Abstract
Objective
To determine intrafollicular hormone levels and characterize mRNA expression of the IGF receptors, IGF binding proteins (IGFBP), and pregnancy-associated plasma protein-A (PAPP-A) in granulosa cells before and after an ovulatory hCG stimulus.
Design
Experimental animal study.
Setting
Academic medical center.
Animal(s)
Adult rhesus macaques.
Intervention(s)
Animals received exogenous FSH to promote the development of multiple preovulatory follicles. Follicles were aspirated before (0 hr), 3, 6, 12, or 24 hr after an ovulatory hCG bolus.
Main Outcome Measure(s)
IGF1, IGF2, and insulin levels in follicular fluid were determined by radioimmunoassay. Messenger RNA (mRNA) levels in granulosa cells were determined by real-time RT-PCR. IGFBPs and PAPP-A in follicular fluid were determined by Western blot analysis and ELISA.
Result(s)
IGF1, IGF2 and insulin in follicular fluid did not change during luteinization. IGF1R, IGFBP1 and IGFBP2 mRNAs were unchanged by hCG. IGF2R, IGFBP3, 5, 6 and PAPP-A mRNAs increased following hCG, while insulin receptor and IGFBP4 mRNAs decreased following hCG treatment. IGFBP 3 and 6 and PAPP-A protein increased following hCG.
Conclusion(s)
Dynamic changes in the expression of the IGFBPs and PAPP-A suggest tight regulation of IGF action during ovulation and corpus luteum formation.
Keywords: luteinization, granulosa cell, IGF, IGFBP, primate
INTRODUCTION
Luteinization of the primate follicle is associated with rapid and dramatic changes in steroidogenesis and differentiation of granulosa cells (1–5). The insulin and IGF families are closely associated with granulosa cell proliferation and differentiation, and may represent key signals in the development of the corpus luteum (6). However, the difficulty in obtaining non-luteinized (pre-LH/hCG) samples in humans and primates has precluded a systematic study of the expression of the insulin and IGF systems during the periovulatory interval in the non-human primate.
The IGF/IGFBP family is comprised of IGF1 and IGF2, IGF1R and IGF2R/mannose 6-phosphate receptor, and at least six IGF binding proteins (IGFBP1–6) with insulin and insulin receptor (IR) forming a closely related signaling system (7–10). IGF1R and insulin receptor are receptor tyrosine kinases signaling predominantly through AKT1-mediated pathways to stimulate cell proliferation, survival and steroidogenesis (11–13) while IGF2R is a candidate tumor suppressor that has been postulated to be important in IGF2 turnover, angiogenesis, and other intra-cellular events (14,15). IGF1 and IGF2 increase proliferation and steroidogenesis in IVF-derived human granulosa cells and bovine preovulatory granulosa cells (16–18).
IGF activity is regulated on several levels: gene expression, receptor expression and the presence of the IGFBPs (14). IGFBPs have a higher affinity for IGFs than does either IGF receptor and thus are capable of binding and inactivating circulating IGFs by preventing interaction with the IGF receptors (8). The IGFBPs also have actions independent of IGFs, including suppression of human granulosa cell steroid synthesis by IGFBP4 (19,20). IGFBPs are regulated at the transcriptional level and through proteolytic degradation, most notably by pregnancy-associated plasma protease-A (PAPP-A) (8,21).
All six IGFBPs are expressed in non-human primate or human follicles at varying stages of development (13,22–28). Despite this, there are no data demonstrating changes in either insulin or the IGF system during luteinization in non-human primates or women. This study seeks to describe follicular fluid concentrations of IGF1, IGF2, and insulin, the mRNA expression of IGF1 and IGF2, the IGF receptors, and granulosa cell mRNA and follicular fluid protein levels of PAPP-A and IGFBP1–6 before and up to 24 hours after an ovulatory hCG bolus given to rhesus monkeys undergoing controlled ovarian stimulation protocols.
MATERIALS AND METHODS
Animals
Adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC) as described (29). Animal protocols and experiments were approved by the University of California, Davis Animal Care and Use Committee, and studies were conducted in accordance with Guide for the Care and Use of Laboratory Animals. Following the onset of menstruation, adult female rhesus monkeys were treated with recombinant human follicle-stimulating hormone for 7 days (r-hFSH, Organon, West Orange NJ, USA; 37.5 IU i.m. bid). The GnRH antagonist Antide (Ares-Serono, Randolph MA, USA, 5 mg/kg body weight, s.c. sid) was administered on days 6–7 to prevent endogenous gonadotropin secretion. Follicles were aspirated the morning after the last dose of r-hFSH by an ultrasound guided procedure (29). The characteristics of the follicular cohort in this model have been described elsewhere (30). The resulting cells are referred to as non-luteinized granulosa cells (NLGC). A subset of animals received an ovulatory bolus of r-hCG on the morning of day 8 and follicles were aspirated before (0h) 3, 6, 12 and 24 hr after hCG (n 3–5/time point). Aspirates represented the pooled contents of multiple follicles from each animal. These were maintained at approximately 35 C within a temperature controlled isolette containing a fixed volume of media at all times. Cumulus oocyte complexes (COC) were removed by transferring the mixture of media, follicular fluid and cellular aspirate to a 24 mm diameter, 70 μm filter (Netwell inserts, #3479, Corning, Inc., Acton, MA, USA); granulosa cells were filtered through the mesh and COC retained. To collect granulosa cells for the corresponding time points, the tube was rinsed with fresh tyrode lactate (TL)-HEPES/polyvinlalcohol (PVA) medium (TL-HEPES/0.1 mg/ml PVA) that was also poured through the filter. The filter was further rinsed with fresh TL-HEPES-PVA medium until blood cells were removed. The rinse from the filter was saved for recovery of granulosa cells for various experiments (see below).
Preparation of macaque granulosa cells
Granulosa cells were recovered from the filter rinse by a modification of the method previously described (31). Briefly, the cell suspension was centrifuged for 5 min at 300g to pellet the red cells; speed was increased to 500g for an additional 5 min, resulting in a thin layer of granulosa cells over the red cell pellet. The supernatant was removed, and the layer of granulosa cells was transferred to a 40% Percoll gradient in medium 199 (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged for 30 min at 500g. The supernatant was removed, and the granulosa cells were recovered from the surface of the Percoll with a Pasteur pipette and washed once with TL-HEPES/PVA before being counted on a hemocytometer. Cells were pelleted and snap frozen for shipment from CNPRC to the University of Maryland Baltimore and stored upon arrival at −80 C pending RNA isolation.
Preparation of macaque follicular fluid
The dilution of each sample was determined by assessing the final volume of the follicular fluid diluted into 1 ml of media. Follicular fluids were stored at −80 C until use.
Radioimmunoassay
IGF1 was measured using a coated-tube radioimmunoassay (Alpco Diagnostics, Salem, NH), and IGF2 by the double-antibody RIA (Alpco Diagnostics). Assay sensitivities were <0.1 ng/ml. Samples were acidified per manufacturer’s instructions to liberate IGFs bound to IGFBPs. Insulin was measured using a coated-tube RIA (Siemens Medical Solutions Diagnostics, Deerfield, IL).
Real time RT-PCR
Total RNA was extracted from cells using RNAqueous Micro kit (Ambion Inc, Austin, TX) and reverse transcribed using MMLV reverse transcriptase (Invitrogen). All probes and primers, including the internal control ribosomal protein L19 (RPL19) were synthesized by Applied Biosystems (Foster city, CA). The target gene and the RPL19 were detected in the same reaction. Semi-quantitative real time experiments using Assays-on-Demand Gene Expression products were also performed according to manuafacturer instruction (Applied Biosystems). The PCR protocol consisted of 40 cycles of denaturing at 95 C for 15 s and annealing/extending at 60 C for 1 min per cycle. Detection of the gene expression was performed during the 2nd step in a two step RT-PCR protocol. To relatively quantify mRNA levels, a standard curve was constructed using pooled macaque granulosa cell cDNA. Data were analyzed as the inverse log ([Ct-Y intercept]/slope of the standard curve) and expressed as a ratio of the target gene to endogenous control (32). Primer and probe sequences are shown in Table 1.
Table 1.
Gene detection primer sequences
| Gene Name | Sequence |
|---|---|
| RPL19 | Forward: CCCCAATGAGACCAATGAAATC Reverse: CAGCCCATCTTTGATGAGCTT Probe: ATGCCAACTCCCGTCAGCAGATC |
| IGF1 | Assay-on-Demand (Applied biosystems) |
| IGF2 | Assay-on-Demand (Applied biosystems) |
| IGF1R | Forward: TGAAAGTGACGTCCTGCATTTC Reverse: TACCGGGTGCCAGGTTATGAT Probe: CACCACCACGTCGAAGAATCGCA |
| IGF2R | Forward: TTTGAGTGGCGAACGCAGTAT Reverse: CCAGCCCCATCTTTGAATGA Probe: CCTGCCCACCTTTCGATCTGACTGAAT |
| Insulin Rec. | Forward: GGGTCCCTGTCCCAAGGT Reverse: GACGTCACCGAGTCGATGGT Probe: CACCTCCTAGAAGGC * targets IR-A and IR-B |
| IGFBP2 | Forward: GCCCTCTGGAGCACCTCTACT Reverse: TCTTGCACTGTTTGAGGTTGTACA Probe: CATCCCCAACTGTGACAAGCATGGC |
| IGFBP3 | Forward: ACGCACCGGGTGTCTGA Reverse: TGCCCTTTCTTGATGATGATTATC Probe: CCCAAGTTCCACCCCCTCCATTCA |
| IGFBP4 | Forward: ACAACAGCTTCAGCCCCTGTA Reverse: CGAATTTTGGCGAAGTGCTT Probe: CGCCCATGACCGCAGGTGC |
| IGFBP5 | Forward: AGGAGACCTACTCCCCCAAGAT Reverse: TTCTTCACTGCTTCAGCCTTCA Probe: CGGCCCAAACACACCCGCA |
| IGFBP6 | Forward: TGCAGAGGAGAATCCTAAGGAGAGT Reverse: TGGTAGAGGTGCCTGGATTCC Probe: CCAAGCAGGCACTGCCCGC |
| PAPP-A | Assay-on-Demand (Applied biosystems) |
Western Blotting
Follicular fluid samples (1 or 2ul) were mixed with 2x loading buffer and resolved on a 12% separating SDS polyacrylamide gel for 45min–1hr depending on the size of the IGFBP. Kaleidoscope markers (Bio-Rad, Hercules CA) were also resolved to determine IGFBP band sizes. Proteins were transferred to PVDF membrane (Millipore, Bedford MA) for 60–90 min at 100V. Membranes were blocked in 5% non-fat dry milk (Carnation) in Tris-buffered saline with 0.05% Tween (TBST) for I hr at room temperature and then incubated overnight with the primary antibody (1:500 dilution for all IGFBPs) in TBST containing 5% milk. After 3 consecutive washes of 15 min each in TBST, the membrane was incubated with the secondary antibody (1:10,000) in TBST containing 5% milk for 60 min at room temperature. The membrane was washed and proteins were visualized using the Western Blue stabilized substrate (Promega, Madison Wi) according to manufacturer’s directions. Antibodies were purchased from the following sources: IGFBP2 (c-18, sc6001, Santa Cruz Biotechnology, Santa Cruz, CA), IGFBP3 (H-98, sc9028 Santa Cruz), IGFBP4 (c-20, sc-6005 Santa Cruz). IGFBP5 (MA-371-5, Austral Biological, San Ramone CA), IGFBP6 (PB-383-9, Austral Biological). Secondary antibodies were conjugated affinipure Donkey anti-Goat and Goat anti-Rabbit (Jackson ImmunoResearch, West Grove, PA) and anti-mouse alkaline phosphatase conjugate (Promega, Madison, WI).
ELISA
PAPP-A was measured using a solid phase sandwich ELISA (Alpco Diagnostics Salem, NH). Assay sensitivity was < 0.133 ug/ml.
Statistical Analysis
Data are presented as mean ± SEM. Bartlett’s χ2 was used to test for heterogeneity of variance, and data were logarithmically transformed (log+2) if variances were significantly different. Data were analyzed by one-way ANOVA followed by Student Newman-Keuls means comparison test. Means were considered significantly different if p<0.05.
RESULTS
Administration of hCG to rhesus monkeys undergoing controlled ovarian stimulation protocols increased intra-follicular concentrations of progesterone have been previously reported in these samples (32) and increased PR mRNA levels (data not presented), indicating a normal progression through the periovulatory interval.
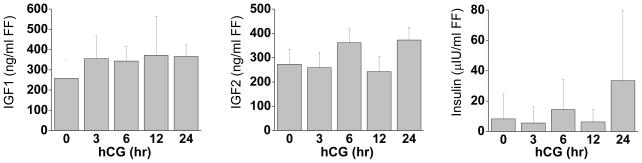
Intra-follicular concentrations of IGF1 and IGF2 were determined by RIA following an acidification step to liberate IGFBP-bound IGFs. Both IGF1 and IGF2 levels in follicular fluid were approximately 250 ng/ml and did not change during luteinization (Fig. 1). Insulin was present at very low levels (<30 μIU/ml) in follicular fluid, and did not change as a result of hCG. Interestingly, insulin was detectable in only 1 of 4 samples prior to hCG, 2/4 at 3 hr, 2/5 at 12 hr, and 6/7 at 24 hr. IGF1 mRNA was not detectable at appreciable levels in any sample, while IGF2 mRNA was present in all samples before and after hCG (data not presented).
Figure 1. Intra-follicular concentrations of IGF1, IGF2, and insulin during luteinization.
IGF1, IGF2, and insulin concentrations were measured using specific radioimmunoassays before (0 hr), 3, 6, 12, and 24 hr after an ovulatory hCG stimulus (n=5, 5, 5, 4, 5). IGF1 and IGF2 samples were acidified prior to assay in order to free IGFs bound to IGFBPs.
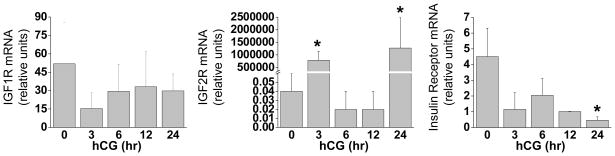
The expression of IGF1 receptor mRNA in granulosa cells did not change with hCG treatment over 24 hr (Fig. 2). However, IGF2 receptor mRNA expression showed a dramatic increase over controls (0 hr) at both 3 and 24 hr post-hCG treatment. Insulin receptor (IR-A and IR-B; (33)) mRNA expression was reduced significantly (p<0.05) 24 hrs after hCG treatment, although a trend of decreasing mRNA expression beginning at 3 hr was evident.
Figure 2. Expression of IGF1R, IGF2R, and Insulin Receptor (IR) mRNAs during luteinization.
Granulosa cells aspirated from rhesus monkeys undergoing controlled ovarian stimulation before (0 hr), 3, 6, 12 and 24 hr following an ovulatory hCG bolus (n=5,5,4,3,3). The mRNA expression of IGF1R (left panel), IGF2R (middle panel) and total insulin receptor (right panel) was determined by real-time RT–PCR. Data were normalized to RPL19 mRNA and expressed as relative units. Asterisk indicates significant differences (P < 0.05) from control (0 hr) samples.
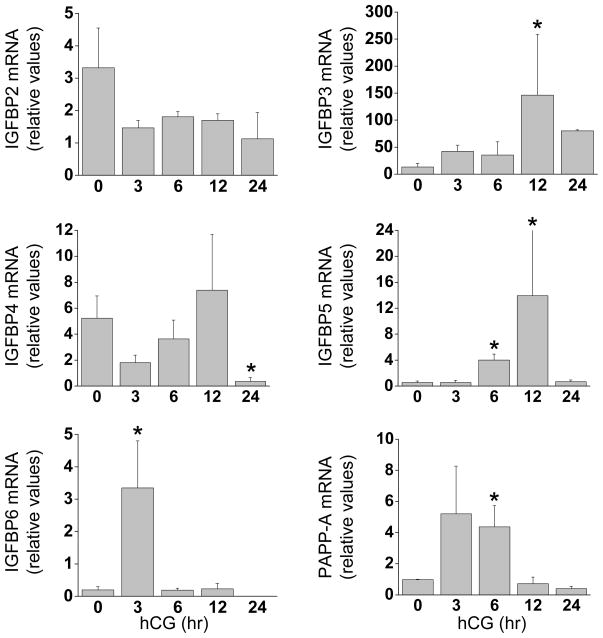
The mRNA expression of IGFBP1–6 was also examined as a part of this study (Fig. 3). Levels of IGFBP1 mRNA were highly variable during luteinization and did not statistically change following hCG (data not presented). IGFBP2 mRNA was present in all samples but did not change following hCG. In contrast, IGFBP3 mRNA increased (11-fold, p<0.05) 12 hr after hCG, while the expression of IGFBP4 declined (15-fold, p<0.05) 24 hr post-hCG. The expression of IGFBP5 mRNA increased at 6 and 12 hr post-hCG relative to controls (7- and 13-fold, respectively; p<0.05). IGFBP6 mRNA increased transiently 3 hr after hCG administration (17-fold, p<0.05).
Figure 3. Granulosa cell expression of IGFBPs during luteinization.
Granulosa cells aspirated from rhesus monkeys undergoing controlled ovarian stimulation before (0 hr), 3, 6, 12 and 24 hr following an ovulatory hCG bolus (n=5,5,4,3,3). The mRNA expression of IGFBP2 (top left panel), IGFBP3 (top right), IGFBP4 (middle left), IGFBP5 (middle right), IGFBP6 (lower left), and PAPP-A (lower right) was determined by real-time RT–PCR. Data were normalized to RPL19 mRNA and expressed as relative units. Asterisk indicates significant differences (P < 0.05) from control (0 hr) samples.
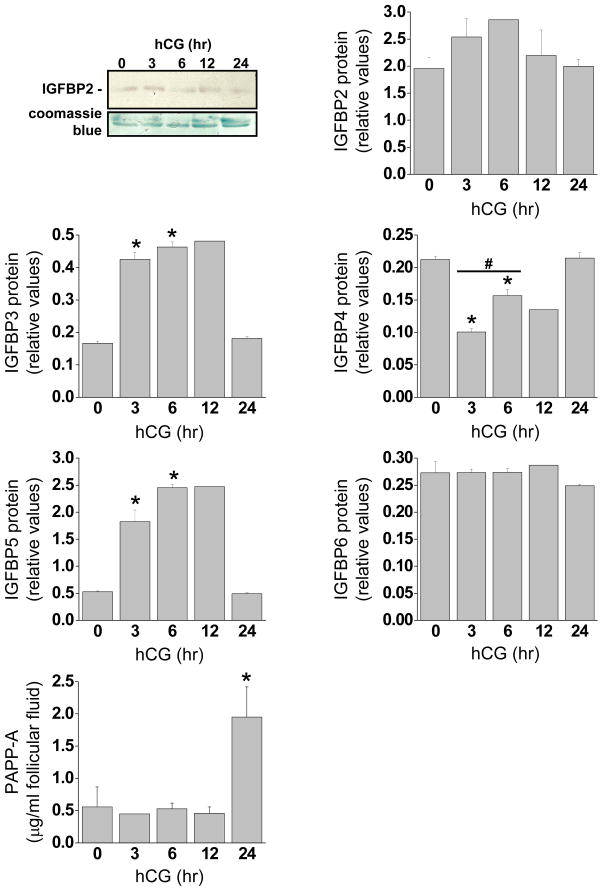
Protein levels of IGFBP 2–6 were determinedusing Western blot (Fig 4). IGFBP2 was present in all samplesand did not change following hCG treatment. In contrast, IGFBP3 increased significantly 3, 6 and 12 hrs post-hCG treatment. IGFBP4 levels decreased significantly at 3, 6 and 12 hrs compared to 0 and 24 hr post-hCG. IGFBP5 protein increased 3, 6 and 12 hr post-hCG. Levels of IGFBP6 protein wereunchanged following hCG.
Figure 4. Follicular fluid levels of IGFBPs and PAPP-A during luteinization.
Follicular fluid was collected from rhesus monkeys undergoing controlled ovarian stimulation before (0 hr), 3, 6, 12 and 24 hr following an ovulatory hCG bolus (N= 3,3,2,3,5). (A) Representative example of a western blot showing the IGFBP2 bands during the time course of hCG treatment, (top panel) normalized to the large proteins remaining in the gel post transfer (bottom panel). (B). The follicular fluid protein profiles of IGFBP2–6 shown in graphical form. The western blots were completed, membranes and gels scanned into a PDF file and IGFBP band density was normalized to coomassie blue stained protein using the program U-SCAN IT (Silk-Scientific Corporation). Asterisk indicates significant differences (P<0.05) from control (0 hr) samples. #, significant differences between 3 and 6 hrs of hCG treatment (IGFBP4). C. follicular fluid levels of PAPP-A determined by ELISA.
Pregnancy-associated plasma protein-A (PAPP-A) is a metalloproteinase that regulates IGF bioavailability through cleavage and inactivation of IGFBPs (22). The expression of PAPP-A mRNA tended to increase 3 hr after hCG (p=0.07) and was 4.4-fold higher (p<0.05) than controls at 6 hr post-hCG (Fig. 3). PAPP-A protein levels determined by ELISA increased significantly 24 hr after an ovulatory hCG bolus (Fig. 4).
DISCUSSION
The objective of this study was to characterize follicular fluid levels of IGF1, IGF2, and insulin, and determine the mRNA and protein expression of the IGF system in granulosa cells and follicular fluid from rhesus monkeys undergoing controlled ovarian stimulation. Intra-follicular concentrations of IGF1 and IGF2 do not change during luteinization, while insulin is present at very low levels. The mRNA and follicular fluid levels of IGF binding proteins (IGFBP) 3, 5, and 6, as well as pregnancy-associated plasma protease-A (PAPP-A), increase following hCG administration. These data suggest tight control of the IGF signal in the luteinizing monkey follicle is exerted through IGFBPs, as well as a possible IGF-independent role for IGFBPs (8).
IGF1 and IGF2
IGFs are important intra-ovarian factors, acting as mitogens, survival factors, and co-gonadotropins supporting steroid synthesis (22). While IGF2 mRNA is expressed by primate granulosa cell, IGF1 mRNA is undetectable, supporting the hypothesis that follicular fluid IGF1 is of seric origin (22,34). However, whether luteinizing theca cells in primates/human contribute to follicular levels remains to be elucidated. The functions of IGF1 and IGF2 are likely diverse, acting as co-gonadotropins to regulate a diversity of processes, including the expression of vascular endothelial growth factor, steroidogenesis, and oocyte maturation (17,18,35,36). Whether IGF1 and IGF2 have roles in periovulatory events distinct from one another, or partially or completely overlapping remains to be determined, but a patient with genetic loss of IGF1 was still capable of spontaneous ovulation (37), suggesting that at least some overlap exists. The levels of IGF1 and 2 determined in the present study, however, represent total rather than bioactive concentrations. The actual IGF signal is predicted to be much lower on the basis of IGFBP expression.
IGF and insulin receptors
The expression of IGF1 receptor mRNA suggests important local roles for the IGFs. Several studies have shown that human and primate granulosa cells express IGF1R and IGF2R mRNA (23,25,38,39), although there are surprisingly few reports of expression in human granulosa cells aspirated after an ovulatory hCG bolus to women undergoing IVF cycles (13). IGF1R can mediate the actions of both IGF1 and IGF2 leading to enhanced steroidogenesis and granulosa cell survival (18), and there is ample evidence to indicate a critical role for IGF1R-mediated signaling through PKB/AKT in granulosa cell function (40). In contrast, most IGF2R protein is intracellular and transports mannose 6-phospate to lysosomes; the limited cell surface protein internalizes IGF2, although there is growing list of potential ligands for this receptor (41). IGF2R is a candidate tumor suppressor (42) and thus the role(s) of this protein leading to ovulation and CL formation are likely very complex. The dynamic nature of IGF2R mRNA expression during luteinization, with distinctive and dramatic peaks at 3 and 24 hr post-hCG, supports the hypothesis that this receptor has important, but undetermined, actions in periovulatory events. However, these changes in IGF2R mRNA are largely driven by very low levels of expression at 0, 6, and 12 hr, and so the consequences of this induction are currently obscure.
The expression of the insulin receptor is reduced 24 hr post-hCG. A role for insulin in normal physiologic processes leading to ovulation and luteal formation is unclear, although insulin has been reported to promote progesterone synthesis in human IVF granulosa cells (14). The low levels of insulin and the declining expression of the insulin receptor may suggest a more limited role in periovulatory events.
IGFBPs
The IGFBPs form a group of proteins that bind IGFs with high affinity and block their interaction with IGF receptors (8). IGFBP1–6 have all been variously reported to be expressed in human IVF-derived granulosa cells and the primate ovary (15,22,23), although the current data are the first to systematically evaluate expression over the course of the ovulatory cascade and luteal formation. While the mRNA expression of IGFBP1–6 is diverse and consistent with complex regulation, there is a generalized increase in mRNA levels of IGFBP3, 5, and 6 during the first 12 hr following hCG, with a concomittent increase in protein for IGFBP3 and 5 in the follicular fluid. This suggests a corresponding reduction in IGF bioavailability facilitating the earliest steps in granulosa cell luteinization. Interestingly, a reduction in mRNA levels of IGFBP4, 5, and 6 occurs at 24 hr as well as a decrease in IGFBP 3 and 5 follicular fluid protein,, consistent with the hypothesis that IGFs are important in luteal steroidogenesis and survival, as well supporting oocyte maturation and embryo development (35,43). The fact that protein levels increase in follicular fluid before observable increases granulosa cell mRNA levels suggests that vesicle-stored IGFBPs in granulosa cells are rapidly secreted after hCG. As the contents of the vesicles are depleted, follicular fluid concentrations decline until new granulosa cell synthesis occurs (new ref: J. Endocrinol. 2001 Feb;168(2):307–315). Alternatively, the rapid increase in follicular fluid levels of IGFBP3 and 5 could originate from serum through increasing vascular permeability associated with luteal formation, although it is unlikely that sufficient changes in the local vascular network occur within the first three hours following hCG to support the observed increases (44,45). The expression of PAPP-A mRNA also increases rapidly following hCG, while PAPP-A protein is highest at 24 hr of hCG treatment. This may represent an additional means to control IGFBP activity.
In summary, high concentrations of total IGF1 and IGF2 are present before and after an ovulatory stimulus in rhesus macaques undergoing controlled ovarian stimulation cycles. IGF1R mRNA is expressed by granulosa cells before and after an ovulatory hCG bolus, while IGF2R is strongly induced by hCG and insulin receptor declines. There is dynamic regulation of the IGFBP mRNAs and follicular fluid protein levels, with a generalized increase in expression apparent during the first 12 hr following hCG. Overall, these data suggest a complex regulation of IGF-I and IGF-II activity during the periovulatory interval and point to a potentially important role for these signals leading to ovulation and corpus luteum formation in primates.
Acknowledgments
The authors would like to thank Jennifer Edwards, Cristy Garmin and Jennifer Scrafford for technical assistance (Department of Biology, Loyola College in Maryland), and George Chaffin for help with laboratory set up. They are grateful to Organon Inc., West Orange, NJ, USA, for the generous supply of recombinant human FSH and Serono laboratories (Ares Advanced Technology), Randolph, MA, USA, for the gift of recombinant hCG.
This research was supported in part by NIH HD043358 (CLC), RR13439 (CAV), RR00169 (CNPRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jirawatnotai S, Moons DS, Stocco CO, Franks R, Hales DB, Gibori G, et al. The cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem. 2003;278:17021–17027. doi: 10.1074/jbc.M301206200. [DOI] [PubMed] [Google Scholar]
- 2.Chaffkin LM, Luciano AA, Peluso JJ. Progesterone as an autocrine/paracrine regulator of human granulosa cell proliferation. J Clin Endocrinol Metab. 1992;75:1404–1408. doi: 10.1210/jcem.75.6.1464640. [DOI] [PubMed] [Google Scholar]
- 3.Chaffkin LM, Luciano AA, Peluso JJ. The role of progesterone in regulating human granulosa cell proliferation and differentiation in vitro. J Clin Endocrinol Metab. 1993;76:696–700. doi: 10.1210/jcem.76.3.8445028. [DOI] [PubMed] [Google Scholar]
- 4.Chaffin CL, Stouffer RL. Local role of progesterone in the ovary during the periovulatory interval. Rev Endocr Metab Disord. 2002;3:65–72. doi: 10.1023/a:1012704903128. [DOI] [PubMed] [Google Scholar]
- 5.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–940. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez ER, Hurwitz A, Vera A, Pellicer A, Adashi EY, LeRoith D, et al. Expression of the genes encoding the insulin-like growth factors and their receptors in the human ovary. J Clin Endocrinol Metab. 1992;74:419–425. doi: 10.1210/jcem.74.2.1309838. [DOI] [PubMed] [Google Scholar]
- 7.Bach LA, Headey SJ, Norton RS. IGF-binding proteins--the pieces are falling into place. Trends Endocrinol Metab. 2005;16:228–234. doi: 10.1016/j.tem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Clay Bunn R, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends in Endocrinology and Metabolism. 2003;14:176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 9.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 10.Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–835. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 11.Adashi EY, Resnick CE, Hurwitz A, Ricciarelli E, Hernandez ER, Roberts CT, et al. Insulin-like growth factors: the ovarian connection. Hum Reprod. 1991;6:1213–1219. doi: 10.1093/oxfordjournals.humrep.a137514. [DOI] [PubMed] [Google Scholar]
- 12.Giudice LC. Insulin-like growth factors and ovarian follicular development. Endocr Rev. 1992;13:641–669. doi: 10.1210/edrv-13-4-641. [DOI] [PubMed] [Google Scholar]
- 13.Chang SY, Tsai MY, Huang FJ, Kung FT. Expression of insulin-like growth factor (IGF), IGF receptor, and IGF-binding protein messenger ribonucleic acids in luteinized granulosa cells from different size follicles after controlled ovarian hyperstimulation. J Assist Reprod Genet. 2002;19:121–126. doi: 10.1023/A:1014732704218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 15.Giudice LC. Insulin-like growth factor family in Graafian follicle development and function. J Soc Gynecol Investig. 2001;8:S26–29. doi: 10.1016/s1071-5576(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 16.Di Blasio AM, Vigano P, Ferrari A. Insulin-like growth factor-II stimulates human granulosa-luteal cell proliferation in vitro. Fertil Steril. 1994;61:483–487. doi: 10.1016/s0015-0282(16)56580-7. [DOI] [PubMed] [Google Scholar]
- 17.Devoto L, Christenson LK, McAllister JM, Makrigiannakis A, Strauss JF., 3rd Insulin and insulin-like growth factor-I and -II modulate human granulosa-lutein cell steroidogenesis: enhancement of steroidogenic acute regulatory protein (StAR) expression. Mol Hum Reprod. 1999;5:1003–1010. doi: 10.1093/molehr/5.11.1003. [DOI] [PubMed] [Google Scholar]
- 18.Spicer LJ, Aad PY. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod. 2007;77:18–27. doi: 10.1095/biolreprod.106.058230. [DOI] [PubMed] [Google Scholar]
- 19.Mason HD, Cwyfan-Hughes S, Holly JM, Franks S. Potent inhibition of human ovarian steroidogenesis by insulin-like growth factor binding protein-4 (IGFBP-4) J Clin Endocrinol Metab. 1998;83:284–287. doi: 10.1210/jcem.83.1.4630. [DOI] [PubMed] [Google Scholar]
- 20.Wright RJ, Holly JM, Galea R, Brincat M, Mason HD. Insulin-like growth factor (IGF)-independent effects of IGF binding protein-4 on human granulosa cell steroidogenesis. Biol Reprod. 2002;67:776–781. doi: 10.1095/biolreprod.101.001511. [DOI] [PubMed] [Google Scholar]
- 21.Firth SM, Baxter RC. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 22.Mazerbourg S, Bondy CA, Zhou J, Monget P. The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles? a comparative species study. Reprod Domest Anim. 2003;38:247–258. doi: 10.1046/j.1439-0531.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 23.Arraztoa JA, Monget P, Bondy C, Zhou J. Expression patterns of insulin-like growth factor-binding proteins 1, 2, 3, 5, and 6 in the mid-cycle monkey ovary. J Clin Endocrinol Metab. 2002;87:5220–5228. doi: 10.1210/jc.2002-020407. [DOI] [PubMed] [Google Scholar]
- 24.Cwyfan Hughes S, Mason HD, Franks S, Holly JM. Modulation of the insulin-like growth factor-binding proteins by follicle size in the human ovary. J Endocrinol. 1997;154:35–43. doi: 10.1677/joe.0.1540035. [DOI] [PubMed] [Google Scholar]
- 25.el-Roeiy A, Chen X, Roberts VJ, LeRoith D, Roberts CT, Jr, Yen SS. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab. 1993;77:1411–1418. doi: 10.1210/jcem.77.5.8077342. [DOI] [PubMed] [Google Scholar]
- 26.el-Roeiy A, Chen X, Roberts V, Shimasakai S, Ling N, LeRoith D, et al. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1–6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab. 1994;78:1488–1496. doi: 10.1210/jcem.78.6.7515389. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Bondy C. Anatomy of the human ovarian insulin-like growth factor system. Biol Reprod. 1993;48:467–482. doi: 10.1095/biolreprod48.3.467. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Wang J, Penny D, Monget P, Arraztoa JA, Fogelson LJ, et al. Insulin-like growth factor binding protein 4 expression parallels luteinizing hormone receptor expression and follicular luteinization in the primate ovary. Biol Reprod. 2003;69:22–29. doi: 10.1095/biolreprod.102.009191. [DOI] [PubMed] [Google Scholar]
- 29.VandeVoort CA, Tarantal AF. The macaque model for in vitro fertilization: superovulation techniques and ultrasound-guided follicular aspiration. J Med Primatol. 1991;20:110–116. [PubMed] [Google Scholar]
- 30.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology. 2003;59:699–707. doi: 10.1016/s0093-691x(02)01129-9. [DOI] [PubMed] [Google Scholar]
- 31.Stewart DR, Vandevoort CA. Simulation of human luteal endocrine function with granulosa lutein cell culture. J Clin Endocrinol Metab. 1997;82:3078–3083. doi: 10.1210/jcem.82.9.4240. [DOI] [PubMed] [Google Scholar]
- 32.Fru KN, Vandevoort CA, Chaffin CL. Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod. 2006;75:568–574. doi: 10.1095/biolreprod.106.053470. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi Y, Flier J, Benecke H, Ransil B, Moller D. Ligand-binding properties of the two isoforms of the human insulin receptor. Endocrinology. 1993;132:1132–1138. doi: 10.1210/endo.132.3.8440175. [DOI] [PubMed] [Google Scholar]
- 34.Giudice LC. Insulin-like growth factor family in Graafian follicle development and function. Journal of the Society for Gynecologic Investigation. 2001;8:S26–S29. doi: 10.1016/s1071-5576(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang TH, Chang CL, Wu HM, Chiu YM, Chen CK, Wang HS. Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil Steril. 2006;86:1392–1401. doi: 10.1016/j.fertnstert.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Chequer JC, Stouffer RL, Hazzard TM, Patton PE, Molskness TA. Insulin-like growth factors-1 and -2, but not hypoxia, synergize with gonadotropin hormone to promote vascular endothelial growth factor-A secretion by monkey granulosa cells from preovulatory follicles. Biol Reprod. 2003;68:1112–1118. doi: 10.1095/biolreprod.102.011155. [DOI] [PubMed] [Google Scholar]
- 37.Dor J, Ben-Shlomo I, Lunenfeld B, Pariente C, Levran D, Karasik A, et al. Insulin-like growth factor-I (IGF-I) may not be essential for ovarian follicular development: evidence from IGF-I deficiency. J Clin Endocrinol Metab. 1992;74:539–542. doi: 10.1210/jcem.74.3.1740488. [DOI] [PubMed] [Google Scholar]
- 38.el-Roeiy A, Chen X, Roberts VJ, Shimasakai S, Ling N, LeRoith D, et al. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1–6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab. 1994;78:1488–1496. doi: 10.1210/jcem.78.6.7515389. [DOI] [PubMed] [Google Scholar]
- 39.Qu J, Godin PA, Nisolle M, Donnez J. Expression of receptors for insulin-like growth factor-I and transforming growth factor-{beta} in human follicles. Mol Hum Reprod. 2000;6:137–145. doi: 10.1093/molehr/6.2.137. [DOI] [PubMed] [Google Scholar]
- 40.Grimberg A. Mechanisms by which IGF-I may promote cancer. Cancer Biol Ther. 2003;2:630–635. [PMC free article] [PubMed] [Google Scholar]
- 41.Scott CD, Firth SM. The role of the M6P/IGF-II receptor in cancer: tumor suppression or garbage disposal? Horm Metab Res. 2004;36:261–271. doi: 10.1055/s-2004-814477. [DOI] [PubMed] [Google Scholar]
- 42.Hebert E. Mannose-6-phosphate/insulin-like growth factor II receptor expression and tumor development. Biosci Rep. 2006;26:7–17. doi: 10.1007/s10540-006-9002-3. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura Y, Ando M, Nagamatsu S, Iwashita M, Adachi T, Sueoka K, et al. Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biol Reprod. 1996;55:152–160. doi: 10.1095/biolreprod55.1.152. [DOI] [PubMed] [Google Scholar]
- 44.Fraser H. Regulation of the ovarian follicular vasculature. Reproductive Biology and Endocrinology. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaffin CL, Stouffer RL. Role of gonadotrophins and progesterone in the regulation of morphological remodelling and atresia in the monkey peri-ovulatory follicle. Hum Reprod. 2000;15:2489–2495. doi: 10.1093/humrep/15.12.2489. [DOI] [PubMed] [Google Scholar]