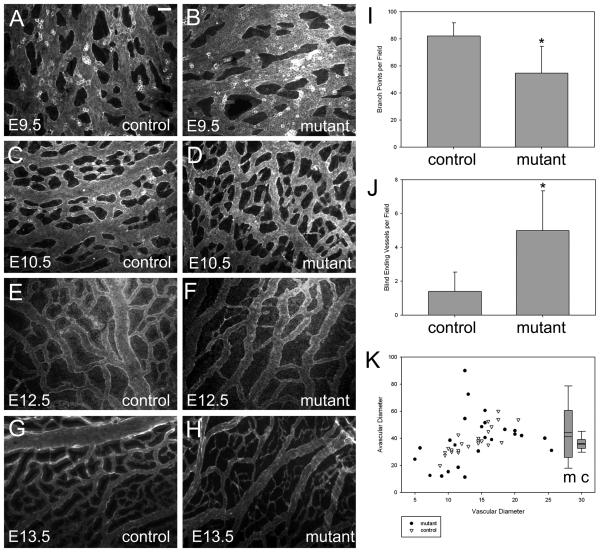
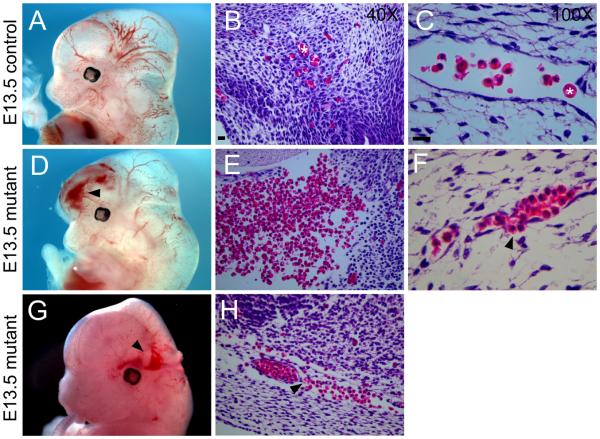
Conditional mutant embryos began to exhibit lethality around E12.5 (Online Figure I). Therefore, embryonic and extraembryonic tissue was examined at midgestational stages. At E11.5, mutant embryos displayed significant defects in placenta vasculature. In the labyrinthine layer, which contains capillaries of fetal origin38, a reduction in vascular density was observed among conditional mutants (Online Fig. III C, D) compared to wild type animals (Online Fig. III A, B). Severe defects in microvascular density were also observed in the embryo proper. By E14.5, conditional mutant embryos were often visibly pale or exhibited a dramatic reduction in vessel density when examined under low power light microscopy (Online Fig. III E-H). PECAM-1 staining of the brain of whole-mounted E13.5 conditional mutant embryos (Online Fig. III J, L) revealed disorganized and less dense vascular networks compared to control littermates (Online Fig. III I, K). Defects were particularly striking in the hyaloid vascular system of the developing eye (Online Fig. III M-N). To further investigate the defects in microvascular patterning resulting from p120 loss, time course experiments were performed by examining vascular plexus formation and microvessel remodeling in the yolk sac. Yolk sacs were isolated at developmental time points from E9.5 to E13.5 and stained for PECAM-1 expression. The formation of the initial vascular plexus appeared normal in p120 conditional mutant yolk sacs (Fig. 2 A, B), whereas subsequent steps in vessel remodeling and expansion were compromised. In control animals, yolk sac vessels remodeled into a well-organized hierarchy of large and small vessels (Fig. 2 A, C, E, and G). In contrast, conditional p120 mutants exhibited reduced vascular branching and increased numbers of blind-ending vessels (Fig. 2 I, J). Furthermore, morphometric analysis of vascular and avascular space revealed that mutant vessels failed to form homogeneous networks. As shown in Figure 2 K, a linear relationship was apparent between vessel diameter and avascular space diameter in control animals (r = 0.82). In contrast, mutant vessels exhibited highly variable avascular space diameter (r = 0.27). Together, these data indicate that loss of p120 results in severe defects in vascular patterning and morphogenesis. In addition to defects in vascular organization, the absence of endothelial p120 also resulted in hemorrhages. In E12.5 embryos, hemorrhages were commonly observed in the brain (Fig. 3 D and G, compared to A) and other organs (not shown) of conditional mutant animals, and histologic staining of brain sections revealed both large hemorrhages (Fig. 3 E) and leaky microvessels (Fig. 3 F, H). These results demonstrate that deletion of endothelial p120 leads to striking defects in mouse microvascular density and patterning as well as compromised vessel integrity in both embryonic and extraembryonic tissue.
Figure 2.
Yolk sac vessels of p120 conditional mutant mice reveal angiogenic remodeling defects. Whole mount yolk sacs from control and mutant embryos at various developmental time points were processed for immunofluorescence microscopy using PECAM-1 antibodies to highlight vessels. Analysis of E12.5 yolk sacs revealed decreased branch points (intersections) in mutant tissues compared to control (p = 0.024) (I), accompanied by an increase in blind-ending vessels (p = 0.015) (J). A plot of vascular vs. avascular diameters in E12.5 yolk sacs revealed a lack of uniformity in blood vessel networks in mutant tissues compared to control (K, scatter plot). The ratio of vascular/avascular diameters showed greater variability in mutant tissues (K, box plot). Scalebar is 100μM.
Figure 3.
Conditional mutant mice exhibit hemorrhages. E13.5 control (A) and mutant (D,G) embryos photographed using light microscopy. Hemorrhages in mutant mice are indicated by arrowheads. Hematoxylin and eosin staining of paraffin-embedded sections reveal normal microvessels in the brains of control embryos (B,C) and hemorrhaging (E) and leaky vessels (F,H – indicated by arrowheads) in the brains of mutant embryos. Asterisks in B and C mark red blood cells in microvessels. Scalebars are 20μM.
Cadherin expression and pericyte recruitment are decreased in p120-null endothelial tissues
Previously, p120 was found to stabilize VE-cadherin expression at the plasma membrane by inhibiting cadherin endocytosis and degradation15. To determine if deletion of the p120 gene caused a corresponding loss of VE-cadherin in mouse vessels, p120 and VE-cadherin colocalization were examined in embryonic vessels of E10.5 animals (Fig. 4). In control vessels, VE-cadherin colocalized extensively with p120 (Fig. 4 A-C). VE-cadherin was also detected in p120-null vessels but appeared to be reduced compared to control vessels (Fig. 4 D-F). To determine if the relative levels of cadherin were reduced in the absence of p120, we took advantage of the mosaic nature of p120 expression in a subset of embryos, thereby allowing comparison of VE-cadherin levels within the same tissue section. Line scan pixel intensity plots revealed that the levels of VE-cadherin were significantly reduced in vessels that were p120-null (Fig. 4, G-I, quantified in M). In contrast to VE-cadherin, no alteration in PECAM-1 levels was observed in vessels lacking p120 (Fig. 4 J-L, quantified in N; see also Fig. 1). Furthermore, we were unable to detect any differences in expression or localization of the tight junction protein claudin-5 (Online Fig. IV).
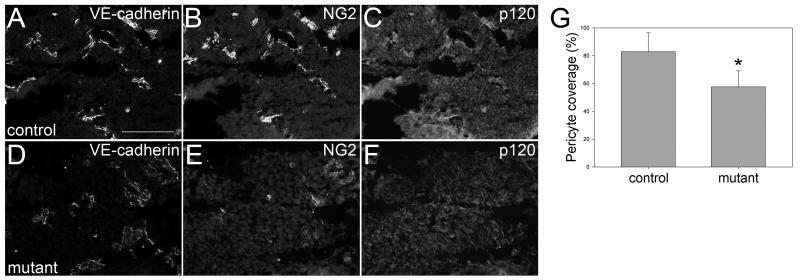
N-cadherin is also expressed in endothelial cells and plays important roles in vascular development39, 40. Previous work has shown that p120 also regulates N-cadherin levels19, 31, 41. Therefore we examined N-cadherin expression levels in E11.5 mouse embryos. In control animals, vessels expressing N-cadherin were readily observed adjacent to the neural tube (Fig. 5 A-D). However, in mutant littermates, N-cadherin was not detected in corresponding p120-null vessels (Fig. 5 E-H, quantified in I and J). N-cadherin has been implicated in the recruitment of pericytes to developing vessels40. These cells associate with endothelial cells and are important regulators of vessel remodeling and stabilization42. Therefore, we surveyed the degree of pericyte coverage of blood vessels in the brains of E11.5 mutant and control embryos using the pericyte antigen NG240, 43. Importantly, pericyte coverage was significantly reduced in mutant embryos (Fig. 6, compare B and E; quantified in G). Thus, loss of p120 leads to a substantial decrease in endothelial cadherin expression levels and is associated with reduced pericyte coverage of developing vessels.
Figure 5.
N-cadherin is absent in endothelial cells lacking p120 in vivo. Cryosectioned E11.5 embryos were fixed and stained for N-cadherin, VE-cadherin (to identify blood vessels) and p120 (to verify its absence in mutant endothelial tissue). In the area surrounding the neural tube, N-cadherin-positive blood vessels were frequently seen in control mice (A-D). Corresponding vessels of mutants lacking vascular p120 also lacked N-cadherin (E-H). N-cadherin expression was quantified in mutant and control blood vessels relative to p120 as described for VE-cadherin (see Fig. 4). The average N-cadherin fluorescence intensity of p120-positive vessels was more than 2.5 times that of p120-negative vessels (J). (Mann-Whitney Rank Sum, p ≤ 0.001). Scalebar is 20μM.
Figure 6.
Reduced pericyte recruitment in brains of mutant mice. 12-μM cryosections were cut from the brains of E11.5 mice and stained for NG2 to identify pericytes, VE-cadherin to identify blood vessels, and p120. Representative fields are shown for control (A-C) and mutant (D-F) mice. To quantify pericyte coverage of blood vessels, multiple images were collected from a series of two mutant and three control mice from the same litter. 500 individual vessels from each population were scored based on their contact with pericytes. The percent pericyte coverage per field was compared between mutant and control (G). Control vessels had pericyte coverage of nearly 83% compared to only 58% pericyte coverage in mutant vessels (t-test, p ≤ 0.001). Scalebar is 100μM.
Primary endothelial cells lacking p120 exhibit proliferation defects
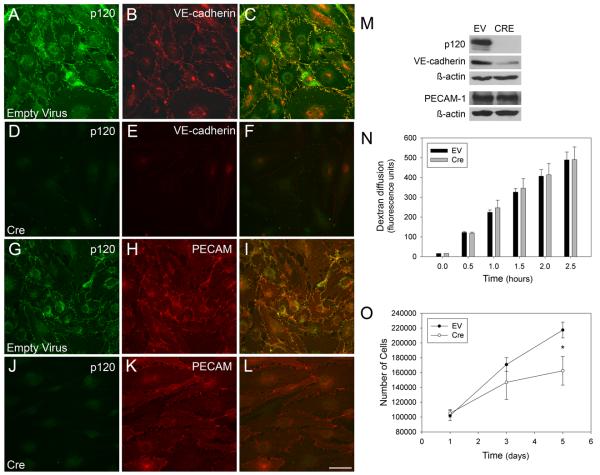
To explore the cellular mechanisms underlying the vascular defects in the conditional p120-null mouse, primary endothelial cells were isolated from newborn p120flox/flox; Cre− mice. To cause p120 deletion, Cre recombinase was introduced using an adenoviral vector44. Following a 72-hour period to allow for turnover of previously transcribed p120, the cells were fixed and examined by immunofluorescent microscopy (Fig. 7). Control cells infected with empty adenoviral vector retained p120 expression (Fig. 7 A, G). Additionally, these cells also expressed both VE-cadherin (Fig. 7 B) and PECAM-1 (Fig. 7 H), confirming the endothelial identity of the cells. Expression of Cre resulted in near complete ablation of p120 (Fig. 7 D, J). Furthermore, loss of p120 resulted in a striking reduction in VE-cadherin (Fig. 7 E) but not PECAM-1 levels (Fig. 7 K) This result was confirmed by western blot analysis (Fig. 7 M). In addition to expression of adhesion molecules, cultured mouse endothelial cells were also examined for barrier function and proliferation potential. For barrier studies, monolayers of endothelial cells from p120flox/flox; Cre− mice were cultured on filter membranes and infected with adenovirus to express either GFP or Cre. Diffusion of Texas red-labeled dextran across the cell layers was monitored as an assay of barrier function (Fig. 7 N). Although a slight increase in dextran diffusion was observed in some experiments, we were unable to demonstrate a statistically significant change in dextran flux in p120-null cells compared to controls. However, endothelial cells lacking p120 exhibited significantly reduced growth rates (Fig. 7 O). These results suggest a role for p120 in endothelial proliferation.
Figure 7.
Ablation of p120 in cultured mouse endothelial cells leads to decreased VE-cadherin levels and reduced proliferation. Immunofluorescence microscopy of primary mouse endothelial cells infected with adenoviral Cre or an empty vector show successful ablation of p120 (compare A and D, G and J). In the absence of p120, VE-cadherin levels are reduced (compare B and E), but PECAM-1 levels are not affected by p120 loss (compare H and K). Western blot analysis confirms the reduction of VE-cadherin, but not PECAM-1, in p120-null cells (M). Measurement of the diffusion of Texas Red-labeled dextran across a confluent monolayer of p120-null cells shows no statistically significant change in barrier function compared to control cells (N) Growth curve analysis desmonstrates that p120 null endothelial cells exhbit slower growth rates compared to control cells (Mann-Whitney Rank Sum, p=0.029 at day 5). Scalebar is 50μM.
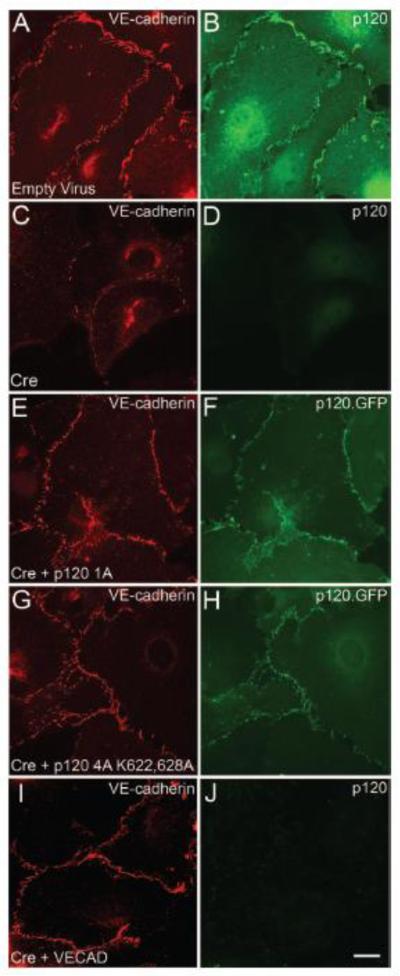
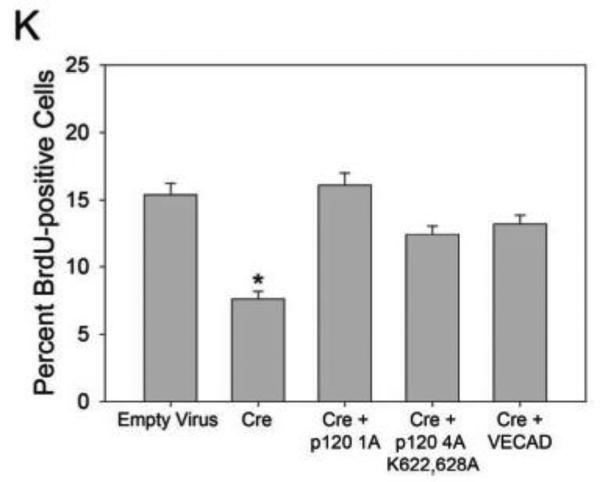
Previous studies have shown that VE-cadherin plays a key role in endothelial growth control 45-47, and more recent studies have shown that p120-null keratinocytes exhibit a growth-arrested phenotype. This latter study also implicated p120 inhibition of RhoA as the mechanism underlying this mitotic defect48. To determine if the endothelial growth defect in p120-null cells is RhoA- or VE-cadherin-dependent, exogenous p120 and VE-cadherin were re-expressed in p120-null primary endothelial cells. In addition to wild type p120 1A, a p120 mutant unable to inhibit RhoA (p120 4A K622,628A)49 was utilized to determine if inhibition of RhoA is involved in regulating VE-cadherin expression levels and/or endothelial proliferation. Similar to the results shown in Fig. 7, loss of endogenous p120 resulted in significantly decreased VE-cadherin levels (Fig. 8 C, D) and a corresponding decrease in endothelial proliferation as measured by BrdU uptake (Fig. 8 K). Expression of exogenous wild type p120 1A rescued both VE-cadherin levels (Fig. 8 E, F) and BrdU uptake. Furthermore, VE-cadherin levels were also restored by the Rho-uncoupled p120 mutant (p120 4A K622,628A). Interestingly, the Rho-uncoupled mutant also rescued the proliferation defect observed in p120-null cells, suggesting that decreased VE-cadherin expression underlies the reduction in proliferation. Consistent with this interpretation, exogenous expression of VE-cadherin in p120-null cells also restored BrdU uptake. These studies indicate that p120 regulates endothelial proliferation through a cadherin-dependent mechanism.
Figure 8.
Re-expression of p120 rescues VE-cadherin expression and restores proliferation rates in p120-null endothelial cells. Immunofluorescence microscopy of primary mouse endothelial cells infected with adenoviral Cre demonstrates ablation of p120 (compare B and D). In the absence of p120, VE-cadherin levels are reduced (compare A and C). Re-expression of wild type p120 (E, F) or the Rho-uncoupled p120 mutant (p120 4A K622,628A) (G, H) restored VE-cadherin expression in p120-null cells. Exogenously expressed VE-cadherin assembled at cell-cell junctions in the absence of p120 (I, J). Deletion of p120 reduced endothelial proliferation rates. Bromodeoxyuridine (BrdU) uptake by p120-null and control cells was measured and compared to total nuclei (stained by DAPI). Cells lacking p120 showed a decrease in proliferation compared to control (N). (Kruskal-Wallis One Way Analysis of Variance on Ranks, p ≤ 0.001). Furthermore, re-expression of wild type p120 restored endothelial proliferation (K). Similarly, both the Rho-uncoupled p120 mutant and exogenously expressed VE-cadherin also rescued endothelial proliferation in p120-null cells (Multiple Comparisons versus Control Group by Dunnett's Method, p < 0.05). Scalebar is 20μM.
Discussion
The findings reported here demonstrate for the first time a central and indispensable role for p120-catenin in mammalian vascular development. Deletion of endothelial p120 results in downregulation of both VE-cadherin and N-cadherin, as well as decreased endothelial proliferation and reduced pericyte coverage of developing microvessels. These alterations are associated with microvascular patterning defects, hemorrhaging and midgestational embryonic lethality.
One of the most striking phenotypes resulting from the selective inactivation of the p120 gene in endothelial cells is the failure of microvessels to properly pattern. The earliest defects in vessel patterning were observed in the placenta at E11.5, where vascular density in the labyrinthine was markedly reduced (Online Fig. III A-D). Analysis of vascular patterning in the yolk sac at different developmental stages suggests that microvessels form normally, but then either regress or are unable to expand with tissue growth (Fig. 2). Morphological analysis of yolk sac microvessels revealed decreased vessel branching and increased avascular space (Fig. 2 I-K). These observations suggest that microvessel growth into avascular areas is insufficient to keep pace with the rapid midgestational growth of the embryo. Consistent with this notion, deletion of p120 in cultured endothelial cells resulted in significantly reduced endothelial cell growth and proliferation (Fig. 7 and 8). It is likely that the inability of endothelial cells to proliferate efficiently in the absence of p120 explains, at least partially, the defect in vascular density observed in vivo.
In addition to microvascular patterning defects, mutants lacking endothelial p120 also exhibited hemorrhaging, particularly in the brain. Tight junction proteins are essential to maintaining the blood-brain barrier50, and recent studies have implicated VE-cadherin in the transcriptional regulation of claudin-551, suggesting that alterations in tight junctions may also underlie some of the p120-null defects. However, we were unable to demonstrate any significant alterations in the expression or localization of claudin-5 in the absence of endothelial p120 (Online Fig. IV). In addition to tight junctions, pericytes associate with small vessels and have been shown to regulate capillary diameter and vascular permeability in the brain52. Loss of pericytes in the developing mouse results in lethality due to microvascular hemorrhaging and edema42. Importantly, deletion of p120 resulted in reduced pericyte coverage in mutant vessels in the brain (Fig. 6). These findings suggest that reduced pericyte recruitment, rather than alteration of tight junctions, is the underlying cause of brain hemorrhaging in the p120 mutant embryos.
Pericyte recruitment and endothelial growth are both regulated by endothelial cadherins6, 39, 40. Previous studies have demonstrated that a central function of p120 is to post-translationally stabilize cadherins53. In cultured endothelial cells, knockdown of p120 using siRNA leads to VE-cadherin endocytosis and degradation18. In the present study, we observed that VE-cadherin levels were significantly reduced in vessels lacking p120 (Fig. 4), demonstrating that p120 also regulates VE-cadherin levels in vivo. Similarly, in endothelial cells isolated from p120flox/flox;Cre− mice, deletion of the p120 gene upon expression of Cre recombinase leads to a significant reduction in both p120 and VE-cadherin (Fig. 7). These cells also exhibit dramatically reduced proliferation (Fig. 8). Loss of endothelial proliferation during embryonic development provides a reasonable explanation for the loss of microvascular density observed in vivo (Fig 2, Online Fig. III). A number of studies have implicated VE-cadherin in regulating endothelial proliferation54, 55. Consistent with these previous studies, the endothelial proliferation defect of p120-null endothelial cells could be rescued by expression of exogenous VE-cadherin (Fig. 8), suggesting that reduced VE-cadherin levels play an important role in both endothelial proliferation and in the loss of vessel density in the mutant embryos. Similar to VE-cadherin, levels of N-cadherin are also reduced in p120-null vessels (Fig. 5). N-cadherin is required for vascular development and has been implicated in pericyte recruitment to developing vessels39, 40. Together, these results suggest that the reduction in VE-cadherin and N-cadherin upon deletion of endothelial p120 contributes to the reduced microvascular density and hemorrhaging that characterizes these mutants.
In addition to regulating cadherin endocytosis, p120 inhibits RhoA activity. Furthermore, recent studies indicate that RhoA inhibition can rescue proliferation defects observed in p120-null keratinocytes48. To determine if RhoA inhibition is important for regulating VE-cadherin levels and/or endothelial cell proliferation, we utilized a p120 mutant that associates with cadherin but is unable to inhibit RhoA. This approach revealed that decreased endothelial VE-cadherin levels and reduced proliferation could both be rescued by re-expression of wild type p120 1A or a mutant p120 defective in RhoA inhibition (Fig. 8). These observations are consistent with our recent report that p120 inhibits cadherin endocytosis in a RhoA-independent manner17. Furthermore, the ability of the Rho-uncoupled mutant to rescue both VE-cadherin levels and endothelial proliferation further couples loss of VE-cadherin to the proliferation defect in the p120-null endothelial cells.
Previous studies demonstrated that VE-cadherin null animals die embryonically due to severe vascular remodeling defects47, 56. However, heterozygous mice in which VE-cadherin protein levels were reduced by approximately 50% were phenotypically normal. In the absence of p120, VE-cadherin levels in vivo were reduced by approximately 50% as assessed by fluorescence intensity measurements of control and p120-null vessels (Figure 4 M). However, it is important to note that deletion of p120 results in reduced levels of both N-cadherin and VE-cadherin, both of which are required for vascular development39, 47, 56. In addition, a recent study using a zebrafish model demonstrated that even a modest reduction in VE-cadherin resulted in brain hemorrhaging29. The severity of the phenotype correlated with the degree to which VE-cadherin levels were reduced, suggesting that loss of VE-cadherin may also contribute directly to the hemorrhaging observed in our conditional p120 mutant animals. Lastly, it is also likely that loss of p120 compromises VE-cadherin adhesive functions independently from control of cadherin expression levels. Likely possibilities include mis-regulation of Rho-family GTPases57. Consistent with this notion, VE-cadherin was recently shown to reduce vessel sprouting by suppressing Rac1 activity and enhancing actomyosin contractility58. Given the role of p120 in regulating Rho-family GTPases, this activity of p120 may also contribute to some aspects of endothelial cell function in developing vessels, including tubule formation and barrier function. Additional mouse genetic models and other approaches will be required to distinguish between these possibilities. Lastly, we should note that the Tie-2 promoter has been shown to be active in hematopoietic cells. Although it is formally possible that functions of p120 are important in this lineage, the data presented here provide clear evidence for a crucial role of p120-catenin in endothelial cells, both in vivo and in vitro. Together, these findings demonstrate a central role for p120 catenin in vascular integrity, microvascular patterning, and endothelial proliferation.
Novelty and Significance.
What is known?
p120-catenin is a widely-expressed adherens junction protein that is essential for vertebrate development.
p120-catenin stabilizes cell surface VE-cadherin in cultured endothelial cells by regulating cadherin endocytosis.
The role of p120-catenin in vertebrate vascular development is not yet known.
What new information does this article contribute?
Loss of endothelial p120-catenin results in midgestational embryonic lethality in the developing mouse.
The vascular p120-null phenotype includes hemorrhaging, angiogenic remodeling defects, endothelial cadherin reduction, and decreased pericyte recruitment.
p120-catenin ablation results in a reduction in endothelial proliferation which is VE-cadherin-dependent.
Summary
p120-catenin is an armadillo family protein that localizes to intercellular adherens junctions in many cell types and is required for vertebrate development. Previous studies from our lab and others have shown that by binding to members of the cadherin family of adhesion receptors, p120-catenin stabilizes their expression at the cell surface. However, the role of p120-catenin during mammalian vascular development is not understood. We reveal here an essential role for endothelial p120-catenin in the developing mouse embryo and demonstrate that ablating endothelial p120-catenin causes hemorrhages and microvascular patterning defects which result in midgestational lethality. Furthermore, we show that the loss of p120-catenin leads to a reduction of endothelial cadherins and a cellular proliferation defect which is VE-cadherin-dependent. This study demonstrates for the first time that p120-catenin is required for mammalian vascular development and that its ablation compromises vascular integrity and microvascular morphogenesis. This work provides novel insights into the regulation of intercellular adhesion during the process of vascular development.
Supplementary Material
Sources of Funding
This work was supported by RO1AR050501 (APK), KO1AR053965 (KX), and T32EY007092 (RGO) from the NIH, a career development award from the Dermatology Foundation (KX), and a predoctoral fellowship from the AHA (CMC).
Non-standard Abbreviations and Acronyms
- VE-cadherin
vascular endothelial cadherin p120, p120-catenin
- PECAM-1
platelet endothelial cell adhesion molecule 1
- MMTV
mouse mammary tumor virus
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bazzoni G, Dejana E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 2.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 3.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm's length. Am J Physiol Cell Physiol. 2004;286:C987–997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 4.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Czirok A, Zamir EA, Szabo A, Little CD. Multicellular sprouting during vasculogenesis. Curr Top Dev Biol. 2008;81:269–289. doi: 10.1016/S0070-2153(07)81009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestweber D. VE-Cadherin: The Major Endothelial Adhesion Molecule Controlling Cellular Junctions and Blood Vessel Formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 7.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 8.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim Biophys Acta. 2008;1778:670–691. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Thoreson MA, Anastasiadis PZ, Daniel JM, et al. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap AS, Niessen CM, Gumbiner BM. The Juxtamembrane Region of the Cadherin Cytoplasmic Tail Supports Lateral Clustering, Adhesive Strengthening, and Interaction with p120ctn. J. Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol. Cell. Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657–663. doi: 10.1016/j.semcdb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Xiao K, Garner J, Buckley KM, et al. p120-Catenin Regulates Clathrin-dependent Endocytosis of VE-Cadherin. Mol. Biol. Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell. 2009;20:1970–1980. doi: 10.1091/mbc.E08-07-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao K, Allison DF, Buckley KM, et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1143–1153. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- 21.Anastasiadis PZ. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Daniel JM. Dancing in and out of the nucleus: p120ctn and the transcription factor Kaiso. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773:59–68. doi: 10.1016/j.bbamcr.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 23.Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J. Cell Biol. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacquelet A, Lin L, Rorth P. Binding site for p120/delta-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol. 2003;160:313–319. doi: 10.1083/jcb.200207160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J. Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciesiolka M, Delvaeye M, Van Imschoot G, et al. p120 catenin is required for morphogenetic movements involved in the formation of the eyes and the craniofacial skeleton in Xenopus. J Cell Sci. 2004;117:4325–4339. doi: 10.1242/jcs.01298. [DOI] [PubMed] [Google Scholar]
- 27.Fang X, Ji H, Kim SW, et al. Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J Cell Biol. 2004;165:87–98. doi: 10.1083/jcb.200307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds AB. p120-catenin: Past and present. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, Dejana E. Stable Vascular Connections and Remodeling Require Full Expression of VE-Cadherin in Zebrafish Embryos. PLoS One. 2009;4:e5772. doi: 10.1371/journal.pone.0005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MA, Reynolds AB. Blocked Acinar Development, E-Cadherin Reduction, and Intraepithelial Neoplasia upon Ablation of p120-Catenin in the Mouse Salivary Gland. Developmental Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 Catenin Regulates Dendritic Spine and Synapse Development through Rho-Family GTPases and Cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-Catenin Mediates Inflammatory Responses in the Skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koni PA, Joshi SK, Temann U-A, Olson D, Burkly L, Flavell RA. Conditional Vascular Cell Adhesion Molecule 1 Deletion in Mice: Impaired Lymphocyte Migration to Bone Marrow. J. Exp. Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 35.Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Ryding AD, Sharp MG, Mullins JJ. Conditional transgenic technologies. J Endocrinol. 2001;171:1–14. doi: 10.1677/joe.0.1710001. [DOI] [PubMed] [Google Scholar]
- 37.Coffin JD, Poole TJ. Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development. 1988;102:735–748. doi: 10.1242/dev.102.4.735. [DOI] [PubMed] [Google Scholar]
- 38.Takata K, Fujikura K, Shin B-C. Ultrastructure of the Rodent Placental Labyrinth: A Site of Barrier and Transport. J. Reprod. Dev. 1997;43:13–24. [Google Scholar]
- 39.Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J. Cell Biol. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tillet E, Vittet D, Feraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400. doi: 10.1016/j.yexcr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Ferreri DM, Minnear FL, Yin T, Kowalczyk AP, Vincent PA. N-cadherin levels in endothelial cells are regulated by monolayer maturity and p120 availability. Cell Commun Adhes. 2008;15:333–349. doi: 10.1080/15419060802440377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 44.Stec DE, Davisson RL, Haskell RE, Davidson BL, Sigmund CD. Efficient Liver-specific Deletion of a Floxed Human Angiotensinogen Transgene by Adenoviral Delivery of Cre Recombinase in Vivo. J. Biol. Chem. 1999;274:21285–21290. doi: 10.1074/jbc.274.30.21285. [DOI] [PubMed] [Google Scholar]
- 45.Caveda L, Martin-Padura I, Navarro P, et al. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin) J Clin Invest. 1996;98:886–893. doi: 10.1172/JCI118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venkiteswaran K, Xiao K, Summers S, et al. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta -catenin. Am J Physiol Cell Physiol. 2002;283:C811–821. doi: 10.1152/ajpcell.00417.2001. [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P, Lampugnani M-G, Moons L, et al. Targeted Deficiency or Cytosolic Truncation of the VE-cadherin Gene in Mice Impairs VEGF-Mediated Endothelial Survival and Angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci U S A. 2008;105:15399–15404. doi: 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanagisawa M, Huveldt D, Kreinest P, et al. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. J Biol Chem. 2008;283:18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiology of Disease. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 52.Kutcher ME, Herman IM. The pericyte: Cellular regulator of microvascular blood flow. Microvascular Research. 2009;77:235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delva E, Kowalczyk AP. Regulation of cadherin trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 Are Differentially Required for Stretch-Mediated Proliferation in Endothelial Versus Smooth Muscle Cells. Circ Res. 2007;101:e44–52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 55.Corada M, Zanetta L, Orsenigo F, et al. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood. 2002;100:905–911. doi: 10.1182/blood.v100.3.905. [DOI] [PubMed] [Google Scholar]
- 56.Gory-Faure S, Prandini MH, Pointu H, et al. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- 57.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abraham S, Yeo M, Montero-Balaguer M, et al. VE-Cadherin-Mediated Cell-Cell Interaction Suppresses Sprouting via Signaling to MLC2 Phosphorylation. Current Biology. 2009;19:668–674. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.