INTRODUCTION
The lumbar puncture (LP) is a common Emergency Department (ED) procedure often used in the diagnosis of subarachnoid hemorrhage (SAH). Although the LP is a relatively simple test, significant diagnostic uncertainty can arise when trauma from the needle causes bleeding into the subarachnoid space. This is usually referred to as a “traumatic tap”. Traumatic LPs are estimated to occur in 10–30% of routine LPs, although the true frequency is unknown and depends on how a traumatic LP is defined. 1 At present, there is no consensus as to what constitutes a traumatic LP. The sensitivity of detecting blood in the cerebrospinal fluid (CSF) via LP is considered to approach 100%. Given an approximate 20% traumatic tap incidence, however, the specificity is only about 80%. 2
Distinguishing a traumatic LP from SAH is critical for the emergency physician. SAH is a devastating condition with high morbidity and mortality; roughly two-thirds of untreated SAH patients die or have serious neurologic disabilities as a consequence. 3 Depending on their neurologic status at initial clinical presentation, many patients with SAH can do well with early diagnosis and treatment. 4 Consequently, low-grade SAHs, often referred to as “sentinel leaks,” that are quickly diagnosed may have excellent outcomes. Conversely, those patients with traumatic LPs who are incorrectly presumed to have SAH are exposed to unnecessary, expensive, and potentially harmful procedures such as angiography.
The usual method of diagnosing SAH begins with computed tomography (CT) of the head without contrast. If this study is negative or equivocal, an LP is performed. 5 CT scan has a sensitivity of 98% within the first 12 hours of the ictus and 93% within 24 hours. Sensitivity decreases to approximately 80% at 72 hours and 50% at 1 week. CT scan findings are positive in 92% of patients who have SAH. Sensitivity is lower when using older second or first-generation scanners. Most North American hospitals have been using third-generation scanners since the mid 1980s. 6
There is consensus in the literature that an LP should be performed when strong clinical suspicicon of SAH exists in the setting of a negative or equivical CT or when CT is not available. 7 The presence of red blood cells (RBCs) in the CSF is diagnostic for SAH. However, there is no consensus in the literature on the number of RBC per cubic millimeter (mm3) needed to make the diagnosis. 8 Although most counts range from a few thousand to a million or more, there are case reports of SAH diagnosed by LP with RBC counts in the low hundreds. 9, 10 Thus, the ability to differentiate between SAH and traumatic LP is crucial even at low numbers of RBCs.
SAH often can be distinguished from traumatic LP by comparing the RBC counts of the first and last tubes of CSF. However, this is not always reliable, especially at higher numbers of RBCs. Theoretically the RBC count will not decrease between the first and last tubes in the setting of SAH; however, multiple case reports of this phenomenon do exist. The method of comparing the first and last tubes of CSF for RBCs has never been validated in a study. 11 Some authors suggest that a decrease in RBC count of more than 30% between the first and last tubes of CSF is diagnostic of a traumatic LP, although this has never been verified. 12
A potentially more reliable method of differentiating SAH from a traumatic LP is to spin down the CSF and examine the supernatant fluid for the presence of xanthochromia, a pink or yellow coloration of the CSF supernatant caused by the breakdown of RBCs and subsequent release of heme pigments. However, this method is sensitive and specific only when spectrophotometry (rather than the naked eye) is used to identify xanthochromia. 13 Unfortunately, many laboratories do not have the capability to identify xanthochromia with spectrophotometry. In addition, although xanthochromia is present 12 hours after the bleed in nearly 100% of patients with an SAH, it typically will not appear until up to 4 hours after the ictus.
The lack of consensus on how to differentiate a traumatic LP from one diagnostic for SAH creates a diagnostic dilemma for the emergency department physician. When there is a low suspicion for SAH, which patients can be safely sent home without extensive and expensive workups? Finding a lower limit of RBCs in the CSF and determining the necessary degree of RBC clearance in consecutive tubes would be useful in excluding a clinically significant SAH and diagnosing a traumatic LP. The goal of this study is twofold. The first aim is to determine whether a CSF RBC cutoff value exists that may safely exclude a radiographically detectable SAH. The second goal is to compare the RBC clearance from tubes 1 and 4 in LPs of subjects identified as positive for SAH to those of radiographically normal subjects.
METHODS
A retrospective medical record review was performed on all adult patients who received a lumbar puncture for the complaint of headache in the emergency department at a university teaching hospital from January 2001 until April 2003. Pediatric patients were excluded because subarachnoid hemorrhage is a relatively rare event in this population and traumatic lumbar punctures are more frequent and difficult to interpret in children. 1 In addition, patients in which the diagnosis of subarachnoid hemorrhage was not entertained were excluded (for instance, those with a previous diagnosis for their headaches such as normal pressure hydrocephalus).
Patients were identified through computerized laboratory records. The original sample size was 594 patients, of whom 305 had RBCs in their CSF after LP. Those included in the study had at least one RBC in the CSF. Six were excluded because of an alternative diagnosis to SAH. Radiographic confirmation of SAH was made in 11 patients (8 by angiogram, 3 by CT initially and then angiogram). Of the 288 radiographically normal subject LPs, tube 1 and tube 4 data was available for 142. The other 146 had tube 4 data only. Tube 1 and 4 data were available for all 11 of the SAH LPs.
The two groups (SAH LPs and radiographically normal subject LPs) were analyzed in two ways: first in terms of absolute RBC count in tube 4 and then with respect to percentage decrease in RBC from tubes 1 to 4. In the first instance, mean RBC counts for tube 4 and 95% confidence intervals were calculated (using the exact binomial method) for each group. In addition, relative positive and negative predictive values for SAH were calculated for the different ranges of RBCs in tube 4.
A second assessment was made based on RBC clearance from tube 1 to 4. The mean percentage of RBC clearance was calculated for both groups and relative negative and positive predictive values were calculated for the different ranges of clearance. In both comparisons, continuous variables were analyzed using Student’s t-test and p values were calculated. The study was approved by the hospital’s institutional review board.
RESULTS
Initial medical record review was performed on 594 LP patients, 299 of whom had CSF RBCs and were included in the study. Of these, 288 (48.5%) were considered not to have a radiographically detectable SAH using the criteria of at least 1 RBC per mm3 in the CSF after LP and a negative imaging study for SAH. Of these 288 traumatic LPs, data for both tubes 1 and 4 were available for 142 (49.3%). SAH was found in 11 patients (3.7%). Radiographic confirmation of the disease by angiogram was performed in each case.
Mean CSF RBC counts for both groups are shown in Table 1. The radiographically normal subject LP group had a mean of 6,793 RBCs in tube 1 (95% CI = 4,029–9,496) and 443 RBCs (95% CI = 292–592) in tube 4. Mean CSF RBC counts for the SAH group were 399,237 in tube 1 and 307,700 in tube 4. The range of values in tube 4 for the SAH group was 990 to 2,234,563 RBCs. Because of the small sample size and grossly divergent values of the SAH set, 95% confidence intervals were not calculated for mean CSF RBCs in this group.
Table 1.
Mean RBCs and mean percent change for traumatic and SAH-positive lumbar punctures. (Only data incorporating both tubes 1 and 4 is included.)
| Traumatic LP (n=142) | SAH (n=11) | |
|---|---|---|
| Mean RBCs | ||
| Tube 1 | 6763 (95% CI 4029–9496) | 399,277 |
| Mean RBCs | ||
| Tube 4 | 443 (95% CI 295–592) | 307,700 |
| Mean Percent | ||
| Change | 82.1% (95% CI +84.9 to 79.9) | −9.1% (95% CI-19.3 to 2.1) |
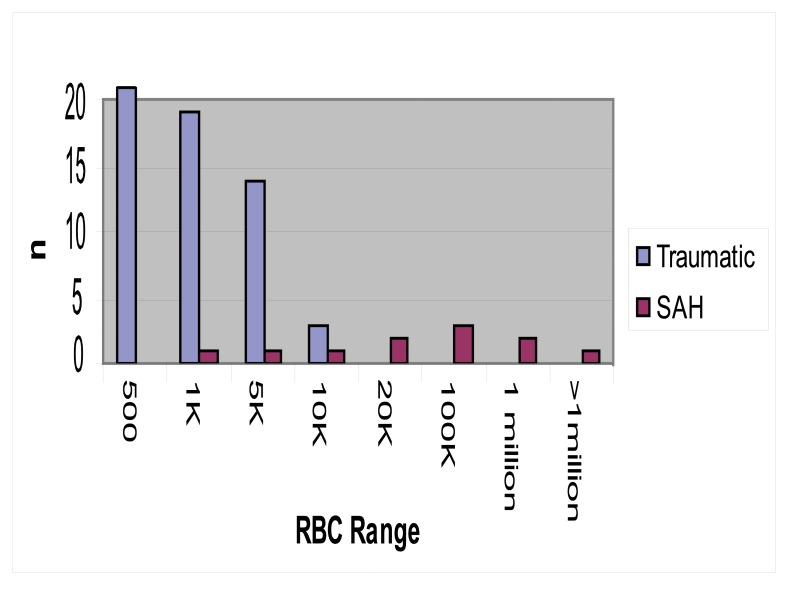
Table 2 and Figure 1 show the distribution of radiographically normal subjects and SAH positive LPs by RBC range. The relative predictive values, sensitivity and specificity for each range are shown in Table 2. Relative, rather than absolute, predictive values are reported in order to better reflect the trends for each RBC range. This was done because the absolute predictive values for SAH are extreme with limited variation between different RBC ranges due to the low prevalence of the disease. This makes the absolute predictive values difficult to interpret.
Table 2.
Relative negative predictive value, relative positive predictive value, sensitivity and specificity at various CSF RBC ranges for traumatic and SAH diagnostic groups. SAH RBC values in parentheses; 95% confidence inter vals in parentheses; p-values < 0.001
| Tube 4 RBCs | SAH (n=11) | Traumatic LPs (n=288) | Negative Predictive Value | Positive Predictive Value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 0–500 | 0 | 252 | 100% | 0% | 100% (89.0–100%) | 87.5% (86.3–87.5%) |
| 500–1,000 | 1 (990) | 19 | 95% | 5% | 90.9% (79.2–99.5%) | 94.1% (92.9–94.4%) |
| 1,000–5,000 | 1 (1434) | 14 | 93.3% | 6.7% | 81.8% (72.8–96.3%) | 87.5% (86.3–87.5%) |
| 5,000–10,000 | 1 (5894) | 3 | 75% | 25% | 72.7% (57.3–72.7%) | 100% (99.0–100%) |
| 10,000–20,000 | 2A (11,665) (12,657) | 0 | 0% | 100% | 54.5% (40.2–54.5%) | 100% (99.1–100%) |
| 20,000–100,000 | 3 (21,345) (62,301) (63,124) | 0 | 0% | 100% | 27.3% (18.8–27.3%) | 100% (99.3–100%) |
| 100,000–1,000,000 | 2 (100,322) (870,412) | 0 | 0% | 100% | 9.1 % (1.5–9.1%) | 100% (99.7–100%) |
| >1,000,000 | 1 (2,223,563) | 0 | 0% | 100% | 0% | 100% |
Figure 1.
Frequency of traumatic and SAH positive LPS by CSF RBC range.
In the radiographically normal subject LP group, 252 of 288 (87.5%) RBC counts in tube 4 were between 0–500. There were no SAH positive LPs in this range. The relative negative predictive value (rNPV) of an LP with a RBC count in tube 4 between 0 and 500 was 100%; the relative positive predictive value (rPPV) was 0%. The sensitivity for SAH in this RBC range was 100% (95% CI = 89–100); the specificity was 87.5 % (86.3–87.5). In contrast, there were no LPs with tube 4 RBC counts of more than 10,000 in the radiographically normal subjects while 8 of 11 (72.7%) SAH positive LPs had counts of more than 10,000 (range =11,665–2,223,563). An RBC count between 10,000–20,000 corresponded to a rNPV of 0% and a rPPV of 100%. Sensitivity and specificity were 54.5% (95% CI = 40.2–54.5) and 100% (99.1–100) respectively. Data for the other RBC ranges is summarized in table 2.
The mean percent change from tube 1 to 4 (table 1, figure 2) was – 82.1% (95% CI = −84.9 to −79.9) for the radiographically normal subject LP group and – 9.1% (95% CI = −19.3 to 2.1) for the SAH group (Table 1). The radiographically normal subject LP group had a tube 1 to 4 RBC clearance of at least 70% in 88% of the sample (125 of 142). In contrast, none of the 11 SAH RBC counts cleared more than 30%, and 4 out of 11 (36.4%) increased in value.
Figure 2.
Mean percentage decrease in RBC counts between tubes 1 and 4 for SAH positive and traumatic LPs
DISCUSSION
The current literature makes no recommendation regarding the CSF RBC cutoff value at which one may exclude clinically significant SAHs in patients with negative CT scans who have had LPs. In addition, there is no consensus on the amount of RBC clearance between tubes 1 and 4 that defines a traumatic LP. The results of this study suggest that it may be possible to use the two parameters to guide the emergency medicine physician in the management and the disposition of a patient who has had a negative CT. These two parameters include the absolute CSF RBC count and RBC clearance from tube 1 to 4.
The study results suggest that there may be a CSF RBC cutoff value at which one can safely exclude a radiographically detectable SAH. In our study, an RBC count in tube 4 of 500 or less corresponded to a negative predictive value for SAH of 100%. This number was statistically significant and independent of clearance from tube 1 to 4. The sensitivity for SAH in this RBC range was 100%. There was an SAH positive LP with a RBC count of 990. Thus the sample size needs to be larger to verify that this range is safe. Preliminarily, however, it appears that an RBC count of less than 500 may safely exclude a radiographically detectable SAH.
Interestingly, there were no LPs with an RBC count of more than 10,000 in the radiographically normal subject group. There were 8 SAH positive LPs in this range. Therefore, the study data suggest that a tube 4 RBC count of more than 10,000, irrespective of clearance from tube 1 to 4, is suspicious for a radiographically detectable SAH. These patients should undergo further radiographic evaluation, including angiography. This is an important preliminary finding, as previous authors have suggested that CSF RBC clearance is a more important determining factor than absolute number of RBCs in tube 4. The study suggests that there may indeed be a cutoff value at which SAH is independent of RBC clearance.
The intermediate RBC ranges are more difficult to interpret. There were 39 samples in the RBC range of 500–10,000; 36 of these had radiographically normal studies. SAH positive LPs accounted for 7.7% (3/39) of this RBC range. This is a high percentage. Although more data is needed before adequate interpretation of these findings can be made, our findings suggest that patients within this CSF RBC range cannot safely be discharged without further study.
The data for RBC clearance from tube 1 to 4 is dramatic. RBC clearance in the radiographically normal subject LP group averaged 82.1% compared to 9.1 % in the SAH group. In addition, a large proportion (88%) of the radiographically normal subject group cleared at least 70% from tube 1 to 4. All of the samples in the radiographically normal subject group cleared by at least 43%. There were no SAH positive LPs that cleared more than 30%, and 4 of the 11 samples actually increased in RBC count from tube to 1 to 4. Thus, there was no overlap between the two groups in terms of CSF RBC clearance. Our study suggests that an RBC count in tube 4 that is at least 70% less than that in tube 1 excludes a radiographically detectable SAH. This is a much more conservative value than previous estimates that an LP may be considered traumatic if the RBC count clears more than 30%. 12 Analysis of this data may guide the emergency medicine physician in the management and disposition of patients in which a radiographically detectable SAH may not be excluded. According to this study, a CSF RBC count of less than 500 coupled with an RBC decrease from tube 1 to 4 of at least 70% may exclude patients with a radiographically detectable SAH.
This pilot study was limited by several factors. The sample size, although adequate in the radiographically normal subject group, consisted of only a small number of subjects in the SAH group. A greater sample population would decrease the chance of a type I error. In addition, LP data was not available for all patients in whom SAH was diagnosed during the study timeframe, and it is possible that this data would change the conclusions derived. The subjects in the radiographically normal group were not followed up to find out if any death occurred from a SAH. Additionally, two-tube data was not available for each LP included in the study. Finally, not all the subjects in the radiographically normal group received an angiogram. The angiogram is the gold standard for diagnosis of a SAH; however, it is expensive and difficult to obtain in an expeditious manner. Despite these deficiencies, this study yields important preliminary information in an area where none currently exists. Based on the study data, it may be possible for emergency medicine physicians to utilize CSF RBC analysis to safely exclude a radiographically detectable SAH.
REFERENCES
- 1.Shah KH, Edlow JA. Distinguishing traumatic lumbar puncture from true subarachnoid hemorrhage. J of Emer Med. 2002;23:67–74. doi: 10.1016/s0736-4679(02)00464-x. [DOI] [PubMed] [Google Scholar]
- 2.Bonita R, Thomson S. Subarachnoid hemorrhage: epidemiology, diagnosis, management, and outcome. Stroke. 1985;16:591–4. doi: 10.1161/01.str.16.4.591. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–8. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 5.Chyatte D, Tindall G, Cooper P. Diagnosis and management of aneurysmal SAH In: The Practice of Neurosurgery. Williams and Wilkins; 1996. [Google Scholar]
- 6.Edlow JA, Wyer PC. Evidence-based emergency medicine/clinical question. How good is a negative cranial computed tomographic scan result in excluding subarachnoid hemorrhage? Ann Emerg Med. 2000;36:507–16. doi: 10.1067/mem.2000.109449. [DOI] [PubMed] [Google Scholar]
- 7.Van der Wee N, Rinkel GJ, Hasan D, van Gijn J. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry. 1995;58:357–9. doi: 10.1136/jnnp.58.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeulen M. Subarachnoid haemorrhage: diagnosis and treatment. J Neurol. 1996;243:496–501. doi: 10.1007/BF00886869. [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern LB, Luna-Gonzales H, Huber JC, Jr, et al. Worst headache and subarachnoid hemorrhage: prospective, modern computed tomography and spinal fluid analysis. Ann Emerg Med. 1998;32:297–304. [PubMed] [Google Scholar]
- 10.Leblanc R. The minor leak preceding subarachnoid hemorrhage. J Neurosurg. 1987;66:35–9. doi: 10.3171/jns.1987.66.1.0035. [DOI] [PubMed] [Google Scholar]
- 11.Lang DT, Berberian LB, Lee S, Ault M. Rapid differentiation of subarachnoid hemorrhage from traumatic lumbar puncture using the Ddimer assay. Am J Clin Pathol. 1990;93:403–5. doi: 10.1093/ajcp/93.3.403. [DOI] [PubMed] [Google Scholar]
- 12.Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnnoid hemorhage without detectable aneurysm. A review of the causes. Stroke. 1993;24:1403–9. doi: 10.1161/01.str.24.9.1403. [DOI] [PubMed] [Google Scholar]
- 13.Stieg PE, Kase CS. Intracranial hemorrhage: diagnosis and emergency management. Neurol Clin. 1998;16:373–90. doi: 10.1016/s0733-8619(05)70069-4. [DOI] [PubMed] [Google Scholar]
- 14.Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36. doi: 10.1056/NEJM200001063420106. [DOI] [PubMed] [Google Scholar]