Abstract
The central nervous system (CNS) utilizes anticipatory (APAs) and compensatory (CPAs) postural adjustments to maintain equilibrium while standing. It is known that these postural adjustments involve displacements of the center of mass (COM) and center of pressure (COP). The purpose of the study was to investigate the relationship between APAs and CPAs from a kinetic and kinematic perspective. Eight subjects were exposed to external predictable and unpredictable perturbations induced at the shoulder level while standing. Kinematic and kinetic data were recorded and analyzed during the time duration typical for anticipatory and compensatory postural adjustments. When the perturbations were unpredictable, the COM and COP displacements were larger compared to predictable conditions with APAs. Thus, the peak of COM displacement, after the pendulum impact, in the posterior direction reached 28 ± 9.6 mm in the unpredictable conditions with no APAs whereas it was 1.6 times smaller, reaching 17 ± 5.5 mm during predictable perturbations. Similarly, after the impact, the peak of COP displacement in the posterior direction was 60 ± 14 mm for unpredictable conditions and 28 ± 3.6 mm for predictable conditions. Finally, the times of the peak COM and COP displacements were similar in the predictable and unpredictable conditions. This outcome provides additional knowledge about how body balance is controlled in presence and in absence of information about the forthcoming perturbation. Moreover, it suggests that control of posture could be enhanced by better utilization of APAs and such an approach could be considered as a valuable modality in the rehabilitation of individuals with balance impairment.
Keywords: postural control, standing, external perturbations, compensatory and anticipatory adjustments
Introduction
The equilibrium of vertical posture is achieved when the center of mass (COM) of the body is positioned over the base of support (BOS) and is aligned with the center of pressure (COP). Any body perturbation, either external such as a sudden translation of the support surface or internal such as fast arm or leg movement, shifts the projection of the COM closer to the borders of the BOS and the alignment between the COM and COP is disrupted: this may result in the loss of body equilibrium. To minimize the danger of losing equilibrium, the central nervous system (CNS) utilizes anticipatory postural adjustments (APAs) by activating the trunk and leg muscles prior to the forthcoming body perturbation. As a result of such anticipatory muscle activity, the observed displacements of the COM and COP are small (Belenkiy et al. 1967; Massion 1992; Aruin and Latash 1995; Li and Aruin 2007). Theoretically, minor equilibrium disturbances could be counteracted with involvement of APAs only. In the real world, however, this happens rarely since the body perturbations are too large to be counteracted using just APAs. Thus, the CNS uses compensatory postural adjustments (CPAs) that are initiated by the sensory feedback signals (Park et al. 2004; Alexandrov et al. 2005). As such, CPAs serve as a mechanism of restoration of the position of the COM after a perturbation has already occurred.
While important information about the individual role of APAs and CPAs in control of posture is available in the literature, to the best of our knowledge, there are no studies investigating systematically the role of APAs in subsequent control of posture after a perturbation has occurred, i.e., during the CPA phase. Understanding the role of APAs in compensatory control of posture is important because activities such as throwing or catching a ball (that induce expected perturbations) or manual trunk disturbs such as pulling or pushing (that might be considered as unexpected perturbations) are commonly used by clinicians to treat individuals with orthopedic and neurologic impairments (Kisner and Colby 2007). Such perturbations are also used for balance control training or physical fitness in the elderly. However, little is known about the role of anticipatory postural adjustments, specifically, its relationship with CPAs in controlling body balance.
In a recent study, we investigated a relationship between the anticipatory and compensatory EMG activity recorded in the trunk and leg muscles prior to and after external perturbations (Santos et al. 2009). It was shown that if present, anticipatory EMG activity in the trunk and leg muscles scales down the magnitudes of compensatory EMGs. In contrast, when no APAs were generated, greater activity and different sequence of muscle activation were seen during the CPA phases.
The principal purpose of the present study was to investigate the relationship between APAs and CPAs from a kinetic and kinematic perspective. This study tested the hypothesis that the predictability of a perturbation influences the relationship between COM and COP displacements. Our second hypothesis was that the joint angular displacements after the perturbation would be larger for the unpredictable as compared to the predictable conditions.
To test these hypotheses we utilized an experimental paradigm that induces external perturbations at the shoulder level; this type of perturbation is commonly used in clinical settings to enhance/restore balance control, for example, catching a ball (Kisner and Colby 2007). The perturbations were applied with the subjects in the standing position, with their eyes open and eyes closed: this introduced identical predictable and unpredictable body perturbations.
METHODS
Subjects
Eight subjects (4 males and 4 females) free from any neurological or musculoskeletal disorders participated in the experiments. The mean age of the subjects was 25±2 years, mean body mass 59.1±6.5 kg, and mean height 1.67±0.08m. They all signed a written informed consent approved by the Institutional Review Board of the University of Illinois at Chicago.
Procedure
The subjects were instructed to maintain upright stance while standing barefoot on the force platform with their feet shoulder width apart. They were positioned in front of an aluminum pendulum attached to the ceiling. The pendulum had a foam covered hand bar attached to its distal end. A load (mass, m = 1.36 kg) was attached to the pendulum next to its distal end and a rope fastened to the pendulum was passed through a pulley system attached to the ceiling. Perturbations consisted of unidirectional forces applied to both the subjects’ extended hands, using the pendulum that was pulled to a fixed distance away from the subjects’ hands (0.8 m). All the trials of the pendulum release were implemented by the same experimenter. The subjects were required to receive each pendulum impact with their hands, while their arms were extended at the shoulder level, and to maintain their balance after the perturbation. Since the perturbation was induced in sagittal plane and applied to both the hands, it did not produce lateral or rotational body perturbations, and as such could be considered as symmetrical. The two experimental conditions were: 1) a series of perturbations were applied with the subjects’ eyes open and as such were predictable in their timing; we call this condition “predictable perturbations” and 2) another series of perturbations were applied with the subjects’ eyes closed; this condition is called “unpredictable perturbations”. No advance warning of the impending perturbation was provided; instead, the subjects wore earphones and listened to music delivered via a mini audio player (iPod, Apple Inc.) to prevent them from obtaining auditory information about the moment of the pendulum release during unpredictable perturbations. In addition, the experimenter released the pendulum at different times during the data collection, thus during the unpredictable conditions, the individuals could not predict when the pendulum would hit on their hands (for more details see (Santos et al. 2009).
For safety purposes in all the experiments, the subjects wore a harness with two straps attached to the ceiling. Five trials were performed in each experimental condition and the order of experimental conditions was randomized for each subject. Since the mass of the pendulum and the distance from which it was released remained the same, perturbations of similar magnitude were induced in both, predictable and unpredictable perturbation conditions. The magnitude of the perturbation was large enough to evoke compensatory feet-in-place reactions.
Data recording
A six-camera VICON 612 system (Oxford Metrics) was used to collect three-dimensional kinematic data. Retroreflective markers were placed over anatomical landmarks bilaterally according to the Plug-In-Gait (PIG) model (Oxford Metrics), which includes: second metatarsal head, calcaneus, lateral malleolus, lateral epicondyle of the femur, anterior/posterior superior iliac spines, second metacarpal, lateral epicondyle of the humerus, acromio-clavicular joint. Also, subjects wore head and wrists bands with 4 and 2 markers attached on them, respectively. Finally, 5 additional markers were attached over: 7th cervical vertebrae, 10th thoracic vertebra, inferior angle of the right scapula, between the 2 sternoclavicular joints, and xiphoid process of the sternum bone.
Ground reaction forces and moments of forces were recorded using a force platform (AMTI, USA) positioned on the floor. An accelerometer (PCB, USA) was attached to the pendulum to register the moment of perturbation.
Kinematic data were collected at 100 Hz, while forces, moments of force, and accelerometer signals were acquired at 1000 Hz. The synchronization was achieved by the Vicon software in a way that for one pulse of kinematic data recorded, ten pulses of analog data were recorded. Data collection was performed using the Vicon 612 system that controlled data collection of all signals.
Data processing
Kinematic and kinetic data were recorded and stored on a PC computer for further analysis. All signals were analyzed off-line using Matlab programs. Individual trials were viewed on a computer screen and aligned according to the first visible onset rise of the accelerometer signal. The alignment time was referred to as time zero (T0=0). Aligned trials within each series were averaged for each subject.
Displacements of the center of pressure (COP) in the anterior-posterior direction were calculated using the following approximation (Winter et al. 1996):
where My is the moment in sagittal plane, Fz and Fx are the vertical and anterior-posterior components of the ground reaction force, and d is the distance from the origin of the force platform to the surface. The perturbations were induced in the sagittal plane and applied simultaneously to both the hands of the subjects positioned orthogonal to the direction of the pendulum impact, and as such they were associated with anterior-posterior displacements of the COP and negligible COP displacements in medial-lateral directions. Hence, data on COP displacements in medial-lateral directions will not be presented.
The joint angles were derived from the Plug-in-Gait (PIG) model (Oxford Metrics). The PIG model consisted of fifteen body segments, including pelvis, femur (2), tibia (2), feet (2), humerus (2), radius (2), hands (2), thorax, and head. The kinematic data were low-pass filtered at 8 Hz and angular displacements of the ankle (ANK), knee (KEE), hip (HIP), spine (SPN), thorax (TOR), and head (HEA) in the tridimensional planes were calculated. For ANK, KEE, and HIP, the angles were calculated for both the right and left body sides. For ANK, KEE, HIP, SPN, and HEA in the sagittal plane, the positive sign corresponds to flexion and the negative to extension. Body mass and height, 7 anthropometrical measures such as leg length, knee, ankle, elbow, and wrist width and shoulder offset and hand thickness for each subject were entered in the PIG model. These measures together with the kinematic data were used to calculate body’s COM position. The COM displacements in the sagittal (COMy) and transverse planes (COMz) were analyzed. As the perturbations were applied to the subject’s both hands simultaneously, the COM displacements in the frontal plane were minimal and were not used for analysis purposes. The onset of COMy, COMz and COP displacements were determined visually by a trained researcher. The peak displacements for COMy (PCOMy), COMz (PCOMz) and COP (PCOP) and their respective times (TCOMy, TCOMz, and TCOP) were also calculated. These measures indicated the individuals’ balance control ability after the perturbations.
The mean displacements of the ANK, KEE, HIP, SPN, TOR, and HEA angles were calculated for each of the 4 epochs of 150 ms in duration. Thus, the following epochs were utilized: 1) −200ms to −50 ms (preparatory reactions, APA1), 2) −50ms to +100ms (anticipatory reactions, APA2), 3) +100ms to 250 ms (compensatory reactions, CPA1), and 4) +250ms to 400 ms (compensatory reactions CPA2). Note that the duration of each of four epochs was the same (150 ms) as the duration of the epochs used to analyze the EMG signals (Santos et al. 2009), however, the starting point of each epoch was shifted 50 ms towards T0 to account for the electromechanical delay (Cavanagh and Komi 1979; Vint et al. 2001; Georgoulis et al. 2005; Rocchi et al. 2006). This shift was applied to account for the kinetic and kinematic changes produced by the muscular activity that occurred before the pendulum impact (APAs) (Santos et al. 2009). For example, the mechanical event of the first epoch (−200 to −50ms) corresponded to the muscular activation that occurred at (−250 to −100ms). The baseline for each kinetic and kinematic variable was calculated as well in the interval from −500 ms to −450 ms. The baseline measure for each variable was multiplied by 3 to account for 3 times difference in the duration of the baseline window and the four epochs. This baseline measure was then subtracted from each of the respective kinetic and kinematic variables. As the perturbations were symmetrical, only the right ANK, KEE, and HIP angles were used for statistical analysis.
Principal component analysis (PCA) was used to better understand the segmental coordination during the APA and CPA phases. Each of these principal components is a linear combination of the original variables. As they are orthogonal to each other, there is no redundant information. This approach has been utilized in the past to interpret EMG activities (Wang et al. 2006) and movements of the body segments (Hughey and Fung 2005; Li and Aruin 2008) associated with self-induced or external body perturbations. The data included the mean raw angles of ANK, KEE, HIP, SPN, TOR, and HEA for each subject in the sagittal plane. Thus, for predictable conditions, the principal components were computed from 4 data matrices of 15 × 6, i.e., 15 samples accounting for 150 ms and 6 angles. For unpredictable conditions, only two data matrices of 15 × 6 were used to calculate the principal components, which corresponded to the two 150 ms CPA time windows. Also, combined PCA values were calculated for each of the APA (APA1 and APA2) and CPA (CPA1 and CPA2) phases. As a result, for predictable conditions, the principal components were computed from 2 data matrices of 30 × 6, i.e., 30 samples accounting for 300 ms and 6 angles. These 300 ms corresponded to the sum of the two 150 ms time windows of APA and CPA. For unpredictable conditions, only one data matrix of 30 × 6 was used to calculate the principal components, which corresponded to the two 150 ms CPA time windows. The percent of total variability explained by each principal component was also calculated for both time windows as well. The mean PCA values of all subjects are presented in absolute values.
Statistical Analysis
Multiple repeated measures ANOVAs with two within-subjects factors (condition (2) and epochs (4)) were used to compare each joint angle (ANK, KEE, HIP, SPN, TOR, HEA). A post hoc analysis was used to further investigate between epoch’s differences in the joint angular positions. The repeated measures ANOVA with two within-subjects factors (condition (2) and time windows (2)) was also used to compare the 4 PCA values. Paired T-Test was used to compare PCOMy, PCOMz, PCOPy as well as TCOMy, TCOMz, and TCOP, between conditions (predictable and unpredictable).
Results
Angular displacements
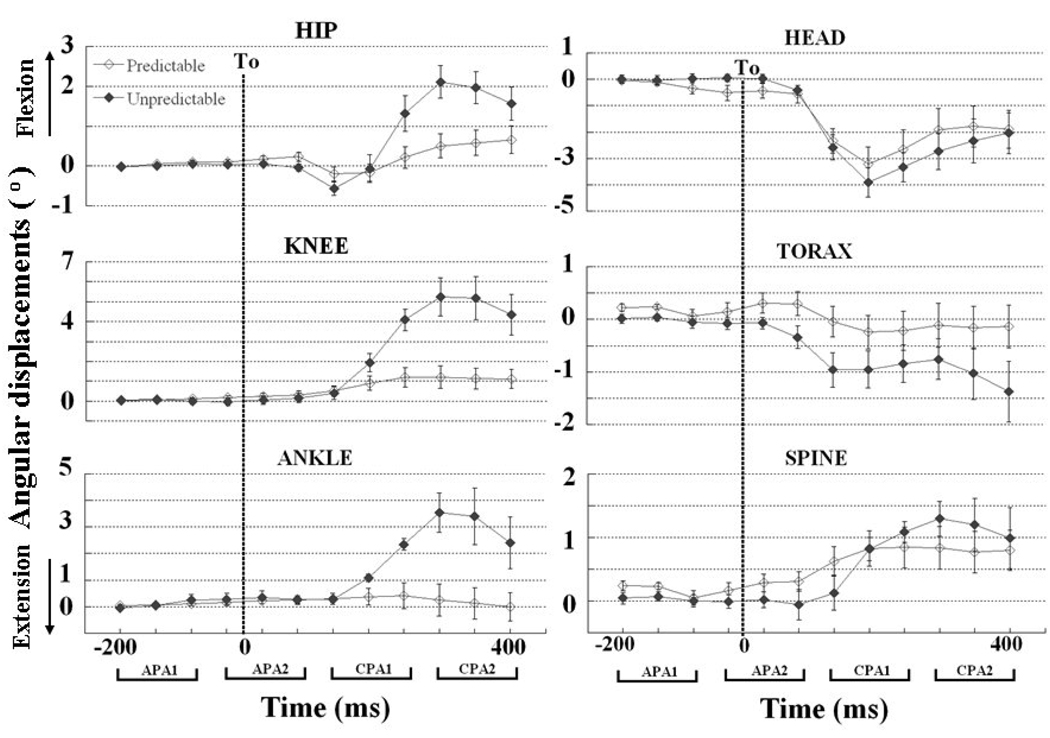
Changes in the angular position of the ankle, knee, hip, spine, thorax, and head are shown in Figure 1. Note that the angular position was calculated during the interval from −200 ms to +400 ms in relation to T0, each point represents the average of 50 ms time window and their respective standard errors, and the four 150 ms epochs are shown. There were no anticipatory displacements in the ankle, knee, and hip joints while small anticipatory displacements could be seen in the spine, thorax, and head angles prior to the perturbation (T0). The observed anticipatory displacements however were not significantly different between predictable and unpredictable conditions. In contrast, during the CPA phases, the displacement of the ankle, knee and hip joints were markedly greater during unpredictable compared to predictable conditions with the largest displacement occurring in the knee and ankle joints. The difference in the angular displacement in each of these two joints between the predictable and unpredictable conditions was statistically significant (ankle, p=0.01 and knee, p<0.01). Also, a significant interaction effect between conditions and epochs was observed for the angular displacements in the ankle, knee, and hip joints (p<0.01, p<0.01, and p<0.01) for all comparisons). No significant differences were observed between conditions (expected and unexpected perturbations) for spine, thorax and head displacements. Overall, the within comparison of the four epochs for the angular displacements showed significant differences for all joints across the time windows. Usually, the angular displacements during the epochs APA1 and APA2 were close to zero and increased (ankle, knee, hip, and spine) or decreased (head and thorax) significantly after the impact (CPA1, CPA2) (Fig. 1).
Figure 1.
Temporal evaluation (from −200 ms to + 400 ms in relation to T0) of the ankle, knee, hip, spine, thorax, and head displacements during predictable and unpredictable conditions. Each point represents the angular displacements in the sagittal plane (flexion (+) and extension (−) of these variables averaged over a 50 ms interval (−201 to −150 ms, −151 to −100, and so on) and its standard error. The 4 time epochs of 150 ms used for the analysis are represented by the brackets on the bottom (APA1, APA2, CPA1, and CPA2). The dotted vertical line shows the moment of body perturbation (T0).
Since there were no significant differences in PCA values between the two APA (APA1 and APA2) phases or between the two CPA phases (CPA1 and CPA2), only the combined CPAs were further analyzed. The analysis of the combined PCAs demonstrated that the first two principal components accounted for approximately 96% of the variance of the six angles for predictable (APA and CPA) and unpredictable (CPA) conditions. Table 1 lists the averaged loading of the two principal components and their percentage of variance. The first principal component during predictable conditions accounted for 79% and 77% of the variance for APA and CPA phases, respectively. Similarly, the principal component during unpredictable conditions (CPA) was responsible for 79% of the variance as well. In addition, according to the PCA loadings, the proximal joints (head and thorax) were the principal joints to change the angular position in the APA phase during predictable conditions. In the experiments with the predictable perturbations, head and hip joint were the most responsible for the movements in the CPA phase. In contrast, for unpredictable conditions, the knee followed by the hip joint accounted for the principal angular changes in the CPA phase (Table 1).
Table 1.
Two principal components (mean and standard deviation) and their corresponding percentage variance.
| Joints | Predictable | Unpredictable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APA | CPA | CPA | ||||||||||
| pc1 | sd | pc2 | sd | pc1 | sd | pc2 | sd | pc1 | sd | pc2 | sd | |
| Head | 0.57 | ± 0.31 | 0.49 | ±0.26 | 0.70 | ±0.27 | 0.47 | ±0.37 | 0.28 | ±0.17 | 0.74 | ±0.19 |
| Thorax | 0.43 | ±0.18 | 0.36 | ±0.25 | 0.27 | ±0.16 | 0.25 | ±0.18 | 0.15 | ±0.09 | 0.30 | ±0.26 |
| Spine | 0.31 | ±0.13 | 0.40 | ±0.26 | 0.14 | ±0.12 | 0.28 | ±0.34 | 0.19 | ±0.18 | 0.19 | ±0.13 |
| Hip | 0.19 | ±0.15 | 0.23 | ±0.22 | 0.29 | ±0.21 | 0.41 | ±0.29 | 0.42 | ±0.11 | 0.23 | ±0.16 |
| Knee | 0.28 | ±0.24 | 0.27 | ±0.17 | 0.24 | ±0.25 | 0.19 | ±0.11 | 0.67 | ±0.10 | 0.25 | ±0.11 |
| Ankle | 0.18 | ±0.24 | 0.21 | ±0.27 | 0.22 | ±0.19 | 0.25 | ±0.23 | 0.36 | ±0.18 | 0.23 | ±0.19 |
| % of variance | 78.78 | ±13.11 | 16.78 | ±12.17 | 76.97 | ±13.10 | 19.12 | ±11.96 | 79.48 | ±12.48 | 16.69 | ±11.48 |
COM and COP displacements
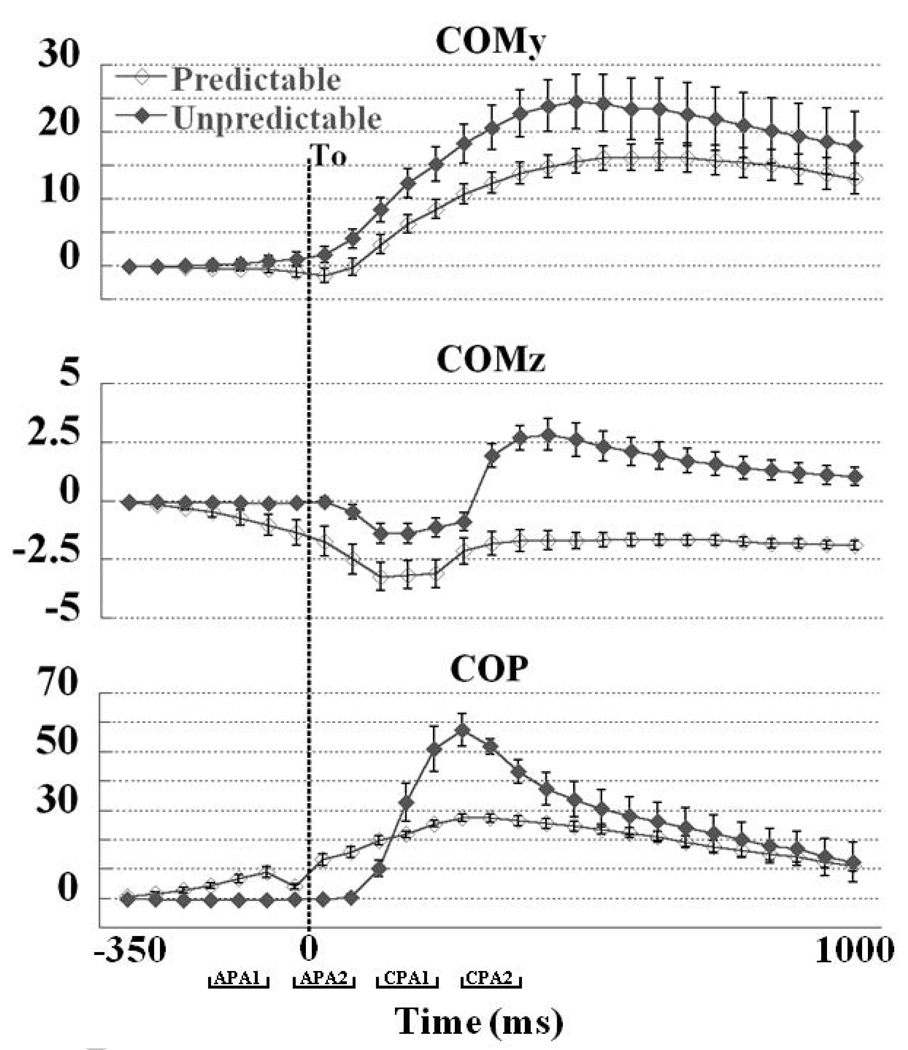
For the predictable conditions, the COP was the first to move at −306 ± 77 ms followed by COMz at −226 ± 98 ms and COMy at −153 ± 39 ms before T0. For unpredictable conditions the COMy was the first to move: the onset time for COMy was 42 ± 16 ms followed by COMz and COP at 46 ± 28 ms and 48 ± 27 ms after To, respectively.
Fig. 2 depicts the mean displacements of COM in the anterior-posterior and vertical directions and COP displacement in the anterior-posterior direction for both predictable and unpredictable conditions. During the APA phase small displacements of COMy in the anterior direction and COMz displacements in the downwards direction, were observed during the predictable conditions. In addition, the COP excursion in the posterior direction prior to the perturbation was observed when the perturbation was predictable. In contrast, for unpredictable conditions no displacements were seen for COMz and COP during anticipatory phase. At the same time, small displacements were observed for COMy in the posterior direction (Fig. 2). After the perturbations, displacements of COMy and COP in the posterior direction and COMz in the downward direction were seen in predictable and unpredictable conditions as well. However, the magnitudes of COMy, COMz, and COP displacements were substantially larger in conditions with unpredictable perturbations compared to predictable perturbations.
Figure 2.
Temporal evaluation (from −350 ms to +1000 ms in relation to T0) of the COMy, COMz, and COP displacements during predictable and unpredictable conditions. Each point represents the mean COMy and COP displacements in anterior (−) and posterior (+) directions and COMz displacements towards downward (−) and upward (+) directions averaged over a 50 ms intervals (−351 to −300 ms, −301 to −250, and so on) and its standard error across 8 subjects. The dotted vertical line shows the moment of body perturbation (T0).
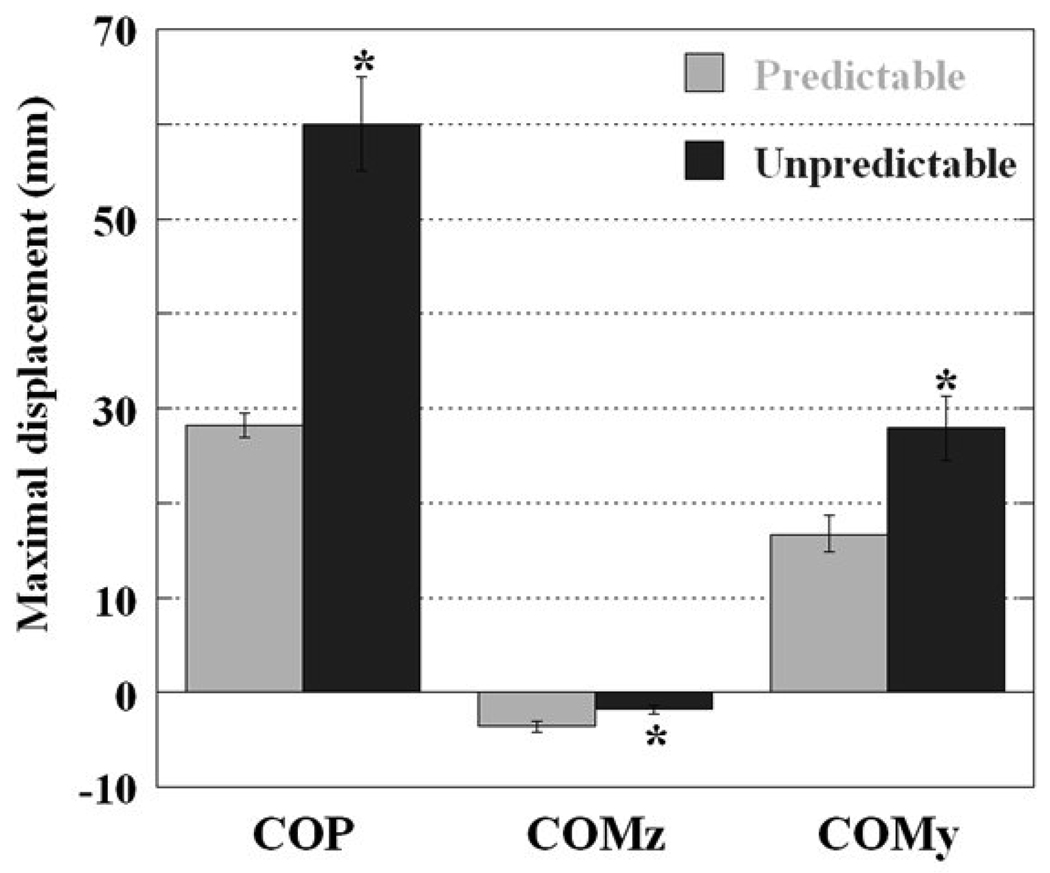
The peak COMy, COMz, and COP displacements calculated after T0 are shown in Fig.3. The peak of COP displacement (PCOP) was 28 ± 3.6 mm for predictable condition and 60 ± 14 mm for unpredictable condition, in the posterior direction. The peak of COMz displacement (PCOMz) in downward direction was 3.6 ± 0.6 mm for predictable and 1.7 ± 0.4 mm for unpredictable conditions. The COMy peak in the posterior direction reached 17 ± 5.5 mm in experiments with predictable perturbations while for unpredictable task it reached 28 ± 9.6 mm. All the three maximal displacements were significantly different between predictable and unpredictable conditions (p<0.01, p=0.01, p<0.01, respectively).
Figure 3.
Differences between predictable and unpredictable conditions in the maximal/peak COMy, COMz, and COP displacements (mean and standard error) after the perturbation. * denotes significant difference at alpha level of 0.05.
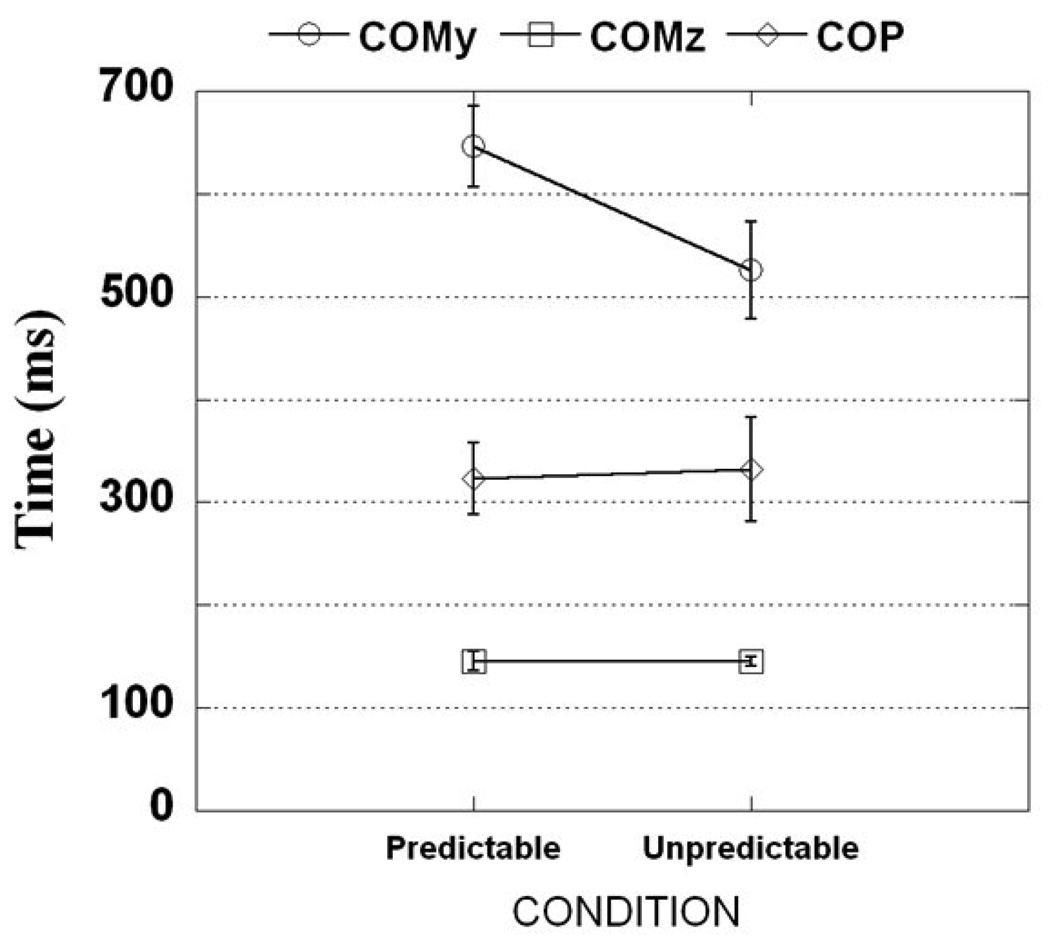
The COMz was the first to reach its peak in both predictable and unpredictable conditions: the times of the TCOMz peaks were 146 ±10 ms and 145 ±5 ms after T0, respectively. The COP reached its peak after COMz, its times (TCOP) were 323 ±98 ms and 332 ± 142ms after the T0 for predictable and unpredictable conditions, respectively (Fig. 4). The COMy reached its peak last: during predictable conditions TCOMy was at 646 ±112 ms after the perturbation impact while for unpredictable conditions TCOMy was observed at 573 ±112 ms after T0. The time when each of the COMy, COMz and COP variables reached their peaks was not significantly different between the predictable and unpredictable conditions.
Figure 4.
Times at which COMy (TCOMy), COMz (TCOMz), and COP (TCOP) reached their maximal/peak displacements (mean and standard error) after the perturbations for predictable and unpredictable conditions.
DISCUSSION
In this study, we analyzed the anticipatory and compensatory postural adjustments triggered by external predictable and unpredictable perturbations of the same magnitude applied at the shoulder level of standing subjects. There were several principal findings. First, the patterns and magnitudes of joint angular displacements differed between the predictable and unpredictable conditions. Thus, small changes in the lower extremities joint angular positions were seen after the predictable pendulum impact. In contrast, large changes in the ankle, knee, and hip angular positions were observed after the unpredictable perturbations. Second, the COP and COM onset sequence changed between predictable and unpredictable perturbations. Third, when APAs were utilized (predictable perturbations), small COP and COM excursions were seen during the CPA phase. In contrast, when the perturbation was unpredictable, large COM and COP displacements were observed during the recovery of body balance. Finally, the timings of the peak of COM and COP displacements were similar for the predictable and unpredictable conditions whereas the magnitudes of the peak displacements were significantly different between the two conditions. These main differences across the postural responses between predictable and unpredictable perturbations and the COM-COP interplay are discussed in details below.
Postural control during predictable and unpredictable perturbations
It was demonstrated recently that the patterns and sequence of activation of muscles used in postural control change depending on the availability of information about the forthcoming perturbation (Santos et al. 2009). When the perturbation was predictable, a distal-to-proximal sequence of anticipatory activation of leg and trunk muscles was observed prior to the pendulum impact (Santos et al. 2009). This pattern of activation, however, might not be associated with large movements in the lower limb and the upper body joints prior to the pendulum impact as is shown in the current study (Fig. 1). Notice that these displacements were similar to those during unpredictable perturbations, in which no anticipatory muscular activity occurred prior to the pendulum impact (Santos et al. 2009). Interestingly, during predictable conditions, small amount of anticipatory EMG activity was associated with smaller joint angular displacements after the perturbations. Quite opposite, during unpredictable conditions, higher compensatory EMG activity with a proximal to distal sequence of activation was associated with larger angular displacements, especially of lower limb joints after the perturbations (Fig. 1).
Although the ankle, knee, and hip angular positions as well as COM and COP excursions were greater during unpredictable perturbations, the movements of the upper part of the body, especially in the CPA phase, were smaller and similar between the two experimental conditions. The small changes in the angular position of the upper body seen as a combination of head extension and the flexion of the thorax, spine, and hip during the APA phase (confirmed by the PCA analysis, Table 1) could be responsible for the anterior and downward displacement of the COM in anticipation to the pendulum impact. Similar small trunk movements and relatively large hip and ankle joint displacements were reported in subjects exposed to multidirectional surface perturbations (Henry et al. 2001). The outcomes of both studies suggest that the changes in the angular position of the lower limb joints play an important role in minimizing the trunk displacement after unpredictable perturbations in standing individuals. In fact, when the lower limbs are not utilized to counteract the perturbation, for example while the seated subjects are exposed to horizontal surface translations, the COM displacements and changes in the angular position of the head, trunk and arms are larger compared to ones recorded in standing subjects (Preuss and Fung 2008). Therefore, the results of the current study taken together with the literature data suggest that regardless of the level at which the unpredictable perturbation is induced to a standing individual (via the surface on which the subjects stand or at the shoulder level), movements of the lower limbs are used to minimize the trunk and head movements. This in turn allows preserving the upper body vertical orientation, especially its orientation in the sagittal plane.
Moreover, the lower limb joint excursions described in the present study seem to be primarily responsible for differences found in COM and COP displacements between the conditions with predictable and unpredictable perturbations. It appears that during predictable perturbations, the CNS strategy was to better arrange the body segments, especially the proximal ones, and as a result, smaller changes in the angular position of the lower limb joints were seen after the perturbation. In contrast, to recover the equilibrium in unpredictable conditions, the subjects used combination of movements in the ankle, knee and hip joints: such a strategy has been described in the literature (Nashner 1977; Horak and Nashner 1986). Furthermore, to restore balance, the subjects in the present study utilized considerable knee flexion rather than hip and ankle movements (confirmed by the magnitudes of pc loadings in Table 1 and Fig. 1). Similar knee flexions associated with forward displacement of the body, induced by a movement of the force platform on which the subjects were positioned have been described previously (Hughes et al. 1995). A possibility of utilization of such a “knee” or “suspensory strategy” has been mentioned in the literature (Nashner 1977). Thus, there is a likelihood of a general rule by the CNS to use knee flexion while counteracting unpredictable perturbations induced in the sagittal plane.
It is important to note that the expectation of the forthcoming perturbation could potentially change the behavior with the focus, for example, to increase the stiffness of the joints by co-activation of certain postural muscles. However, it was not the case in our study since the exact timing of the unpredictable pendulum impact was not known to the subject and as such no feedforward postural adjustments were generated.
Finally, even though the peak displacements of the COM and COP (PCOMy, PCOMz, and PCOP) between predictable and unpredictable conditions were considerably different, the times at which they reached their peaks of displacement (TCOMy, TCOMz, and TCOP) after the perturbations were consistently similar between the conditions (Fig 4). It is important to point out that no specific instructions were given to the participants with respect to maintaining balance while performing the experimental tasks. Also, it is interesting to note that the COP was the first to move and reached its peak earlier than COMy in the predictable condition; on the other hand, in experiments with unpredictable conditions the COP displacement began after the COMy, however, it still reached its peak before COMy. It maybe suggested that instead of controlling only the magnitude of the COP and COM peak displacements the CNS tightly controls the timing of the peak displacement to achieve the functional goal of maintaining balance. As such, during the unpredictable perturbations, the COP moves from its onset to the peak in a very short time as compared to the predictable conditions. On the other hand, when the perturbation could be predicted, more time is available for the COP to reach its peak (refer Fig. 2). It is quite possible that the CNS estimates the “ideal” amount of time needed for the COP to respond to a perturbation and reach its peak. This could be achieved by using factors such as a life-long experience, environmental context, information obtained during practice trials, or a combination of these factors. This idea is supported by the outcome of the studies that showed that in experiments involving unpredictable perturbations, in contrast to predictable perturbations, there was a low correlation between magnitude of perturbation and intensity of responses in terms of COM displacement (Rietdyk et al. 1999), EMG activity and ankle torques (Horak et al. 1989). In others words, the CNS is set to give the initial response and correct it during the course of perturbations, which in the present study corresponded to the CPA1 and CPA2 phases, respectively.
Interplay between COP and COM
It has been shown experimentally that unexpected multidirectional surface tilts (Hughey and Fung 2005) or surface translations (Henry et al. 2001; Horak et al. 2005) are associated with the COM shift in the direction of the perturbations. This COM shift is then followed by the COP shift in order to catch up with the COM and recover body equilibrium. Indeed, in the current study the COM displacement during unpredictable perturbations was in the posterior direction (which coincides with the direction of the perturbation) and was followed by the COP displacement in the same direction. Corresponding results were observed in experiments involving standing individuals exposed to unexpected lateral perturbations induced at the shoulder level (Rietdyk et al. 1999). Therefore, one can conclude that any type of unpredictable perturbation (e.g. that induced by a moving surface or pushing at the shoulder level) generates a COM displacement (which coincides with the direction of the perturbation), which is followed by the displacement of the COP in the same direction.
Quite the opposite, during predictable perturbations, the COP was the first to shift, followed by COM movements. Similar order of COP and COM displacement was previously observed in the studies utilizing voluntary leg lifts (Hughey and Fung 2005), rising on tiptoe (Ito et al. 2004), or whole body reaching movements (Stapley et al. 1999). In addition, it looks like the initial displacement of the COP in the posterior direction in the present study created the momentum that resulted in forward (COMy) and downward (COMz) COM displacements (Fig. 2). Comparable interplay between the COP and COM displacements was observed in experiments using whole-body reaching task (Crenna et al. 1987; Stapley et al. 1998; Stapley et al. 1999). For example, it was reported that while performing a whole body reaching task in the sagittal plane, the anticipatory backward COP displacement created a negative COM momentum allowing all body segments to move in forward direction: this was confirmed by changes in the ground reaction forces in relation to the position of COM (Stapley et al. 1999). The existence of such a relationship between COP and COM displacements during the APA phase (observed in the present study and described in the literature) suggests that, in the case of predictable perturbations, the CNS utilizes anticipatory activation of muscles to better arrange the body segments; this provides some mechanical advantage while controlling posture. Another possible explanation to the existence of such a relationship could be related to the utilization of adjustment in body balance sway as coined by Cordo and Nashner (Cordo and Nashner 1982). Indeed, small joint movements and COM-COP displacements observed prior to the pendulum impact support the suggestion that the CNS could utilize such balance sway adjustments. Both strategies allow better body stability to counteract the external perturbation and as a consequence, result in smaller EMG activity (see (Santos et al. 2009) and substantially smaller displacements of COMy, COMz and COP observed within the CPA time windows (Fig. 2). In turn, smaller peaks of COM and COP displacements (PCOMy, PCOMz and PCOP) can be observed after the perturbations (Fig. 3).
Conclusion
Unpredictable perturbations, that were not associated with any anticipatory corrections, induced large compensatory changes in the angular positions of the ankle, knee, and hip joints, and larger displacements of the COM followed by displacements of the COP. In contrast, APAs seen in conditions with predictable perturbations initiated COP displacements, resulting in better arrangement of the body position prior to the impact; this led to smaller compensatory COP-COM excursions and smaller displacements in the lower extremities joint angles after the impact. The outcome of this study provides additional knowledge about how body balance is controlled in presence or in absence of information about the forthcoming perturbation. It also suggests ways of enhancement of postural control by better utilization of APAs and such an approach could be considered as a valuable modality in the rehabilitation of individuals with balance impairment.
Acknowledgement
This work was supported in part by NIH grants HD-50457 and HD-51628 and NIDRR grant H133P060003.
Biographies

Alexander S. Aruin received his Ph.D. in Medical Cybernetics from the Moscow Institute of Artificial Organs and Transplantation in 1978 and a D.Sc. (Ph.D.) in Biomechanics from the Latvian Institute of Traumatic Injuries and Orthopedics in 1990. He was affiliated with the Moscow Institute of Electronic Engineering as Full Professor and Director of the Laboratory of Ergonomics and Biomechanics. After moving to the United States in 1992, he became a faculty at Rush-Presbyterian St. Luke’s Medical Center in Chicago and later a faculty member at Pennsylvania State University. He is currently a Professor of Physical Therapy, Bioengineering, and Kinesiology and the Director of the Knecht Movement Science Laboratory at the University of Illinois at Chicago, a Professor of Physical Medicine & Rehabilitation at Rush University, Chicago, and a Senior Clinical Researcher at Marianjoy Rehabilitation Hospital in Wheaton, Illinois. He has co-authored more than 100 referred papers and two monographs in the fields of biomechanics, kinesiology, motor control and neuromuscular disorders. His primary research interests have been focused on motor disorders and rehabilitation, biomechanics, and motor control. Dr. Aruin develops new technologies for training healthy people and for providing physical therapy and rehabilitation to injured and disabled individuals. He is a member of the American Society for Biomechnaics, Society for Neuroscience, and International Society of Motor Control.

Neeta Kanekar received her Bachelor’s degree in Physiotherapy from Maharashtra University of Health Sciences, Seth G. S. Medical College & K.E.M Hospital, India and her Master’s degree in Physical Therapy from University of Illinois at Chicago (UIC), USA. After practicing as a PT for a few years, she is now pursuing her PhD degree in the program of Kinesiology, Nutrition, and Reh abilitation with a major in Rehabilitation at UIC. She is currently working in the Knecht Mov ement Science Laboratory in the Department of Physical Therapy at UIC. Her primary area of rese arch is related to rehabilitation, biomechanics, and motor control. Presently her research projects are focused on: (1) feedforward and feedback mechanisms of balance control, (2) effect of fatigue on post ural control, (3) effect of balance training in healthy individuals, the elderly, and individuals with mus culoskeletal and neurological disorders. She is a member of the Indian Association of Physiotherapists and the National Honor Society of Phi Kappa Phi, USA.

Marcio J. Santos graduated in Physical Therapy (PT) from the Universidade Estadual de Londrina (UEL), Brazil. He obtained his Master Degree (M.S.) in Human Physiology from the Universidade Estadual de Campinas (UNICAMP), Brazil, and his Ph.D. in Rehabilitation Science from the University of Kansas Medical Center (KUMC), USA. He worked as a postdoctoral research associate in the Knecht Movement Science Laboratory at the Department of Physical Therapy at the University of Illinois at Chicago (UIC), USA. He is currently assistant professor at the Department of Physical Therapy at the Universidade Estadual de Santa Catarina (UDESC), Brazil. He has co-authored various scientific papers in the motor control, biomechanics and rehabilitation fields, testing healthy individuals and those with disabilities. His present research projects focused on: (1) feedforward and feedback postural control and (2) grip force control in healthy individuals and orthopedic and neurologic patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biol Cybern. 2005;93:309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res. 1995;103:323–332. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Belenkiy V, Gurfinkel V, Pal’tsev Y. Elements of control of voluntary movements. Biofizika. 1967;10:135–141. [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Crenna P, Frigo C, Massion J, Pedotti A. Forward and backward axial synergies in man. Exp Brain Res. 1987;65:538–548. doi: 10.1007/BF00235977. [DOI] [PubMed] [Google Scholar]
- De Carlo MS, Talbot RW. Evaluation of ankle joint proprioception following injection of the anterior talofibular ligament*. J Orthop Sports Phys Ther. 1986;8:70–76. doi: 10.2519/jospt.1986.8.2.70. [DOI] [PubMed] [Google Scholar]
- Georgoulis AD, Ristanis S, Papadonikolakis A, Tsepis E, Moebius U, Moraiti C, Stergiou N. Electromechanical delay of the knee extensor muscles is not altered after harvesting the patellar tendon as a graft for ACL reconstruction: implications for sports performance. Knee Surg Sports Traumatol Arthrosc. 2005;13:437–443. doi: 10.1007/s00167-005-0656-3. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J Neurophysiol. 2001;85:559–570. doi: 10.1152/jn.2001.85.2.559. [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62:841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson's disease. Exp Neurol. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Hughes MA, Schenkman ML, Chandler JM, Studenski SA. Postural responses to platform perturbation: kinematics and electromyography. Clin Biomech (Bristol, Avon) 1995;10:318–322. doi: 10.1016/0268-0033(94)00001-n. [DOI] [PubMed] [Google Scholar]
- Hughey LK, Fung J. Postural responses triggered by multidirectional leg lifts and surface tilts. Exp Brain Res. 2005;165:152–166. doi: 10.1007/s00221-005-2295-9. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res. 1994;101:159–164. doi: 10.1007/BF00243226. [DOI] [PubMed] [Google Scholar]
- Ito T, Azuma T, Yamashita N. Anticipatory control related to the upward propulsive force during the rising on tiptoe from an upright standing position. Eur J Appl Physiol. 2004;92:186–195. doi: 10.1007/s00421-004-1088-3. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a 'dynamometric map' for human balance control. Neuroreport. 1998;9:3247–3252. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- Kisner C, Colby LA. Therapeutic exercise : foundations and techniques. Philadelphia: F.A. Davis; 2007. [Google Scholar]
- Li X, Aruin AS. The effect of short-term changes in the body mass on anticipatory postural adjustments. Exp Brain Res. 2007;181:333–346. doi: 10.1007/s00221-007-0931-2. [DOI] [PubMed] [Google Scholar]
- Li X, Aruin AS. The effect of short-term changes in body mass distribution on feed-forward postural control. J Electromyogr Kinesiol. 2008 doi: 10.1016/j.jelekin.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Fixed patterns of rapid postural responses among leg muscles during stance. Exp Brain Res. 1977;30:13–24. doi: 10.1007/BF00237855. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–427. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Perry SD, Santos LC, Patla AE. Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res. 2001;913:27–34. doi: 10.1016/s0006-8993(01)02748-2. [DOI] [PubMed] [Google Scholar]
- Preuss R, Fung J. Musculature and biomechanics of the trunk in the maintenance of upright posture. J Electromyogr Kinesiol. 2008;18:815–828. doi: 10.1016/j.jelekin.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Rietdyk S, Patla AE, Winter DA, Ishac MG, Little CE. NACOB presentation CSB New Investigator Award. Balance recovery from medio-lateral perturbations of the upper body during standing. North American Congress on Biomechanics. J Biomech. 1999;32:1149–1158. doi: 10.1016/s0021-9290(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Mancini M, Chiari L, Cappello A. Dependence of anticipatory postural adjustments for step initiation on task movement features: a study based on dynamometric and accelerometric data. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1489–1492. doi: 10.1109/IEMBS.2006.260731. [DOI] [PubMed] [Google Scholar]
- Ruget H, Blouin J, Teasdale N, Mouchnino L. Can prepared anticipatory postural adjustments be updated by proprioception? Neuroscience. 2008;155:640–648. doi: 10.1016/j.neuroscience.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Santos M, Kanekar N, Aruin A. The effect of anticipatory postural adjustments on compensatory control of posture: 1. Electromyographic data. Journal of Electromyography and Kinesiology. 2009 doi: 10.1016/j.jelekin.2009.06.006. in press. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Role of lateral muscles and body orientation in feedforward postural control. Exp Brain Res. 2008;184:547–559. doi: 10.1007/s00221-007-1123-9. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Liu W. Possible factors related to functional ankle instability. J Orthop Sports Phys Ther. 2008;38:150–157. doi: 10.2519/jospt.2008.2524. [DOI] [PubMed] [Google Scholar]
- Simoneau GG, Ulbrecht JS, Derr JA, Becker MB, Cavanagh PR. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17:1411–1421. doi: 10.2337/diacare.17.12.1411. [DOI] [PubMed] [Google Scholar]
- Stal F, Fransson PA, Magnusson M, Karlberg M. Effects of hypothermic anesthesia of the feet on vibration-induced body sway and adaptation. J Vestib Res. 2003;13:39–52. [PubMed] [Google Scholar]
- Stapley P, Pozzo T, Grishin A. The role of anticipatory postural adjustments during whole body forward reaching movements. Neuroreport. 1998;9:395–401. doi: 10.1097/00001756-199802160-00007. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Pozzo T, Cheron G, Grishin A. Does the coordination between posture and movement during human whole-body reaching ensure center of mass stabilization? Exp Brain Res. 1999;129:134–146. doi: 10.1007/s002210050944. [DOI] [PubMed] [Google Scholar]
- Vint PF, McLean SP, Harron GM. Electromechanical delay in isometric actions initiated from nonresting levels. Med Sci Sports Exerc. 2001;33:978–983. doi: 10.1097/00005768-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Wang Y, Asaka T, Zatsiorsky VM, Latash ML. Muscle synergies during voluntary body sway: combining across-trials and within-a-trial analyses. Exp Brain Res. 2006;174:679–693. doi: 10.1007/s00221-006-0513-8. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]