Abstract
This retrospective cohort study of HIV/hepatitis C virus (HCV) coinfected patients evaluated time trends and rates of HCV evaluation for patients seen between January 1, 1997 and October 30, 2004. Survival analysis and Cox proportional hazards modeling were used to describe the time to evaluation and covariates associated with this outcome. Patients were predominantly white and male. Of 248 eligible patients, 108 (44%) were evaluated for HCV treatment. The median time to evaluation was 2.98 years. Of 108 evaluated, 17 (16%) received at least one dose of interferon and/or ribavirin. The median time to treatment after being evaluated was 1.39 years. Of the 17 (35%) treated 6 patients had a sustained virologic response, but only 2.4% of the original number of patients were cured. Approximately one half of patients in an HIV-specialty clinic were evaluated for HCV therapy and 16% received treatment, but the median time to treatment from the time of HCV diagnosis was over 4 years. Further efforts to identify and to overcome barriers to HCV treatment are warranted.
Introduction
Up to 33% of HIV-infected individuals in the United States are also infected with hepatitis C virus (HCV).1 Liver disease from HCV infection has emerged as a leading cause of morbidity and mortality among HIV-infected individuals.2 Fortunately, recently published trials of pegylated interferon and ribavirin have yielded substantial advances in the field, with 26%–40% of HIV/HCV coinfected patients treated successfully.3–5 Despite these encouraging results, most HIV/HCV coinfected individuals may not be evaluated for or receive therapy.6,7 A recent guideline on HIV quality of care measures, however, has recommended that all newly diagnosed HIV-infected patients receive baseline viral hepatitis testing.8 In this study, the time trends, rates, and predictors of HCV therapy evaluation and HCV therapy initiation were determined in an urban HIV clinic.
Methods
Study population
This was a retrospective cohort study of HIV/HCV coinfected patients, conducted on the University of Washington (UW) HIV cohort, a longitudinal observational study of HIV-infected patients receiving primary care at a public hospital (Harborview Medical Center, Seattle, WA) from 1995 to the present.9 Study subjects were identified from the University of Washington HIV Information System (UWHIS), which captures comprehensive clinical data from all patients seen at the HIV specialty clinic since 1995. The UWHIS contains standardized information about demographics, prior antiretroviral treatment history and prior AIDS-defining illnesses, laboratory test results, clinical encounter data (including all inpatient and outpatient diagnoses), and medications (over 90% of patients obtain their medications from a UW pharmacy; outside pharmacy information is integrated into UWHIS by chart review). Patients were included if they had confirmed HIV infection (reactive HIV enzyme-linked immunosorbent assay [ELISA] followed by either a positive Western blot for HIV or a positive HIV RNA test), had their first visit to the clinic between January 1, 1997 and October 30, 2004, had at least two visits over a 12-month period and had at least 3 months of follow-up clinical visits after the first positive HCV RNA test, and tested positive for HCV RNA (VERSANT bDNA v 2 or 3, Bayer Corp, Tarrytown, NY; or Amplicor 2.0, Roche Diagnostics, Indianopolis, IN; or real-time polymerase chain reaction [PCR] using analyte-specific reagents from Roche). This study was approved by the Human Subjects Division at the University of Washington and all subjects gave written consent for the inclusion of their clinical data in the UWHIS.
HIV specialty clinic
The HIV specialty clinic provides comprehensive medical care for patients infected with HIV. There were over 50 physicians with advanced training in infectious diseases and/or HIV and 10 mid-level providers (physician assistants and nurse practitioners) who provided care during the study time period. As per clinic protocol, all new clinic patients are tested for serologic evidence of viral hepatitis; for patients with risk factors for HCV and CD4 counts less than 200 cells per microliter, HCV RNA testing is also routinely performed (92% of those patients reporting IDU and with a baseline CD4 count less than 200 had an HCV RNA test within 6 months). Referral to hepatitis C treatment specialists is available within the hospital.
Measurements and outcomes
Because most providers will determine the genotype of a patient's HCV infection if they are considering initiating HCV therapy, evaluation for HCV treatment was defined as the provider's decision to order an HCV genotype assay. Patients were excluded if the genotype was done prior to HCV RNA testing. Time to evaluation was defined as the time from the first positive HCV RNA test until HCV genotyping. The number of patients evaluated in each calendar year from 1999–2005 was ascertained. Chart review was performed to determine if providers were anticipating treatment of HCV (but no genotype was performed) and the primary reason for nonevaluation. If a provider explicitly stated why a subject was not being evaluated for HCV treatment, this reason was documented. If no explicit reason was made, judgment of the primary reason for nonevaluation was made by an abstractor who is a physician who routinely treats HIV/HCV coinfected patients (J.S.).
Provider decision to initiate HCV treatment was defined as the dispensation of 1 or more dose of any interferon containing regimen. Time to treatment was calculated from the date of the genotype test to dispensation of antiviral treatment.
Statistical methods
Kaplan-Meier survival analysis was used to describe the time to evaluation. Predictors of evaluation and treatment were assessed by the construction of a Cox proportional hazards model. Age and nadir CD4 count were defined at the time of the first positive HCV RNA assay. Substance abuse and mental health diagnosis were defined as ever having the condition, on or before the date of the first HCV RNA test. Patients were censored at death, the last clinical visit if there was no contact with the clinic for more than 18 months, or on May 20, 2005, whichever occurred earliest. Stata statistical software was used for all analyses (V9.0, StataCorp, College Station, TX).
Results
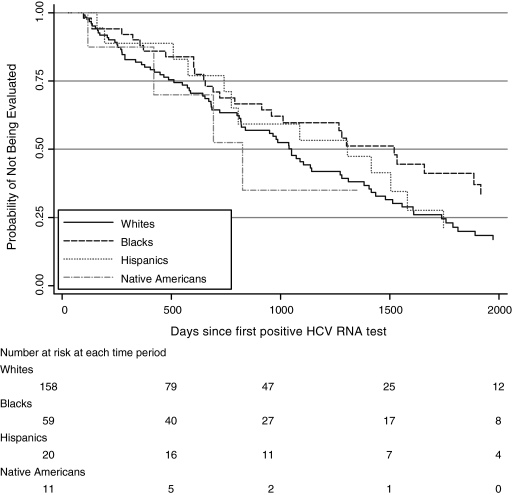
Of 248 eligible HIV/HCV coinfected patients, 108 (44%) were evaluated for possible HCV treatment. Among these 108 who had genotyping, 33 (31%) received at least one liver biopsy. Nine (27%) had stage 3 or 4 fibrosis (Batts-Ludwig scale). There were 43 patients who died during the study. The patients that died were more likely to be evaluated than the patients who lived, 74% versus 54%. The median time to evaluation was 2.98 years (interquartile range [IQR], 1.61–5.12 years; Fig. 1). The evaluation rate peaked in 2001 and 2003–2004, corresponding to the years of publication of the registration trials for pegylated interferon in HCV monoinfected10,11 and HIV/HCV coinfected patients, respectively (Table 1).3–5 Similarly, the number of patients treated peaked in 2003, shortly after pegylated interferon alfa-2b and 2a were approved.
FIG. 1.
Time to evaluation of hepatitis C virus (HCV) among HIV-positive patients by race/ethnic group in University of Washington HIV Information System (UWHIS) cohort, 1997–2004.
Table 1.
Year of Evaluation and Treatment Initiation
| Year | No. evaluated | No. treated |
|---|---|---|
| 1999 | 2 | 0 |
| 2000 | 8 | 2 |
| 2001 | 22 | 1 |
| 2002 | 14 | 3 |
| 2003 | 25 | 7 |
| 2004 | 27 | 3 |
| 2005a | 10 (26) | 1 (3) |
Study censored at May 20, 2005, figures in parentheses are projections based on rate up to the censoring date.
No significant associations were found between patient characteristics and evaluation rates in univariate and multivariate analyses (Table 2). However, we noted a decreased rate of evaluation among African American patients compared to whites (HR = 0.68, 95% confidence interval [CI] 0.45, 1.02), as well as among patients with a nadir CD4 count less than 200 per microliter (HR = 0.83, 95% CI 0.58, 1.18).
Table 2.
Time to Evaluation and Predictors of Being Evaluated for Hepatitis C Virus
| Covariate | n (%) | Median time to evaluation (years) (interquartile range) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Age | ||||
| <40 years old | 110 (44) | 3.12 (1.78–4.86) | Ref | |
| ≥40 years old | 138 (56) | 2.88 (1.57–5.48) | 1.01 (0.98–1.04) | |
| Gender | ||||
| Female | 52 (21) | 3.25 (2.04–4.66) | Ref | |
| Male | 196 (79) | 2.94 (1.40–5.12) | 1.02 (0.68–1.52) | |
| Race/Ethnicity (%) | ||||
| White | 158 (64) | 2.88 (1.40–4.76) | Ref | Ref |
| African American | 59 (24) | 4.16 (1.79–5.54) | 0.66 (0.44–0.99) | 0.68 (0.45–1.02) |
| Hispanic | 20 (8) | 3.58 (2.03–4.78) | 0.75 (0.43–1.30) | 0.80 (0.45–1.41) |
| Alaska Native/American Indian | 11 (4) | 2.26 (1.15–n/a) | 1.25 (0.45–3.42) | 1.35 (0.49–3.74) |
| CD4 cell count (cells/μL)a | ||||
| ≥200 | 141 (57) | 2.70 (1.65–4.71) | Ref | Ref |
| <200 | 106 (43) | 3.79 (1.34–5.39) | 0.81 (0.58–1.13) | 0.83 (0.58–1.18) |
| HIV RNA level (copies/mL)b | ||||
| <500 | 75 (30) | 3.47 (1.65–4.45) | Ref | |
| 500–10,000 | 55 (22) | 3.47 (1.57–5.33) | 0.89 (0.52–1.51) | |
| >10,000 | 117 (47) | 2.77 (1.58–5.17) | 0.93 (0.59–1.46) | |
| Antiretroviral therapyc | ||||
| No | 160 (65) | 2.84 (1.57–5.12) | Ref | |
| Yes | 85 (35) | 3.51 (1.86–4.90) | 0.84 (0.56–1.26) | |
| History of substance abuse | ||||
| No | 62 (25) | 3.68 (1.33–5.20) | Ref | |
| Yes | 186 (75) | 2.88 (1.61–4.97) | 0.99 (0.67–1.42) | |
| History of mental health diagnosis | ||||
| No | 124 (50) | 2.77 (1.61–4.65) | Ref | |
| Yes | 124 (50) | 3.49 (1.51–5.17) | 1.04 (0.74–1.46) | |
Nadir CD4 count considered at time of first HCV RNA test, data not available for 1 patient.
Most recent HIV RNA test considered at time of first HCV RNA test. Data available within 6 months for 201/248 (83%) of patients. Data not available for 1 patient.
On highly active antiretroviral therapy within 6 months of first HCV RNA test; data not available for 3 patients.
n/a, not available; HCV, hepatitis C virus.
The most common reason for not evaluating a patient was ongoing or recent drug abuse (29%), followed by severe concurrent disease (28%), in particular advanced HIV disease or hepatic disease. Depression or other psychiatric condition was the third most common reason (12%).
Of the 108 evaluated patients, 17 (16%) received at least one dose of antiviral therapy for HCV. The median time elapsed time between evaluation and treatment was 1.39 years (IQR, 0.53–2.64 years). No covariates were predictive of being treated, although there was a trend showing patients with a history of substance abuse to be less likely to start treatment (HR = 1.66, 95% CI 0.85–3.22). Four patients who received a liver biopsy were not treated because they had a low fibrosis score (0–1). Of 17 treated patients, 6 (35%) achieved a sustained virologic response (SVR). Thus, of 248 coinfected patients, only 2.4% were successfully treated for hepatitis C infection.
Discussion
In this urban HIV clinic, fewer than half of HCV/HIV coinfected patients were evaluated for possible interferon-based HCV treatment. Only 16% received treatment, and only 2.4% of the entire cohort achieved an SVR. The median time to evaluation for treatment was almost 3 years, a significant period of delay in patients who may have a much more rapid progression to cirrhosis, compared to patients without HIV.12 Similarly, the median time elapsing between evaluation and treatment initiation was 1.4 years. Substance abuse and advanced HIV infection were the primary reasons for nonevaluation. Contrary to what may be expected, patients who died during the study were actually evaluated at a higher rate (74%) than those who lived (54%). This suggests that those in closer, more frequent follow-up were more likely to be evaluated.
The conclusions are limited by the reliance on genotyping as a surrogate measure of being evaluated. Other studies examining this issue have used referral to a liver specialty clinic as a measure of being evaluated.6,7 This outcome measure was not used in the current study because there were five providers in the HIV clinic who managed HCV therapy without specialty consultation. Alternative measures such as liver biopsy were considered, but would have resulted in an even lower “evaluation rate” (33/248, 13%). Furthermore, chart review did not identify additional patients in whom HCV therapy was being contemplated.
Other reasons for nonevaluation of HCV, such as provider turnover and financial barriers, could not be ascertained. However, the hospital has made a commitment to providing HCV therapy in which no other source of funding can be located.
These data provide insights into provider approaches to the management of HCV in HIV-infected patients. The prolonged time intervals between initiation of clinical care and evaluation for HCV treatment may reflect the competing priorities that providers face when first taking over care of HIV/HCV coinfected patients. Issues such as ongoing drug and alcohol use and the need to initiate antiretroviral treatment likely take precedence over evaluation for HCV treatment in this population, as determined in chart review. Indeed, our data suggest that if a patient engages in HIV care long enough, evaluation for HCV treatment eventually occurs. The findings reinforce a recent study that certain subgroups (African Americans, most notably) are slower to link into HIV care and highlights the need to target this group for outreach and engagement into care.13
The numbers of patients evaluated for care peaked during years corresponding to the publication of major clinical trials involving pegylated interferon, as well as Federal Drug Administration approval of pegylated interferon-alfa 2b in 2001 and alfa 2a in 2002. Although our data do not extend beyond 2005, the number of patients treated has leveled off. Thus, these data suggest that providers caring for HIV/HCV coinfected patients are prepared to explore HCV treatment options for their patients, but many barriers to care remain in the patient population. The low rates of treatment initiation and sustained virologic response in this study are similar to the rates reported in veteran,6 homeless,14 and primarily African American HIV/HCV coinfected populations.7
The main implications of our findings are twofold. First, our study suggests that for many HIV providers, evaluation for HCV treatment and initiation of HCV therapy takes time. Second, the low rate of treatment reported by this and other studies reflects the suboptimal nature of currently available therapy for the treatment of HCV in HIV-infected patients. While pegylated interferon and ribavirin therapy are challenging for HCV infected patients without HIV coinfection, the barriers to successful treatment are even more substantial in HIV coinfected patients, in part because of underlying social disruption, ongoing substance use, and issues associated with polypharmacy. Developments in polymerase and protease inhibitor agents hold promise for newer, more effective therapy. However, rapid viral resistance to new HCV treatments make it likely that interferon will continue to be included in HCV treatment regimens. Therefore, strategies to effectively engage coinfected patients in primary care, to integrate substance use treatment into HIV primary care, and to promote adherence to antiretroviral therapy for HIV infection remain priorities in the effective management of HCV/HIV patients.
Acknowledgments
This publication was made possible by Grant Numbers T32 AI 7044-28 (J.S.), K23 AI 51523 (C.W.), K24 AI071113 (A.W.), and P30 AI027757 (M.K.), from the National Institutes of Health, Allergy and Infectious Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
J.S. was also supported by a research grant from Bristol Myers-Squibb Virology. J.S. has received speaking honoraria and research funding from Hoffman La-Roche Pharmaceuticals and speaking honoraria from Gilead.
References
- 1.Staples C., Jr Rimland D. Dudas D. Hepatitis C in the HIV Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): The effect of coinfection on survival. Clin Infect Dis. 1999;29:150–154. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 2.Tedaldi EM. Baker RK. Moorman AC, et al. Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2003;36:363–367. doi: 10.1086/345953. [DOI] [PubMed] [Google Scholar]
- 3.Carrat F. Bani-Sadr F. Pol S, et al. Pegylated inteferon alfa-2b vs standard interferon alfa-2b, plus ribavirin for chronic hepatitis C in HIV-infected patients. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 4.Chung RT. Andersen J. Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torriani FJ. Rodriguez-Torres M. Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 6.Fultz SL. Justice AC. Butt AA. Rabenack L. Weissman R. Rodriguez-Barradas M. Testing, referral, and treatment patterns for hepatitis C virus coinfection in a cohort of veterans with human immunodeficiency virus infection. Clin Infect Dis. 2003;36:1039–1046. doi: 10.1086/374049. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SH. Lucas GM. Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 8.Yehia BR. Gebo KA. Hicks PB, et al. Structures of care in the clinics of the HIV Research Network. AIDS Patient Care STDs. 2008;22:1007–1013. doi: 10.1089/apc.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane HM. Van Rompaey SE. Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20:1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 10.Fried MW. Shiffman ML. Reddy R, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 11.Manns MP. McHutchison JG. Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 12.Benhamou Y. Bochet M. Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 13.Ulett KB. Willig JH. Lin H-Y, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs. 2009;23:41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall CS. Charlebois ED. Hahn JA. Moss AR. Bangsberg DR. Hepatitis C virus infection in San Francisco's HIV-infected urban poor. J Gen Intern Med. 2004;19:357–365. doi: 10.1111/j.1525-1497.2004.30613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]