Abstract
Water consumption acutely reduces meal energy intake (EI) among middle-aged and older adults. Our objectives were to determine if premeal water consumption facilitates weight loss among overweight/obese middle-aged and older adults, and to determine if the ability of premeal water consumption to reduce meal EI is sustained after a 12-week period of increased water consumption. Adults (n = 48; 55–75 years, BMI 25–40 kg/m2) were assigned to one of two groups: (i) hypocaloric diet + 500 ml water prior to each daily meal (water group), or (ii) hypocaloric diet alone (nonwater group). At baseline and week 12, each participant underwent two ad libitum test meals: (i) no preload (NP), and (ii) 500 ml water preload (WP). Meal EI was assessed at each test meal and body weight was assessed weekly for 12 weeks. Weight loss was ~2 kg greater in the water group than in the nonwater group, and the water group (β = −0.87, P < 0.001) showed a 44% greater decline in weight over the 12 weeks than the nonwater group (β = −0.60, P < 0.001). Test meal EI was lower in the WP than NP condition at baseline, but not at week 12 (baseline: WP 498 ± 25 kcal, NP 541 ± 27 kcal, P = 0.009; 12-week: WP 480 ± 25 kcal, NP 506 ± 25 kcal, P = 0.069). Thus, when combined with a hypocaloric diet, consuming 500 ml water prior to each main meal leads to greater weight loss than a hypocaloric diet alone in middle-aged and older adults. This may be due in part to an acute reduction in meal EI following water ingestion.
INTRODUCTION
If recent trends continue, 86% of US adults will be overweight or obese by the year 2030 (1). Middle-aged and older adults (aged ≥40 years) are at increased risk for obesity and ~70% in this segment of the population are currently overweight or obese (2). Age-related weight gain may be attributed to several factors including a reduction in energy expenditure, a reduction in energy requirements, and an increased susceptibility to energy overconsumption (3–6). Obesity among older adults is associated with impaired physical function, increased morbidity and mortality, and greater health care costs (7–9). Thus, identifying successful weight management strategies for middle-aged and older adults has significant public health implications.
Increasing daily water consumption is widely recognized as a weight loss strategy in the general public, yet there is surprisingly little data supporting this practice. Epidemiological studies suggest that energy intake (EI) is significantly lower (~9%, or 194 kcal/d) in water drinkers compared with nonwater drinkers (10), and that sweetened beverage consumption is associated with weight gain and obesity (11). Recently, investigators reported that substituting water for energy-containing beverages decreases self-reported EI (12), and that increasing self-reported daily water consumption by ≥1l in overweight women is associated with increased weight loss of ~2 kg over a 12-month diet intervention compared with women who consumed <1l water daily (13). Laboratory-based test meal studies have demonstrated that water consumed with a meal reduces ratings of hunger and increases rating of satiety (14,15), though no differences in meal EI were observed when compared to a no beverage condition (14). We (16,17) have recently demonstrated that both normal-weight and overweight/obese middle-aged and older adults ingest less energy at an ad libitum meal when given a water preload (WP) (500 ml, ~16 fl oz) 30 min prior to the meal compared with a no-preload meal condition. However, a reduction in meal EI following water ingestion has not been observed in studies of young adults (16,18), suggesting there may be age-related differences in the ability of water to acutely reduce EI. Other studies reporting no effect of water ingestion on EI in young adults (19–21) have used water as a control condition; a no-preload condition is not available for comparison. It is unknown if increased water consumption facilitates weight loss over time.
We tested the hypothesis that premeal water consumption would lead to greater weight loss in older overweight and obese individuals consuming a hypocaloric diet. Given previous findings (16,17), a secondary objective was to determine if the ability of premeal water consumption to reduce ad libitum EI is sustained after a 12-week period of increased water consumption in older overweight and obese adults.
METHODS AND PROCEDURES
Subject characteristics
Overweight or obese (BMI 25–40 kg/m2) men and women between the ages of 55–75 years were recruited from the local community though newspaper advertisements. For inclusion in the study, individuals were required to be weight stable (± 2 kg, >1 year) and non-smokers. Individuals were excluded if they reported a history of depression, eating disorders, diabetes, uncontrolled hypertension (>159/99 mm Hg), heart, lung, kidney disease; cancer, food allergies/intolerances to items used in the laboratory test meals; or current use of medications known to alter food intake or body weight. Individuals were blinded to the specific purpose of the study, and were informed that the study involved examination of dietary factors believed to influence weight loss. The study protocol was approved by the Institutional Review Board of Virginia Polytechnic Institute and State University. All participants provided written informed consent prior to study enrollment.
Protocol
Initial screening procedures and baseline assessments
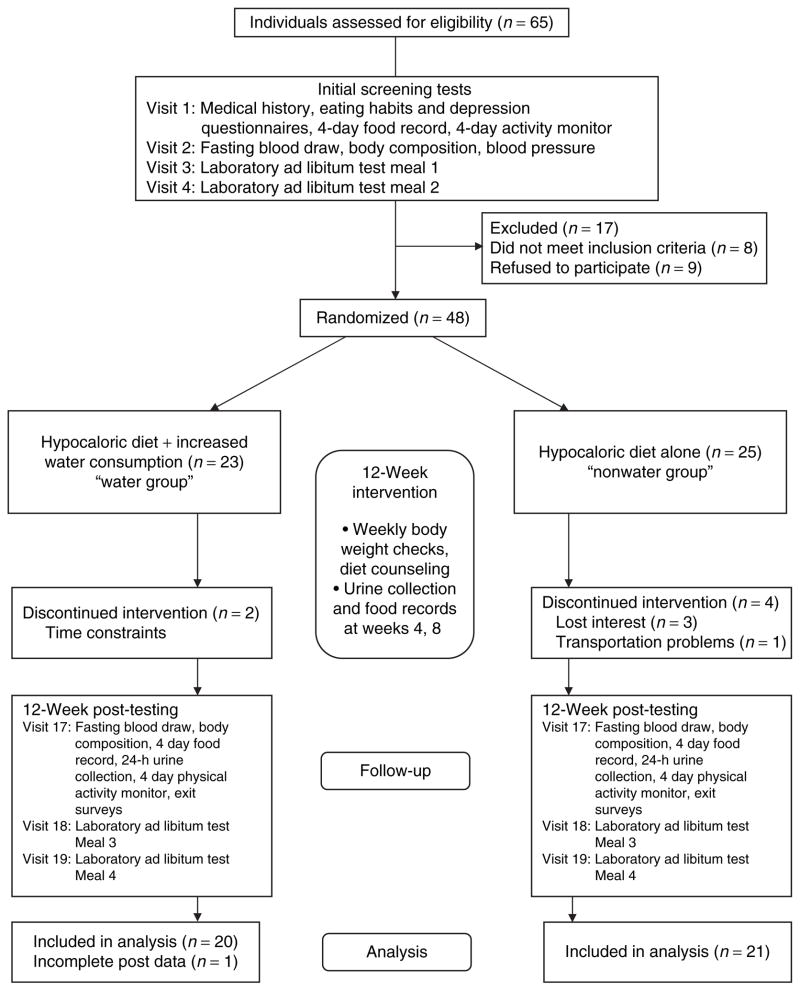
An overview of the study protocol is depicted in Figure 1. Individuals meeting initial enrollment criteria completed baseline laboratory assessments over a series of four visits. Height was measured in meters without shoes using a wall-mounted stadiometer, and body weight was measured to the nearest 0.1 kg using a digital scale with participants wearing light street clothing and no shoes (Scale-Tronix model 5002, Wheaton, IL). Percentage body fat, absolute fat mass, fat-free mass, and total body bone mineral content were measured using dual-energy X-ray absorptiometry (GE Lunar Prodigy; GE Healthcare, Madison, WI). Waist circumference was measured to the nearest 0.5 cm at the umbilicus, using a Gulick tape measure (Gulick, Country Technology, Gays Mill, WI). Resting blood pressure was measured in the seated position using a mercury sphygmomanometer after a 15-min period of rest; the average of three measurements ±6 mm Hg was used. To assess habitual dietary intake and beverage consumption, participants were instructed in proper methods to record their food and beverage intake (including water consumption) for 4 consecutive days, which included 3 weekdays and 1 weekend day, and provided with food models to assist in portion size determination. Records were reviewed for completeness upon their return, and analyzed using diet analysis software (NDS-R 4.05; University of Minnesota, Minneapolis, MN). A second trained technician reviewed all diet analyses for data entry errors. To assess habitual beverage consumption, baseline and week 12 food intake records were manually reviewed to calculate mean daily amounts (kcal, g) of water and other beverages consumed. Dietary energy density (ED; kcal/g) was calculated from the food and beverage intake records and was expressed in four ways (22): total ED including all foods and beverages consumed; beverage ED including water; beverage ED excluding water; and ED from food only, excluding all beverages. When comparing ED (food + beverages) between individuals or over time, excluding water from the calculation could lead to higher ED values among water consumers or those increasing water intake, compared to those consuming energy-free beverages (diet sodas, coffee, and tea) (22). Thus, multiple ED calculations were performed. Participants collected urine for one 24-h period for assessment of total urine volume, and specific gravity was determined using a refractometer (Fisher UriSystem; Fisher Scientific, Hampton, NH). Blood was sampled from an antecubital vein for assessment of lipid and lipoprotein concentrations, which were performed using a SynchronLX20 (Beckman Coulter, Fullerton, CA). Total cholesterol and triglyceride concentrations were determined using the timed endpoint method, high-density lipoprotein cholesterol was determined by homogenous assay, and low-density lipoprotein cholesterol was determined by calculation. Habitual physical activity (steps/day) was measured using GT1M activity monitors for a 4-day period (ActiGraph, Pensacola, FL).
Figure 1.
Study design.
Following initial assessments, each participant underwent two laboratory test meal conditions within a 2-week period, separated by a minimum of 2 days, in a random order as follows: (i) 30-min waiting period (no preload (NP)) followed by an ad libitum breakfast meal, and (ii) preload consisting of 500 ml (~16 fl oz) chilled bottled water followed within 30 min by an ad-lib meal. Condition 1 served as the “baseline” EI for comparison. A 30-min time interval between the preload and ad libitum meal is the most effective time interval to study EI compensation using preloads (23). Subjects were instructed not to eat or drink for at least 12 h prior to arriving for the test meal. The meal consisted of typical breakfast items (cinnamon raisin bagel, cream cheese, margarine, jelly, vanilla yogurt, banana, mozzarella cheese stick, cereal bar, orange juice, coffee, cream, and sugar) provided in excess of what would normally be consumed, from which the participants were allowed to self-select during a 20-min meal period. All foods used in the breakfast meals were evaluated for palatability prior to study initiation. Foods were presented on a meal tray and arranged in the same manner (i.e., location on tray, temperature) on both testing days, and meals were served in individuals cubicles under standardized laboratory conditions (i.e., quiet, temperature controlled). All foods were covertly weighed (±0.1 g) before being served and again after the completion of the meal to determine the amount consumed. Meal energy and nutrient intake were calculated using diet analysis software (NDS-R; University of Minnesota, Minneapolis, MN). Participants completed visual analog scales during the test meal procedure at times 0, 30, 60, 90, 120, and 150 min to subjectively rate their feelings of hunger, satiety (fullness) and thirst (24–26). Time 0 represented arrival for the meal and time 30 represented the time immediately prior to receiving the meal.
Intervention period
Following completion of all baseline assessments (Figure 1), participants were randomly assigned to one of two diet groups for 12 weeks: (i) hypocaloric diet + 16 fl oz (500 ml) bottled water prior to each of the three daily meals (“water group”), or (ii) hypocaloric diet alone (“nonwater group”). Individuals assigned to the water group were provided with cases of bottled water (Aquafina; Pepsico, Purchase, NY), and were instructed to consume one bottle prior to each meal (3 × 16 fl oz bottles/day). Water group participants were provided with a daily tracking form to record their premeal water consumption, which was returned to the study personnel at weekly visits for calculation of weekly water consumption (%) compliance. Nonwater group participants were offered bottled water, but were not given instructions or recommendations on water consumption. Both groups were provided with a variety of additional foods consistent with their meal plans, in order to keep participants blinded to the study purpose. Consumption of these items was not mandatory. Participants received one “provided” food per week in addition to the bottled water, and all participants received the same food item during that week (e.g., seven red delicious apples, 55 kcal each; seven navel oranges, 62 kcal each; one box of microwave popcorn, Orville Redenbacher’s Smart Pop 94% Fat-Free, four Butter-Flavored 100-calorie packs; ConAgra Foods, Omaha, NE). Both groups received individualized instruction by a registered dietitian on a hypocaloric diet (women: 1,200 kcal, men: 1,500 kcal), which was developed using United States Department of Agriculture food guide pyramid guidelines (27). Consumption of fruits, vegetables, lean sources of protein, lowfat/nonfat dairy products, and whole grains was emphasized; both groups were instructed to moderate their consumption of high-fat snack foods, sweetened energy-containing beverages, and alcohol. Meal plan booklets with sample menus were also provided. Average energy and macronutrient content (% energy from fat/carbohydrate/protein, ED) of the 1,200 and 1,500 kcal sample menus, not including optional energy-free beverages (e.g., water, diet soft drinks) were as follows: 1,191 kcal (30/52/21, 1.28 kcal/g); 1,425 kcal (28/53/22, 0.93 kcal/g). Participants were instructed to maintain their current level of physical activity throughout the intervention.
Participants returned weekly to the laboratory for body weight measurement and dietary counseling, and dietary intake records were repeated at weeks 4 and 8 to encourage compliance.
Post-testing
Following the 12-week intervention, participants repeated all baseline measurements (body weight and composition, 4-day dietary intake record and activity monitoring, fasting blood draw, resting blood pressure, 24-h urine collection, two ad libitum laboratory test meal studies), completed an exit survey, and were compensated $50.
Statistical analyses
Power calculations (α = 0.05, power = 0.8) were performed based upon expected differences in weight loss between hypocaloric diets groups (2.0 ± 2.5 kg) to determine the targeted final sample size (n = 40). Baseline group demographic characteristics were assessed using independent samples t-test and Pearson’s χ2-tests (SPSS vs. 12.0 for windows). To assess group difference in weight loss over 12 weeks, a random coefficients (mixed) model (i.e., growth curve analysis) was used, which includes all available data from an individual, corrects for unreliability of measurement and emphasizes individual growth trajectories rather than average values at each occasion (28,29). The growth curve model was fitted using STATA 9.1 xtmixed function. Full-information maximum likelihood estimation, which uses all available data (i.e., weekly body weight measurements) on the 48 participants enrolled into the intervention, was used to address partially observed data. To capture potential variations in the effect of increased water consumption on weight loss over the 12-week intervention, a quadratic effect of time (week-squared) was included in the model as a covariate. The intercept was specified at the first occasion of measurement (i.e., week = 0). Follow-up occasions occurred weekly for 12 weeks, and time was coded as 0–12. All main effects and their interactions with the linear and quadratic effects remained in the model regardless of the significance of the effect.
For secondary outcome variables, repeated measures ANOVA was used to assess group and time differences for subjects completing the 12-week intervention; analysis of covariance was used to adjust for baseline differences when present. When significant interactions were detected, t-tests were used for post hoc analyses. Group differences in pre-to-post change values (Δ) were analyzed using independent samples t-test. The trapezoidal model was used to calculate area under the curve (AUC) for each visual analog scale variable (30), and differences in visual analog scale ratings during the test meal period were assessed using repeated measures ANOVA. Associations among variables were assessed by simple correlational analyses (Pearson’s r). The α-level was set a priori at P < 0.05. Data are expressed as mean ± s.e.m.
RESULTS
Baseline characteristics
In the study, 48 individuals were enrolled and randomized, and 41 completed the 12-week intervention and all post-testing measurements (Figure 1). Baseline group demographic characteristics are shown in Table 1. Most participants were white (~92%), and remaining participants were African American (n = 2), and “other” (n = 2). There were no group differences at baseline in age, body weight, BMI, body composition, urinary specific gravity, systolic blood pressure, total cholesterol and triglyceride concentration, or physical activity level; however, 24-h urine volume and high-density lipoprotein cholesterol concentration was lower and diastolic blood pressure and low-density lipoprotein cholesterol concentration was higher in the water group at baseline (Table 2). There were no baseline group differences in mean daily intake of water, total beverage volume, or beverage energy content (Table 3).
Table 1.
Baseline group demographic characteristics: hypocaloric diet with increased daily water consumption (“water group”) and hypocaloric diet alone (“nonwater group”)
| Water group (n = 23) | Nonwater group (n = 25) | |
|---|---|---|
| Men/women, na | 12/11 | 6/19 |
| Race, white/nonwhite, n | 21/2 | 23/2 |
| Age, years | 62.6 ± 1.2 | 62.2 ± 1.0 |
| Height, m | 1.69 ± 0.02 | 1.65 ± 0.02 |
| Weight, kg | 93.2 ± 2.8 | 89.9 ± 3.4 |
| BMI, kg/m2 | 32.6 ± 0.8 | 32.9 ± 1.3 |
Data are presented as mean ± s.e.m.
Group difference, P < 0.05.
Table 2.
Body composition and other clinical characteristics in the water and nonwater groups before and after the 12-week intervention
| Water group |
Nonwater group |
|||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| BMI, kg/m2 a | 32.1 ± 1.1 | 29.5 ± 1.1 | 31.8 ± 1.1 | 29.9 ± 1.1 |
| Waist circumference, cma | 105.5 ± 2.7 | 99.4 ± 2.8 | 106.1 ± 2.6 | 100.6 ± 2.6 |
| % Body fata | 39.9 ± 1.8 | 36.5 ± 2.0 | 41.0 ± 1.7 | 38.9 ± 1.9 |
| Total fat mass, kgb | 35.1 ± 2.2 | 29.7 ± 2.3 | 34.3 ± 2.1 | 31.0 ± 2.2 |
| Total fat-free mass, kg | 52.4 ± 2.6 | 51.2 ± 2.5 | 49.4 ± 2.5 | 48.1 ± 2.5 |
| Total bone mineral content, kg | 3.1 ± 0.1 | 3.1 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 |
| Systolic blood pressure, mm Hga | 126 ± 2 | 118 ± 2 | 120 ± 2 | 112 ± 2 |
| Diastolic blood pressure, mm Hga,c | 80 ± 1 | 73 ± 1 | 74 ± 1 | 69 ± 1 |
| Total cholesterol, mg/dla | 221 ± 8.7 | 201 ± 7.9 | 196 ± 8.7 | 177 ± 7.9 |
| HDL-C, mg/dlb,c | 42 ± 2.7 | 42 ± 2.6 | 51 ± 2.7 | 47 ± 2.6 |
| LDL-C, mg/dla | 153 ± 7.4 | 139 ± 6.6 | 123 ± 7.4 | 108 ± 6.6 |
| Triglycerides, mg/dld | 132 ± 15.4 | 101 ± 15.3 | 113 ± 15.4 | 110 ± 15.3 |
| Urine volume, mla,c | 1,594 ± 171 | 2,233 ± 168 | 1,951 ± 153 | 2,214 ± 150 |
| Specific gravity, UGb | 1.015 ± 0.001 | 1.009 ± 0.001 | 1.013 ± 0.001 | 1.011 ± 0.001 |
Data are presented as mean ± s.e.m.
Significant main effect of time, P < 0.01.
Significant group by time interaction, P < 0.05.
Group difference at baseline, P < 0.05.
Significant main effect of time, P < 0.05.
Table 3.
Self-reported dietary intake and physical activity in water and nonwater groups before and after the 12-week intervention
| Water group |
Nonwater group |
|||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Total diet | ||||
| Energy, kcal/da | 1,991 ± 131 | 1,454 ± 95 | 2,085 ± 134 | 1,511 ± 98 |
| Weight, g/db | 2,616 ± 148 | 3,226 ± 228 | 2,884 ± 152 | 2,699 ± 234 |
| Carbohydrate (% energy) | 48.5 ± 2.0 | 51.1 ± 2.6 | 47.1 ± 2.1 | 48.7 ± 2.7 |
| Protein (% energy)a | 16.1 ± 0.7 | 18.0 ± 0.6 | 15.6 ± 0.7 | 17.4 ± 0.6 |
| Fat (% energy) | 35.2 ± 1.4 | 31.5 ± 2.0 | 33.8 ± 1.4 | 32.4 ± 2.1 |
| Energy density, kcal/gcd | 0.78 ± 0.05 | 0.48 ± 0.05 | 0.74 ± 0.05 | 0.63 ± 0.05 |
| Beverages only | ||||
| Energy, kcal/da | 235 ± 30 | 148 ± 24 | 292 ± 31 | 156 ± 25 |
| Weight, g/db | 1,588 ± 121 | 2,287 ± 157 | 1,762 ± 124 | 1,372 ± 161 |
| Water consumption, g/db | 306 ± 98 | 1,291 ± 111 | 446 ± 100 | 323 ± 114 |
| Weight, excluding water, g/da | 1,283 ± 116 | 996 ± 108 | 1,316 ± 119 | 1,048 ± 111 |
| Energy density, including water, kcal/ga | 0.15 ± 0.03 | 0.07 ± 0.02 | 0.20 ± 0.03 | 0.13 ± 0.02 |
| Energy density, excluding water, kcal/ga | 0.20 ± 0.03 | 0.17 ± 0.03 | 0.24 ± 0.03 | 0.15 ± 0.03 |
| Food only | ||||
| Energy, kcal/da | 1,756 ± 126 | 1,306 ± 85 | 1,793 ± 129 | 1,355 ± 88 |
| Weight, g/d | 1,027 ± 78 | 939 ± 145 | 1,123 ± 80 | 1,327 ± 149 |
| Energy density, kcal/ga | 1.74 ± 0.08 | 1.44 ± 0.10 | 1.63 ± 0.08 | 1.26 ± 0.10 |
| Physical activity, steps/d | 7,073 ± 717 | 7,349 ± 682 | 6,749 ± 807 | 7,051 ± 767 |
Data are presented as mean ± s.e.m.
Significant main effect of time, P < 0.01.
Significant group by time interaction, P < 0.05.
Calculated with all foods and beverages, including water.
Significant group by time interaction, P < 0.01.
Intervention
As depicted in Figure 2, weight declined significantly over the 12 weeks for both groups (β = −0.27, P < 0.01), although the water group (β = −0.87, P < 0.001) showed a 44% greater decline (i.e., greater rate of weight loss) over the 12 weeks than the nonwater group (β = −0.60, P < 0.001). There was also a significant quadratic trend in weight loss (β = 0.01, P < 0.05), indicating that the linear decline in weight leveled off toward the end of the study period. This abatement was greater for the water group (β = 0.03, P < 0.001) than for the nonwater group (β = 0.02, P < 0.001).
Figure 2.
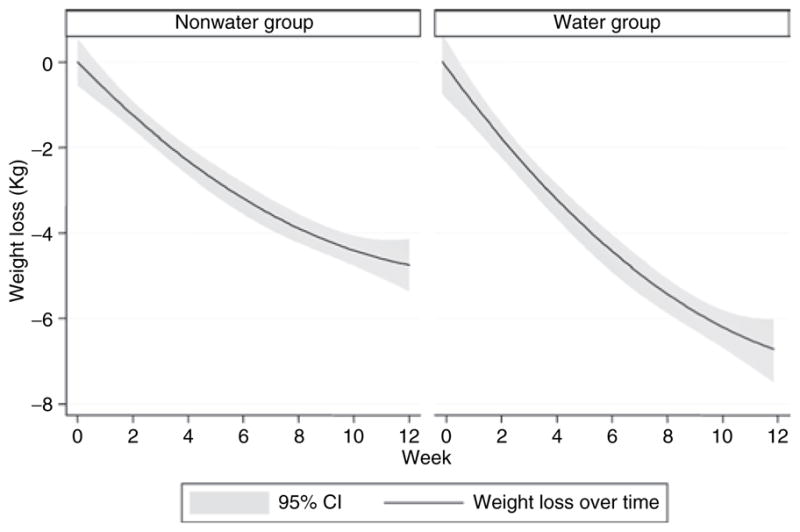
Weight loss among water and nonwater group participants over the 12-week intervention.
Body composition and other clinical outcome variables at baseline and postintervention are presented in Table 2. Decline in total fat mass was greater in the water than nonwater group (water: Δ–5.4 ± 0.6 kg; nonwater: Δ–3.3 ± 0.5 kg; P = 0.01); however, percent of initial body weight lost (7.8 ± 0.7% vs. 6.5 ± 0.7%, water vs. nonwater, respectively; P = 0.17) and reduction in percent body fat (water: Δ–3.4 ± 0.5%; nonwater: Δ–2.1 ± 0.6%; P = 0.08) were not different between groups. Reductions in BMI, waist circumference, systolic and diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, and triglyceride concentrations were observed over the 12-week intervention, but there were no group differences in changes in these outcomes (Table 2). There was no change over time or between groups in bone mineral content during the 12-week intervention. The reduction in high-density lipoprotein cholesterol concentration was smaller in the water group compared with the nonwater group following the 12-week intervention (water: Δ–0.6 ± 0.9 mg/dl; nonwater: Δ–3.9 ± 0.9 mg/dl; P = 0.01).
Average weekly water intake compliance among water group participants was reported to be 90 ± 2%, and an objective indicator of compliance, urinary specific gravity, declined over time in the water group as compared to the nonwater group (Table 2). The increase in urine volume over time was not different between groups.
Due to an unintended greater random allocation of men to the water group than nonwater group (Table 1), additional analyses were performed to determine if weight loss outcomes differed between men and women in two groups. Total weight loss was not different (all P > 0.05) among men and women in each diet group (water: men −7.7 kg, women −7.0 kg, both ~8% of initial weight lost; nonwater: men −6.7 kg, women −5.0 kg, both ~6% of initial weight lost) or in the pooled sample (men −7.3 kg, women −5.7 kg, ~7% of initial weight).
Dietary intake and physical activity outcomes over the 12-week intervention are presented in Table 3. There were no baseline group differences in mean daily EI or dietary ED, but several differences were detected in dietary outcomes at baseline compared to week 12. Mean daily EI declined similarly in both groups. Total dietary ED (food + all beverages, including water) declined more in the water group as compared to the nonwater group. After 12 weeks, both groups had significantly reduced EI from beverages to ~10% of total EI, and water group participants demonstrated greater increases in water and total fluid consumption than the nonwater group participants. Beverage ED, both including and excluding water, declined in both groups but no group differences in beverage ED were detected. Similarly, energy and ED from food alone decreased in both groups, but no group differences were found. Dietary changes associated with reductions in body weight included changes in water intake (r = 0.35, P = 0.03), and absolute and relative fat intake (fat grams: r = −0.36, P = 0.03; percent energy from fat: r = −0.44, P = 0.005). No other significant associations of dietary intake variables with weight changes were found. Physical activity level did not change during the 12-week intervention.
Of the 31 participants completing the exit survey, 11 (water group, n = 8; nonwater group, n = 3) believed that water was involved some aspect of the study, and of those, eight (water group, n = 7; nonwater group, n = 1) accurately identified the purpose of the study.
Ad libitum test meals
In the pooled sample, mean ad libitum breakfast meal EI was lower in the WP condition as compared to the NP condition at baseline (WP 498 ± 25 kcal, NP 541 ± 27 kcal, P = 0.009) but not at week 12 (WP 480 ± 25 kcal, NP 506 ± 25 kcal, P = 0.069). No significant group by condition differences were found in breakfast meal EI, when expressed in either in absolute (kcal) or relative (% change) terms.
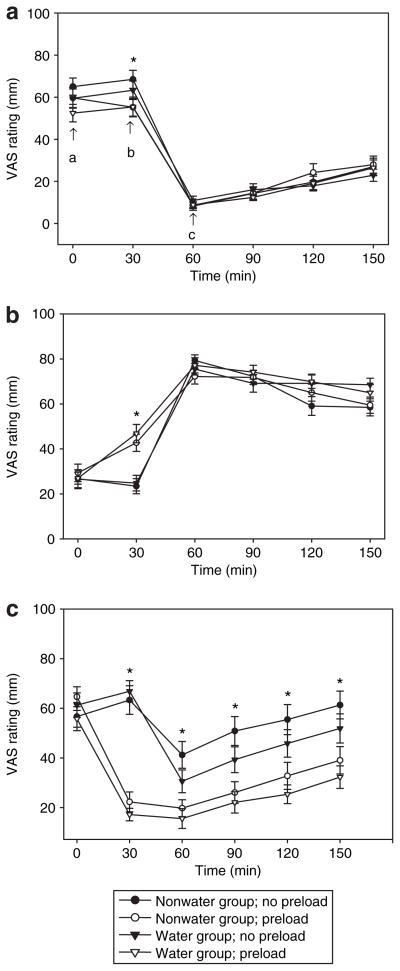
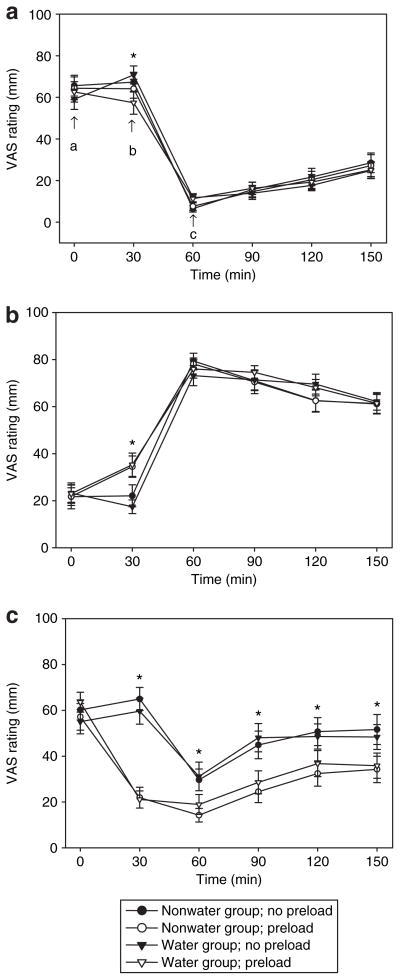
Subjective ratings of hunger, fullness, and thirst during the two test meal conditions at baseline and at 12 weeks are shown in Figures 3 and 4, respectively. Hunger AUC ratings did not differ significantly between groups, conditions, or over time. Fullness AUC ratings were higher in the WP compared to NP condition (8,975 ± 258 vs 8,296 ± 275 mm min, respectively; P = 0.002), but there were no differences between groups or over time. As would be expected, thirst AUC ratings were lower in the WP compared to NP condition (4,090 + 342 vs. 7,297 mm min, respectively; P < 0.001), and no differences were noted between groups or over time. Hunger and thirst AUC values were correlated in the WP condition (r = 0.496, P < 0.001) but not the NP condition (r = 0.149, P = 0.312).
Figure 3.
Visual analog scale (VAS) ratings of (a) hunger, (b) fullness, and (c) thirst among water and nonwater group participants at baseline in the water preload and no-preload ad libitum meal conditions. Following completion of the 0 min VAS scale, the water preload was provided (water preload condition) (a); subjects completed the next VAS scale at 30 min, and were immediately provided with the ad libitum meal (b). VAS scales were completed following the ad libitum meal at 60 min (c), and at subsequent 30-min intervals until the completion of the 150-min testing period. *Significant difference between preload conditions, P < 0.05. No group differences were detected.
Figure 4.
Visual analog scale (VAS) ratings of (a) hunger, (b) fullness, and (c) thirst among water and nonwater group participants following the 12-week intervention in the water preload and no-preload ad libitum meal conditions. Following completion of the 0 min VAS scale, the water preload was provided (water preload condition) (a); subjects completed the next VAS scale at 30 min, and were immediately provided with the ad libitum meal (b). VAS scales were completed following the ad libitum meal at 60 min (c), and at subsequent 30-min intervals until the completion of the 150-min testing period. *Significant difference between preload conditions, P < 0.05. No group differences were detected.
DISCUSSION
To our knowledge, this is the first randomized controlled trial investigating the influence of increased water consumption on weight loss. Our results indicate that when combined with a hypocaloric diet, consuming 500 ml (~16 fl oz) of water prior to each of the three main daily meals (1.5 l/d) leads to ~2 kg greater weight loss over 12 weeks as compared to a hypocaloric diet alone (Figure 2), among middle-aged and older adults. This difference was attributed to a 44% greater rate of weight loss among water group participants compared to nonwater participants over the 12-week period. This effect may be due in part to an acute reduction in meal EI following water ingestion, which we observed at the baseline laboratory test meal studies. A reduction in meal EI following water consumption is accompanied by increased sensations of fullness, which may facilitate a lower meal EI following water ingestion. However, it is not clear from our findings how long this effect is sustained, as we did not observe significant differences between meal conditions after the 12-week weight loss intervention.
Our data are consistent with prior reports. In a secondary analysis of a trial comparing several weight loss diets, Stookey et al. (13) found that overweight women who reported drinking ≥1 l/d of water over a 12-month period increased weight loss by ~2 kg compared to those who did not increase water consumption. However, intentionally water consumption was not manipulated, and water consumption data was self-reported. Nonetheless, our data are in agreement with these findings in that they support a beneficial role of increasing water consumption while consuming a hypocaloric diet.
Though the exact mechanism responsible for the greater weight loss with increased water consumption is presently unknown, consuming water before a meal or with a meal reduces sensations of hunger, and increases satiety (15–17). First, changes in subjective sensations of hunger and satiety are associated with an acute reduction in meal EI (16,17), but prior to our study it was unknown if this acute reduction in meal EI could facilitate weight loss while consuming a hypocaloric diet. Advancing age is also associated with delayed gastric emptying (31) that may play a role in reducing meal EI following a WP in middle-aged and older adults; this possibility warrants further investigation. We did not detect group differences in self-reported EI over the 12-week intervention, possibly due to the limitations associated with utilizing self-reported dietary intake measures (32). Studies including objective measures of daily EI, such as those conducted on an in-patient metabolic research unit, are needed to more accurately quantify the potential daily reduction in EI associated with increased water ingestion.
Second, replacing energy-containing beverages in the diet with water may lead to a reduction in overall EI, as epidemiological data suggests that total beverage energy contributes >400 kcal to daily EI (33). In our sample, beverage EI declined by ~100 kcal over the 12-week intervention, but did not differ between groups and is thus unlikely to explain our findings. As both groups were instructed to moderate their consumption of sweetened energy-containing beverages and alcohol, the lack of a group difference in beverage EI and nonwater beverage consumption is not unexpected. However, in the entire sample, a greater increase in water intake was positively associated with weight loss. In addition, overall dietary ED (food + beverages, including water) decreased significantly more in the water group than the nonwater group which may be attributed to an increased water intake among water group participants; reducing dietary ED is thought to be an effective weight loss strategy (34).
Finally, it is possible that daily self-monitoring of water intake contributed to a greater weight loss in our water group participants, as others have demonstrated benefits of daily self-monitoring behaviors associated with weight management (i.e., daily self-weighing) (35). Further research is warranted to determine the relative contributions of each of these possible physiological and behavioral mechanisms related to water consumption promoting weight loss.
There are some limitations that should be acknowledged. First, the sample size was small. However, this sample size provided sufficient power to detect physiologically and statistically significant effects in many outcome variables which were consistent with our hypothesis. Second, no standardized laboratory test is available to objectively assess compliance with the water intervention. We utilized urinary specific gravity, 24-h urine collections, self-reported daily compliance logs, and food intake records. These procedures provided reasonable indicators of compliance when comparing the two groups over time and there was consistency among most of these measures. Finally, these results may not apply to the general population, in that our study only included primarily white, middle-aged and older adults. Rolls et al. (18) did not observe a difference in meal EI in young, normal-weight men who were given 8 and 16 oz of water 30 min prior to a meal as compared to no beverage. is observation is consistent with our findings in young adults (16). Future studies examining premeal water intake in younger populations could address methodological changes such as increasing the quantity of the WP, or reducing the time between the preload ingestion and the ad libitum meal.
These findings may have clinical implications. Our prior work (16,17) led us to hypothesize that premeal water consumption could reduce daily EI by ~225 kcal, and over a 12-week period, could produce an energy deficit of ~18,900 kcal and lead to ~2.5 kg weight loss. Although we recognize this is an extrapolation, it is consistent with our findings. Dietitians and other weight management practitioners often advise individuals desiring weight loss to increase their water consumption, and this strategy is often recommended in popular weight loss programs (36–38). These findings provide an evidence-basis for this strategy among middle-aged and older adults. In addition, increasing water consumption is a simple, inexpensive behavioral change which can be recommended as a component of a hypocaloric diet to possibly enhance weight loss outcomes. Another potential health benefit of this strategy is improved hydration status, as habitual fluid intake among our population (Table 2) was well below current guidelines (39). us, our findings suggest benefits of increasing water consumption for weight management and health among middle-aged and older adults.
We conclude that for overweight or obese middle-aged and older adults, consuming ~2 cups of water prior to each of the three main daily meals may increase weight loss when combined with a hypocaloric diet, as compared to a hypocaloric diet alone. This strategy may aid in increasing fullness, thereby promoting a reduction in meal EI. Future studies, with larger sample sizes, are needed to confirm our findings as well as to determine how long the acute reduction in meal EI following water ingestion is sustained; if this increased weight loss with water consumption is maintained over time; and if increased water consumption facilitates long-term weight loss maintenance.
Acknowledgments
Funding for this investigation was provided by a research grant from the Institute for Public Health and Water Research.
Footnotes
DISCLOSURE
The senior author (B.M.D.) has received research funding for this investigation from the Institute for Public Health and Water Research
References
- 1.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Van Walleghen EL, Orr JS, Gentile CL, Davy KP, Davy BM. Habitual physical activity differentially affects acute and short-term energy intake regulation in young and older adults. Int J Obes (Lond) 2007;31:1277–1285. doi: 10.1038/sj.ijo.0803579. [DOI] [PubMed] [Google Scholar]
- 4.Rolls BJ, Dimeo KA, Shide DJ. Age-related impairments in the regulation of food intake. Am J Clin Nutr. 1995;62:923–931. doi: 10.1093/ajcn/62.5.923. [DOI] [PubMed] [Google Scholar]
- 5.Roberts SB, Fuss P, Heyman MB, Young VR. Influence of age on energy requirements. Am J Clin Nutr. 1995;62:1053S–1058S. doi: 10.1093/ajcn/62.5.1053S. [DOI] [PubMed] [Google Scholar]
- 6.Roberts SB, Dallal GE. Energy requirements and aging. Public Health Nutr. 2005;8:1028–1036. doi: 10.1079/phn2005794. [DOI] [PubMed] [Google Scholar]
- 7.Leigh JP, Hubert HB, Romano PS. Lifestyle risk factors predict healthcare costs in an aging cohort. Am J Prev Med. 2005;29:379–387. doi: 10.1016/j.amepre.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 9.Sharkey JR, Ory MG, Branch LG. Severe elder obesity and 1-year diminished lower extremity physical performance in homebound older adults. J Am Geriatr Soc. 2006;54:1407–1413. doi: 10.1111/j.1532-5415.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 10.Popkin BM, Barclay DV, Nielsen SJ. Water and food consumption patterns of U.S. adults from 1999 to 2001. Obes Res. 2005;13:2146–2152. doi: 10.1038/oby.2005.266. [DOI] [PubMed] [Google Scholar]
- 11.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stookey JD, Constant F, Gardner CD, Popkin BM. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity (Silver Spring) 2007;15:3013–3022. doi: 10.1038/oby.2007.359. [DOI] [PubMed] [Google Scholar]
- 13.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008;16:2481–2488. doi: 10.1038/oby.2008.409. [DOI] [PubMed] [Google Scholar]
- 14.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44:187–193. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Lappalainen R, Mennen L, van Weert L, Mykkänen H. Drinking water with a meal: a simple method of coping with feelings of hunger, satiety and desire to eat. Eur J Clin Nutr. 1993;47:815–819. [PubMed] [Google Scholar]
- 16.Van Walleghen EL, Orr JS, Gentile CL, Davy BM. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity (Silver Spring) 2007;15:93–99. doi: 10.1038/oby.2007.506. [DOI] [PubMed] [Google Scholar]
- 17.Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc. 2008;108:1236–1239. doi: 10.1016/j.jada.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav. 1990;48:19–26. doi: 10.1016/0031-9384(90)90254-2. [DOI] [PubMed] [Google Scholar]
- 19.Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159–1164. doi: 10.1093/ajcn/53.5.1159. [DOI] [PubMed] [Google Scholar]
- 20.Holt SH, Sandona N, Brand-Miller JC. The effects of sugar-free vs sugar-rich beverages on feelings of fullness and subsequent food intake. Int J Food Sci Nutr. 2000;51:59–71. doi: 10.1080/096374800100912. [DOI] [PubMed] [Google Scholar]
- 21.Rodin J. Comparative effects of fructose, aspartame, glucose, and water preloads on calorie and macronutrient intake. Am J Clin Nutr. 1990;51:428–435. doi: 10.1093/ajcn/51.3.428. [DOI] [PubMed] [Google Scholar]
- 22.Ledikwe JH, Blanck HM, Khan LK, et al. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr. 2005;135:273–278. doi: 10.1093/jn/135.2.273. [DOI] [PubMed] [Google Scholar]
- 23.Rolls BJ, Kim S, McNelis AL, et al. Time course of effects of preloads high in fat or carbohydrate on food intake and hunger ratings in humans. Am J Physiol. 1991;260:R756–R763. doi: 10.1152/ajpregu.1991.260.4.R756. [DOI] [PubMed] [Google Scholar]
- 24.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 25.Parker BA, Sturm K, MacIntosh CG, et al. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58:212–218. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- 26.Porrini M, Crovetti R, Testolin G, Silva S. Evaluation of satiety sensations and food intake after different preloads. Appetite. 1995;25:17–30. doi: 10.1006/appe.1995.0038. [DOI] [PubMed] [Google Scholar]
- 27.Dietary Guidelines for Americans 2005. US Department of Health and Human Services. 6. US Department of Agriculture; Washington, DC: 2005. [Google Scholar]
- 28.Raudenbush S, Bryk A. Hierarchical linear model: Applications and data analysis methods. 2. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- 29.Singer J, Willett J. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 30.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 31.Parker BA, Chapman IM. Food intake and ageing–the role of the gut. Mech Ageing Dev. 2004;125:859–866. doi: 10.1016/j.mad.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Johnson R, Hankin J. Dietary Assessment and Validation. In: Monsen E, editor. Successful Approaches. 2. American Dietetic Association; 2003. pp. 227–242. [Google Scholar]
- 33.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007;15:2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- 34.Ledikwe JH, Rolls BJ, Smiciklas-Wright H, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. 2007;85:1212–1221. doi: 10.1093/ajcn/85.5.1212. [DOI] [PubMed] [Google Scholar]
- 35.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 36.Atkins R. Dr. Atkins’ NEW Diet Revolution. Avon Books; New York: 1992. [Google Scholar]
- 37.Miller-Kovach K. Water and Health. Weight Watchers International; New York: 2008. [Google Scholar]
- 38.Sears B, Lauren W. The Zone. Harper Collins; New York: 1995. [Google Scholar]
- 39.Panel on Dietary Reference Intakes for Electrolytes and Water. Institute of Medicine. The Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Dietary Reference Intakes for Water, Potassium, Sodium Chloride, and Sulfate. National Academy Press; Washington, DC: 2004. pp. 146–147. [Google Scholar]