Abstract
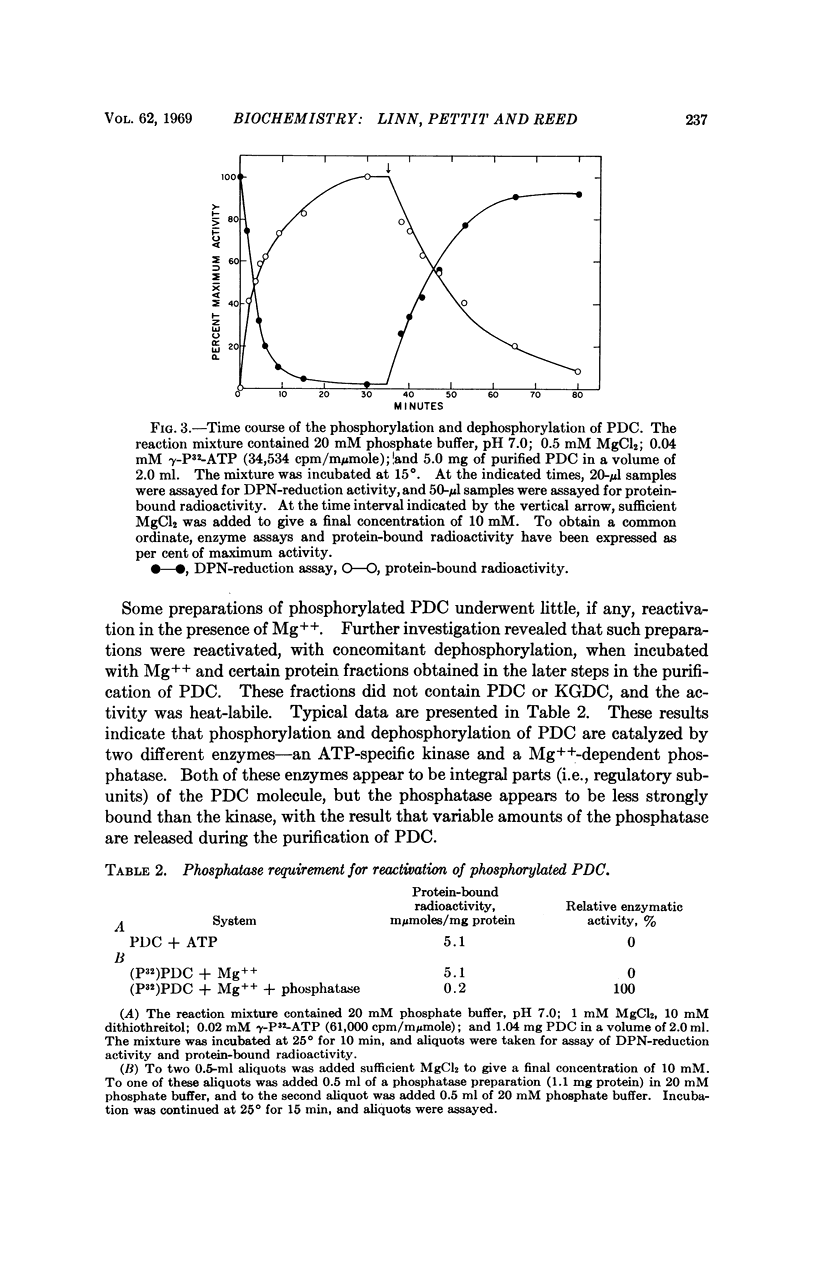
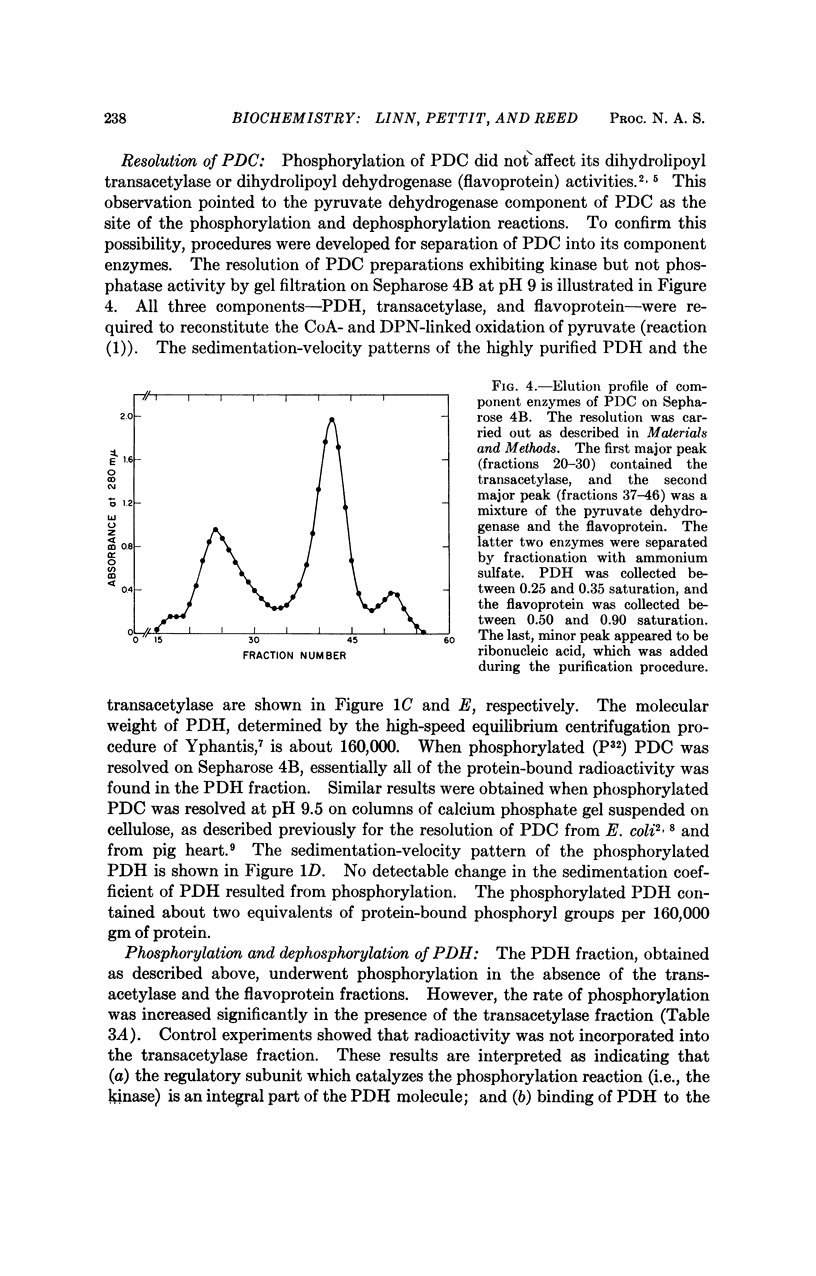
This paper reports the discovery that the activity of the multienzyme pyruvate dehydrogenase complex from beef kidney mitochondria is regulated by a phosphorylation-dephosphorylation reaction sequence. The site of this regulation is the pyruvate dehydrogenase component of the complex. Phosphorylation and concomitant inactivation of pyruvate dehydrogenase are catalyzed by an ATP-specific kinase (i.e., a pyruvate dehydrogenase kinase), and dephosphorylation and concomitant reactivation are catalyzed by a phosphatase (i.e., a pyruvate dehydrogenase phosphatase). The kinase and the phosphatase appear to be regulatory subunits of the pyruvate dehydrogenase complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN D. L., LARNER J. STUDIES ON UDPG-ALPHA-GLUCAN TRANSGLUCOSYLASE. III. INTERCONVERSION OF TWO FORMS OF MUSCLE UDPG-ALPHA-GLUCAN TRANSGLUCOSYLASE BY A PHOSPHORYLATION-DEPHOSPHORYLATION REACTION SEQUENCE. Biochemistry. 1963 Jul-Aug;2:669–675. doi: 10.1021/bi00904a009. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. 3. Resolution and reconstitution of the pig heart pyruvate dehydrogenase complex. J Biol Chem. 1967 Mar 25;242(6):1356–1358. [PubMed] [Google Scholar]
- Ishikawa E., Oliver R. M., Reed L. J. Alpha-Keto acid dehydrogenase complexes, V. Macromolecular organization of pyruvate and alpha-ketoglutarate dehydrogenase complexes isolated from beef kidney mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):534–541. doi: 10.1073/pnas.56.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. II. PROPERTIES. J Biol Chem. 1963 Aug;238:2609–2614. [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Shepherd D., Garland P. B. A continuous recording technique for the measurement of carbon dioxide, and its application to mitochondrial oxidation and decarboxylation reactions. Biochem J. 1967 Jun;103(3):677–691. doi: 10.1042/bj1030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. R., Old L. O., Reed L. J. Regulatory properties of pyruvate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1968 May 10;31(3):495–500. doi: 10.1016/0006-291x(68)90504-4. [DOI] [PubMed] [Google Scholar]
- Walter P., Paetkau V., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. 3. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J Biol Chem. 1966 Jun 10;241(11):2523–2532. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Westermann B., Wieland O. Suppression of pyruvate oxidation in liver mitochondria in the presence of long-chain fatty acid. Eur J Biochem. 1968 Feb;3(4):512–518. doi: 10.1111/j.1432-1033.1967.tb19561.x. [DOI] [PubMed] [Google Scholar]




