Abstract
CVT-10216 is a highly selective, reversible inhibitor of ALDH-2 that reduces excessive alcohol drinking. Anxiety plays a role in alcoholism. The present study asks whether CVT-10216 has anxiolytic properties, as reflected in social interaction behavior in four unrelated rodent models: endogenous anxiety-like behavior in naïve Fawn-Hooded rats, repeated alcohol-withdrawal-induced anxiety, restraint stress-induced anxiety and drug-induced anxiety. CVT-10216 counteracted anxiety in all models except that produced by the 5-HT2C agonist, mCPP. CVT-10216 exhibited both acute and prophylactic inhibitions of repeated alcohol-withdrawal-induced anxiety. Importantly, anxiogenic behavior induced by the benzodiazepine receptor inverse agonist, DMCM, was counteracted dose-dependently by CVT-10216. Thus, a non-addictive selective inhibitor of ALDH-2 has both anxiolytic and antidipsotropic properties, which may be dependent, in part on the involvement of the GABA–benzodiazepine system.
Keywords: ALDH-2 inhibitor, Social interaction test, Fawn-Hooded rats, Anxiogenic behavior, Ethanol withdrawal, DMCM, mCPP, Restraint stress
1. Introduction
There has been renewed interest in aldehyde dehydrogenase-2 (ALDH-2) as a target for therapeutic agents in alcoholism. Daidzin, an isoflavone isolated from kudzu, inhibits alcohol intake (Keung and Vallee, 1993a) and selectively inhibits ALDH-2 (Keung, 2001; Keung and Vallee, 1993b). CVT-10216 (see Fig. 1) was developed as a highly selective, reversible inhibitor of ALDH-2, guided by the interaction of daidzin with human ALDH-2. The IC50 is about 29 nM for ALDH-2 but 1300 nM for ALDH-1; further details are given in Arolfo et al. (2009). CVT-10216 reduces excessive alcohol drinking of alcohol-preferring rats and prevents self-administration in Long–Evans rats (Arolfo et al., 2009; Overstreet et al., 2007).
Fig. 1.

Structure of CVT-10216.
Despite the availability of several medications approved for treating alcoholism, the majority of patients continue to relapse at high rates. It is believed that stress can be a factor in these relapses and that anxiety states are the intermediate states that lead eventually to the increased drinking (Sinha 2001, Sinha et al., 2009). Yet, there is no evidence that the three main drugs used in the treatment of alcoholism, acamprosate, naltrexone, and disulfiram, have anxiolytic properties (e.g. Anton and Swift, 2003; Bayard et al., 2004; anonymous, 2007). In addition, drugs that are used to counteract alcohol-withdrawal symptoms such as the benzodiazepines (BZDs), are not considered long-term treatments against alcohol drinking, in part because of their addictive potential (Anton and Swift, 2003; Bayard et al., 2004).
Given the excellent profile of CVT-10216 for preventing or reducing self-administration or relapse to alcohol (Arolfo et al., 2009), it was decided to explore whether CVT-10216, a selective inhibitor of ALDH-2 (see Fig. 1), might also have anxiolytic effects. There is no known literature on the potential anxiolytic effects of ALDH-2 inhibitors (Snyder and Keeler, 1981) nor has a link been established between ALDH-2 inhibitors and benzodiazepines. Thus, this study is breaking new ground. Here we describe the anxiolytic properties of CVT-10216 in four rodent model systems exhibiting anxiety-like behavior.
2. Methods
2.1. Animals
FH and iP rats (selected from breeding colonies at the University of North Carolina) were about 70 days of age (300 g) and Sprague–Dawley (SD) rats (Charles-River, Raleigh, NC) were about 50 days of age (210 g) at the beginning of the study and 70 days of age (300 g) at the end. Rats were housed in a standard animal environment with temperatures about 22 °C and humidity about 50%. The light:dark cycle was 0700–1900, with lights on at 0700. All procedures were approved by the UNC Institutional Animal Care and Use Committee.
2.2. FH rats
After selection from the breeding colony, FH rats were randomly assigned to one of four treatment groups, each containing 9 rats. One group received 1 ml/kg of 0.5% carboxymethylcellulose (CMC), the vehicle for CVT-10216 (CV Therapeutics, Palo Alto, CA). The other three groups received an ip injection of 3.75, 7.5, or 15 mg/kg CVT-10216 in CMC vehicle. Thirty min after the injection, the rats were placed in the open field arena for the recording of social interaction and line crosses in a 5-min session (see details later).
2.3. iP rats
The alcohol-preferring iP rats were selected from the breeding colonies maintained at UNC—Chapel Hill at 70 days of age and used in a study of CVT-10216 on locomotor activity (see below).
2.4. Ethanol exposure studies
Individually housed SD rats (N=8 per group) were given a complete nutritious liquid diet (Knapp et al., 2004; Overstreet et al., 2002) after 5 days to adapt to the local conditions. Three days later, most of the rats received a 4.5% ethanol diet and the others remained on the control liquid diet without ethanol. Rats consumed ethanol for 15 days, in three cycles of 5 days each separated by 2-day withdrawal periods between the cycles. This design enabled us to test two treatment regimens with CVT-10216. Acute effects of CVT-10216 (3.75 and 15 mg/kg, ip) were determined 5 h into the third withdrawal, 30 min after dosing. Prophylactic effects were determined 5 h into the 3rd withdrawal in rats that had received CVT-10216 (1.875–15 mg/kg) ip into each of the 1st and 2nd withdrawals but not the third. Thus, prophylactically treated rats had not received CVT-10216 for 5 days before social interaction testing.
2.5. Stress study
Sprague–Dawley rats (N=8 in each group) were injected ip with either CMC vehicle or 15 mg/kg CVT-10216 and 30 min later subjected to restraint stress in decapicones for 1 h. Only the single high dose of CVT-10216 was used in this study because other works found this dose to be the highest dose with selective effects on alcohol intake (Arolfo et al., 2009) or anxiety-like behavior(other experiments in this study). After a 30-min recovery period, the rats were exposed to the open field arena for 5 min and social interaction and line crosses were recorded.
2.6. Locomotor activity
Although many previous studies have concluded that social interaction and line crosses recorded in the social interaction test are independently regulated (File and Seth, 2003; Overstreet et al., 2002, 2003a,b), a test of CVT-10216 on locomotor activity in individual animals was obtained to get rid of any doubt. Both the FH and alcohol-preferring iP rats were used. Rats received either vehicle or 15 mg/kg CVT-10216 and the rats were placed in the open field apparatus 30 min after the injections for a 5-min session. Once again, only the single high dose was used because of other data with multiple doses suggested that this dose would be adequate to detect differences, if they were any.
2.7. Chemically-induced anxiogenic effects
Experimental Sprague–Dawley rats received one of four treatments (N=8 per group): CMC vehicle or 3.75, 7.5 or 15 mg/kg CVT-10216 in CMC vehicle. Fifteen min later all of these rats received a second injection of 0.5 mg/kg DMCM (methyl 6,7-demethoxy-4-ethyl-β-carboline-3-carboxylate). A fifth, control group received two injections of vehicle (CMC, acidified saline) 15 min apart. The rats were placed in the open field arena 15 min after the second injection for the recording of social interaction and locomotor activity for 5 min. Approximately one week later the rats were treated with CVT-10216 (3.75, 7.5, 15 mg/kg) or CMC vehicle. The 5-HT2C receptor agonist mCPP (m-chlorophenylpiperazine; 0.5 mg/kg) or vehicle (saline) was given 15 min later and the social interaction test was carried out 15 min after this injection.
2.8. Social interaction
Social interaction has been validated repeatedly as an index of anxiety-related behavior (See File and Seth, 2003) because it is decreased following anxiety-provoking stimuli such as bright lights or exposure to cat odor (File, 1980; File and Hyde, 1978), after administration of anxiogenic drugs (e.g., Bhattacharya et al., 1997; File and Lister, 1984; Guy and Gardner, 1985), or following withdrawal from drugs of abuse, including alcohol (Andrews et al., 1997; Costall et al., 1990, File et al., 1989; Irvine et al., 2001; Kampov-Polevoy et al., 2000; Overstreet et al., 2002). Conversely, social interaction can be increased by prior exposure to the test arena (File 1980; File and Hyde, 1978) or the administration of anxiolytic drugs at doses that have little effect on locomotor activity (File, 1980; Lightowler et al., 1994).
Experienced observers blinded to the experimental conditions carried out the social interaction test in a square open field (60 cm by 60 cm, with 16 15×15 cm squares marked out on the floor). Pairs of rats receiving the same treatment were placed in the arena and social interactions recorded. Rats were unfamiliar with the open field and the lighting conditions were low in order to generate an intermediate level of anxiety-related behavior. Rat pairs were matched on the basis of body weights and treatment conditions and placed simultaneously in the open field. During the 5-min session, line crosses (by two forepaws) and time spent in social interaction (grooming, sniffing, following) were scored individually for each rat (Kampov-Polevoy et al., 2000; Overstreet et al., 2002, 2003a). This procedure has been validated by previous studies in our laboratory (Overstreet et al., 2002, 2003a).
2.9. Statistical analyses
The data were analyzed by statistical analyses that were appropriate for the research designs. The FH studies and the alcohol-withdrawal-, the restraint- and the chemically-induced anxiogenic effects were analyzed by one-way ANOVAs, with Tukey–Kramer tests being used to identify the significant group differences. The locomotor activity studies were analyzed by a 2-way ANOVA, with strain and treatment as the two main effects.
3. Results
3.1. Counteraction of FH innate anxiety
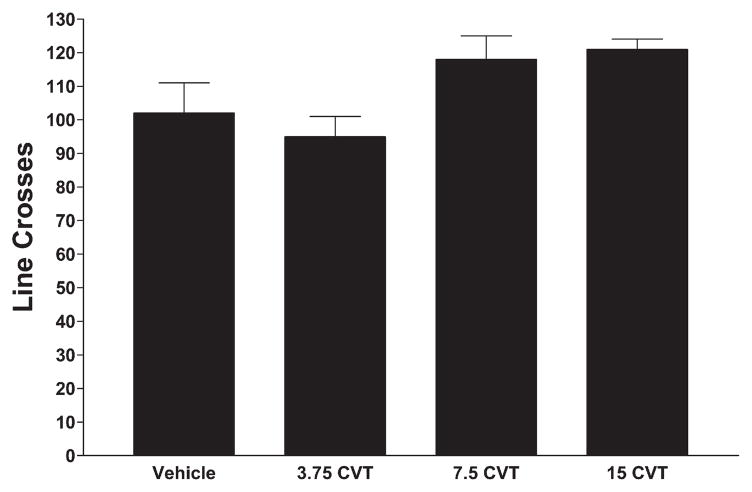
Fawn-Hooded rats are characterized by innate increased anxiety in the social interaction test compared to other rat strains (Kantor et al., 2000). The FH rats treated with vehicle exhibited very low social interaction behavior (Fig. 2), as expected (Kantor et al., 2000). However, the FH rats treated with CVT-10216 exhibited a dose-dependent increase in social interaction (less anxiety), punctuated by a 2-fold increase in social interaction after 15 mg/kg (Fig. 2; F[3,32] = 22.18, p<0.0001). In contrast, CVT-10216 did not significantly affect line crosses (Fig. 3; F[3,32]=3.09, p<0.05). Although the ANOVA was significant, none of the CVT-10216-treated groups was different from the vehicle-treated group, according to Tukey’s tests.
Fig. 2.
Effects of CVT-10216 or vehicle on social interaction behavior in FH rats. Rats were injected ip with vehicle (2 ml/kg) or CVT-10216 (3.75, 7.5 or 15 mg/kg) 30 min prior to being placed in the open field for a 5-min session. Data represent the mean ± s.e.m. (s) for 9 rats in each group. *Significantly different from vehicle, p<0.001.
Fig. 3.
Effects of CVT-10216 or vehicle on line crosses in the social interaction test in FH Rats. See legend to Fig. 2 for details.
3.2. Reduction in anxiety induced by alcohol withdrawal
Repeated exposure to ethanol induced an anxiety response as reflected by a reduction in social interaction (Fig. 4), supporting previous findings (Overstreet et al., 2002, 2003a). CVT-10216 injected 30 min before testing increased the time of social interaction in the ethanol-withdrawn rats (Fig. 4), with the higher doses completely normalizing the behavior. The one-way ANOVA was statistically significant (F[3,43]=11.63, p<0.001) and confirmed the anxiolytic effect of 15 mg/kg CVT-10216 in this model. No significant effects on locomotor activity were detected (F[3,43]=2.16, NS; data not shown).
Fig. 4.
Effects of acute CVT-10216 treatment on social interaction behavior of alcohol-withdrawn Sprague–Dawley rats. After being exposed to 15 days of 4.5% ethanol diet in three cycles of 5 days, rats were withdrawn from alcohol on a third occasion. Vehicle or CVT-10216 (3.75, 15 mg/kg) was injected ip 4.5 h after the alcohol was removed and 0.5 h before the social interaction test was carried out. The data represent the mean±s.e.m. (s) for 8 rats.
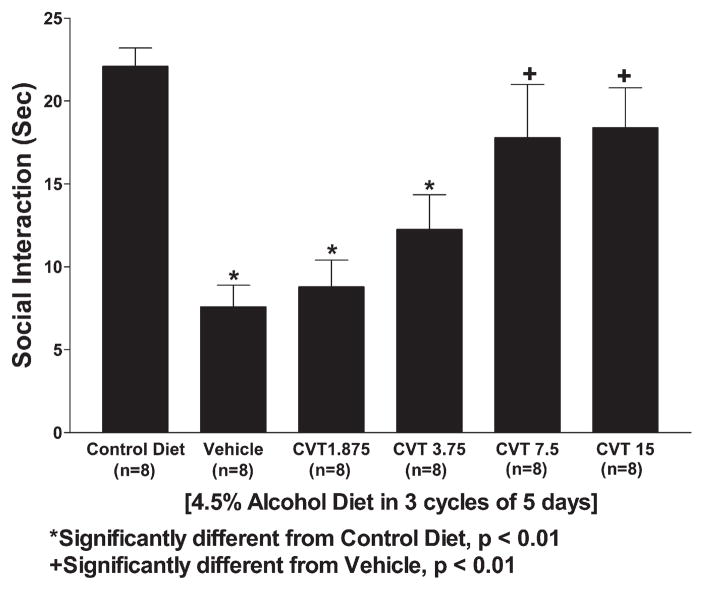
Importantly, CVT-10216 (3.75–15 mg/kg) increased social interaction behavior prophylactically during the third withdrawal even though the drug has been given only during the 1st and 2nd withdrawals (Fig. 5). Thus, even though testing was carried out 5 days after the last dose of CVT-10216, the significant 1-way ANOVA confirmed group differences (F[5,57]=11.02, p<0.001). This finding suggests that CVT-10216 was interfering with the adaptive changes in the brain that occur during ethanol withdrawals. There were significant differences among the groups for locomotor activity (F[5,57]=4.66, p<0.01), but the pattern of differences was different from those for social interaction (Fig. 6).
Fig. 5.
Effects of pretreatment with CVT-10216 on social interaction behavior of alcohol-withdrawn Sprague–Dawley rats. Rats were exposed to an ethanol-containing diet for 15 days in three cycles of 5 days. Vehicle or CVT-10216 was injected 4 h after the ethanol was withdrawn after the 1st and 2nd cycles only. The social interaction test was carried out 5 h after the ethanol was removed on the last cycle. The data represent the mean±s.e.m. (s) for 8 rats.
Fig. 6.
Effects of pretreatment with CVT-10216 on line crosses in the social interaction test in alcohol-withdrawn Sprague–Dawley rats. See legend for Fig. 5 for details.
3.3. Counteraction of stress-induced anxiety
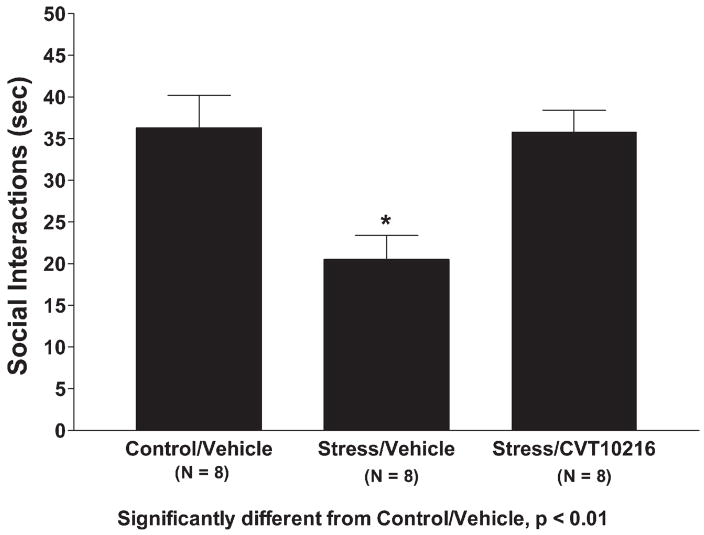
Restraint stress for 60 min reduced social interaction behavior, as expected (Breese et al., 2004), and this anxiogenic effect was prevented by pretreatment with CVT-10216 (Fig. 7). The one-way ANOVA was statistically significant (F[2,19]=6.82, p<0.01) and Tukey–Kramer tests confirmed significant differences between the controls and stressed rats as well as the vehicle- and CVT-10216-treated rats. There were no significant (F[2,19]=2.12, NS) differences in locomotor activity (data not shown).
Fig. 7.
Effects of CVT-10216 on social interaction behavior in rats subjected to restraint stress. Injection of CVT-10216 or vehicle was given 30 min prior to the 1-h session of restraint stress. The social interaction test was carried out 30 min after the end of the session on restraint stress. The data represent the mean±s.e.m. (s) for 8 rats. *Significantly different from control/vehicle, p<0.01.
3.4. Reduction in DMCM-induced anxiogenic effects
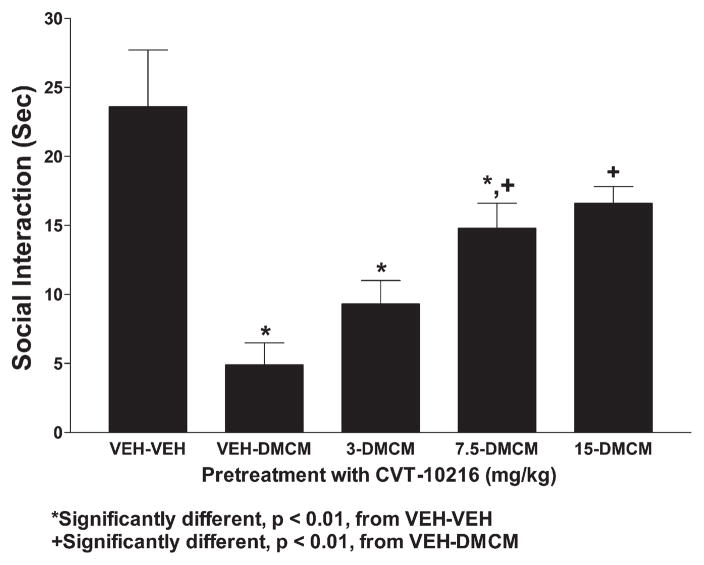
As expected, DMCM, a BZD receptor inverse agonist, substantially reduced social interaction behavior (Fig. 8). This anxiogenic effect was prevented by CVT-10216 in a dose-dependent manner, as revealed by the significant one-way ANOVA (F[4,35]=9.57, p<0.0001) and subsequent Tukey’s tests. On the other hand, the reduced locomotor activity induced by DMCM was not counteracted by CVT-10216 except for the dose of 7.5 mg/kg (Fig. 9). There were significant group effects (F[4,35]=7.74, p<0.0001) and Tukey’s tests confirmed that DMCM reduced locomotor activity when compared to controls; only the group receiving 7.5 mg/kg CVT-10216 was not different from control.
Fig. 8.
Effects of CVT-10216 on social interaction behavior in rats treated with BZD inverse agonist, DMCM. Injection of CVT-10216 was given 15 min prior to the injection of DMCM, which occurred 15 min prior to the social interaction test. The data represent the mean±s.e.m. (s) for 8 rats. *Significantly different from VEH–VEH, p<0.01; +significantly different from VEH–DMCM, p<0.01.
Fig. 9.
Effects of CVT-10216 on line crosses in rats treated with BZD inverse agonist, DMCM. See legend for Fig. 8 for details.
3.5. Lack of effect of CVT-10216 on mCPP-induced anxiogenic effects
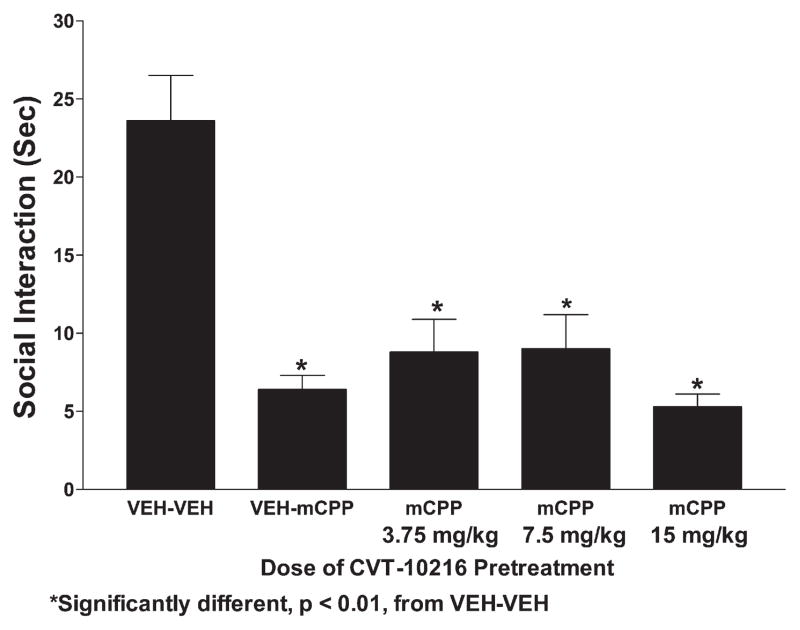
There was also a substantial anxiogenic effect of mCPP, a 5-HT2C agonist, as indicated by a low social interaction score (Fig. 10). CVT-10216 did not prevent this mCPP-induced anxiogenesis. There were significant group differences (F[4.35]=21.71, p<0.0001) and all groups receiving mCPP were similar to each other and different from the controls (Fig. 6). There was also a substantial effect of mCPP on locomotor activity (Fig. 11) and none of the CVT-10216 treatments had any effect. There were significant group differences (F[4,35]=8.92, p<0.0001) and Tukey’s tests confirmed that each group treated with mCPP had significantly lower activity than controls.
Fig. 10.
Effects of CVT-10216 on social interaction behavior in rats treated with 5-HT2C agonist, mCPP. Injection of CVT-10216 was given 15 min prior to the injection of mCPP, which occurred 15 min prior to the social interaction test. The data represent the mean±s.e.m. for 8 rats. *Significantly different from VEH–VEH, p<0.01.
Fig. 11.
Effects of CVT-10216 on line crosses in rats treated with 5-HT2C agonist, mCPP. See legend to Fig. 10 for details.
3.6. Effect of CVT-10216 on locomotor activity
The 15 mg/kg dose of CVT-10216 was given to two alcohol-preferring rat strains because both the iP and FH rats showed a substantial reduction in alcohol intake at this dose (Arolfo et al., 2009). As illustrated in Table 1, although the iP rat was more active than the FH rat, CVT-10216 did not affect activity in either strain. The results of a 2-way ANOVA confirmed these impressions; the strain effect was significant (F[1,20]=34.58, p<0.0001) but the treatment effect was not (F[1,20]=2.53, NS).
Table 1.
Effects of CVT-10216 on locomotor activity in FH and iP rats.
| Treatment group | Line crosses |
|---|---|
| FH-Veh | 97.0±2.0 |
| FH-CVT | 89.0±8.0 |
| iP-Veh | 139.0±8.0 |
| iP-CVT | 127.0±8.0 |
4. Discussion
The major finding in this report is that the highly selective and reversible ALDH-2 inhibitor CVT-10216 exhibits unequivocal anxiolytic properties in four different rat models of anxiety-like behavior. The social interaction test was used in this study to measure the anxiolytic properties of CVT-10216 against endogenous anxiety-like behavior in the naïve FH rats, alcohol-withdrawal-induced anxiety, stress-induced anxiety and drug-induced anxiety. Locomotor activity was also assayed as a control for nonspecific effects of the treatments on neural function. The usefulness of the social interaction test as an index of anxiety-like behavior is well established (File and Seth, 2003) and locomotor activity is mostly independent from social interaction behavior (File and Seth, 2003; Overstreet et al., 2002, 2003a, 2004). Here we find that CVT-10216 has virtually no effect on locomotor activity when assayed in all of the models tested and in independent tests of locomotor activity. Indeed, changes in locomotor activity were rarely observed when CVT-10216 exhibited clear dose-dependent anxiolytic effects.
Naïve FH rats exhibit endogenous anxiety-like behavior characterized by a reduced amount of social interaction behavior (Kantor et al., 2000). CVT-10216 significantly and dose-dependently increases the social interaction behavior of the FH rats, without affecting locomotor activity. These findings suggest that the anxiolytic properties of CVT-10216 appear to selectively suppress inherited CNS mechanisms required for anxiety without producing sedation or affecting the control of locomotor activity. Because the development of anxiety is modulated by drugs that influence multiple systems, such as flumazenil, a BZD receptor antagonist, buspirone an 5-HT1A receptor agonist, CRF1 receptor antagonists and baclofen, a GABA-B receptor agonist (Knapp et al., 2004, 2005, 2007; Overstreet et al., 2003a, 2004), it is not possible to suggest which mechanisms underlie the effects of CVT-10216. Kantor et al. (2000) reported that the FH rat was more sensitive to the anxiogenic effects of the 5-HT2C agonist mCPP. However, the failure of CVT-10216 to counteract the anxiogenic effects of mCPP in SD rats argues against this mechanism underlying its anxiolytic effects.
In order to identify potential receptor mechanisms underlying the anti-anxiety properties of CVT-10216, we next used an alcohol-withdrawal model of anxiety in SD rats because of the identified role of specific neurotransmitter receptors in response to alcohol withdrawal. We asked whether CVT-10216 would also prevent the development of alcohol-withdrawal-induced anxiety, a well-recognized event that often precipitates relapse drinking (Breese et al., 2005b). Here we show that CVT-10216 suppresses anxiety-like behavior in rats withdrawn from ethanol. In general, our results suggest that anxiolytic properties of CVT-10216 were greatest after acute treatment, with the 15 mg/kg dose completely normalizing the abnormal behavior produced by repeated withdrawal during 3 cycles of forced alcohol consumption. Moreover, there was also an additional prophylactic effect of CVT-10216 which appeared to prevent the development of anxiogenic behavior during the course of the repeated withdrawals. Thus, CVT-10216 administered during the first two cycles of alcohol withdrawal reduced the degree of anxiety when social interaction was measured after the 3rd cycle of withdrawal 5 days after the last dose of CVT-10216. This finding suggests that the anxiolytic properties of CVT-10216 not only appear to immediately suppress manifestations of anxiety, but also prophylactically prevent the development of neurochemical changes required for the expression of anxiety in the future. Importantly there was no effect of CVT-10216 on locomotor activity in these experiments, an observation that suggests that CVT-10216 targets anxiogenic behavior selectively.
Restraint-induced stress is another model system for producing anxiety-like behavior and searching for anxiolytic agents. CVT-10216 prevented the development of stress-induced anxiety-like behavior in restrained SD rats. A variety of other compounds counteract stress-induced anxiety-like behavior in this model. These compounds include CRF1 receptor antagonists, BZD receptor antagonists, and 5-HT1A receptor agonists (Breese et al., 2004, 2005a). One notable exception to this list is the 5-HT2C receptor antagonist that does not counteract stress-induced anxiety-like behavior (Breese et al., 2004) but is effective as an anxiolytic agent in FH rats (Bagdy et al., 2001; Kantor et al., 2000) and in alcohol-withdrawal-induced anxiety (Overstreet et al., 2003a). These observations raised the possibility that CVT-10216 may not target 5-HT2C mechanisms. Indeed, we find that CVT-10216 is not effective against anxiety produced by mCPP, a 5-HT2C agonist. Moreover, CVT-10216 also does not affect the suppression of locomotor activity produced by mCPP.
The GABA–BZD receptor system is a well-recognized mechanism that appears to be involved in human anxiety and in rat models of anxiety-like behavior, including those used in this study. Therefore we asked whether CVT-10216 would be therapeutically effective against anxiety-like behavior produced by DMCM, a BZD receptor inverse agonist (Stephens et al., 1984). These results suggest that the GABA–BZD receptor system is implicated in the anti-anxiety properties of CVT-10216. Consistent with this possibility, flumazenil, a BZD receptor antagonist, is effective in both stress and multiple alcohol-withdrawal protocols (Breese et al., 2004; Knapp et al., 2007). Thus, our findings suggest that CVT-10216 has anxiolytic properties that may involve GABA/BZD pathways, even though we have not observed any direct interactions between CVT-10216 and GABA or BZD receptors (unpublished observations, 2007).
Disulfiram, an irreversible ALDH-1 and ALDH-2 inhibitor, does not appear to have anxiolytic properties in humans (Goyer et al., 1984; Snyder and Keeler, 1981). This difference may be related, in part, to the fact that disulfiram and its metabolites inhibit many sulfhydryl and metal-containing enzymes while CVT-10216 is a highly selective, reversible inhibitor of ALDH-2 only. Fortunately, unlike the BZD receptor agonists, CVT-10216 does not appear to be an addictive substance (Arolfo et al., 2009). Studies are underway to determine whether some of the metabolic substrates of ALDH-2 in the brain might play a role in mediating the anxiolytic properties of CVT-10216.
Because the social interaction test was the exclusive test used in these studies, we do not know how general the anxiolytic properties of CVT-10216 are. In particular, anxiety is inferred in the social interaction test from an inhibition of behavior. Whether CVT-10216 will be effective in a model where anxiety is inferred from an increase in behavior, such as the defensive burying test, cannot be answered at this point. It should be emphasized, however, that anxiety tests do not necessarily provide identical information. The FH rats and several other strains with innate low social interaction do not exhibit any differences in the elevated plus maze (Overstreet et al., 1998; Rezvani et al., 2007).
The pattern of effects on anxiety-related behavior resembles those seen for CRF1 receptor antagonists (Breese et al., 2004; Overstreet et al., 2004). However, little is known about the effects of CVT-10216 on the HPA axis. It is unlikely that a modulation of this system plays a role in the effects of CVT-10216 because we have previously shown that corticosterone does not modify alcohol-withdrawal-induced anxiety-like behavior (Breese et al., 2004).
In summary, CVT-10216 exhibits anxiolytic effects in several well-established rodent model systems. These findings provide a nice complement to the recently established anti-drinking effects of CVT-10216 (Arolfo et al., 2009). The GABA–BZD system mediates the anxiety-like behavior seen in these models and it is possible that CVT-10216 may be interacting with this system to produce its anxiolytic effects. However, these findings are also consistent with recent reports that a GABA-B receptor agonist appears to have anxiolytic effects in animals (Knapp et al., 2007), to counteract alcohol-withdrawal signs in humans (Addolorato et al., 2006), to reduce alcohol drinking in animals and humans (Addolorato et al., 2000, 2002; Flannery et al., 2004; Colombo et al., 2000) and to reduce alcohol operant self-administration in alcohol-dependent rats (Walker and Koob, 2007). Studies are underway to investigate the role of GABA-B receptors in the mechanism of action of CVT-10216.
Acknowledgments
We acknowledge the technical support of Robert Angel and Kui-Ling. We thank Jeff Zablocki for the copy of the structure of CVT-10216. This work was supported, in part, by funding from NIAAA and CV Therapeutics.
References
- Addolorato G, Caaputo E, Capristo E, Domenicalli M, Bernardi M, Janin L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind, randomized controlled study. Alcohol Alcohol. 2002;37:504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capuo F, Capisto E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake II — preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, et al. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006;119:276.e13–8. doi: 10.1016/j.amjmed.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphe nucleus–dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology. 1997;130:228–34. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- Anonymous Alcohol withdrawal syndrome: how to predict, diagnose and treat it. Prescribe Int. 2007;16:24–31. [PubMed] [Google Scholar]
- Anton RF, Swift RM. Current pharmacotherapies of alcoholism: a U.S. perspective. Am J Addict. 2003;12(suppl 1):S53–68. doi: 10.1111/j.1521-0391.2003.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Overstreet DH, Yao L, Fan P, Lawrence AJ, Tao G, et al. Suppression of heavy drinking and addictive drug-seeking by a novel ALDH-2 inhibitor. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.01031.x. [Electronic publication ahead of print, Aug 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with 5-HT2C receptor antagonist SB242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Psychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bayard M, McIntyre J, Hill KR, Woodside J. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69:1443–50. [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakraharti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–24. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology. 2004;29:470–82. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF(1)- and benzodiazepine receptor antagonists and a 5-HT(1a)-receptor agonist. Neuropsycho-pharmacology. 2005a;30:1662–9. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005b;29:185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MAM, Lobnia AC, Pan M, Reali R, et al. Ability of baclofen in reducing alcohol intake and withdrawal severity. I. — preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Ondansetron inhibits a behavioural consequence of withdrawing from drugs of abuse. Pharmacol Biochem Behav. 1990;36:339–44. doi: 10.1016/0091-3057(90)90414-d. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Lister RG. Do the reductions in social interaction produced by picrotoxin and pentylenetetrazole indicate anxiogenic actions? Neuropharmacology. 1984;23:793–6. doi: 10.1016/0028-3908(84)90113-8. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcot PK. Flumazenil but not nitrendipine reverses the increased anxiety during alcohol withdrawal in the rat. Psychopharmacology. 1989;98:262–4. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, et al. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–23. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Goyer PF, Brown GL, Minichiello MD, Major LF. Mind-altering effects of disulfiram in alcoholics. J Stud Alcohol. 1984;45:209–13. doi: 10.15288/jsa.1984.45.209. [DOI] [PubMed] [Google Scholar]
- Guy AP, Gardner CR. Pharmacological characterisation of a modifled social interaction model of anxiety in the rat. Neuropsychobiology. 1985;13:194–200. doi: 10.1159/000118187. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology. 2001;153:315–20. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–84. [PubMed] [Google Scholar]
- Kantor S, Anheuerer ZF, Bagdy G. High social anxiety and low aggression in Fawn-Hooded rats. Physiol Behav. 2000;71:551–7. doi: 10.1016/s0031-9384(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Keung WM. Biogenic aldehyde(s) derived from the action of monoamine oxidase may mediate the antidipsotropic effect of daidzin. Chemico-Biol Inter. 2001;130–132:919–30. doi: 10.1016/s0009-2797(00)00245-3. [DOI] [PubMed] [Google Scholar]
- Keung W-M, Vallee BL. Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc Natl Acad Sci USA. 1993a;90:10008–12. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Vallee BL. Daidzin: a potent selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proc Nat Acad Sci USA. 1993b;90:1247–51. doi: 10.1073/pnas.90.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res. 2005;29:553–63. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–11. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–95. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowler S, Kennett GA, Williamson U, Blackburn TP, Tulloch IF. Anxiolytic-like effect of paroxetine in the rat social interaction test. Pharmacol Biochem Behav. 1994;49:281–5. doi: 10.1016/0091-3057(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Daws LC, Schiller GD, Orbach J, Janowsky DS. Cholinergic/serotonergic interactions in hypothermia: Implications for rat models of depression. Pharmacol Biochem Behav. 1998;59:777–85. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–69. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–13. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003a;167:344–52. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Keung WM, Tao G, Arolfo MP, Yao L, Zablocki J, et al. Novel selective inhibitors of mitochondrial aldehyde dehydrogenase (ALDH2) reduce alcohol intake. Presented at the 30th annual meeting of the Research Society on Alcoholism; July 7–11; 2007; p. #530. [Google Scholar]
- Overstreet DH, Kralic JE, Morrow AL, Ma ZZ, Zhang YW, Lee DYW. NPI-031G (puerarin) reduces anxiogenic effects of alcohol withdrawal or benzodiazepine inverse or 5-HT2C agonists. Pharmacol Biochem Behav. 2003b;75:619–25. doi: 10.1016/s0091-3057(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Cleaves M, Parsian A. Further genetic characterization of the fawn-hooded (FH/Wjd) rat, an animal model of comorbid depression and alcoholism. Psychiat Genet. 2007;17:77–83. doi: 10.1097/YPG.0b013e328012d7c3. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergqist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S, Keeler M. Acute effects of disulfiram on anxiety levels of chronic alcoholics. Int Pharmacopsychiatry. 1981;16:49–56. doi: 10.1159/000468474. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Kehr W, Schneider HH, Schmiechen R. Beta-carbolines with agonistic and inverse agonistic properties at benzodiazepine receptors of the rat. Neurosci Lett. 1984;47:333–8. doi: 10.1016/0304-3940(84)90535-4. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–8. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]