Abstract
Mortality in HIV-positive persons is increasingly due to non-HIV–related medical comorbidities. There are limited data on the prevalence and patient awareness of these comorbid conditions. Two hundred subjects at an urban HIV clinic were interviewed in 2005 to assess their awareness of 15 non-HIV–related medical comorbidities, defined as medical problems that are neither AIDS-defining by standard definitions, nor a direct effect of immune deficiency. Medical charts were subsequently reviewed to establish prevalence and concordance between self-report and chart documentation. Eighty-four percent of subjects self-reported at least 1 of 15 medical comorbidities and 92% had at least 1 condition chart-documented. The top 5 chart-documented conditions were hepatitis C (51.5%), pulmonary disease (28.5%), high blood pressure (27%), high cholesterol (24.5%), and obesity (22.5%). In multivariate analysis, higher number of non-HIV–related medical comorbidities was associated with older age, female gender, and intravenous drug use as route of HIV transmission. Across self-reported non-HIV–related medical comorbidities, the absolute concordance rate ranged from 67% to 96%, the sensitivity ranged from 0% to 79%; the positive predictive value ranged from 0% to 100%. While the vast majority of largely urban minority HIV-positive subjects were diagnosed with non-HIV–related medical comorbidities, there is significant room for improvement in patient awareness. In order to help patients optimally access and adhere to medication and medical care for these non-HIV–related medical comorbidities, interventions and educational campaigns to improve patient awareness that take cultural background, literacy, and educational level into account should be developed, implemented, and evaluated.
Introduction
In resource-rich countries, HIV/AIDS is a chronic disease that can be well managed in medically adherent patients. Mortality in HIV-positive persons is increasingly due to non-HIV–related medical comorbidities1–5 defined as medical problems that are neither AIDS-defining by standard definitions,6 nor a direct effect of immune deficiency.
While AIDS-defining opportunistic infections continue to be diagnosed, conditions comorbid with HIV (e.g., hepatitis C virus infection, renal disease, peripheral neuropathy, and cancer) or associated with HIV antiretroviral therapy (e.g., hyperlipidemia, cardiovascular disease, and diabetes) are prevalent among patients receiving HIV treatment in outpatient clinics. Increasing numbers of HIV-infected patients face end-organ disease from comorbid conditions such as hepatitis B or C and HIV nephropathy.7,8 Furthermore, as the HIV-positive population ages, age-related illnesses (e.g., arthritis, cancer, and osteoporosis) are also on the rise.
There are high rates of comorbid psychiatric and substance use disorders among HIV-positive persons9 and these factors have been found to be associated with poorer adherence to medication and medical care.10, 11 It is therefore essential to take psychiatric and substance use disorders into account when examining non-HIV–related medical comorbidities in HIV-positive persons. HIV disproportionately affects members of ethnic minority groups who have significant health disparities in terms of access to medical care and medical outcomes.12
Prevalence of non-HIV–related medical comorbidities in HIV-positive persons
While numerous studies focus on the relationship between HIV infection and one specific comorbid medical condition, only three studies have investigated non-HIV–related medical comorbidities in HIV-positive persons more comprehensively. These studies provide data on different populations at different time points: New York City clinic patients age 55 or older between 1996–1998,13 U.S. veterans receiving care from 1999 to 2000,14 and U.S. veterans receiving care from 1997 to 2004.15 In a sample of 165 HIV-positive clinic patients aged 55 or older,13 89% had at least one comorbid condition. The most frequently occurring were hypertension (41%), chronic airway disease (29%), diabetes mellitus (22%), arthritis (18%), hepatitis C virus infection (16%), and coronary artery disease (14%).13 The most prevalent medical comorbidities in a sample of 881 U.S. veterans receiving HIV care14 were hepatitis (53%), hypertension (24%), hyperlipidemia (17%), diabetes (11%), and pulmonary disease (10%). In a very large sample of over 33,000 HIV-positive veterans receiving care, the most prevalent non-HIV–related medical comorbidities were hypertension (20%), liver disease (13%), diabetes (8%), and pulmonary disease (8%).15 All three of these studies report on predominantly male samples (71%, 99%, and 98% respectively) and data on the prevalence of non-HIV–related medical comorbidities in HIV-positive women are needed.
Awareness of non-HIV–related medical comorbidities in HIV-positive persons
Studies that report on concordance between self-report and medical record data in non-HIV populations have found a wide range in sensitivity depending on the populations studied (general population,16–18 elderly,19–21 end-stage renal disease22). Studies in HIV-positive patient populations have examined concordance between self-report and medical record data on HIV test results,23 health care utilization,24 and stimulant drug use.25 In a sample of 628 HIV-positive persons in New York City, Patel and colleagues26 found consistency between the medical concerns expressed and the actual health care situation and health status of these individuals.
While medical comorbidities, such as hepatitis C, obesity, arthritis, cardiovascular disease, diabetes mellitus, and malignancies are emerging as important targets of treatment as the longevity of HIV-positive patients increases, limited knowledge exists about the incidence, severity, and health outcomes of HIV-positive individuals with multiple morbidities. We also lack information about patient awareness of these conditions and access to care for treatment of these conditions. The awareness HIV-infected patients have of their medical comorbidities must be studied in order to formulate programs to improve patient awareness, as well as to promote “chronic disease” prevention and self-management.
As the HIV-positive population ages, a major challenge for the primary provider is to broaden the focus of intervention beyond HIV illness and treatment, to encompass other comorbid conditions.27 The nature of this broadened focus depends upon the complexity of the comorbidity. Some illnesses can be prevented or ameliorated by improving health behaviors (such as hypertension, diabetes, and obesity) and some can be ameliorated, once diagnosed, by improving access to and adherence to additional treatment (such as hepatitis C virus infection).
This study investigates the prevalence of non-HIV–related medical comorbidities in a sample of 200 predominantly racial and ethnic minority HIV-positive patients receiving care at a New York City HIV clinic in 2005, the HIV-positive patients' awareness of their non-HIV–related medical comorbidities, and whether their self-report was concordant with the medical record. As a secondary aim, we examined the relationship between the prevalence of non-HIV–related medical comorbidities and demographics, clinical profile, and psychiatric profile. This study is the first that we are aware of to comprehensively report on HIV-positive patients' awareness of their non-HIV–related medical comorbidities.
Methods
Study setting
The study was conducted at Jack Martin Fund Clinic, the HIV clinic at Mount Sinai Medical Center New York City, providing comprehensive, colocated primary and specialty care (psychiatry, gynecology, neurology, nephrology, and a hepatitis coinfection clinic), nursing, social work, mental health, and nutrition services. At the time of the study, the summer of 2005, approximately 1000 patients were in active care. The majority of clinic patients reside in Harlem and the Bronx, national epicenters of the HIV epidemic, are Medicaid insured or in the AIDS Drug Assistance Program, and are members of ethnic minority groups (over 90% Hispanic and/or black).
Participant selection
Once permission from the clinic medical providers and ancillary staff was obtained, the study coordinators approached all patients as they arrived at the clinic for their scheduled appointments. If the patient expressed interest, the coordinators then described the study and obtained written informed consent in a private room. There were no exclusion criteria other than the ability to provide informed consent. All patients who were interested in participating were consented. Subjects were then given a set of 15 paper cards each asking whether they had one of the following 15 non-HIV–related medical comorbidities: high blood pressure, high cholesterol, pulmonary disease, renal insufficiency, obesity, arthritis, cardiovascular disease, diabetes mellitus, cancer (non-HIV), stroke, hepatitis C, chronic hepatitis B, circulatory problems, thyroid problems, and osteoporosis. The 15 cards were identical except for the fact that each one had a different medical condition listed on the front. The three possible answer categories for each condition were: “I have it,” “I don't have it,” “I'm not sure.” In addition, subjects completed another card of the same format asking about “Drug Use Problems.” The order of the card administration was varied across subjects to eliminate any response order effects.
Subjects were encouraged to fill in answers on the cards with minimal support from the study coordinators to minimize bias. Names for conditions commonly used by patients were included and someone was present to explain the condition if a subject was unfamiliar with the medical term. Each interview took approximately 15 minutes and participants were given a $4 MetroCard as reimbursement for their time and effort.
Demographic information (gender, age, race/ethnicity), height, and current use of cigarettes and alcohol were obtained at the time of the interview on a one page form subjects were asked to complete after filling in the cards. Permission to access their inpatient and outpatient records and to be re-contacted in the future was also obtained from each subject. The study and all materials were approved by the Institutional Review Board at Mount Sinai School of Medicine.
Subsequently, the outpatient medical charts (paper) along with the electronic medical records of laboratory and radiology records for each subject were reviewed by a physician (G.O.) who was blinded to subject's self-report to determine concordance between subject self-report and chart documentation. A codebook specifying the criteria for coding each medical condition as present that was developed by the physician (G.O.) in collaboration with the senior author (D.F.) was used.
Each subject's paper medical record was reviewed for documented non-HIV–related medical comorbidities and medication profiles. Since a one-page problem list at the front of a medical chart does not always adequately reflect all current problems and diagnoses, the provider progress notes were reviewed for completeness. Laboratory studies were reviewed from the subject's initial visit to the hospital to further document the existence of high cholesterol, renal insufficiency, diabetes mellitus, chronic hepatitis B, thyroid problems, and hepatitis C antibody and viremia. When available, diagnostic tests (chest x-rays, pulmonary function tests, electrocardiograms, echocardiograms, dual-energy x-ray absorptiometry [DEXA] scans, computed tomography/magnetic resonance imaging [CT/MRI] scans, and arterial brachial index scans) were reviewed in order to confirm the presence of provider-diagnosed pulmonary and cardiovascular disease (including cerebrovascular events), osteoporosis, and circulatory problems and in all cases did so. Pathology reports were reviewed for documented non-HIV malignancy. For each subject the most recent HIV markers (viral load and CD4) were obtained along with their current highly active antiretroviral therapy regimen. Attendance to scheduled appointments at the HIV clinic for the 1 year prior to the research interview was determined through medical chart review and used as a marker of adherence to care. The following variables were also coded from chart review: route of HIV transmission, years since HIV-positive diagnosis, presence of psychiatric diagnosis, use of psychotropic medication, colocated psychiatric treatment, history of intravenous drug use, current attendance to methadone maintenance treatment program, total number of medications prescribed daily, and total number of pills prescribed daily. Twenty subjects were randomly selected for the initial chart review done by the physician (G.O.). The first author (J.W.) independently did a chart review on these 20 subjects. Discrepancies between the two chart reviews were minor and were used to arrive at a consensus methodology and the chart abstraction codebook was modified to assure consistency in subsequent reviews. The primary source of discrepancy was how to code a condition in the absence of any information relevant to the condition in the chart. It was decided that if there was no information relevant to a particular condition (e.g., non-HIV cancer) in the chart, it was considered to be absent.
In this study, we take the chart documentation as the gold standard and compare subject self-report to this. This decision was based on the clinical observation that led to initiation of this study, namely that significant numbers of HIV-positive patients in the clinic were unaware of chart-documented non-HIV–related medical comorbidities.
Statistical analysis
Descriptive analyses were performed to document the frequencies of each index condition (15 non-HIV–related medical comorbidities). χ2 tests, Kruskal-Wallis tests, and Mann-Whitney U tests were used to compare groups. Spearman's rank coefficients were used for correlational analyses. The prevalence of each index condition based on self-report was compared with the prevalence based on medical chart review, taking the chart documentation as the gold standard. The absolute concordance rate (proportion of those whose self-report of the presence of the index condition agreed with the chart documentation), sensitivity (probability in those with the chart documented condition of self-reporting it), and positive predictive value (probability of having the chart documented condition in those who self-report it) for each of the 15 conditions were also calculated, as these values provide the most clinically relevant information regarding patient awareness. Multivariate linear regression analyses were used for modeling of the number of comorbidities as a linear function of those variables found to be related to the number of chart-documented conditions in univariate analyses. Given the large number of variables examined in univariate analyses, only those that were significant at the p < 0.05 level were included in the multivariate analysis.
Results
Subject recruitment and characteristics
Two hundred subjects were serially recruited (approximately 20% of the active clinic patient population at the time). The demographics of the recruited subjects were representative of the entire clinic population (Table 1). Women were well represented in the sample (45%) of predominantly ethnic minority subjects (91% Hispanic and/or black). While 78% of subjects were on antiretroviral medication, only 41% had an undetectable HIV viral load. The subjects had a mean age of almost 48 years and had been diagnosed with HIV infection for a mean of over 11 years. Sixty-three percent of the subjects had a chart-documented psychiatric diagnosis and 45% were on psychotropic medication. Of those with a psychiatric diagnosis, 86% received colocated psychiatric care at the HIV clinic. The most common psychiatric conditions treated at the HIV clinic are mood and anxiety disorders. Sixty-nine percent of subjects self-reported never using alcohol. Twenty-four percent of subjects self-reported that they currently had a “drug use problem.” Twenty percent of subjects were in a methadone maintenance treatment program. Subjects were on a mean of almost 7 different medications and were prescribed an average of 11 pills per day. They attended a mean of almost 14 appointments at the HIV clinic in the last year and adhered to a mean 78% of their scheduled appointments in the prior year.
Table 1.
Sample Characteristics of the Study Sample (n = 200)
| Demographics | ||
| Gender | Male (%) | 55 |
| Female (%) | 45 | |
| Age | Mean Years (s.d.) | 47.7 (7.7) |
| Race/ethnicity | Black, non-Hispanic (%) | 42.5 |
| Hispanic, non-black (%) | 38.5 | |
| Hispanic, black (%) | 10.0 | |
| White, non-Hispanic (%) | 6.0 | |
| Other (%) | 3.0 | |
| Clinical profile | ||
| Median CD4+ [cells/μL] (range) | 372 (4–1459) | |
| % undetectable HIV viral load (<50 copies/mL) | 41 | |
| Median detectable HIV viral load (copies/mL) (range) | 9297 (52–>750,000) | |
| Antiretroviral medication (%) | 78 | |
| Route of transmission (%) | ||
| Heterosexual | 43 | |
| Intravenous drug use | 40 | |
| Homosexual | 13 | |
| Other | 4 | |
| Mean years since HIV diagnosis (SD) | 11.4 (5.3) | |
| Mean total # medications (SD) | 6.9 (3.6) | |
| Mean total # pills daily (SD) | 11.0 (6.5) | |
| Mean # appointments attended last year (SD) | 13.6 (10.5) | |
| Mean % kept appointments attended last year (SD) | 78 (16) | |
| Psychiatric profile | ||
| Psychiatric diagnosis in chart (%) | 63 | |
| On psychotropic medication (%) | 45 | |
| Colocated psychiatric care at HIV clinic | 54 | |
| Current cigarette use (%) | 64 | |
| Current alcohol use (%) | 31 | |
| Current illicit substance use (%) | 24 | |
| Methadone maintenance program (%) | 20 | |
| Past history of intravenous drug use (%) | 40 | |
SD, standard deviation.
Self-reported non-HIV–related medical comorbidities
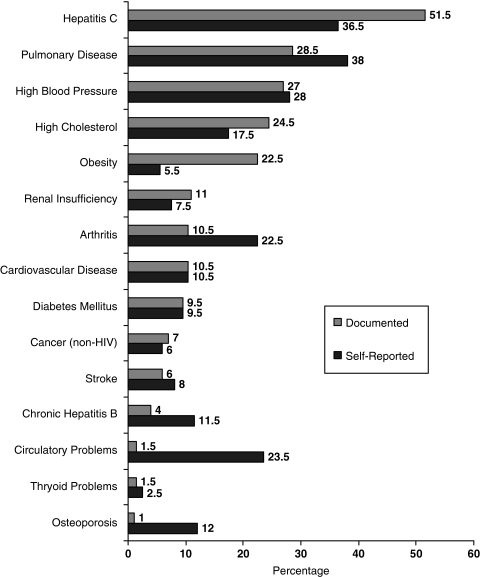
The prevalence rates of each self-reported condition is graphically represented in Figure 1 in order of decreasing documented prevalence. Eighty-four percent of subjects self-reported having at least 1 of the 15 non-HIV–related medical comorbidities. The median number of self-reported conditions was 2. The 5 most commonly self-reported non-HIV medical comorbidities were pulmonary disease (38%), hepatitis C (36.5%), high blood pressure (28%), circulatory problems (23.5%), and arthritis (22.5%).
FIG. 1.
Prevalence of self-reported and chart-documented comorbid conditions.
Chart-documented non-HIV–related medical comorbidities
The prevalence of each chart documented condition is graphically represented in Figure 1 in order of decreasing documented prevalence. Ninety-two percent of subjects had at least one comorbid medical condition chart documented of the 15 conditions examined (median = 2). The top five chart-documented non-HIV related medical comorbidities were hepatitis C (51.5%), pulmonary disease (28.5%), high blood pressure (27%), high cholesterol (24.5%), and obesity (22.5%).
Given that approximately half of the sample had hepatitis C, a post hoc analysis was conducted to determine how those with hepatitis C coinfection compared to those without hepatitis C coinfection on the presence of the other 14 conditions. This analysis found that those with hepatitis C had a greater number of comorbid conditions than those without hepatitis C (U = 2486.5; p < 0.01). The four most prevalent comorbid conditions for both the group coinfected with hepatitis C virus (HCV) and the group not coinfected with HCV remain pulmonary disease, high blood pressure, high cholesterol, and obesity. The HCV-coinfected sample was largely infected through intravenous drug use (71%) whereas those without HCV were predominantly infected through heterosexual transmission (64%; χ2 = 79.3; p < 0.01).
Relationship between demographics and non-HIV–related medical comorbidities
Women had a higher mean number of chart documented comorbid conditions than did men (2.5 in women versus 1.9 in men; U = 3871.0; p = 0.01). When the prevalence rates of men and women are compared on the 15 individual chart documented conditions, only obesity reaches statistical significance (37.5% in women versus 11.3% in men; χ2 = 18.50; p < 0.001); the absolute proportions are higher but not statistically significant for all conditions in women than in men with the exception of hepatitis C, cardiovascular disease, stroke, and circulatory problems. Age was positively correlated with number of non-HIV related medical comorbidities (Spearman's ρ = 0.35; p < 0.01). Race/ethnicity was not related to number of non-HIV related medical comorbidities (χ2 = 6.34; p = 0.18).
Relationship between clinical profile and non-HIV related medical comorbidities
Subjects with undetectable HIV viral load had more chart documented non-HIV–related medical comorbidities than those with detectable HIV viral load (U = 3702.5; p = 0.01). Neither CD4+ cell count (Spearman's ρ = 0.12; p = 0.10) nor being on antiretroviral medication (U = 3249.5; p = 0.67) was related to number of non-HIV–related medical comorbidities. Route of HIV transmission was related to number of non-HIV–related medical comorbidities (χ2 = 18.7; p < 0.01). Those with intravenous drug use had more non-HIV–related medical comorbidities than those with heterosexual or homosexual routes of transmission. The number of years since diagnosis with HIV infection was positively related to number of non-HIV–related medical comorbidities (Spearman's ρ = 0.15; p = 0.03); as was the number of medications being taken (Spearman's ρ = 0.38; p < 0.01). The number of chart documented non-HIV–related medical comorbidities was positively correlated with the number of appointments attended in the prior year (Spearman's ρ = 0.25; p < 0.01) but not with the percent of appointments that were kept in the prior year (Spearman's ρ = 0.04; p = 0.62).
Relationship between psychiatric profile and non-HIV–related medical comorbidities
Subjects with a psychiatric diagnosis self-reported more non-HIV–related medical comorbidities than did those without one (U = 3504.5; p = 0.04) but did not have more chart-documented non-HIV related medical comorbidities than did those without one (U = 4021.0; p = 0.52). Subjects with a psychiatric diagnosis were less likely to have an undetectable HIV viral load (<50 copies per milliliter) than those without a psychiatric diagnosis (36% versus 54%, χ2 = 5.8; p = 0.02), but they were not less likely to be on antiretroviral medication (χ2 = 1.1; p = 0.30).
Multivariate analysis
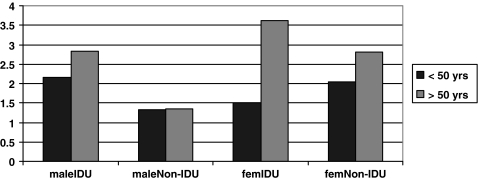
The seven variables that were found to be related to number of chart documented non-HIV–related medical comorbidities on univariate analysis were grouped into those which are predictive of the number of comorbidities (age, gender, route of transmission, years since HIV diagnosis) and those likely to be a consequence of the number of comorbidities (number of medications, number of kept appointments in last year, HIV viral load). The four potentially causal variables were entered in a multivariate analysis. The three variables that remain in the model are age, gender, and intravenous drug use as route of transmission. These three variables were entered into a new regression analysis with all three interaction terms. All three variables and three interaction terms are significant (Table 2). As illustrated in Figure 2, it is the older females who are most strongly driving the relationship between both age and gender with higher numbers of non-HIV–related medical comorbidities.
Table 2.
Multivariate Analysis of Predictive Factors Associated with Number of Non-HIV–Related Medical Comorbidities
| Variable | B | SE B | β | t | p |
|---|---|---|---|---|---|
| Age (A) | 0.083 | 0.022 | 0.419 | 3.851 | 0.000 |
| Gender (G) | 2.706 | 1.269 | 0.883 | 2.132 | 0.034 |
| Intravenous drug use as route of transmission (I) | −4.089 | 1.508 | −1.314 | −2.712 | 0.007 |
| Interaction AG | −0.080 | 0.027 | −1.285 | −2.916 | 0.004 |
| Interaction AI | 0.085 | 0.030 | 1.407 | 2.859 | 0.005 |
| Interaction GI | 0.953 | 0.421 | 0.275 | 2.264 | 0.025 |
SE, standard error.
FIG. 2.
Observed mean number of chart comorbidities by age, gender, and route of HIV transmission.
Relationship between self-report and chart documentation
The absolute concordance rate, sensitivity, and positive predictive value of the conditions are listed in Table 3. The highest rates of agreement between subject self-report and chart documentation were found for thyroid problems (96%), diabetes mellitus (93%) stroke (91%), cancer (non-HIV) (90%), and cardiovascular disease (86%). The lowest rates of agreement between subject self-report and chart documentation were found for circulatory problems (67%), arthritis (67%), high cholesterol (71%), pulmonary disease (75%), and hepatitis C (78%). Subjects with the index condition showed the highest awareness of having it (sensitivity) for diabetes mellitus (79%), high blood pressure (78%), pulmonary disease (74%), circulatory problems (67%), and thyroid problems (67%). Subjects with the index condition showed the lowest awareness of having it for osteoporosis (0%), obesity (24%), high cholesterol (37%), arthritis (38%), and renal insufficiency (41%). Subjects who reported an index condition were most likely to actually have it (positive predictive value) in the case of obesity (100%), hepatitis C (93%), diabetes mellitus (79%), high blood pressure (76%), and non-HIV cancer (67%). Subjects who reported an index condition were least likely to actually have it in the case of osteoporosis (0%), circulatory problems (4%), arthritis (18%), chronic hepatitis B (22%), and stroke (38%).
Table 3.
Agreement Between Subject Report and Chart Documentation of Fifteen Non-HIV–Related Medical Comorbidities
| Medical comorbidity | Absolute concordance ratea(%) | Sensitivityb(%) | Positive predictive valuec(%) |
|---|---|---|---|
| High blood pressure | 84 | 78 | 76 |
| High cholesterol | 71 | 37 | 51 |
| Pulmonary disease | 75 | 74 | 56 |
| Renal insufficiency | 84 | 41 | 60 |
| Obesity | 82 | 24 | 100 |
| Arthritis | 67 | 38 | 18 |
| Cardiovascular disease | 86 | 52 | 51 |
| Diabetes mellitus | 93 | 79 | 79 |
| Cancer (non-HIV) | 90 | 57 | 67 |
| Stroke | 91 | 50 | 38 |
| Hepatitis C | 78 | 66 | 93 |
| Chronic hepatitis B | 79 | 63 | 22 |
| Circulatory problems | 67 | 67 | 4 |
| Thyroid problems | 96 | 67 | 40 |
| Osteoporosis | 80 | 0 | 0 |
Absolute concordance rate = the percentage of those whose self-report of the presence of the index condition agreed with the chart documentation.
Sensitivity = the percentage of those with the chart documented index condition who self-reported the condition.
Positive predictive value = the percentage of those self-reporting the index condition that had it according to the chart documentation.
Discussion
The vast majority of largely urban minority HIV-positive subjects in care in this study were diagnosed with non-HIV–related medical comorbidities, with prevalence rates being higher in women than in men and associated with older age and intravenous drug use as route of HIV transmission. The comorbidities found to be most prevalent (hepatitis C, pulmonary disease, high blood pressure, and high cholesterol) were largely consistent with earlier studies of HIV-positive persons.13–15 Those coinfected with hepatitis C were found to have a greater number of other non-HIV–related comorbid conditions than those without hepatitis C coinfection.
The high prevalence rates of non-HIV–related medical comorbidities found in this population are noteworthy given that persons with HIV are living longer and increasingly dying of medical problems that are no longer AIDS defining. Patients should no longer be educated solely about their HIV disease; they must also be made aware of their other medical problems in order to provide effective comprehensive care. Preventive care is an essential part of providing HIV care. This includes the promotion of behavioral change (e.g., smoking cessation, exercise, weight loss, dietary modification) as well as ensuring that patients undergo recommended screening tests.28 This is consistent with recently issued primary care guidelines for the management of HIV-positive persons.29
While this is the first study to comprehensively investigate the concordance between self-report and medical record data of non-HIV–related medical comorbidities in HIV-positive persons, three previous studies of HIV-positive persons have investigated this specifically as it relates to hepatitis C,11,30 and hepatitis B and C.31 In a sample of 182 marginally housed HIV-positive persons with comorbid hepatitis C infection, 64% self-reported having hepatitis C infection.11 In a sample of 681 HIV-positive women with comorbid hepatitis C infection enrolled in the Women's Interagency HIV Study, 77% reported knowing their hepatitis C diagnosis.30 In a sample of 970 HIV-positive patients in clinical care, the sensitivity of self-reported hepatitis B infection was 27% (correct self-report by those who do have hepatitis B infection) and the sensitivity of self-reported hepatitis C infection was 72%.31 Our finding of sensitivity of 66% for self-reported hepatitis C infection is in line with the results of these three studies and our finding of 63% for self-reported hepatitis B infection is considerably higher than in the one other study to date.
While absolute concordance rates were 67% or higher for all 15 non-HIV–related medical comorbidities, there is still much room for improvement. The most clinically relevant metric of patient awareness is the sensitivity value per non-HIV– related medical comorbidity. Fully one third (34%) of those with hepatitis C, the most prevalent comorbid medical non-HIV–related medical comorbidity, were unaware that they had this viral infection. Ideally we would like HIV-positive persons to be fully aware of all of their non-HIV–related medical comorbidities. Given this lack of adequate awareness on the part of patients, it is important to develop strategies to better integrate HIV care into the broader context of care for other non-HIV–related medical comorbidities.
Priorities need to be set to begin developing programs and interventions to increase patient awareness of non-HIV–related medical comorbidities. In establishing priorities, the following dimensions can be taken into account: (1) prevalence; (2) associated morbidity and mortality; and (3) chronicity. It is most important that patients become aware of the non-HIV–related medical comorbidities, which are: (1) most prevalent; (2) most likely to increase morbidity and mortality; and (3) require chronic disease management. Of the 15 non-HIV–related medical comorbidities examined, the 7 comorbidities that best meet these criteria are: hepatitis C, pulmonary disease, high blood pressure, high cholesterol, renal insufficiency, cardiovascular disease, and diabetes mellitus. Among these 7 non-HIV–related medical comorbidities, high cholesterol, renal insufficiency, cardiovascular disease, and hepatitis C had the lowest sensitivity and are important disease states to target in educational programs for HIV-positive persons if choices need to be made.
The predictive variables found to be related to having a greater number of comorbid conditions in multivariate analyses are older age, female gender, and intravenous drug use as route of HIV transmission, with the group of women older than 50 years strongly driving these associations. The finding that age is associated with increased non-HIV related medical comorbidity replicates previous case-control studies conducted pre-highly active antiretroviral therapy (HAART)32 and post-HAART.33 While intravenous drug use is highly associated with coinfection with hepatitis C, the most prevalent non-HIV–related comorbidity in the study, we reported on post hoc analyses that found that the hepatitis C coinfected subjects also had significantly more of the other 14 non-HIV–related medical comorbidities than did those without hepatitis C coinfection. This finding is thus likely explained by multiple aspects of intravenous drug use that negatively impact overall health status including food insecurity and nutritional deficits.34,35 This is the first study to document higher rates of non-HIV–related medical comorbidities in HIV-positive women than in men. Further research is needed to investigate whether this finding can be replicated in other HIV-positive patient populations and what the underlying reasons are for these gender differences. A direction for such research on gender disparities is provided by a recent study of over 400 severely disadvantaged HIV-positive persons in New York City, which found that women were less likely to use HIV primary care services and more likely to have an emergency department visit than were men.36 Our findings may reflect a tendency for women to wait until they are more ill to come to HIV primary care as compared to men.
This study has several limitations. First, we have taken the medical record as the gold standard for each index condition. As is the case in most chart review studies, it is likely that there are non-HIV–related medical comorbidities that were not documented in the medical records as well as the possibility that there were incorrectly documented non-HIV–related medical comorbidities. It could be that medical documentation was incomplete in our studies or that providers minimized a medical complaint and did not pursue further diagnostic evaluation for it. It would have been more complete to interview each provider regarding each individual subject to further supplement the information obtained from the chart review. Furthermore, there are reasons why a subject's self-report may differ from the medical record other than lack of knowledge of having a medical condition. These may include the subject failing to report a diagnosis due to social stigma and the subject's lack of familiarity with the terms used.
Another limitation of this study is that we did not collect information on educational level, literacy, or health literacy. These unexplored constructs may play a key role in determining subject awareness37 and we recommend that this be examined in future research. We did not undertake substudies on the subjects who were in specialized integrated care programs to analyze if there was improved awareness. Future studies should investigate whether integrated programs (e.g., hepatitis C coinfection clinics) empower patients with problem-specific health literacy, and whether this has an effect on disease outcomes. This study was conducted at only one site limiting generalizability and the potential for sampling bias exists as clinic patients who did not attend any appointments during the summer of 2005 were not eligible for participation. Finally, while absolute concordance rate, sensitivity, and positive predictive value were used to compare the accuracy of self-report across the 15 non-HIV–related medical comorbidities, all three of these values are affected by the prevalence of each condition as well as the accuracy of the chart documentation for each condition.
In order for patients to optimally access and adhere to care for a medical condition, awareness of having the condition is essential. Interventions and educational campaigns to increase the awareness of HIV-positive patients of their non-HIV–related medical comorbidities that provide them with culturally appropriate information aimed at their level of literacy and education should be developed, implemented, and evaluated. Future studies should investigate the complex relationship between multiple medical morbidities, patient awareness, and adherence to care and medication. It is important to study adherence to antiretroviral medication and HIV care in the context of the entire medication and treatment regimen of the HIV-positive patient.
In order to guide the development of educational materials, strategies, and campaigns to increase accurate patient awareness of non-HIV–related medical comorbidities, research is needed on the underlying reasons for the discordance between patient self-report and chart documentation. This study did not investigate the patient, provider, or clinic factors that are related to the level of patient awareness of non-HIV–related medical comorbidities. This is a vitally important topic for future research to address. Such research should be designed to control for the potentially confounding impact of the number of comorbid conditions on level of awareness. Guidance for such research comes from a recent study of Sohler and colleagues38 investigating the factors related to disagreement between self-report of number of HIV primary care visits and chart documentation in a sample of marginalized, HIV-infected patients in 10 sites across the United States. The factors they found to be associated with disagreement included younger age, non-Hispanic black race/ethnicity, lower education, and substance use. These factors should be investigated to see if they also predict low levels of HIV-positive patient awareness of non-HIV–related medical comorbidities.
Future research should also investigate the relationship between patient awareness of non-HIV–related medical comorbidities and adherence to medication and medical care and whether medication adherence differs for antiretroviral medication, psychotropic medication, and medication regimens for other non-HIV–related medical comorbidities in HIV-positive patients with multiple morbidities, particularly those with psychiatric and substance use disorders. As HIV-positive patients live longer and present with increasing numbers of non-HIV–related medical comorbidities that are being treated, it is imperative to educate patients about these non-HIV–related medical comorbidities in order to improve their adherence to comprehensive health care and effectively provide chronic disease management.
Acknowledgments
The project described was supported by grant number K23MH071177 (J.J.W.) and grant number K23MH071181 (E.R.) from the National Institute of Mental Health and grant number K23DA018623 from the National Institute on Drug Abuse (D.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute on Drug Abuse, or the National Institutes of Health. We are grateful to the 200 research subjects who voluntarily participated in this study.
Parts of the data were presented at the following meetings: NIMH/IAPAC International Conference on HIV Treatment Adherence, Jersey City, New Jersey, March 2006 and XVI International AIDS Conference, Toronto, Canada, August 2006.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sackoff JE. Hanna DB. Pfeiffer MR. Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 2.Crum NF. Riffenburgh RH. Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 3.French AL. Gawel SH. Hershow R, et al. Trends in Mortality and Causes of Death Among Women With HIV in the United States: A 10-Year Study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bica I. McGovern B. Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Lacombe K. Pacanowski J. [HIV infection and comorbidities] Rev Prat. 2006;56:995–1004. [PubMed] [Google Scholar]
- 6.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 7.Roland ME. Havlir DV. Responding to organ failure in HIV-infected patients. N Engl J Med. 2003;348:2279–2281. doi: 10.1056/NEJMp030074. [DOI] [PubMed] [Google Scholar]
- 8.Buchacz K. Baker RK. Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22:1345–1354. doi: 10.1097/QAD.0b013e328304b38b. [DOI] [PubMed] [Google Scholar]
- 9.Hutton H. Santora PB. Weiss JJ. Addiction and Multiple Morbidities in HIV-Positive Patients. In: Henningfield JE, editor; Santora PB, editor; Bickel WK, editor. Addiction Treatment: Science and Policy for the Twenty-first Century. Baltimore: The Johns Hopkins University Press; 2007. pp. 113–119. [Google Scholar]
- 10.Weiss J. Bangsberg D. Psychiatric aspects of adherence to medical care and treatment. In: Cohen M, editor; Gorman J, editor. Comprehensive Textbook of AIDS Psychiatry. New York; Oxford University Press: 2008. pp. 281–295. [Google Scholar]
- 11.Hall CS. Charlebois ED. Hahn JA. Moss AR. Bangsberg DR. Hepatitis C virus infection in San Francisco's HIV-infected urban poor. J Gen Intern Med. 2004;19:357–365. doi: 10.1111/j.1525-1497.2004.30613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu C. Selwyn PA. Current health disparities in HIV/AIDS. AIDS Read. 2008;18:144–146. 152–148, C143. [PubMed] [Google Scholar]
- 13.Shah SS. McGowan JP. Smith C. Blum S. Klein RS. Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clin Infect Dis. 2002;35:1238–1243. doi: 10.1086/343048. [DOI] [PubMed] [Google Scholar]
- 14.Kilbourne AM. Justice AC. Rabeneck L. Rodriguez-Barradas M. Weissman S. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epidemiol. 2001;54:S22–S28. doi: 10.1016/s0895-4356(01)00443-7. [DOI] [PubMed] [Google Scholar]
- 15.Goulet JL. Fultz SL. Rimland D, et al. Aging and infectious diseases: Do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klungel OH. de Boer A. Paes AH. Seidell JC. Bakker A. Cardiovascular diseases and risk factors in a population-based study in The Netherlands: Agreement between questionnaire information and medical records. Neth J Med. 1999;55:177–183. doi: 10.1016/s0300-2977(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Okura Y. Urban LH. Mahoney DW. Jacobsen SJ. Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.St Sauver JL. Hagen PT. Cha SS, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clin Proc. 2005;80:203–210. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]
- 19.Haapanen N. Miilunpalo S. Pasanen M. Oja P. Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145:762–769. doi: 10.1093/aje/145.8.762. [DOI] [PubMed] [Google Scholar]
- 20.Skinner KM. Miller DR. Lincoln E. Lee A. Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: Experience from the Veterans Health Study. J Ambul Care Manage. 2005;28:102–110. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bush TL. Miller SR. Golden AL. Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkin SS. Cavanaugh K. Longenecker JC. Fink NE. Levey AS. Powe NR. Agreement of self-reported comorbid conditions with medical and physician reports varied by disease among end-stage renal disease patients. J Clin Epidemiol. 2007;60:634–642. doi: 10.1016/j.jclinepi.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher DG. Reynolds GL. Jaffe A. Johnson ME. Reliability, sensitivity and specificity of self-report of HIV test results. AIDS Care. 2007;19:692–696. doi: 10.1080/09540120601087004. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham CO. Li X. Ramsey K. Sohler NL. A comparison of HIV health services utilization measures in a marginalized population: Self-report versus medical records. Med Care. 2007;45:264–268. doi: 10.1097/01.mlr.0000250294.16240.2e. [DOI] [PubMed] [Google Scholar]
- 25.Reinhard MJ. Hinkin CH. Barclay TR, et al. Discrepancies between self-report and objective measures for stimulant drug use in HIV: Cognitive, medication adherence and psychological correlates. Addict Behav. 2007;32:2727–2736. doi: 10.1016/j.addbeh.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel S. Weiss E. Chhabra R, et al. The Events in Care Screening Questionnaire (ECSQ): A new tool to identify needs and concerns of people with HIV/AIDS. AIDS Patient Care STDs. 2008;22:381–393. doi: 10.1089/apc.2007.0105. [DOI] [PubMed] [Google Scholar]
- 27.Henry K. Internal medicine/primary care reminder: What are the standards of care for HIV-positive patients aged 50 years and older? Curr HIV/AIDS Rep. 2009;6:153–161. doi: 10.1007/s11904-009-0021-0. [DOI] [PubMed] [Google Scholar]
- 28.Buchacz K. Rangel M. Blacher R. Brooks JT. Changes in the clinical epidemiology of HIV infection in the United States: Implications for the clinician. Curr Infect Dis Rep. 2009;11:75–83. doi: 10.1007/s11908-009-0011-9. [DOI] [PubMed] [Google Scholar]
- 29.Aberg JA. Kaplan JE. Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MH. Grey D. Cook JA, et al. Awareness of hepatitis C infection among women with and at risk for HIV. J Gen Intern Med. 2007;22:1689–1694. doi: 10.1007/s11606-007-0395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo Re V., 3rd Frank I. Gross R, et al. Self-reported hepatitis B and C virus infections had low sensitivity among HIV-infected patients. J Clin Epidemiol. 2007;60:294–299. doi: 10.1016/j.jclinepi.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Skiest DJ. Rubinstien E. Carley N. Gioiella L. Lyons R. The importance of comorbidity in HIV-infected patients over 55: A retrospective case-control study. Am J Med. 1996;101:605–611. doi: 10.1016/S0002-9343(96)00329-4. [DOI] [PubMed] [Google Scholar]
- 33.Tumbarello M. Rabagliati R. De Gaetano Donati K, et al. Older HIV-positive patients in the era of highly active antiretroviral therapy: Changing of a scenario. AIDS. 2003;17:128–131. doi: 10.1097/00002030-200301030-00020. [DOI] [PubMed] [Google Scholar]
- 34.Chen CY. Lin KM. Health consequences of illegal drug use. Curr Opin Psychiatry. 2009;22:287–292. doi: 10.1097/yco.0b013e32832a2349. [DOI] [PubMed] [Google Scholar]
- 35.Hendricks K. Gorbach S. Nutrition issues in chronic drug users living with HIV infection. Addict Sci Clin Pract. 2009;5:16–23. doi: 10.1151/ascp095116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohler NL. Li X. Cunningham CO. Gender dDisparities in HIV health care utilization among the severely disadvantaged: Can we determine the reasons? AIDS Patient Care STDS. 2009;23:775–783. doi: 10.1089/apc.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drainoni ML. Rajabiun S. Rumptz M, et al. Health literacy of HIV-positive individuals enrolled in an outreach intervention: Results of a cross-site analysis. J Health Commun. 2008;13:287–302. doi: 10.1080/10810730801985442. [DOI] [PubMed] [Google Scholar]
- 38.Sohler NL. Coleman SM. Cabral H, et al. Does self-report data on HIV primary care utilization agree with medical record data for socially marginalized populations in the United States. AIDS Patient Care STDs. 2009;23:837–843. doi: 10.1089/apc.2009.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]