Abstract
Syphilis outbreaks in the United States have been reported since 2000 with highest rates in the South and many cases among HIV-infected individuals. We evaluated incident syphilis cases and concurrent gonorrhea and chlamydia screening at a southern HIV clinic. A retrospective cohort study included HIV-infected patients with at least one reactive plasma reagin (test for serum reagin antibodies to cardiolipin-cholesterol-lecithin antigen) and primary care visit from July 2004 to June 2007. Primary, secondary, and early latent syphilis cases were identified as incident syphilis and evaluation for gonorrhea and chlamydia within 1 month were described. Logistic regression was performed to determine factors associated with incident syphilis. Among 1544 patients, 40 incident syphilis cases were identified (5 primary, 29 secondary, and 6 early latent). The majority of patients were not virologically suppressed and only 25% had gonorrhea and chlamydia testing. In adjusted analyses, younger age (0.57 per 10 years, 95% confidence interval [CI] 0.41–0.80) and minority race (2.26, 95% CI 1.12–4.59) were associated with incident syphilis. Among 40 incident syphilis cases, only 1 in 4 were further tested for gonorrhea and chlamydia. These low rates are concerning as concurrent sexually transmitted infections (STIs) increase risk for HIV transmission. HIV care provider education with emphasis on STI testing in the setting of incident syphilis is key in prevention.
Introduction
In the year 2000, the lowest documented rates of primary and secondary syphilis cases were recorded in the United States since reporting began in 1941, and the goal of syphilis elimination appeared to be within reach. However, from 2001 to 2007 an upswing in syphilis cases was noted. Based on 2007 Centers for Disease Control and Prevention (CDC) data, primary and secondary syphilis cases increased nearly 18% from 2006 to 2007 with more cases occurring in the southern United States (48.8%) than in other regions. The University of Alabama at Birmingham (UAB) 1917 HIV/AIDS Clinic (1917 Clinic) is located in Jefferson County, Alabama, the county with the highest rates of primary and secondary syphilis among the top 61 counties and independent cities that accounted for 70% of primary and secondary syphilis cases in 2007.1
The increase in primary and secondary syphilis cases over the last 7 years in the United States has disproportionately affected males (from 3.0 to 6.6 cases per 100,000 population) and in particular men who have sex with men (MSM). MSM were estimated to account for 65% of syphilis cases in 2007 based on information from 44 states and Washington, D.C., increasing from an estimated 4% of cases in 2000. Furthermore, an increasing number of primary and secondary syphilis cases have been observed in HIV coinfected MSM.1 At the same time, patients with syphilis are also at high risk for other sexually transmitted infections (STIs) such as gonorrhea and chlamydia. Comprehensive STI screening in the setting of a new diagnosis of syphilis is recommended.2,3
As an HIV clinic with a large population of MSM in Jefferson County, Alabama, the 1917 Clinic was poised to experience the full impact of the domestic syphilis epidemic that has continued to grow since 2000. The objectives of this retrospective cohort study of HIV-infected patients in continuing care were to determine factors associated with syphilis risk by comparing sociodemographic and clinical characteristics of individuals who developed syphilis (primary, secondary or early latent) to those who screened negative and to describe both relevant laboratory markers and the frequency of timely screening for concurrent STIs in those who developed syphilis during the study period.
Methods
The UAB 1917 Clinic Cohort Observational Database Project (UAB 1917 Clinic Cohort) is a prospective cohort study that contains detailed sociodemographic, psychosocial, and clinical information from over 6000 clinic patients dating back to 1988 (www.uab1917cliniccohort.org). Currently, over 1600 patients receive primary and subspecialty HIV care at the clinic and are followed in the Institutional Review Board (IRB)-approved prospective study. The 1917 Clinic uses a locally programmed electronic medical record that imports all laboratory values from the central UAB laboratory, requires electronic prescription for all medications, and contains detailed encounter notes. The electronic medical record and database are 100% quality controlled, with all provider notes reviewed within 72 hours of entry into the system to ensure appropriate data capture regarding diagnoses and medications, including start and stop dates for antiretrovirals and all other prescribed drugs. This retrospective cohort study nested in the UAB 1917 Clinic Cohort was approved by the UAB IRB.
Study sample and procedures
Patient data were retrieved through a combination of UAB 1917 Clinic Cohort Database queries supplemented by manual medical record abstraction. Initial electronic (Microsoft SQL query) data extraction for clinical and sociodemographic variables was performed on HIV-infected patients aged 19–75 years old who had undergone rapid plasma reagin (RPR) testing for syphilis (routine care or otherwise) and had attended at least one primary care visit at the UAB 1917 Clinic from July 2004 to June 2007. Additional targeted medical record abstraction was undertaken for patients with reactive RPR values to confirm new syphilis cases and to retrieve additional data. Data on Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) testing (urine, urethral, cervical, rectal, and/or pharyngeal tests utilizing nucleic acid amplification tests or cultures) within 1 month of syphilis diagnosis were extracted via manual chart abstraction in order to examine the comprehensiveness of concurrent STI testing. The STI testing interval (within 30 days of a positive RPR that lead to the diagnosis of incident syphilis) was chosen for the reasons that follow. While testing of high-risk HIV-infected individuals without STI symptoms is recommended every 3–6 months, testing for concurrent STIs in the setting of an active STI such as syphilis is a cornerstone of STI management and control.3 Furthermore, the testing interval definition was liberal in that it allowed for the fact that the provider may not test for concurrent STIs on the specific day that the positive RPR test was performed and provided for the opportunity to call the patient back into clinic for syphilis treatment and additional testing. Additional STI testing data collected within the context of two ongoing studies at the 1917 Clinic during the same study period were extracted from the study databases and counted as STI testing for the purposes of this analysis as providers of individuals participating in these studies may have deferred routine STI testing in favor of testing through the study.
Study variables
Patient-level characteristics including age, race, sex, sexual preference, years in care at the 1917 Clinic, and CD4 +T lymphocyte nadir were recorded. Patient sexual preference was abstracted from charts with male patients defined as MSM if they reported a history of sex with men only or sex with men and women. Men were classified as heterosexual if they reported sex with women only. Women were classified as heterosexual regardless of sexual preference as data related to female homosexual activity is limited. Patients with unknown sexual preference were excluded from analyses (n = 28). Provider diagnoses of affective mental health disorders (depression, anxiety, bipolar disease), substance abuse disorders (cocaine/crack, methamphetamines, opiates, injection drug use [IDU], or polysubstance abuse), and alcohol abuse disorder were extracted from the cohort database. The primary outcome measure was the diagnosis of incident syphilis, defined as a confirmed new diagnosis of primary, secondary, or early latent syphilis during the study period. Primary and secondary syphilis cases included individuals with a positive RPR and clinically compatible signs documented on physical examination. Early latent syphilis cases included individuals with a positive RPR, without signs or symptoms of syphilis documented in the chart and a documented negative RPR within the previous 12 months.4 Those with previously treated syphilis or syphilis of unknown duration were excluded from the incident syphilis population and included with the syphilis negative group as time of infection could not be confirmed, and they did not meet the study definition for incident syphilis.
Statistical analysis
Descriptive statistics were used to evaluate overall patient-level characteristics and bivariate analyses (χ2, t tests) were used to compare patient-level characteristics between the incident syphilis and syphilis negative groups. Descriptive statistics for HIV disease markers (CD4 count, plasma HIV viral load value ± 180 days from incident syphilis diagnosis) and additional testing for GC and CT (≤30 days from syphilis diagnosis) were reported in the incident syphilis group. Unadjusted and adjusted logistic regression models were performed to characterize factors associated with incident syphilis diagnosis during the study period using SAS V9.1.3 software (SAS Institute, Cary, NC).
Results
Among 1544 patients meeting study inclusion criteria, 40 cases (2.6%) of incident syphilis were identified and compared to 1504 syphilis-negative patients. Overall, the majority of patients were male (76%) and MSM (57%) with the following race/ethnicity distribution (49% white, 49% black, and 2% other). The “other” group consisted of the following: American Indian (n = 1), Asian (n = 2), Hispanic (n = 17), Multiracial (n = 2) and other/unknown (n = 8). Average age was 39.4 ± 9.8 years, and 45% had received HIV care at the 1917 Clinic for more than 5 years, while 17% had been in care for less than a year. A provider diagnosis of an affective mental health disorder was present in 56%, substance abuse in 25%, and 17% had alcohol abuse documented. The mean CD4 count nadir for the overall sample was 190 ± 184 cells/mm3. Bivariate analyses revealed statistically significant differences (p < 0.05) among age, gender, and race between the incident syphilis and syphilis negative groups (Table 1). New syphilis cases were found with greater frequency among MSM of black/other race (5.6%) compared to minority race/ethnicity heterosexuals (2.0%), white MSM (2.0%), and white heterosexuals (1.0%; p < 0.05, data not shown). Logistic regression modeling was utilized to determine factors associated with an increased risk for incident syphilis diagnosis during the study period (primary, secondary, or early latent cases). In the unadjusted analyses, those who were younger (odds ratio [OR]: 0.57 per 10 years, 95% CI: 0.41–0.80), male (OR: 2.88, 95% CI: 1.02–8.15), and black or other minority race/ethnicity (OR: 2.07, 95% CI: 1.06–4.04) were at higher risk for incident syphilis. In the multivariable logistic regression model, black/other race (adjusted odds ratio [AOR]: 2.26, 95% CI: 1.12–4.59) was independently associated with an increased risk for incident syphilis, whereas increasing age (AOR: 0.62 per 10 years, 95% CI: 0.44–0.87) was associated with a lower risk. No other variables were statistically significant (Table 2).
Table 1.
Characteristics Associated with Incident Syphilisa Diagnosis in HIV-Infected Individuals Receiving Care at UAB 1917 Clinic from July 2004 to June 2007
| Characteristics | Overall | Syphilis+ | Syphilis− | p Value |
|---|---|---|---|---|
| Ageb | 39.4 ± 9.8 | 34.4 ± 10.2 | 39.5 ± 9.7 | 0.001 |
| Gender | ||||
| Male | 1175 (76.1) | 36 (90.0) | 1139 (75.7) | 0.04 |
| Female | 369 (23.9) | 4 (10.0) | 365 (24.3) | |
| Race | ||||
| White | 763 (49.4) | 13 (32.5) | 750 (49.9) | 0.03 |
| Black/otherc | 781 (50.6) | 27 (67.5) | 754 (50.1) | |
| Sexual preference | ||||
| Heterosexual | 661 (42.8) | 11 (27.5) | 650 (43.2) | 0.05 |
| MSM | 883 (57.2) | 29 (72.5) | 854 (56.8) | |
| Gender/race | ||||
| Fem_black/other | 261 (16.9) | 4 (10.0) | 257 (17.1) | |
| Fem_white | 108 (7.0) | 0 (0) | 108 (7.2) | 0.01 |
| Male_black/other | 520 (33.7) | 23 (57.5) | 497 (33.0) | |
| Male_white | 655 (42.4) | 13 (32.5) | 642 (42.7) | |
| Time in care | ||||
| <1 yr | 256 (16.6) | 6 (15.0) | 250 (16.6) | |
| 1–5 yrs | 594 (38.5) | 20 (50.0) | 574 (38.2) | 0.30 |
| >5 yrs | 694 (44.9) | 14 (35.0) | 680 (45.2) | |
| Affective mental health disorder | ||||
| Yes | 862 (55.8) | 17 (42.5) | 845 (56.2) | 0.09 |
| No | 682 (44.2) | 23 (57.5) | 659 (43.8) | |
| History of substance abuse | ||||
| Yes | 387 (25.1) | 11 (27.5) | 376 (25.0) | 0.72 |
| No | 1157 (74.9) | 29 (72.5) | 1128 (75.0) | |
| History of alcohol abuse | ||||
| Yes | 256 (16.6) | 5 (12.5) | 251 (16.7) | 0.48 |
| No | 1288 (83.4) | 35 (87.5) | 1253 (83.3) | |
| CD4 nadir | 190 ± 184 | 224 ± 151 | 189 ± 184 | 0.23 |
Incident syphilis included primary, secondary, or early latent syphilis.
Age was defined for all study participants at the beginning of the study period, July 1, 2004.
Forty-nine percent of individuals in this group were black, while 2% were in the other group, which included patients with American Indian, Asian, Hispanic, multiracial, and other/unknown race.
MSM, men who have sex with men.
Table 2.
Adjusted and Unadjusted Logistic Regression Analyses of Characteristics Associated with Incident Syphilisa Diagnosis in HIV-Infected Persons in 1917 Clinic from July 2004 to June 2007
| Characteristics | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age (OR per 10 yr)b | 0.57 (0.41–0.80) | 0.62 (0.44–0.87) |
| Gender | ||
| Male vs. female | 2.88 (1.02–8.15) | 2.50 (0.72–8.68) |
| Race | ||
| Black/otherc vs. white | 2.07 (1.06–4.04) | 2.26 (1.12–4.59) |
| Sexual preference | ||
| MSM vs. heterosexual | 2.01 (1.00–4.05) | 1.58 (0.67–3.75) |
| Time in care | ||
| <1 yr | 1.0 | — |
| 1–5 yrs | 1.45 (0.58–3.66) | |
| >5 yrs | 0.86 (0.33–2.26) | |
| Affective mental health disorder | ||
| Yes vs. no | 0.58 (0.31–1.09) | — |
| History of substance abuse | ||
| Yes vs. no | 1.14 (0.56–2.30) | — |
| History of alcohol abuse | ||
| Yes vs. no | 0.71 (0.28–1.84) | — |
| CD4 nadir (OR per 50 cells) | 1.05 (0.97–1.13) | — |
Incident syphilis included primary, secondary, or early latent syphilis.
Age was defined for all study participants at the beginning of the study period, July 1, 2004.
Forty-nine percent of individuals in this group were black, while 2% were in the other group, which included patients with American Indian, Asian, Hispanic, Multiracial, and other/unknown race.
OR, odds ratio; CI, confidence interval; MSM, men who have sex with men.
Of the 40 incident syphilis cases, secondary syphilis (n = 29) was most common followed by primary (n = 5) and early latent (n = 6). In those with primary syphilis, chancres were identified as penile (n = 3) and perianal (n = 2).The majority of cases occurred in patients of black/other race (n = 27). Patients with early latent syphilis were older (38 ± 10.2 years). Those with primary and early latent syphilis were more commonly on concurrent antiretroviral therapy (100% and 83%, respectively) than individuals diagnosed with secondary syphilis. Median RPRs for each group were as follows: primary (1:16), secondary (1:64), and early latent (1:64). Median CD4 values were highest in those with primary syphilis (524 cells/mm3) and lowest in those with early latent disease (278 cells/mm3). Median plasma HIV viral load was highest in the secondary syphilis group (2235 copies per milliliter) and lowest in the primary syphilis group (49 copies per milliliter; Table 3). The majority of patients were not virologically suppressed below 50 copies per milliliter (total not suppressed = 67%; 30% [n = 12] 50–5000 copies per milliliter, 22% [n = 9] 5001–50,000 copies per milliliter, 15% [n = 6] > 50,000 copies per milliliter).
Table 3.
Selected Characteristics of HIV-Infected Individuals Diagnosed with Incident Syphilis
| Syphilis stage (number) | Mean age ± SD | Median RPR | Percent taking ART | Median CD4 (cells/mL3)a | Virologic suppression(< 50 copies/mL3)a | Median viral load (copies/mL3)a |
|---|---|---|---|---|---|---|
| Primary (5) | 34 ± 10.5 | 1:16 | 100% | 524 | 60% (n = 3) | 49 |
| Secondary (29) | 33 ± 10.4 | 1:64 | 48% | 336 | 34% (n = 10) | 2235 |
| Early latent (6) | 38 ± 10.2 | 1:64 | 83% | 278 | 0% (n = 0) | 283 |
CD4 count and viral load ± 180 days from syphilis diagnosis.
SD, standard deviation; RPR, rapid plasma reagin; ART, antiretroviral therapy.
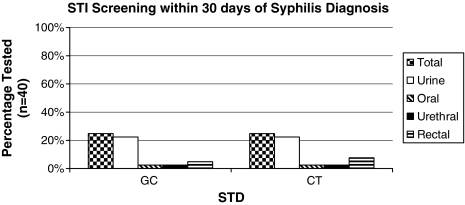
Only 10 of the 40 incident syphilis cases (25%) were tested for gonorrhea (GC) and/or chlamydia (CT) at one or more anatomic sites (rectum, pharynx, and/or genitalia) within 30 days of syphilis diagnosis. All of those tested were male and MSM with equal race distribution, including 50% white (n = 5) and 50% black (n = 5). During our study period, STI tests could have been performed independently by the primary care provider or through one of two studies providing additional STI testing at the 1917 Clinic, both of which had overlapping enrollment periods. Of the 10 patients tested, 2 were referred for GC or CT testing as part of one of these research protocols, rather than routine clinical care. Urine tests were most commonly utilized for GC and CT evaluation (25%) followed by rectal (5% for GC, 8% for CT) and oral (3%; Fig. 1). Of note, all of those in our study population tested for GC and CT at various anatomic sites were negative.
FIG. 1.
Sexually transmitted infection (STI) for gonorrhea and chlamydia at various sites within 30 days of new syphilis diagnosis in HIV-infected persons at 1917 Clinic from July 2004 to June 2007. All patients tested (n = 10; 25%) were male and men who have sex with men (MSM). STI testing was performed by clinicians in 8 cases, whereas 2 individuals were tested through a research study. A total of 3 patients (2 clinicians, 1 research study) were tested in more than one anatomic site of exposure. GC, Neisseria gonorrhoeae; CT, Chlamydia trachomatis; STD, sexually transmitted disease.
Discussion
Our study confirms a concerning number of new syphilis cases among persons receiving continuing care for HIV in a county with the highest rate of syphilis in the nation. Despite the clear risk for additional STIs, in our study only 25% of HIV-infected patients with a new diagnosis of syphilis were tested for gonorrhea or chlamydia within 30 days. As the majority of incident syphilis cases presented with active signs and symptoms of newly acquired syphilis, we suspected that providers would be more likely to consider and test for concurrent STIs in this setting. However, this turned out not to be the case. These outcomes mirror alarming trends seen in other studies regarding poor STI testing rates observed in HIV-infected individuals.5–7 The 1917 Clinic has served the HIV-infected population of Alabama and surrounding states since 1988. With the clinic staff's wealth of experience in secondary HIV and STI prevention and concurrent studies focusing on enhanced STI screening at the clinic during our study period, ideal conditions for aggressive STI screening would have been expected. Our results belie this conclusion, as despite these advantages, in the month following a new diagnosis of syphilis only 25% of cases were tested for GC and/or CT. With a new diagnosis of syphilis, testing for other STIs such as GC and CT is important not just for primary case identification and secondary prevention of these infections but also due to their association with increases in HIV viral load and/or shedding that increases the transmission risk of HIV from coinfected patients.8–12
Among the 40 cases of syphilis diagnosed during the study period, more than two thirds were on antiretroviral therapy (ART). Near the time of syphilis diagnosis (±180 days), only one third had undetectable viral loads. Stated another way, the majority of patients were not virologically suppressed, underscoring the risk for HIV transmission. In the setting of active syphilis infection and absence of viral suppression, risk of HIV transmission is even higher as syphilis may enhance and facilitate transmission.10,11,13–15 Syphilis as well as gonorrhea and chlamydia have been shown to increase the chance of HIV transmission.11 Genital ulceration seen in primary syphilis infections has been shown to increase the risk of HIV transmission between twofold and ninefold.16 Evidence has shown that syphilis may increase viral load of HIV and decrease CD4 counts, increasing the risk of transmissibility especially in secondary syphilis and in those not receiving antiretroviral therapy, while treatment reverses this.13–15 At least one study disputes this notion.17 Furthermore, such low STI screening rates in an HIV-infected population serves to underscore the disquieting gap that exists between the STI testing guidelines endorsed by several national organizations3 and the reality of current clinical practice. Therefore, provider education with an emphasis on the implementation of STI screening guidelines in individuals with HIV should be a vital component of national STI prevention policy.
As is the case nationally, the higher rates of syphilis seen in our black patients demonstrate continuing health disparities. Black race was found to double the odds for incident syphilis in the study period. In our population, while MSM sexual preference did not reach statistical significance in the adjusted model, MSM of black/other race bore the highest burden of new syphilis cases (5.6%). This is consistent with previous studies, including a report from North Carolina of increased risk of syphilis in HIV-infected young black men who were MSM, bisexual or reported anonymous sex.18 Increased syphilis risk in blacks has also been reported in the general population per CDC data, with the rate of syphilis transmission in black men compared to whites noted to be at least fivefold higher in 200619 and more than 6.0 times higher in 2007.1 Interestingly, the high burden of STIs and HIV found in the black MSM population, when compared to other MSM populations, cannot simply be explained by increased sexual risk behaviors practiced by this population and will require innovative approaches to STI prevention in order to address this problem.20,21
Younger age was also associated with incident syphilis in this study. Over the past several years, CDC data has reported the highest syphilis rates in those in the 30–39 year old age range. From 2005 to 2006, CDC data reveal the largest increase in primary and secondary syphilis to be found in 20–29 year olds with the highest rates in those aged 25–29.19 The latest CDC data from 2007 reveals highest syphilis rates continuing in those aged 20–29 years old.1 A similar trend was described in the recent MMWR report of the Jefferson County, Alabama, syphilis epidemic in which the proportion of cases in persons aged less than 30 years increased from 22.4% during 2002–2004 to 37.4% during 2005–2007 (p = 0.016).22 In concordance with the decreasing age of individuals newly diagnosed with syphilis nationally as well as within the county in which the clinic is located, our data appear to follow this trend as every 10-year increase in age was found to at least halve the risk of acquiring syphilis infection in our study population. These data suggest an additional population in which expanded educational and prevention efforts may afford higher returns for secondary prevention.
The findings of our study should be interpreted within the context of the study limitations. As a single academic HIV treatment center in the southeastern United States, the results may not be generalizable to other regions of the country or to international locations. As with all retrospective observational studies, our findings can point to associations, but not prove causality between these and the outcome of interest. Regarding sexual preference, we did not account for female homosexual activity; all of the females were classified as heterosexual as risk data related to female homosexuality is more limited. Additionally, we acknowledge that gathering such sensitive information as sexual preference may lead to bias as declaration of same gender sexual behavior may be less accepted in particular cultures/regions, leading to an underestimation of the proportion of cases attributable to MSM. Furthermore, limitations may exist regarding the documentation of incident syphilis cases and STI testing as patients may have had tests for syphilis and/or other STIs performed at an outside clinic that would potentially lead to an underestimate of the numbers of incident syphilis cases as well as an underestimate of the proportion of patients with incident syphilis who had received concurrent STI testing. The fact that STI testing results are not readily available among sites presents an additional challenge to the primary care providers in trying to coordinate the care of these individuals.
In summary, our study suggests that in this HIV-infected population, younger individuals of minority race/ethnicity were at greatest risk of acquiring syphilis and represent a priority population for prevention counseling in clinical settings. The fact that only one in four patients with incident syphilis received concurrent STI testing in our subspecialty HIV clinic that includes a research focus on STI testing and prevention should serve as a call to action as it highlights the disparity that exists between STI guideline recommendations and implementation. More aggressive implementation of STI guidelines and prevention efforts are needed to address the exponential growth in domestic syphilis rates, particularly among HIV-infected patients in whom concurrent STIs such as syphilis, gonorrhea or chlamydia increase the risk of HIV transmission.8–14 Data from one study suggest that at least semiannual testing among all HIV-infected individuals regardless of reported sexual activity or behavioral factors is effective and at minimal cost by increasing early diagnoses, thereby reducing the infectious period of those with STIs.23 In areas where outbreaks have occurred, routine screening every 3 months along with CD4 lymphocyte count and HIV viral load testing identified significant numbers of asymptomatic early syphilis.24 Given the success of routine screening, the resurgence of syphilis, and the paucity of testing for other STIs, we support the integration of routine periodic STI screening in HIV primary care.
Acknowledgments
We thank the University of Alabama at Birmingham 1917 Clinic HIV/AIDS Clinic Cohort management team for their assistance with this project. We thank the 1917 Clinic Informatics team, especially Eugene Gibson, for their invaluable assistance in data extraction. We also thank Dr. Terri Forney for her contribution to the project. J.H.W. has received research funding from the Bristol-Myers Squibb Virology Fellows Research Program for the 2006–2008 Academic Years. M.J.M. has received recent research funding from Tibotec Therapeutics and has consulted for Bristol-Myers Squibb and Gilead Sciences.
Supported by the University of Alabama at Birmingham Center for AIDS Research (grant P30-AI27767), the Mary Fisher CARE Fund, and the CFAR Network of Integrated Clinical Sites (CNICS; grant 5R24-AI 067039).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Division of STD Prevention. Atlanta: Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; c2009. [Jun 18;2009 ]. Sexually Transmitted Disease Surveillance 2007 Supplement: Syphilis Surveillance Report [Internet] [Google Scholar]
- 2.Guidance for STD clinical preventive services for persons infected with HIV. Sex Transm Dis. 2001;28:460–463. doi: 10.1097/00007435-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2003;52:1–24. [PubMed] [Google Scholar]
- 4.Holmes KK. Sparling PF. Stamm WE, et al. Sexually Transmitted Diseases. 4th. New York: McGraw Hill Medical; 2008. [Google Scholar]
- 5.Hutchinson J. Goold P. Wilson H. Jones K. Estcourt C. Sexual health care of HIV-positive patients: An audit of a local service. Int J STD AIDS. 2003;14:493–496. doi: 10.1258/095646203322025821. [DOI] [PubMed] [Google Scholar]
- 6.Kahle E. Zhang Q. Golden M. Goldbaum G. Buskin S. Trends in evaluation for sexually transmitted infections among HIV-infected people, King County, Washington. Sex Transm Dis. 2007;34:940–946. doi: 10.1097/olq.0b013e31813e0a48. [DOI] [PubMed] [Google Scholar]
- 7.Lister NA. Fairley CK. Read T. Mijch A. Screening for STIs in individuals with HIV infection. Sex Transm Infect. 2002;78:387–388. doi: 10.1136/sti.78.5.387-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghys PD. Fransen K. Diallo MO, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d'Ivoire. AIDS. 1997;11:F85–F93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 9.McClelland RS. Wang CC. Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 10.Johnson LF. Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–959. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 11.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 12.Cohen CE. Winston A. Asboe D, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect. 2005;81:217–219. doi: 10.1136/sti.2004.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchacz K. Patel P. Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–2079. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palacios R. Jimenez-Onate F. Aguilar M, et al. Impact of syphilis infection on HIV viral load and CD4 cell counts in HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44:356–359. doi: 10.1097/QAI.0b013e31802ea4c6. [DOI] [PubMed] [Google Scholar]
- 15.Kofoed K. Gerstoft J. Mathiesen LR. Benfield T. Syphilis and human immunodeficiency virus (HIV)-1 coinfection: Influence on CD4 T-cell count, HIV-1 viral load, and treatment response. Sex Transm Dis. 2006;33:143–148. doi: 10.1097/01.olq.0000187262.56820.c0. [DOI] [PubMed] [Google Scholar]
- 16.Fleming DT. Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadiq ST. McSorley J. Copas AJ, et al. The effects of early syphilis on CD4 counts and HIV-1 RNA viral loads in blood and semen. Sex Transm Infect. 2005;81:380–385. doi: 10.1136/sti.2004.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sena AC. Torrone EA. Leone PA. Foust E. Hightow-Weidman L. Endemic early syphilis among young newly diagnosed HIV-positive men in a southeastern U.S. state. AIDS Patient Care STDs. 2008;22:955–963. doi: 10.1089/apc.2008.0077. [DOI] [PubMed] [Google Scholar]
- 19.Division of STD Prevention. Atlanta, GA: Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; 2007. [May 29;2009 ]. Sexually Transmitted Disease Surveillance 2006. [Google Scholar]
- 20.Millett GA. Flores SA. Peterson JL. Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: A meta-analysis of HIV risk behaviors. AIDS. 2007;21:2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann LH. Grimley DM. Chen H, et al. Risk behaviors in HIV-positive men-who-have-sex-with-men (MSM) participating in an intervention in a primary care setting. Int J STD/AIDS. 2009;20:607–612. doi: 10.1258/ijsa.2009.009030. [DOI] [PubMed] [Google Scholar]
- 22.Primary, secondary syphilis—Jefferson county, Alabama, 2002–2007. MMWR Morb Mortal Wkly Rep. 2009;58:463–467. [PubMed] [Google Scholar]
- 23.Rieg G. Lewis RJ. Miller LG. Witt MD. Guerrero M. Daar ES. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDs. 2008;22:947–954. doi: 10.1089/apc.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winston A. Hawkins D. Mandalia S. Boag F. Azadian B. Asboe D. Is increased surveillance for asymptomatic syphilis in an HIV outpatient department worthwhile? Sex Transm Infect. 2003;79:257–259. doi: 10.1136/sti.79.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]