Abstract
CD4+ T cell responses and macrophage activation are essential components of schistosome egg-induced granuloma formation. Previous studies implicated tumour necrosis factor (TNF) as a potential mediator of macrophage recruitment and activation during schistosome infection. Here we demonstrate that signalling by TNF and its receptors can influence granuloma formation, but is ultimately dispensable for granuloma formation in this system. However, we identify a previously unrecognised role for TNF in limiting hepatocellular damage in response to schistosome eggs. Further, we show that this activity of TNF is independent of TNF receptors (TNFR1 and TNFR2). Taken together, these data suggest that additional, as yet unrecognised receptors exist for TNF and that these receptors are capable of mediating important pathological effects in the liver. Finally, we provide evidence that TNF plays an unexpected role in maintaining adult schistosome viability in the portal system.
Keywords: Schistosome, Schistosoma, Granuloma, Cytokines, Cytokine receptors, Tumour necrosis factor
1. Introduction
Infection by trematode parasites of the genus Schistosoma induces severe tissue and organ damage in infected hosts. The pathology associated with schistosomiasis is largely attributed to the intense granulomatous reactions and subsequent fibrosis induced by parasite eggs that become trapped in host organs such as the liver and intestine. There is also evidence that eggs themselves cause tissue damage by elaborating toxins, as a failure to encapsulate eggs within granulomas results in damage to surrounding tissues, with accompanying morbidity and mortality (Amiri et al., 1992; Cheever et al., 1993). Egg-induced granulomas thus represent a compromise between limiting tissue damage and prolonging survival, sacrificing long-term integrity of tissue architecture for prolonged survival in the short term. Antibody responses to soluble egg factors may also contribute to protecting host tissues from damage by egg toxins (Murare et al., 1992).
Granulomatous inflammation is defined by the presence of numerous activated macrophages that differentiate into epithelioid and giant cells and perform specialised barrier functions. In other infectious diseases where granulomas form the primary defence against a persistent infectious agent, e.g. mycobacterial infections, the macrophage component of the cellular response is essential for host defence, preventing dissemination of the pathogen and providing a barrier that protects surrounding healthy tissue. This situation exhibits obvious parallels with the granulomatous response to schistosome eggs and indeed, schistosome egg-induced granulomas contain numerous activated macrophages, epithelioid and giant cells, presumably recruited to protect surrounding healthy tissue from egg toxins. Further, granuloma formation in schistosomiasis is dependent on CD4+ T cell responses (Warren et al., 1967; Iacomini et al., 1995; Hernandez et al., 1997), as is the case for Mycobacterium infections (Kaufmann and Ladel, 1994). In both cases, therefore, the primary effector cells are macrophages, while an adaptive immune response is required for effective cellular recruitment and organisation at the site of granuloma formation.
While there is general agreement that CD4+ T cell responses are essential to granuloma formation in schistosomiasis, controversy has surrounded the nature of the Th cell response that is required. In Mycobacterium infections, the situation appears straightforward: IFN-γ and tumour necrosis factor (TNF) are essential for effective intracellular killing and containment of mycobacteria by macrophages in vivo (Kindler et al., 1989; Cooper et al., 1993; Flynn et al., 1993, 1995), and emergence of type 1 T cells that express these cytokines is therefore appropriate. In murine models of schistosomiasis however, the situation is complicated by the fact that schistosome eggs and egg-derived antigens are potent and independent inducers of type 2 T cell responses (Grzych et al., 1991; Vella and Pearce, 1992). Induction of type 2 responses by parasite eggs after the onset of oviposition thus accounts for a skewing of systemic T cell responses during schistosome infection, from a type 1 response during prepatency to a type 2 response by 8 weeks post-infection (p.i.; Pearce et al., 1991). Granuloma formation therefore occurs in an environment that is initially proinflammatory and type 1-like, but which subsequently polarises rapidly to one that is predominantly type 2-like. There is now a clear consensus that type 2 responses contribute substantially to mediating formation of egg-induced granulomas, as abrogation of type 2 responses by ablation of STAT6 (Kaplan et al., 1998) or IL-4 receptor α (Jankovic et al., 1999) expression greatly reduces granuloma formation. However, molecular mechanisms for how type 2 cytokines activate macrophages for granuloma formation and induce differentiation into epithelioid and giant cells have yet to be identified. Such activities are usually ascribed to proinflammatory signals such as TNF and IFN-γ.
Previous work from our laboratory demonstrated that exogenous TNF alone was sufficient to mediate granuloma formation around schistosome eggs, in the absence of an adaptive immune response (Amiri et al., 1992). However, this work did not address the issue of whether TNF contributes to the CD4+ T cell-mediated granuloma formation observed in hosts with intact adaptive immune systems. In this study, we specifically sought to determine what role TNF plays in schistosome egg-induced granuloma formation by analysing Schistosoma mansoni infections in immunocompetent mice where TNF signalling alone has been specifically disrupted. We also analysed the recently described phenomenon of egg-induced hepatocyte apoptosis (Brunet et al., 1999) in these animals to determine whether TNF is implicated in this process, as TNF signalling via TNFR1 has previously been shown to induce apoptosis of hepatocytes under some circumstances (Leist et al., 1995). Further, we analysed the effects of absolute TNF deficiency on schistosome fecundity, as this cytokine had previously been shown to modulate parasite egg production (Amiri et al., 1992).
2. Materials and methods
2.1. Infection with S. mansoni
Puerto Rican strain S. mansoni was maintained in the laboratory using Biomphalaria glabrata snails and golden hamsters Mesocricetus auratus as intermediate and definitive hosts, respectively (Smithers and Terry, 1965). Cercariae harvested from infected B. glabrata were used to infect age-, sex- and background-matched groups of mice via the tail skin. Three to six mice of each genotype were used per experiment. Cercarial dose varied from 100 to 150 cercariae per mouse, depending on the experiment. At the time of euthanasia, parasites were recovered by perfusion from the portal system of experimental animals, immediately fixed in 4% buffered formaldehyde and counted. To determine the hepatic egg burden in each animal and assess the rate of egg production by the parasites, 100 mg samples of liver tissue were homogenised and digested in 50 ml of 0.7% porcine trypsin in PBS for 1 h at 37 °C in an orbital shaker; the eggs were then sedimented at 4 °C and counted under a dissecting microscope. Where appropriate, the significance of differences between groups was tested using χ2 tests, T-tests or Fisher’s exact tests. Statistical analyses were performed with GraphPad Prism Version 4.00 software (GraphPad Software, Inc., San Diego, CA). P values of less than 0.05 were considered significant. All results presented are representative of at least two independent experiments.
2.2. Experimental animals
Breeding pairs of TNFR1−/−/R2−/− mice on a mixed B6/129 genetic background were provided by Dr George Yap, National Institutes of Health, Bethesda, MD (Yap et al., 1998). Homozygosity for mutant alleles of both TNFRs was confirmed by PCR (data not shown). TNFR1−/− mice with a C57BL/6 background (C57BL/6-Tnfrsf1a tm1Mak) were purchased from Jackson Laboratory (Bar Harbor, ME). TNF−/− and TNF−/−/lymphotoxin-α (LT-α)−/− mice, generated directly on a C57BL/6 background using B6 ES cells, are described elsewhere (Korner et al., 1997). Wild type C57BL/6 mice were obtained from Charles River (Wilmington, MA). Wild type B6/129 F1 hybrids were obtained from Taconic Farms (Germantown, NY). In some experiments, the body weight of each animal was monitored weekly throughout the course of infection. All animals were maintained at the Animal Care Facility, Veterans Affairs Medical Center San Francisco, in accordance with protocols approved by the VAMC Institutional Animal Care and Use Committee.
2.3. Histology
Infected mice were euthanised at day 42 and 56 p.i. to evaluate granuloma formation in the liver. Following recovery of the parasites from the portal circulation by perfusion, tissues were removed and fixed in 4% buffered formaldehyde, embedded in paraffin and sectioned to 5 μm before staining with haematoxylin and eosin (H&E). For detection of apoptotic cells, sections were stained by the TdT-mediated dUTP nick end labelling (TUNEL) method (In Situ Cell Death Detection Kit, Roche Molecular Biochemicals, Indianapolis, IN), as recommended by the manufacturer, using aminoethyl carbazole (AEC Single Solution, Zymed Laboratories, Inc., South San Francisco, CA) as a substrate. Sections were then counterstained with haematoxylin.
2.4. Assessment of hepatic granuloma volume
Representative H&E-stained liver sections from each animal were systematically scanned at 100 × magnification and all eggs present in the section were examined for the presence of a granuloma or apoptosis. For every granuloma containing a single egg, the mean radius and granuloma volume was calculated from two measurements of granuloma diameter obtained with an ocular micrometer.
2.5. Plasma alanine aminotransferase activity
Heparinised blood samples were obtained by cardiac puncture at the time of euthanasia and alanine aminotransferase (ALT) activity in the plasma was measured using a colorimetric assay (GP-Transaminase Kit, Sigma Diagnostics, Inc., St Louis, MO), as recommended by the manufacturer.
2.6. In vitro stimulation of splenocytes and cytokine assays
To evaluate systemic immune responses to schistosome egg antigens, spleens were removed from infected animals at day 56 p.i. and single cell suspensions produced by forcing splenic tissue through nylon cell strainers. After lysis of erythrocytes with 144 mM NH4Cl/17 mM Tris (pH 7.2) cells were cultured at a density of 10 × 106 cells ml−1 in RPMI/10% fetal bovine serum/25 mM HEPES/100 μg ml−1 streptomycin/100 units ml−1 penicillin for 72 h, either in the presence or absence of S. mansoni soluble egg antigens (SEA; Elliott, 1996). Cytokine concentrations in conditioned supernatants were then determined using a cytometric bead array (Mouse Th1/Th2 Cytokine Cytometric Bead Array (CBA) Kit, BD Biosciences, San Diego, CA) as described by the manufacturer, which allows for simultaneous analysis of IL-2, IL-4, IL-5, IFN-γ and TNF concentrations. CBA data were acquired using a FACScan flow cytometer (BD Biosciences). Medium conditioned by cells from uninfected mice was used as a negative control.
3. Results
3.1. Granuloma formation is delayed in the absence of TNF receptors but not in the absence of TNF ligand
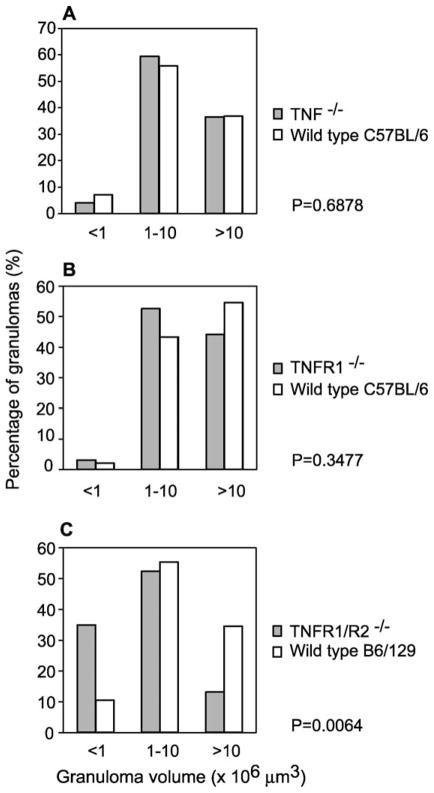
Quantitative histological analysis of egg-induced hepatic granulomas from TNF−/−, TNFR1−/− and TNFR1−/−/R2−/− mice at day 56 p.i. revealed no major differences compared to those induced in wild type animals (data not shown), indicating that TNF and its receptors are ultimately dispensable for granuloma formation in immunocompetent hosts. As egg production by S. mansoni commences at approximately day 35 p.i. in the mouse, liver pathology at day 42 p.i. is therefore representative of early events in granuloma formation. To evaluate whether the early pathogenesis of granulomas proceeded with similar kinetics in each of the deficient strains, we examined granulomas from TNF−/−, TNFR1−/− and TNFR1−/−/R2−/− mice at day 42 p.i. In all animals examined, we observed broad variation in the volume of inflammatory reactions associated with parasite eggs (Fig. 1A–C), ranging from no granulomatous reaction whatsoever (presumably around eggs that had only recently arrived in the liver), to robust macrophage-rich granulomatous reactions in more mature lesions. At least some relatively mature lesions (>10 × 106 μm3) were evident in all three deficient strains at this early time point (Fig. 1A–C), again emphasising that TNF and its receptors are ultimately dispensable for granuloma formation. Likewise, there was no difference in the percentage of hepatic eggs associated with granulomas (data not shown). However, examination of the distribution of granuloma volumes in each gene-targeted strain revealed that TNFR1−/−/R2−/− mice (Fig. 1C), but not TNFR1−/− (Fig. 1B) or TNF−/− (Fig. 1A) mice, consistently showed significantly more small granulomas and fewer large granulomas compared to wild type animals (e.g. P = 0.0064 returned by χ2 test). These observations suggest that nascent granulomas in TNFR1−/−/R2−/− mice remain small and take longer to mature than equivalent lesions in TNF−/− and TNFR1−/− mice. This effect was independent of mouse genetic background, as the distribution of granuloma volumes was identical in wild type C57BL/6 and B6/129 animals (Fig. 1).
Fig. 1.
Size distribution of early granulomas in TNFR1/R2−/−, TNFR1−/− and TNF−/− mice. Livers from Schistosoma mansoni-infected TNF−/− (A), TNFR1−/− (B) and TNFR1−/−/R2−/− (C) mice at 6 weeks p.i. were sectioned and stained with H&E. Sections were systematically scanned and all granulomas with a visible central egg were measured with an ocular micrometer to determine granuloma volume. Granulomas were then assigned, depending on volume, to one of three categories: <1 × 106, 1–10 × 106 and >10 × 106 μm3. Differences in the distribution of granuloma volumes in each genotype when compared to the appropriate wild type mice were assessed using χ2 tests and the P values returned by these tests are indicated.
3.2. TNF signalling is not required for egg-induced hepatocyte apoptosis
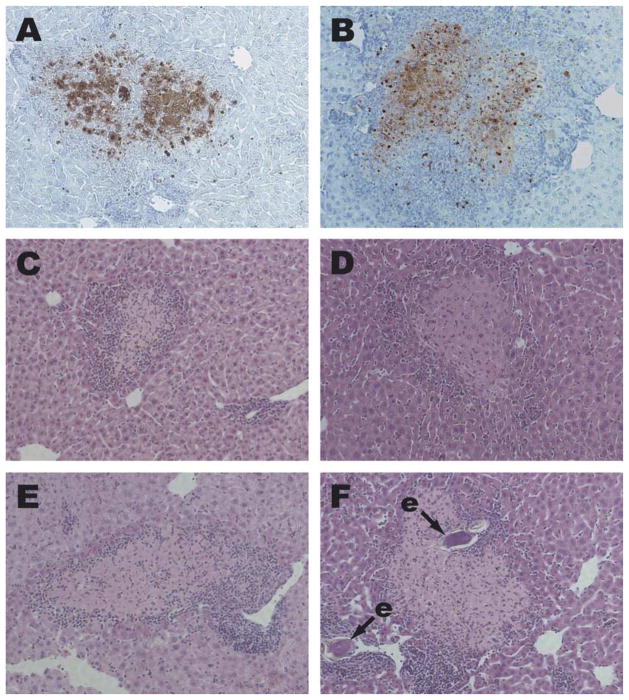
Hepatocyte apoptosis was recently recognised as a feature of schistosome egg-induced pathology at 6 weeks p.i. (Brunet et al., 1999). The apoptotic state of these cells can be confirmed by specific staining with the TUNEL method (Brunet et al., 1999). Areas of apoptotic hepatocytes are also readily visible in H&E-stained sections, as foci of pale, eosinophilic cells (Brunet et al., 1999), that frequently exhibit signs of nuclear fragmentation. TNF signalling via TNFR1 has previously been shown to induce apoptosis of hepatocytes under some circumstances (Leist et al., 1995) and it is reasonable to hypothesise that this mechanism might underlie the apoptosis observed in schistosome-infected mice. However, we observed hepatocyte apoptosis at comparable levels in infected TNFR1/R2−/− (Fig. 2B and D), TNF−/− (Fig. 2E) and TNF/LT−/− (Fig. 2F) mice, indicating that hepatocyte apoptosis occurs independently of TNFRs and their ligands during schistosome infection.
Fig. 2.
Hepatocyte apoptosis in Schistosoma mansoni-infected TNFR1/R2−/−, TNF−/− and TNF/LT−/− mice. Liver sections from S. mansoni-infected wild type (A, C), TNFR1−/−/R2−/− (B, D), TNF−/− (E) and TNF−/−/LT−/− (F) mice were stained by the TUNEL method (A, B) or with H&E (C–F). Apoptotic nuclei in A and B are stained dark red. Areas of apoptotic hepatocytes in C–F appear paler and slightly more eosinophilic than nearby healthy tissue, and are surrounded by smaller, darker-staining inflammatory cells. e, schistosome egg. 100 × magnification.
3.3. Schistosome egg deposition induces cachexia in TNF−/− mice
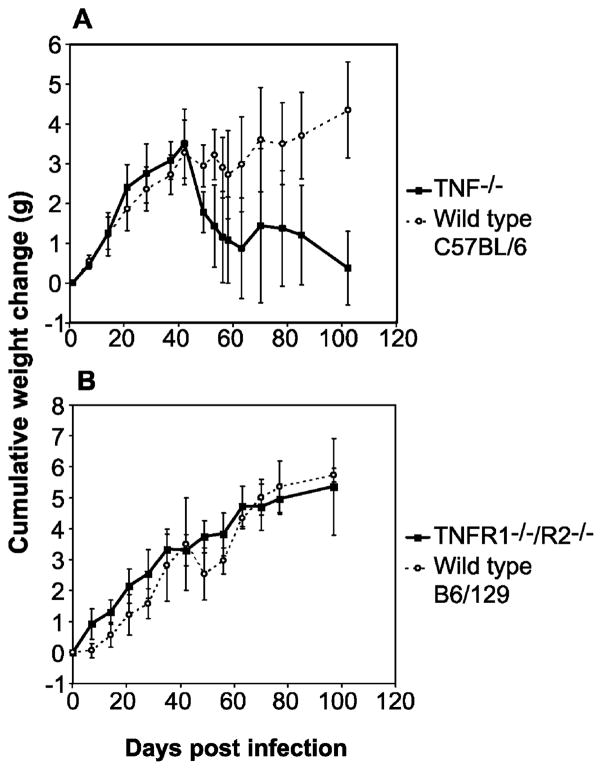
While deletion of the TNF gene had little effect on the morphology of granulomas in schistosome-infected mice (Fig. 1A), clinical evaluation of experimental animals revealed that, paradoxically, TNF−/− mice became cachectic during infection, beginning at around day 42 p.i. Clinical signs associated with cachexia included inactivity, shivering, piloerection, inappetance, dehydration and weight loss. Cachexia could be quantified by monitoring body weight of infected animals through the acute phase of infection (Fig. 3). As shown in Fig. 3A, both wild type C57BL/6 and TNF−/− mice, infected at 8 weeks of age, continued to gain weight during the first 5 weeks of infection. However, from 6 weeks p.i. onwards, TNF−/− mice progressively lost body weight, while wild type mice either maintained or continued to gain body weight. In contrast to TNF−/− mice, TNFR1−/−/R2−/− animals did not present with cachexia and did not lose body weight (Fig. 3B). These results were independent of mouse genetic background, as cachexia was not observed in either wild type C57BL/6 or B6/129 mice.
Fig. 3.
Weight loss in TNF−/− and TNFR1−/−/R2−/− mice during acute schistosomiasis. Age- and sex-matched cohorts of wild type and TNF−/− (A) or TNFR1−/−/R2−/− (B) mice were infected with S. mansoni at day 0 and the body mass of each animal was monitored at weekly intervals. Loss of body mass in TNF−/− mice begins at 6 weeks p.i., coincident with onset of parasite egg laying.
These data lead to two surprising conclusions. First, TNF, initially identified as the primary mediator of cachexia and hence originally known as ‘cachectin’, is paradoxically required to prevent cachexia during murine schistosomiasis. Second, the protective effects of TNF in the context of murine schistosomiasis are independent of either of its known receptors.
3.4. Schistosome infection induces hepatocellular damage in TNF−/− and TNF−/−/LT−/− mice
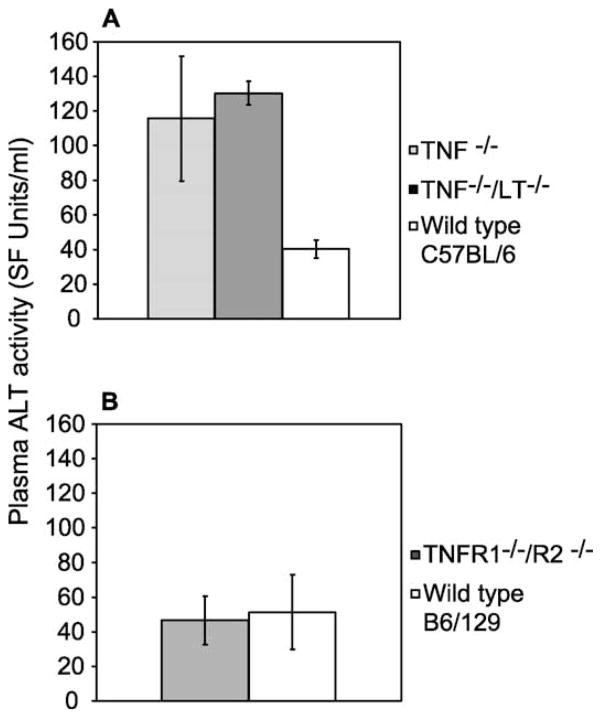
No major histological differences in egg-induced pathology were observed in schistosome-infected TNF−/− or TNFR1−/−/R2−/− mice, both in terms of granuloma formation or hepatocyte apoptosis (Figs. 1 and 2). However, assays for ALT activity in freshly recovered plasma from infected mice revealed significantly higher concentrations of this hepatocyte-specific enzyme in the plasma of TNF−/− mice at day 42 p.i. (Fig. 4A), in excess of those observed in S. mansoni-infected wild type mice at this time point, indicating that increased hepatocellular damage occurs in TNF−/− animals. Similar results were also obtained with TNF−/−/LT−/− mice (Fig. 4A). In contrast, plasma ALT concentrations in TNFR1/R2−/− at day 42 p.i. were similar to those observed in wild type animals (Fig. 4B). These differences in hepatocellular damage were not a function of differences in mouse genetic background, as levels of hepatocellular damage were identical in wild type C57BL/6 and B6/129 mice (Fig. 4). Interestingly, and in parallel with the induction of cachexia demonstrated in Fig. 3, hepatocellular damage resulted from a deficiency in TNF but not its receptors (Fig. 4).
Fig. 4.
Lack of TNFR ligands, but not TNFRs, results in hepatocellular damage in Schistosoma mansoni-infected mice. Plasma from S. mansoni-infected TNF−/−, TNF−/−/LT−/− and wild type C57BL/6 mice (A) and TNFR1−/−/R2−/− and wild type B6/129 mice (B) were quantitatively assayed for the presence of alanine aminotransferase activity at 6 weeks p.i.
3.5. Type 2 responses to schistosome egg antigens are normal in animals deficient in TNF or its receptors
Granuloma formation and subsequent defence against egg toxins has been shown to be largely dependent on induction of type 2 responses to egg antigens (Kaplan et al., 1998; Jankovic et al., 1999). Further, the key type 2 cytokine IL-4 is known to play a central role in preventing excessive liver pathology during acute schistosomiasis by limiting nitric oxide production (Brunet et al., 1999). Therefore, we examined the ability of TNF−/− and TNFR1−/−/R2−/− mice to mount type 2 responses to parasite egg antigens, to determine whether a defect in type 2 response induction might account for the cachexia (Fig. 3A) and increased hepatocellular damage (Fig. 4A) observed in TNF−/− mice. While slightly more IL-2 and less IL-5 were produced by splenocytes from infected TNF−/− mice in response to egg antigens when compared to wild type controls, production of IL-4 by TNF−/− splenocytes was unaltered (data not shown), indicating that schistosome-infected TNF−/− mice mount essentially normal type 2 responses to parasite egg antigens. Egg antigen-specific IL-2, -4 and -5 responses in TNFR1−/−/R2−/− mice were identical to those in wild type control animals (data not shown). Further, no significant differences in IFN-γ production by splenocytes from infected TNF−/− and TNFR1−/−/R2−/− mice in response to egg antigens were detected when compared to wild type controls (data not shown).
3.6. TNF deficiency compromises parasite survival in the portal system
It was previously shown that exogenous TNF stimulated egg production by S. mansoni when administered to parasites in vivo or in vitro, indicating that TNF can influence parasite egg production directly (Amiri et al., 1992). Further, we have recently shown that the adaptive immune system profoundly influences schistosome development during prepatency, resulting in expression of different developmental phenotypes by the parasite (Davies et al., 2001). It was therefore of considerable interest to examine parasite development and egg production by S. mansoni in a TNF-deficient host. However, determination of hepatic egg burdens in TNF−/− mice failed to reveal any impairment of schistosome fecundity (Blank et al., submitted), indicating that TNF is not essential for parasite egg production. This result also demonstrates that TNF deficiency did not adversely affect parasite development during prepatency (Davies et al., 2001).
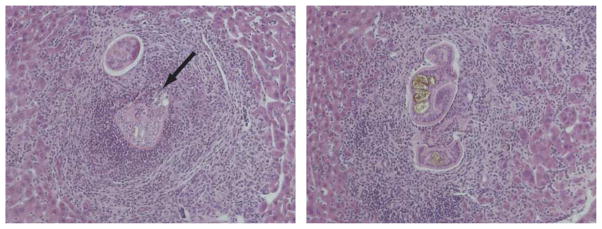
Interestingly, systematic analysis of random liver sections from TNF−/− and wild type control mice infected for 6 weeks or more provided evidence that parasite attrition frequently occurred in the portal systems of TNF−/− mice, but not in wild type controls (Table 1). In many TNF−/− mice, schistosomes could still be found in the liver after 6 weeks p.i. (Table 1), where they elicited an intense local inflammatory response, consisting of lymphocytes and eosinophils (Fig. 5). In some cases, these intrahepatic parasites appeared to be dead or dying, with inflammatory cells infiltrating the parasite tissues (Fig. 5). In contrast, parasites were only occasionally found in the livers of wild type mice after 6 weeks p.i. (Table 1), and were probably the result of post-mortem shunting of worms from the mesenteric veins to the larger branches of the portal vein within the liver, as these parasites were found in the lumina of large vessels, were not accompanied by intense inflammatory infiltrates and appeared healthy. In addition to the obvious morphological differences between parasites in the livers of TNF−/− and wild type mice, analysis of liver sections from a large sample of animals revealed that worms were 3.5 times more likely to be detected in the livers of TNF−/− mice than wild type mice (Table 1). The higher frequency of worms in the livers of TNF−/− mice was statistically significant, as determined by Fisher’s exact test.
Table 1.
Histologic examination of TNF−/− and wild type mouse livers for the presence of embolised schistosomes at 6 weeks p.i.
| Genotype | No. livers examined | Livers containing worms | Percentage containing worms |
|---|---|---|---|
| TNF−/− | 18 | 6 | 13.333 |
| Wild type C57BL/6 | 26 | 1 | 3.846 |
Random H&E-stained sections from the livers of 6-week infected TNF−/− and wild type C57BL/6 mice were examined by light microscopy for the presence of adult schistosomes. Fisher’s exact test, applied to the numbers of livers containing or not containing parasites for each genotype, returned a P value of 0.0134, indicating that the increase in intrahepatic worms detected in TNF−/− mice is statistically significant.
Fig. 5.
TNF deficiency results in attrition of schistosomes in the portal system. Representative dying schistosomes observed in H&E-stained sections from livers of Schistosoma mansoni-infected TNF−/− mice at 6 weeks p.i. Arrow identifies area where inflammatory cells are invading parasite tissue. In the micrograph on the left, a dissociated male–female pair appears to be the focus of the inflammatory reaction, while what appears to be a male with a developed gynecophoric canal is visible in the micrograph on the right. 100 × magnification.
4. Discussion
Our data from animals deficient in TNF or its receptors clearly demonstrate that TNF signalling is ultimately dispensable for granuloma formation in response to schistosome eggs. These results stand in stark contrast to those obtained with other granuloma-inducing stimuli such as mycobacteria (Flynn et al., 1995; Bean et al., 1999) and emphasise the unique nature of the schistosome egg-induced granuloma. Our results are also at odds with previous reports where pharmacological inhibition (Joseph and Boros, 1993) or supplementation (Amiri et al., 1992) of TNF was shown to either negatively or positively affect granuloma formation around schistosome eggs, respectively. Induction of compensatory mechanisms in the genetically deficient strains we have examined may account for these discrepancies. Cheever et al. (1999) also failed to find a role for TNF in granuloma formation during schistosome infection when mice with severe combined immunodeficiency (SCID) were supplemented with exogenous TNF. However, SCID mice express a somewhat ‘leaky’ immunodeficient phenotype that is particularly prevalent when the scid mutation is present on certain genetic backgrounds, including the CB17 SCID mouse (Nonoyama et al., 1993) utilised by Cheever et al. (1999). Hence, granulomas and near normal levels of TNF mRNA were found in their infected SCID animals (Cheever et al., 1999). Under these circumstances, evaluating the effect of administering additional exogenous TNF is complicated by the fact that the supposedly immunodeficient recipient is already expressing normal levels of this cytokine.
Interestingly, combined deficiency of TNFR1 and TNFR2 does appear to retard or delay granuloma formation (Fig. 1C). This effect was not observed in singly deficient TNFR1−/− animals (Fig. 1B), implying that TNFR2 plays a role in granuloma maturation around schistosome eggs. This delay in granuloma formation was not a result of impaired type 2 responses in TNFR1−/−/R2−/− mice, as these animals mounted normal type 2 responses to egg antigens (data not shown). A similar phenomenon has been observed in experimental mycobacterial infection (Jacobs et al., 2000), where TNFR2 deficiency resulted in a modest increase in susceptibility to infection, but did not alter T cell responses to mycobacterial antigen. TNFR2 is known to mediate upregulated expression of C–C chemokines such as macrophage-inflammatory protein (MIP)-1α, MIP-1β, and regulated by activation, normal T cell expressed and secreted (RANTES) chemokine by T cells in an NF-κB-dependent manner (Hornung et al., 2000). Impaired expression of these chemokines as a result of TNFR2 deficiency might negatively impact recruitment of cells to granulomas, explaining the delayed granuloma phenotype observed in TNFR1−/−/R2−/− mice. This possibility is currently under investigation. Importantly however, delayed granuloma formation in TNFR1−/−/R2−/− mice appeared not to adversely affect the outcome of infection, as there were no obvious clinical differences between these animals and wild type mice (Figs. 3 and 4).
It is notable that a delayed granuloma phenotype was observed in TNFR1−/−/R2−/− mice but not in TNF−/− mice. Continued signalling through TNFRs by LT-α might explain this discrepancy, although LT-α was not found to compensate for loss of TNF expression during mycobacterial infection (Bean et al., 1999). While granuloma formation also appeared to proceed normally in infected TNF−/−/LT−/− mice, we were unable to quantify granuloma formation in these animals due to idiopathic accumulation of perivascular lymphoid aggregates in many organs, including the liver (Davies and McKerrow, unpublished).
It has recently been shown that an important function of the egg-induced type 2 response is to limit immunopathology induced by overexpression of proinflammatory cytokines (Brunet et al., 1997, 1999), including apoptosis of hepatocytes. As TNF is expressed during egg deposition (Haseeb et al., 2001) and is capable of inducing hepatocyte apoptosis via TNFR1 (Leist et al., 1995), it is reasonable to expect TNF to play a role in this process during schistosome infection. To test this hypothesis, we examined livers from infected TNFR1/R2−/−, TNFR1−/−, TNF−/− and TNF/LT−/− mice for the presence of hepatocyte apoptosis. As shown in Fig. 2, hepatocyte apoptosis was readily detected in livers from infected animals of all these strains. This result indicates that hepatocyte apoptosis in schistosomiasis is independent of the TNFRs and their ligands. The mechanism by which hepatocyte apoptosis is induced during schistosome infection therefore remains open to debate, but we hypothesise that other pro-apoptotic factors such as IFN-γ (Ishida et al., 2002) or Fas (Leist et al., 1996) may be implicated.
The role of TNF in the development of destructive immunopathology associated with diseases such as rheumatoid arthritis is widely appreciated, as are the benefits of emerging anti-TNF therapies in the treatment of such diseases (reviewed in Graninger and Smolen, 2002). Elevated TNF levels occur in human patients with acute schistosomiasis (de Jesus et al., 2002) and severe pathology in patients is associated with elevated circulating levels of TNF and TNFRs (Mwatha et al., 1998). In experimentally infected mice, elevated levels of circulating TNF and TNFRs also correlate with onset of oviposition and granuloma formation (Haseeb et al., 2001) and sustained production of TNF in the liver correlates with severe chronic disease (Adewusi et al., 1996). Further, elevated TNF production with accompanying cachexia and mortality was observed in S. mansoni-infected IL-4−/− mice, a consequence of the impaired type 2 response mounted by these animals to parasite egg antigens and the subsequent failure to regulate production of proinflammatory cytokines (Brunet et al., 1997). Anti-TNF therapy might therefore be indicated in treatments aimed at ameliorating schistosomiasis-associated immunopathology. With this possibility in mind, we undertook a thorough examination of infection outcome in TNF−/− mice.
Surprisingly, TNF−/− mice developed cachexia and a wasting syndrome (Fig. 3A) similar to that previously described in IL-4−/− mice (Brunet et al., 1997). Disease onset correlated with onset of parasite oviposition (Fig. 3A) and with the appearance of elevated levels of ALT activity in the plasma (Fig. 4A). Taken together, these data indicate that the underlying cause of cachexia is excessive hepatocellular damage in response to parasite egg deposition. Increased liver damage in TNF−/− mice was not a result of increased egg accumulation in the livers of these animals, as hepatic egg burdens were identical to those of wild type controls (Blank et al., submitted). Hepatocellular damage was not a result of upregulated expression of LT-α either, as similar pathology was observed in TNF−/−/LT−/− mice (Fig. 4A). Insufficient numbers of TNF−/−/LT−/− mice were available to determine whether these animals displayed a similar pattern of weight loss upon infection with S. mansoni, but clinical examination and plasma ALT assays indicated that the progression of disease in TNF−/−/LT−/− animals was identical to that in singly deficient TNF−/− mice (Fig. 4A). In schistosome-infected IL-4−/− mice, excessive NO production, stimulated by elevated levels of TNF, appears to be the cause of cachexia and hepatocellular damage (Brunet et al., 1999). However, some amount of NO is believed to be important in protecting against excessive liver pathology (Brunet et al., 1999). One possible explanation for the occurrence of hepatocellular damage in TNF−/− mice might be that insufficient NO is produced during the response to parasite eggs. This possibility is under investigation. Taken together, our data indicate that TNF plays a previously unappreciated role in protecting against hepatocellular damage during schistosomiasis in mice and that anti-TNF therapies aimed at ameliorating schistosomiasis-associated immunopathology may be contraindicated.
Significantly, cachexia, weight loss and hepatocellular damage were not observed in TNFR1−/−/R2−/− mice (Figs. 3B and 4B), highlighting a major discrepancy between the phenotypes of ligand-deficient and receptor-deficient animals with regard to the TNF signalling system. The logical explanation for these disparities is that as yet uncharacterised components of this signalling system exist. In another study where TNF/LT- and TNFR1/R2-deficient animals were compared (Hayder et al., 1999), similar discrepancies were observed and a similar hypothesis was invoked. Therefore a growing body of biological evidence suggests that additional components of the TNF signalling system should be sought.
In light of previously published data showing that TNF essentially functions as a trophic factor for S. mansoni (Amiri et al., 1992), and our recent work demonstrating the dependence of schistosome development on adaptive immune signals (Davies et al., 2001), it was of considerable interest to examine parasite development and egg production in TNF-deficient hosts. Contrary to these results, careful quantification of parasite egg production in TNF−/− mice revealed no impairment of parasite development or fecundity (Blank et al., submitted). Again, upregulation of compensatory mechanisms in TNF−/− mice may account for these differences, as in the case of granuloma formation in TNF−/− animals (Fig. 1). However, upregulation of LT-α does not appear be a factor, as normal egg production and parasite growth were also observed in TNF−/−/LT−/− mice (data not shown). Studies utilising pharmacological inhibition of TNF signalling (Ritter and McKerrow, unpublished) support the previous conclusion that TNF does indeed influence schistosome egg production. An interpretation that reconciles these two findings is that TNF is not important for parasite development during the prepatent period, but does influence schistosome egg production later in the life cycle, as a separate phenomenon, once parasites have matured and commenced reproduction. Cheever et al. (1999) also failed to demonstrate any effect of TNF on schistosome fecundity, although the limitations of their study, discussed above, should be borne in mind when considering the significance of their data. Haseeb et al. (1996) failed to find any direct effect of recombinant TNF on schistosome egg production in vitro, although increased tyrosine uptake by female worms was stimulated by TNF under some conditions. The difficulty associated with maintaining schistosomes in a fully viable state under in vitro conditions should be considered when interpreting these negative results (Haseeb et al., 1996).
Intriguingly, histologic examination of livers from infected animals at 6 weeks or more p.i. revealed that many livers from TNF−/− mice contained worms that appeared to be trapped in liver tissue (Table 1). Many of these worms elicit an intense local inflammatory reaction, composed of lymphocytes and eosinophils, and in some instances inflammatory cells can be seen invading parasite tissue (Fig. 5). To our knowledge, such verminous emboli have not previously been observed in schistosome-infected mice that had not first been treated with a schistosomicidal agent. While morphologic examination of parasites and determination of egg burdens in TNF−/− mice (Blank et al., submitted) suggest that parasite development and fecundity early in infection are not affected by a lack of TNF, this observation suggests that parasite survival after arrival in the portal system may be compromised by a deficiency of this cytokine. Consistent with our hypothesis regarding the later effects of TNF on egg production, it appears that fully developed parasites, rather than developing stages, are affected by TNF deficiency (Fig. 5). Interestingly, a similar embolisation of schistosomes to the liver is consistently observed in animals that are pharmacologically depleted of TNF by treatment with anti-TNF antibodies (Ritter and McKerrow, unpublished).
In conclusion, genetic disruption of TNF signalling reveals that TNF is ultimately dispensable for granuloma formation in response to schistosome eggs but that TNFR2 may play a minor role in maturation of egg-induced granulomas. More importantly, TNF does appear to mediate protection from excessive hepatocellular damage and cachexia, and is not the cause of hepatocyte apoptosis. Finally, our results demonstrate that parasite fecundity is not dependent on this cytokine, but that TNF does play a complex role in propagating the intramammalian stages of the schistosome life cycle.
Acknowledgments
We thank Christopher Franklin for technical support and Katherine A. Feldman for advice on statistical analyses. This work was supported by the National Institutes of Health (F32 AI10424 and P30 DK26743/UCSF Liver Center to S.J.D.) and by the Sandler Family Foundation (to J.H.M). DNAX is supported by Schering Plough Corp., NJ, USA.
References
- Adewusi OI, Nix NA, Lu X, Colley DG, Secor WE. Schistosoma mansoni: relationship of tumor necrosis factor-alpha to morbidity and collagen deposition in chronic experimental infection. Exp Parasitol. 1996;84:115–123. doi: 10.1006/expr.1996.0097. [DOI] [PubMed] [Google Scholar]
- Amiri P, Locksley RM, Parslow TG, Sadick M, Rector E, Ritter D, McKerrow JH. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- Brunet LR, Beall M, Dunne DW, Pearce EJ. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol. 1999;163:4976–4984. [PubMed] [Google Scholar]
- Cheever AW, Eltoum IA, Andrade ZA, Cox TM. Biology and pathology of Schistosoma mansoni and Schistosoma japonicum infections in several strains of nude mice. Am J Trop Med Hyg. 1993;48:496–503. doi: 10.4269/ajtmh.1993.48.496. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Poindexter RW, Wynn TA. Egg laying is delayed but worm fecundity is normal in SCID mice infected with Schistosoma japonicum and S. mansoni with or without recombinant tumor necrosis factor alpha treatment. Infect Immun. 1999;67:2201–2208. doi: 10.1128/iai.67.5.2201-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4 + lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- Elliott DE. Methods used to study immunoregulation of schistosome egg granulomas. Methods. 1996;9:255–267. doi: 10.1006/meth.1996.0032. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Graninger W, Smolen J. Treatment of rheumatoid arthritis by TNF-blocking agents. Int Arch Allergy Immunol. 2002;127:10–14. doi: 10.1159/000048164. [DOI] [PubMed] [Google Scholar]
- Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine Schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- Haseeb MA, Solomon WB, Palma JF. Schistosoma mansoni: effect of recombinant tumor necrosis factor alpha on fecundity and [14C]-tyrosine uptake in females maintained in vitro. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:265–269. doi: 10.1016/s0742-8413(96)00137-5. [DOI] [PubMed] [Google Scholar]
- Haseeb MA, Shirazian DJ, Preis J. Elevated serum levels of TNF-alpha, sTNF-RI and sTNF-RII in murine schistosomiasis correlate with schistosome oviposition and circumoval granuloma formation. Cytokine. 2001;15:266–269. doi: 10.1006/cyto.2001.0925. [DOI] [PubMed] [Google Scholar]
- Hayder H, Blanden RV, Korner H, Riminton DS, Sedgwick JD, Mullbacher A. Adenovirus-induced liver pathology is mediated through TNF receptors I and II but is independent of TNF or lymphotoxin. J Immunol. 1999;163:1516–1520. [PubMed] [Google Scholar]
- Hernandez HJ, Wang Y, Tzellas N, Stadecker MJ. Expression of class II, but not class I, major histocompatibility complex molecules is required for granuloma formation in infection with Schistosoma mansoni. Eur J Immunol. 1997;27:1170–1176. doi: 10.1002/eji.1830270518. [DOI] [PubMed] [Google Scholar]
- Hornung F, Scala G, Lenardo MJ. TNF-alpha-induced secretion of C–C chemokines modulates C–C chemokine receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000;164:6180–6187. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- Iacomini J, Ricklan DE, Stadecker MJ. T cells expressing the gamma delta T cell receptor are not required for egg granuloma formation in schistosomiasis. Eur J Immunol. 1995;25:884–888. doi: 10.1002/eji.1830250404. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. Fed Am Soc Exp Biol J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Brown N, Allie N, Chetty K, Ryffel B. Tumor necrosis factor receptor 2 plays a minor role for mycobacterial immunity. Pathobiology. 2000;68:68–75. doi: 10.1159/000028116. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Ward JM, Cheever AW, Paul WE, Sher A. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- de Jesus AR, Silva A, Santana LB, Magalhaes A, de Jesus AA, de Almeida RP, Rego MA, Burattini MN, Pearce EJ, Carvalho EM. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- Joseph AL, Boros DL. Tumor necrosis factor plays a role in Schistosoma mansoni egg-induced granulomatous inflammation. J Immunol. 1993;151:5461–5471. [PubMed] [Google Scholar]
- Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- Kaufmann SH, Ladel CH. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Korner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, Kontgen F, Fazekas de St Groth B, Sedgwick JD. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- Leist M, Gantner F, Kunstle G, Bohlinger I, Tiegs G, Bluethmann H, Wendel A. The 55-kD tumor necrosis factor receptor and CD95 independently signal murine hepatocyte apoptosis and subsequent liver failure. Mol Med. 1996;2:109–124. [PMC free article] [PubMed] [Google Scholar]
- Murare HM, Dunne DW, Bain J, Doenhoff MJ. Schistosoma mansoni: control of hepatotoxicity and egg excretion by immune serum in infected immunosuppressed mice is schistosome species-specific, but not S. mansoni strain-specific. Exp Parasitol. 1992;75:329–339. doi: 10.1016/0014-4894(92)90218-y. [DOI] [PubMed] [Google Scholar]
- Mwatha JK, Kimani G, Kamau T, Mbugua GG, Ouma JH, Mumo J, Fulford AJ, Jones FM, Butterworth AE, Roberts MB, Dunne DW. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- Nonoyama S, Smith FO, Bernstein ID, Ochs HD. Strain-dependent leakiness of mice with severe combined immune deficiency. J Immunol. 1993;150:3817–3824. [PubMed] [Google Scholar]
- Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Vella AT, Pearce EJ. CD4 + Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- Warren KS, Domingo EO, Cowan RBT. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967;51:735–756. [PMC free article] [PubMed] [Google Scholar]
- Yap GS, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]