Abstract
Proinsulin, a single-chain precursor of insulin, has been detected in biosynthetic studies in vitro,1-4 and in crystalline preparations of insulin from several species.1, 5-7 It appears that proinsulin normally serves neither as a major storage nor secretory form of the hormone.8 Its primary functions seems to be to facilitate the efficient formation of the disulfide bonds of insulin.9
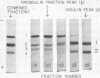
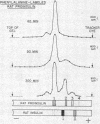
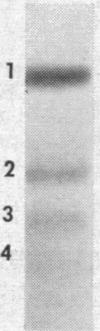
Although several species of fish have two insulins differing slightly in primary structure,10-12 the rat is the only mammal where this is known to be the case.13, 14 As the two rat insulins are nonallelic14 and presumably coded by two distinct genes, a separate proinsulin would be expected to precede each insulin. We have demonstrated the biosynthesis of both insulins15 and of a proinsulin corresponding to each in isolated islets of Langerhans, and have detected probable intermediates in the conversion of these proinsulins to insulin. Labeled peptide material corresponding to the free connecting segment of proinsulin (C-peptide) was detected in insulin-containing fractions isolated from incubated rat islets.
It was noted that islets incubated in vitro secreted small amounts of newly synthesized proinsulin as well as the two insulins and essentially equivalent amounts of C-peptide. Increasing the glucose content of the medium selectively enhanced the incorporation of radioactivity into proinsulin and insulin and also seemed to increase the relative proportion of labeled proinsulin appearing in the medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Grant P. T., Reid K. B. Biosynthesis of an insulin precursor by cod islet tissue and the occurrence of intermediates in the conversion to insulin. Biochem J. 1968 Sep;109(3):32P–32P. doi: 10.1042/bj1090032pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- HUMBEL R. E. Studies on isolated islets of Langerhans (Brockmann bodies) of teleost fishes. II. Evidence for insulin biosynthesis in vitro. Biochim Biophys Acta. 1963 Jul 2;74:96–104. doi: 10.1016/0006-3002(63)91333-7. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Parry D. G., Taylor K. W. Secretion of newly synthesized insulin in vitro. Nature. 1965 Oct 30;208(5009):487–487. doi: 10.1038/208487a0. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of glucose concentration on incorporation of [3H]leucine into insulin using isolated mammalian islets of Langerhans. Biochim Biophys Acta. 1966 Dec 28;130(2):519–521. doi: 10.1016/0304-4165(66)90250-9. [DOI] [PubMed] [Google Scholar]
- Mirsky I. A., Kawamura K. Heterogeneity of crystalline insulin. Endocrinology. 1966 Jun;78(6):1115–1119. doi: 10.1210/endo-78-6-1115. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- SMITH L. F. ISOLATION OF INSULIN FROM PANCREATIC EXTRACTS USING CARBOXYMETHYL AND DIETHYLAMINOETHYL CELLULOSES. Biochim Biophys Acta. 1964 Feb 10;82:231–236. doi: 10.1016/0304-4165(64)90293-4. [DOI] [PubMed] [Google Scholar]
- Schmidt D. D., Arens A. Proinsulin vom Rind Isolierung, Eigneschaften und seine Aktivierung durch Trypsin. Hoppe Seylers Z Physiol Chem. 1968 Sep;349(9):1157–1168. [PubMed] [Google Scholar]
- Smith L. F. Species variation in the amino acid sequence of insulin. Am J Med. 1966 May;40(5):662–666. doi: 10.1016/0002-9343(66)90145-8. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Steiner D. F. Evidence for a precursor in the biosynthesis of insulin. Trans N Y Acad Sci. 1967 Nov;30(1):60–68. doi: 10.1111/j.2164-0947.1967.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung A. K., Yip C. C. The biosynthesis of insulin and "proinsulin" in foetal bovine pancreas. Diabetologia. 1968 Jan;4(1):68–70. doi: 10.1007/BF01241035. [DOI] [PubMed] [Google Scholar]
- WANG S. S., CARPENTER F. H. A COMPOSITIONAL ASSAY FOR INSULIN APPLIED TO A SEARCH FOR "PROINSULIN". J Biol Chem. 1965 Apr;240:1619–1625. [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Lin B. J. Amino acid composition of bovine 'proinsulin'. Biochem Biophys Res Commun. 1967 Nov 17;29(3):382–387. doi: 10.1016/0006-291x(67)90467-6. [DOI] [PubMed] [Google Scholar]