Abstract
EGFR and other ErbB-family tyrosine kinases are overexpressed in many human tumors, and their aberrant expression and mutational activation is associated with the development, progression and aggressiveness of a number of malignancies. Thus the EGFR kinase has long been recognized as a potential drug target in oncology, and small-molecule inhibitors have been under development for more than two decades. As a result of their effectiveness in treating non-small cell lung cancers (NSCLCs) driven by somatic mutations in the EGFR kinase, gefitinib and erlotinib were the first EGFR tyrosine kinase inhibitors (TKIs) approved for clinical use. Ironically, these drugs found their target against mutant forms of the EGFR kinase, which have altered enzyme active sites, and not against the wild type (WT) kinase against which their potency and selectivity was carefully honed. Here we review recent structural and enzymological studies that explore the exquisite sensitivity of a subset of these lung cancer mutants to gefitinib and erlotinib. We discuss available structural evidence for the mechanisms of activation of the EGFR kinase by these mutants, and compare it to physiologic activation of the kinase by ligand-induced dimerization. Finally, we consider the mechanisms by which the secondary T790M “gatekeeper” mutation confers resistance to gefitinib and erlotinib.
Keywords: tyrosine kinase, epidermal growth factor receptor, x-ray crystallography, gefitinib, lung cancer, drug resistance
1. Introduction
Non-small cell lung cancer (NSCLC) is the most common cause of cancer-related death in both men and women. A subset of NSCLC is caused by activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) [1–3]. These somatic mutations are far from rare, as they occur in 10–15% of all NSCLC patients in the North America and Western Europe, and at much higher frequencies in women, in patients of Asian descent, and in non-smokers who develop NSCLC (greater than 50%) 4–6]. The discovery of these mutations in 2004 and the revelation that their presence correlates with clinical responsiveness to small molecule EGFR inhibitors gefitinib and erlotinib has fueled an intense interest in understanding their pathophysiology and drug sensitivity, and also stimulated the search for additional kinase mutations in lung cancer and other solid tumors.
Several distinct EGFR mutations have been described in NSCLC, including small, in-frame deletions in exon 19, insertions in exon 20, and point mutations in exons 18 and 21. Structurally, these mutations map to the vicinity of the active site cleft of the kinase (Figure 1). The various exon 19 deletions remove from 3 to 8 residues from the loop leading into the αC-helix, while the exon 20 insertions add from 1 to 4 residues at the opposite end of this key regulatory element in the N-lobe of the kinase (the architecture of the tyrosine kinase domain is briefly described in the legend to Figure 1). The most common point mutation substitutes Leu858 with arginine; the L858R mutation lies in the activation loop of the kinase, and accounts for over 40% of EGFR mutations. Mutations in residue Gly 719 (to serine, alanine or cysteine) in the P-loop of the kinase are much rarer, accounting for less than 5% of observed mutations [1, 7, 8]. A number of studies demonstrate that these somatic mutations activate the EGFR kinase and drive malignant transformation in vivo [2, 9–13].
Figure 1. Structure of the EGFR kinase domain, highlighting the sites of oncogenic mutations.
The kinase domain fold consists of a smaller N-terminal lobe and a larger C-terminal lobe. The active site lies in the cleft between the two lobes. The kinase is shown in the active conformation. Locations of activating mutations are indicated in red. The regulatory C-helix is colored pink, the phosphate coordinating P-loop is shown in magenta, and the activation loop (A-loop) is colored orange.
Given that the mutations surround the ATP-binding site (Figure 1), which is also the target of EGFR TKIs, it is not surprising that tumors (and tumor cell lines) harboring the different mutations vary in their inhibitor responsiveness. The L858R and exon19 deletion mutants are exquisitely sensitive, while the exon20 insertion mutants are resistant to both gefitinib and erlotinib [1–4, 11]. Likewise, structurally different inhibitors can be expected to vary in their effectiveness against a particular mutant. A number of EGFR inhibitors have been developed; representative examples discussed here are shown in Figure 2. Most share a common anilinoquinazoline core, including gefitinib, erlotinib, and lapatinib (or a closely similar analog as seen in EKB-569 and HKI-272). Addition of a reactive Michael-acceptor group to this core (such as the crotonamide in EKB-569 and HKI-272) confers the ability to bind covalently to the kinase via reaction with Cys 797, which lies at the edge of the active site cleft.
Figure 2. Chemical structures of EGFR TKIs discussed here.
Compounds are shown in a consistent orientation and in a conformation suggestive of their binding modes. EKB-569 and HKI-272 are representative examples of irreversible inhibitors; they incorporate a Michael-acceptor that forms a covalent bond with Cys 797. The remaining compounds bind reversibly to the kinase. While most of the compounds can likely bind the kinase in either its active or inactive conformation, lapatinib and HKI-272 likely require the inactive conformation to accommodate an additional aromatic group appended to their aniline moiety.
Unfortunately, the dramatic responses initially achieved in patients with gefitinib and erlotinib are not durable. Drug resistance inevitably arises in the course of treatment with these TKIs. In approximately 50 % of cases, resistance is due to a secondary somatic mutation in the EGFR kinase domain, T790M [14, 15]. This mutation of the threonine “gatekeeper” residue is analogous to the T315I resistance mutation in imatinib-resistant BCR-Abl. In addition to conferring drug resistance, this mutation also activates the kinase and a germline T790M mutation has been reported in a family with a heritable predisposition to lung cancer [16, 17]. Amplification of the gene encoding the c-Met tyrosine kinase has been recently identified as the cause of approximately one-fourth of treatment acquired resistance to gefitinib and erlotinib. The c-Met amplification activates a parallel oncogenic signaling pathway that bypasses the EGFR, thereby leading to resistance to EGFR TKIs [18].
The dramatic drug responsiveness of NSCLCs bearing mutant alleles of the EGFR kinase stands in stark contrast with the relative insensitivity of a host of tumors that overexpress the WT EGFR. The efficacy of gefitinib and erlotinib in EGFR-mutant NSCLC has both “biological” and “chemical” components; a useful cancer drug must hit its target, and the target must be more important for the growth and survival of the tumor than for that of normal tissues to avoid unacceptable toxic side effects. From a biological perspective, the concept of “oncogene addiction” has been proposed to account for the massive apoptosis that ensues upon inhibition of the mutationally activated EGFR in NSCLC [19, 20]. These tumors depend upon the mutant kinase for survival, and so its inhibition leads to cell death and tumor shrinkage. From a chemical perspective, gefitinib and erlotinib are effective in EGFR-mutant NSCLC because they are more potent inhibitors of the EGFR mutants than of the WT kinase – inhibition of WT EGFR in the normal tissues contributes to the dose limiting toxicity of EGFR TKIs. In the following sections, we review the structural and enzymological work from our group and elsewhere that has illuminated the activity and inhibitor sensitivity of the oncogenic EGFR mutations and the T790M resistance mutation.
2. Structure and catalytic properties of the NSCLC-associated EGFR mutations
A number of groups have undertaken in vitro studies of the catalytic and drug binding properties of oncogenic EGFR mutants. Additionally, the Cole and Leahy laboratories have recently described an enzymatic study of the nearly full-length wild type EGFR protein in active (EGF-bound) and inactive (cetuximab-bound) states [21]. This careful study of the intact, transmembrane receptor provides a valuable point of reference for studies of the mutant proteins, which have been conducted with the isolated cytoplasmic kinase domain. Selected measurements for WT and mutant kinases are presented in Table 1. Carey and colleagues kinetically characterized a number of mutant EGFR kinases, including the common L858R point mutant and the Del(746–750) deletion mutant [22]. They found that both of these mutants are active in vitro, but that both exhibit significantly increased Michaelis constants for ATP (Km[ATP]). Strikingly, they also discovered an enhanced affinity of erlotinib for the mutants, and pointed out the effect that the altered Km[ATP] was expected to have on the sensitivity of the mutants to inhibitor in a cellular context [22]. While the finding that the mutants are catalytically active may have been expected given the clear cancer association of the mutations, they could not be taken for granted. Indeed from a structural perspective, it is not easy to understand how a 5-residue deletion in the heart of the well-conserved tyrosine kinase domain can be tolerated without disrupting the fold of the domain.
Table 1.
Enzyme kinetic parameters and inhibitor affinities of WT and mutant EGFR kinases*
| EGFR studied: | Km[ATP] (µM) | kcat (s−1) | Kd (nM) | Kd / Km[ATP] (×10−3)† | ||
|---|---|---|---|---|---|---|
| Yun et al. (ref. [17,23]) | gefitinib | AEE788 | gefitinib | AEE788 | ||
| Wild type | 5.2 | 0.026 | 35.3±0.4 | 5.3±0.3 | 6.8 | 1.0 |
| T790M | 5.9 | 0.137 | 4.6±0.1 | 27.6±0.7 | 0.78 | 4.7 |
| L858R | 148 | 1.484 | 2.4±0.1 | 1.1±0.1 | 0.016 | 0.0074 |
| L858R/T790M | 8.4 | 0.456 | 10.9±0.6 | 18.6±0.5 | 1.3 | 2.2 |
| G719S | 94.7 | 0.143 | 123.6±5.9 | 11.3±1.5 | 1.31 | 0.12 |
| Ki (nM) | Ki / Km[ATP] (×10−3) † | |||||
| Carey et al. (ref. [22]) | erlotinib | erlotinib | ||||
| Wild type | 5.0 | 17.5 | 3.5 | |||
| L858R | 10.9 | 6.25 | 0.57 | |||
| Del(746–750) | 129.0 | 3.3 | 0.025 | |||
| L861Q | 1.1 | 48.7 | 44.3 | |||
| IC50 (µM) | IC50 (µM) | |||||
| Qiu et al. (ref. [21]) | erlotinib | lapatinib | ||||
| WT EGF/EGFRt | 3.6 | 18 | 0.486±0.089 | 0.281±0.015 | ||
| Cetuximab/EGFRt | 25 | 0.13 | 4.4±2.1 | 0.056±0.010 | ||
The Kd values reported by Yun et al. were determined by equilibrium binding, while the Ki values reported by Carey et al. were determined kinetically. The IC50 values reported by Qiu et al. were determined with 25 nM enzyme, 10 µM ATP, 30 µM peptide substrate and 2mM Mn2+. The Yun and Carey studies were conducted with the WT or indicated mutants introduced into the isolated kinase domain of EGFR, while the Qiu study was carried out with the nearly full-length EGFR (EGFRt) complexed with either activating ligand (EGF) or inhibitory antibody FAB fragment (cetuximab).
The ratios Kd / Km[ATP] or Ki / Km[ATP] provide a relative estimate of inhibitor potency.
We studied the L858R and G719S point mutations. We found approximately 50 fold activation of the L858R mutant, relative to WT EGFR, and a more modest 10-fold activation of the G719S mutant [23]. Zhang et al. reported a similar increase in activity of the L858R mutant kinase [24]. We also found that the mutants had a compromised affinity for ATP. Depending on reaction conditions and choice of phosphoacceptor substrate, the measured Km[ATP] of the L858R mutant ranged from ~30 to 150 µM, roughly 5 to 30 times that of the WT enzyme [17, 23]. For the G719S mutant, we observed Km[ATP]= 95 µM. Given that cellular concentrations of ATP are expected to be in the millimolar range, these changes in Km[ATP] are not likely to significantly impair catalytic activity – available ATP concentrations are still ~10 times that of the measured Km[ATP]. However they can be expected to dramatically impact the apparent Ki of inhibitors, as discussed more fully below.
Given the proximity of the mutations to the ATP binding pocket, we also sought to examine directly the effect of the mutations on inhibitor affinity. We developed a simple direct binding assay that measures quenching of intrinsic tryptophan fluorescence of the kinase upon titration with inhibitor. The assay is very sensitive, requiring only nanomolar concentrations of protein, and is therefore well suited to studying tight-binding inhibitors. For gefitinib and the research compound AEE788, we found much tighter binding to the L858R mutant than to the WT EGFR (Kd=~2.5nM vs. 35 nM for gefitinib and ~1 nM vs. 5 nM for AEE788). In contrast, the G719S mutant bound more weakly to gefitinib (~100nM vs. ~35nM for WT). However, the modestly lower affinity of this mutant for gefitinib does not render it insensitive to the drug. The roughly 3-fold lower affinity of the G719S mutant for gefitinib is more than compensated the ~10-fold higher Km[ATP]. Indeed, tumors bearing the G719S mutation do respond to treatment with gefitinib and erlotinib [4]. Mulloy et al. used a similar approach to measure the affinity of gefitinib for the L858R and Del(747–753) mutants, and found a 5 and 13 fold tighter binding to gefitinib, respectively, of these mutants as compared with WT EGFR [25].
Because the mutations alter the equilibrium between active and inactive states, it is reasonable to ask to what extent the observed differences in ATP and inhibitor affinities of the mutant vs. WT EGFR proteins might stem from different properties of these two conformations, rather than from differences due to the mutations per se. Differences due to the active versus inactive conformation of the enzyme cannot be discounted, particularly in the case of inhibitors that require the inactive conformation of the kinase (as discussed further in Section 3 below). However, mutant-specific alterations are likely to be the dominate factor in alterations of Km[ATP]. It is likely that with the WT enzyme, one is measuring a Km[ATP] for the population of molecules that assume the active conformation, however transiently, as it is unlikely that the bona fide inactive conformation is catalytically competent. Additionally, there is no clear correlation between the extent of activation by particular mutants and alteration of Km[ATP], as would be expected if a change in the equilibrium between active and inactive conformations was also responsible for alterations in ATP affinity.
The significance of these results cannot be overemphasized; in effect, these mutant-specific changes in inhibitor affinity and Km[ATP] are the underlying basis for the success of EGFR-directed TKIs in treating lung cancers driven by these mutations. The compromised affinity for ATP, as reflected in an increased Km[ATP], appears to be particularly important and can be considered the common theme in mutants that respond to TKI therapy.
3. Structural insights into activation and inhibitor sensitivity
Stamos and colleagues provided the first direct structural information for the EGFR kinase in 2002 with the publication of the crystal structure of the kinase domain in complex with erlotinib [26]. This work showed that the kinase could adopt an active conformation in the absence of activation loop phosphorylation, unlike most other protein kinases, and also allowed an intimate view of the binding mode of erlotinib. Although it was not appreciated until more recently, the crystal lattice interactions in this structure also held the key to dissecting the mechanism of activation of the enzyme by receptor dimerization. A second structure was provided by Wood et al. in 2004 in complex with the TKI lapatinib, a potent inhibitor of both EGFR and ErbB2, and now an approved agent for treatment of breast cancer [27]. In contrast to the active conformation of EGFR in the erlotinib complex, the lapatinib-bound enzyme adopted a distinctly different inactive conformation, quite reminiscent of that previously observed in Src family kinases and Cdk2. This inactive state is characterized by an outward rotation of the C-helix, such that the salt-bridge interaction between a key lysine in the active site (K745) and a glutamic acid residue on the C-helix (E762) is disrupted. Additionally, a helical turn in the N-terminal portion of the activation loop helps to stabilize the displaced, inactive position of the C-helix. The striking similarity of this conformation to inactive Src and Cdk2 led the authors to hypothesize that it represented an authentic inactive conformation of the enzyme, although it was apparently stabilized in this structure by the bound inhibitor. As discussed below, this hypothesis has been subsequently confirmed.
In a normal, physiological setting, conversion of the inactive form to the active state is accomplished by receptor dimerization induced by binding of ligands to the extracellular portion of the receptor. Considerable structural information is available to explain how ligand binding induces reorganization and dimerization in the extracellular portion of the receptor; this work has been recently reviewed [28]. Zhang et al. have recently discovered that the ensuing activation of the kinase domain on the cytoplasmic side of the receptor involves an asymmetric mode of dimerization in which the C-terminal lobe of one molecule packs against the N-lobe of the other to push the C-helix into the active position (Figure 3A) [24]. This discovery arose from systematic analysis of the contacts in the crystal structure of the active EGFR reported by Stamos [26]. This high symmetry crystal form suggested multiple possible dimer interactions – both symmetric and asymmetric – and via an insightful series of structure function studies Zhang and coworkers deduced the mode of interaction that was relevant for kinase activation. Interestingly mutations that disrupted that activating dimer – and crystal contact – also allowed crystallization of the kinase in the inactive state in the absence of the inhibitor lapatinib, thereby confirming that the Src-like inactive conformation observed by Wood et al. was indeed the relevant inactive conformation of the EGFR kinase [24]. More recent structural work reveals that the juxtamembrane region of the kinase also plays a role in promoting this active dimeric state [29, 30].
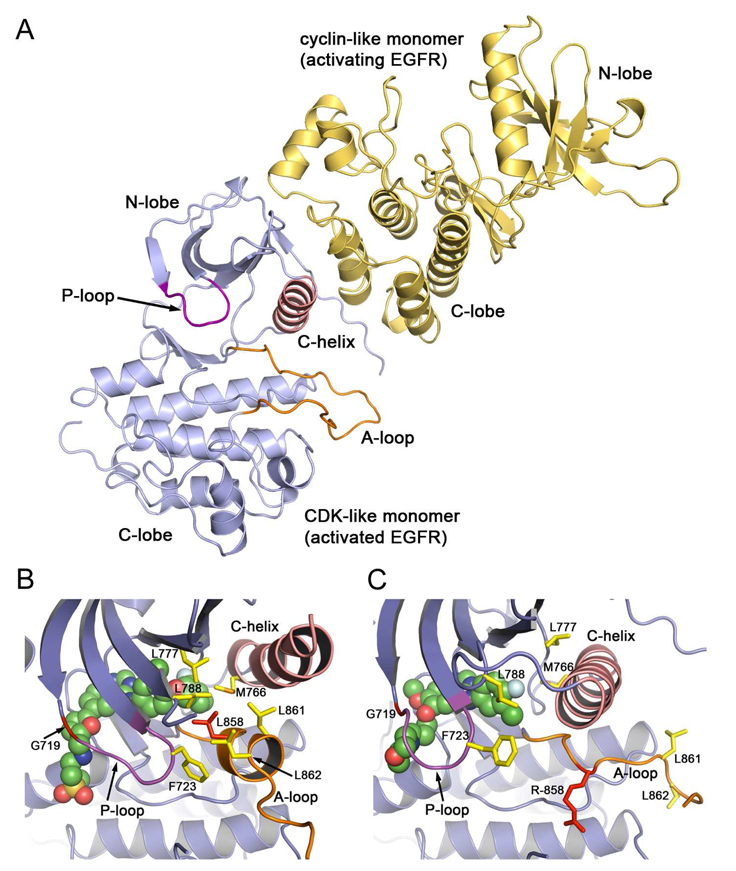
Figure 3. Structural basis for activation of the EGFR kinase by ligand induced dimerization or oncogenic mutation.
A, Upon ligand binding to the extracellular portion of the receptor (not shown), the intracellular kinase domain adopts an asymmetric dimer configuration in which the C-lobe of one molecule binds the N-lobe of the other, effectively “pushing” the C-helix into the inner, active position. This mechanism of activation is reminiscent of activation of cyclin dependent kinases by cyclins; the C-lobe of the “activating” kinase (yellow) essentially assumes the role of the cyclin, activating its partner in the dimer (blue). B and C, Detailed views of the active site cleft of WT EGFR in the inactive state in complex with lapatinib (B) and of the L858R mutant in the active conformation in complex with gefitinib (C). In the L858R and other oncogenic mutants, the mutations destabilize the inactive state to promote the inward, active conformation of the C-helix. The dimerization-dependence of this activation is an active area of investigation, and likely differs among the mutants. B, In the inactive state, the N-terminal portion of the activation loop (orange) forms a short helix centered on Leu 858 that helps lock the C-helix in its outwardly rotated, inactive position. In this state, Leu 858 (shown in red) lies in the center of a group of hydrophobic residues. Mutation of this residue to arginine cannot be accommodated in this conformation, but it is compatible with the rearranged active configuration, as shown in panel C. The G719S mutation is expected to alter the conformation of the P-loop, which contributes Phe 723 to the hydrophobic cluster. The oncogenic L861Q mutation is adjacent to Leu 858, and may similarly destabilize the inactive state. C, In the active state, Arg 858 is solvent exposed and forms a hydrogen bond that may also stabilize the active conformation. Note that in this state, the C-helix assumes its catalytically competent inward position.
How then do the mutations activate the kinase in the absence of this ligand-induced dimerization? The two available structures of WT EGFR – active and inactive – set the stage for understanding the activation of the kinase by mutations (Figure 3,B and C). Residue Leu 858 lies in the helical turn in the activation loop in the inactive state of the kinase, and is part of a cluster of hydrophobic residues that impede the active position of the C-helix and stabilize the inactive conformation of the kinase. Mutation to arginine cannot be tolerated in this conformation, thus the L858R substitution destabilizes the inactive conformation, thereby promoting enzyme activation [23, 24]. In the active state, this residue is relatively solvent exposed, and so the arginine mutation is not disruptive (Figure 3C). In fact, as the crystal structure of the L858R mutation shows, the arginine sidechain makes apparently favorable hydrogen bond interactions that may further stabilize the active configuration [23]. The structure of the G719S mutation indicates a similar, if less direct, mechanism of activation. Gly 719 is within the P-loop, which also contributes to the hydrophobic cluster surrounding Leu 858 in the inactive state, and the inactive conformation of the loop appears to require backbone torsion angles that favor a glycine in this position. Thus mutations to Ala, Ser or Cys may “tweak” the P-loop in a manner that promotes activation [23]. Although crystal structures of the deletion mutants and exon20 insertion mutants have not been reported, it seems likely that shortening of the loop leading into the C-helix will restrain the helix sufficiently to preclude (or at least dramatically disfavor) it from adopting the outward, inactive position, for which a longer or more extended loop would be required. If the deletion mutations can be conceptualized as “pulling” the C-helix into the active position, the exon20 insertion mutations may provide a “push” from the C-terminal end of the helix. A clearer understanding of the effects of these mutations will require elucidation of their crystal structures.
Structural analysis of the WT, L858R and G719S EGFR kinases in complex with inhibitors has also shed light on possible sources of differences in inhibitor binding affinities of the mutants. For example, in the L858R mutant in complex with gefitinib, we have observed two distinct conformations of the aniline ring; one that is identical to that seen in the WT kinase, and another in which the aniline ring is rotated by 180° such that the chlorine substituent interacts with Asp 855 (the first residue of the DFG motif), which in turn participates in a network of hydrogen bonds that extends back to the site of the mutant Arg 858 (Figure 4). In the G719S mutant in complex with a staurosporine analog, we observe a marked reorientation of the bound inhibitor, induced by introduction of the serine sidechain at this position [23]. Structural considerations also suggest that inhibitors such as lapatinib and HKI-272 should not be potent inhibitors of the mutationally activated mutants [17, 27]. These compounds include an additional aromatic group appended to the aniline ring (Figure 2), and as shown by the Wood et al. structure, this group is accommodated only by the outward displacement of the C-helix in the inactive state. Thus their binding should be less favored in the mutants, in which the equilibrium is shifted to the active conformation (see ref. [31] for insights of potential clinical relevance gained from a comprehensive review of available EGFR structures).
Figure 4. Structures of WT and mutant EGFR kinases in complex with inhibitors.
A, The WT kinase in complex with gefitinib. The quinazoline core of the inhibitor is positioned to hydrogen bond with Met 793 in the “hinge” region of the kinase. B, The EGFR L858R mutant in complex with gefitinib. We have observed two modes of binding in the L858R mutant, one that is essentially identical to that in the WT EGFR, and another (pictured here) in which the aniline ring is rotated by ~180° such that the chlorine substituent interacts with Asp 855. Asp 855 in turn participates in a network of water-mediated hydrogen bonds that extends to the site of the mutant Arg 858 (dashed lines). C, Superimposed structures of the resistance-conferring T790M mutant EGFR in the presence (yellow ribbon) and absence (green ribbon) of the inhibitor AEE788. Note that reorientation of the mutant gatekeeper residue Met 790 allows binding of the inhibitor (shown in a ball and stick representation) in essentially the same manner observed in the WT enzyme. D, Structure of T790M EGFR in complex with the irreversible inhibitor HKI-272. The compound binds the inactive conformation of the kinase, with the C-helix displaced from the active site. The aniline portion of the compound arches around the mutant gatekeeper Met 790, and the crotonamide group forms the expected covalent bond with Cys 797 at the edge of the active site cleft.
4. An unexpected twist to the mechanism by which T790M confers resistance
The discovery of a secondary mutation in the EGFR kinase as a cause of acquired resistance to gefitinib and erlotinib – substitution of Thr790 with methionine – was something of a déjà vu experience for those who follow the clinical application of kinase inhibitors. Thr 790 is the so-called gatekeeper residue, which lies at the back of the ATP binding pocket. The description of this residue as the gatekeeper stems from the observation among kinase drug developers that this residue is a major determinant of inhibitor specificity. In kinases that have a small gatekeeper residue such as threonine, a hydrophobic pocket that extends from the ATP site is readily accessible, whereas in kinases with a large gatekeeper residue, access to this pocket is impeded. Clinical resistance to TKIs conferred by mutation of the gatekeeper residue has been observed in a number of kinases, most famously the T315I mutation in BCR-Abl, which confers resistance to imatinib and other Abl inhibitors in the course of treatment of chronic myelogenous leukemia [32, 33]. The corresponding mutation has also been observed clinically in gastrointestinal stromal tumors (GIST) treated with imatinib (the T670I mutation in c-Kit) [34] and in hypereosinophilic syndrome (T674I in PDGFRα) [35]. Thus the discovery of the T790M mutation in EGFR led many to assume that the mutation would sterically block drug binding, as had been observed for the corresponding gatekeeper mutations in Abl and c-Kit.
One clue that the mechanism was not so straightforward was the observation that while T790M conferred resistance to the reversible inhibitors gefitinib and erlotinib, it did not confer resistance to a number of irreversible inhibitors [11, 36–39]. These irreversible compounds have a “core” that is similar to that of the reversible anilinoquinazolines, but additionally contain a Michael acceptor that is posed to react with a cysteine residue (Cys 797) at the edge of the active site cleft when the compound binds the kinase. In particular, the compound EKB-569 has exactly the same aniline substituent as gefitinib (Figure 2). Although the aniline group is the portion of the inhibitor that extends into the gatekeeper pocket, EKB-569 maintains activity against the T790M mutant [36]. This observation suggested that the mutation does not directly block inhibitor binding. Indeed, our direct binding assay revealed that the L858R/T790M maintains low nanomolar binding to gefitinib and AEE788 (Table 1, ref [17]).
The crystal structure of the T790M mutant in complex with AEE788 shows how the compound can be accommodated in the context of the larger gatekeeper residue. A superposition of the unliganded T790M mutant with the AEE788 complex is shown in Figure 4C. This comparison shows that an alternate sidechain rotamer of Met 790 allows for binding of the compound with little loss of affinity as compared with the WT or L858R kinase. We have also determined the structure of the HKI-272 compound in complex with the T790M mutant (Figure 4D). This structure revealed a binding mode in the mutant that is essentially identical to that expected with this compound in the context of a WT threonine gatekeeper residue. In this structure, the kinase adopts the inactive conformation, with the C-helix displaced. The covalent bond with Cys 797 formed by the crotonamide group of this irreversible inhibitor is also observed in the structure [17].
As the structures and binding studies show, altered affinity of the T790M mutants for inhibitor is not sufficient to explain the profound clinical resistance to gefitinib and erlotinib. Of course affinity for drug is only one part of the equation when considering the potency of an ATP-competitive compound. As alluded to above, affinity for ATP can be equally important in a regime where ATP concentrations are two to three orders of magnitude above Km[ATP]. Indeed, increased affinity of the L858R/T790M mutant for ATP appears to be the major factor in conferring resistance to reversible ATP-competitive inhibitors. As shown in Table 1, the T790M mutation decreases the Km for ATP to 8µM, comparable to that of the WT EGFR. In comparing the potency of gefitinib on the very sensitive L858R mutant versus the resistance L858R/T790M mutant, the combination of the ~4 fold decrease in affinity for gefitinib (2.5 nM in L858R vs. 10 nM for L858R/T790M) and the ~18 fold increase in affinity for ATP can be expected to dramatically decrease the potency of the drug at cellular concentrations of ATP, which are on the order of ~1mM (Figure 5) [17].
Figure 5. The increased ATP affinity of the L858R/T790M mutant leads to gefitinib resistance at cellular concentrations of ATP.
A, The calculated potency (Kiapp) of gefitinib for the L858R and L858R/T790M mutants is plotted versus ATP concentration according to the equation Kiapp=Ki (1 + [S]/Km[ATP]), where [S] is the concentration of ATP. We set Ki = Kd as measured in our direct binding assays (see text). The drug resistant mutant is expected to lose potency as ATP concentrations approach the ~1 mM level found in the cell. B and C, experimental inhibition curves for the L858R and L858R/T790M mutants, respectively. The in vitro kinase activity of each mutant was measured in the presence of the indicated concentration of gefitinib at either 0.01 mM ATP (black squares) or 1.0 mM ATP (grey circles). The vertical arrows indicate the approximate serum concentration of gefitinib (~ 1µM) that is achievable in patients. (Figure adapted from Yun et al. [17])
5. Conclusions and Perspectives
Collectively, the studies we review here show that modulation of the inhibitor affinity and ATP affinity (or more precisely, the Km for ATP) of the EGFR kinase by the various lung cancer mutations dictates their sensitivity, and indeed their clinical responsiveness to currently available TKIs directed against the EGFR. The dose-limiting toxicities of EGFR TKIs, primarily skin rash and diarrhea, are thought to stem at least in part from inhibition of WT EGFR in the skin and GI tract. Thus the mutations create a “therapeutic window”, the difference in the drug concentrations required for a therapeutic versus toxic effect, by rendering the NSCLC mutants as much as two orders of magnitude more sensitive to gefitinib or erlotinib that the WT EGFR kinase [3, 11, 40]. (Note that this is true for the L858R, G719S and deletion mutants that have been studied to date, but not true for the exon20 insertion mutants, which are intrinsically drug resistant.) The T790M mutation closes this therapeutic window by restoring the ATP–affinity of the L858R mutant to essentially the level of the WT kinase.
Although current irreversible inhibitors maintain the ability to inhibit both the L858R/T790M and Del19/T790M mutants, they can be expected to inhibit the WT EGFR with equal or even greater potency. For this reason, it appears unlikely that current anilinoquinazoline-based irreversible inhibitors will succeed clinically in patients who develop resistance in the course of treatment with gefitinib or erlotinib. Effective treatment of tumors bearing the T790M mutation will likely require development of a new class of agents, reversible or irreversible, that are more potent against the T790M–bearing mutants than against the WT EGFR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 5.Janne PA, Johnson BE. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2006;12 doi: 10.1158/1078-0432.CCR-06-0555. 4416s–4420s. [DOI] [PubMed] [Google Scholar]
- 6.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28 Suppl 1:S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SK, Gullick WJ, Hill ME. Mutations of the epidermal growth factor receptor in non-small cell lung cancer -- search and destroy. Eur J Cancer. 2006;42:17–23. doi: 10.1016/j.ejca.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 9.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 10.Arao T, Fukumoto H, Takeda M, Tamura T, Saijo N, Nishio K. Small in-frame deletion in the epidermal growth factor receptor as a target for ZD6474. Cancer Res. 2004;64:9101–9104. doi: 10.1158/0008-5472.CAN-04-2360. [DOI] [PubMed] [Google Scholar]
- 11.Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR, Meyerson M. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, Peyton M, Juroske D, Huang Y, Stuart Salmon J, Kim YH, Pollack JR, Yanagisawa K, Gazdar A, Minna JD, Kurie JM, Carbone DP. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- 13.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, Tsuboi M, Tada H, Kuwano H, Mitsudomi T. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12:5764–5769. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 15.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 16.Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, Sharma SV, Brannigan BW, Mohapatra G, Settleman J, Haber DA. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 17.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, Classon M, Haber DA, Settleman J. A common signaling cascade may underlie "addiction" to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazdar AF, Shigematsu H, Herz J, Minna JD. Mutations and addiction to EGFR: the Achilles 'heal' of lung cancers? Trends Mol Med. 2004;10:481–486. doi: 10.1016/j.molmed.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Qiu C, Tarrant MK, Boronina T, Longo PA, Kavran JM, Cole RN, Cole PA, Leahy JD. In vitro enzymatic characterization of near full length EGFR in activated and inhibited states. Biochemistry. 2009;48:6624–6632. doi: 10.1021/bi900755n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, Park F, Haley JD, Gibson N, Sliwkowski MX. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 23.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Mulloy R, Ferrand A, Kim Y, Sordella R, Bell DW, Haber DA, Anderson KS, Settleman J. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–2330. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- 26.Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem. 2002;277:46265–46272. doi: 10.1074/jbc.M207135200. [DOI] [PubMed] [Google Scholar]
- 27.Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K, Alligood KJ, Rusnak DW, Gilmer TM, Shewchuk L. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 28.Lemmon MA. Ligand-induced ErbB receptor dimerization. Exp Cell Res. 2009;315:638–648. doi: 10.1016/j.yexcr.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Red Brewer M, Choi SH, Alvarado D, Moravcevic K, Pozzi A, Lemmon MA, Carpenter G. The juxtamembrane region of the EGF receptor functions as an activation domain. Mol Cell. 2009;34:641–651. doi: 10.1016/j.molcel.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26:1742–1751. doi: 10.1200/JCO.2007.12.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 33.Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, Lai JL, Philippe N, Facon T, Fenaux P, Preudhomme C. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–1018. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- 34.Tamborini E, Bonadiman L, Greco A, Albertini V, Negri T, Gronchi A, Bertulli R, Colecchia M, Casali PG, Pierotti MA, Pilotti S. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:294–299. doi: 10.1053/j.gastro.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, Cross NC, Tefferi A, Malone J, Alam R, Schrier SL, Schmid J, Rose M, Vandenberghe P, Verhoef G, Boogaerts M, Wlodarska I, Kantarjian H, Marynen P, Coutre SE, Stone R, Gilliland DG. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 36.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR, Fidias P, Lynch TJ, Rabindran SK, McGinnis JP, Wissner A, Sharma SV, Isselbacher KJ, Settleman J, Haber DA. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 38.Carter TA, Wodicka LM, Shah NP, Velasco AM, Fabian MA, Treiber DK, Milanov ZV, Atteridge CE, Biggs WH, 3rd, Edeen PT, Floyd M, Ford JM, Grotzfeld RM, Herrgard S, Insko DE, Mehta SA, Patel HK, Pao W, Sawyers CL, Varmus H, Zarrinkar PP, Lockhart DJ. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A. 2005;102:11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sequist LV. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Oncologist. 2007;12:325–330. doi: 10.1634/theoncologist.12-3-325. [DOI] [PubMed] [Google Scholar]
- 40.Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z, Cantley LC, Tenen DG, Johnson BE, Janne PA. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]