Abstract
Autism prevalence studies have often relied on administrative prevalence or clinical diagnosis as case identification strategies. We report the incidence of clinical diagnoses of autism spectrum disorders, versus research-identified autism among residents of Olmsted County, Minnesota, age ≤ 21 years, from 1976–1997.
The incidence of clinically diagnosed ASD (with 95 % CI) was 1.5 per 100,000 (0.0–3.7) in 1980–1983 and 33.1 (22.8–43.3) in 1995–1997, a 22.1-fold increase. In contrast, the incidence of research-identified autism increased from 5.5 (1.4–9.5) per 100,000 to 44.9 (32.9–56.9), an 8.2-fold increase. Only 46.8% of research-identified cases received a clinical diagnosis of ASD.
These findings demonstrate the potential for misleading interpretation of results from epidemiologic studies that rely on clinical diagnosis of autism to identify cases.
Keywords: autism, epidemiology, autistic disorder, incidence, population-based
There is widespread concern about an apparent increase in the prevalence of autism. Studies from the 1980’s and early 1990’s reported prevalence of approximately 4 to 10 per 10,000 children, while recent studies have reported prevalences of 30 to 60 per 10,000 (Bertrand et al., 2001; Burd, Fisher, & Kerbeshian, 1987; Centers for Disease Control and Prevention, 2007b; Fombonne, 2003; Fombonne & Du Mazaubrun, 1992; Fombonne, Simmons, & Ford, 2001; Gillberg, 1984; Ritvo, Freeman, & Pingree, 1989; Steffenburg & Gillberg, 1986; Wing & Potter, 2002; Yeargin-Allsopp et al., 2003) Recently, a multisite study sponsored by the Centers for Disease Control reported prevalence of Autism Spectrum Disorders (ASD) of 6.6 per 1,000 children aged 8 years (Centers for Disease Control and Prevention, 2007a). It has been suggested that the apparent increase in ASD prevalence may be due to the introduction of less restrictive diagnostic criteria, beginning with the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition in 1987 (DSM-III-R) (1980; 1987; 1994; Caronna, 2003; Fombonne, 2003; Wing & Potter, 2002). Furthermore, as highlighted by Gernsbacher et al, there has been an increase in the number of recognized, specific diagnoses in the autism spectrum (Gernsbacher, Dawson, & Goldsmith, 2005). The Centers for Disease Control study clearly showed that reliance on clinical diagnoses and/or eligibility for special education services as a method to identify children with ASD leads to inaccurate prevalence estimates (Centers for Disease Control and Prevention, 2007a). There have also been significant changes in the availability of special educational services, with subsequent, significant increases in the prevalence of children receiving services under the ASD special education category (Newschaffer, Falb, & Gurney, 2005; Shattuck, 2006).
Thus, comparing prevalence estimates obtained in different eras, based on changing diagnostic categories, changing diagnostic criteria and varying sources of information, may yield inaccurate and misleading estimates of change in the number of children affected by autism. In order to assess the potential impact of changing diagnostic criteria on estimates of the number of children affected by autism, it is necessary to compare estimates that are based on clinical diagnosis to estimates based on research identification independent of clinical diagnosis, in the same population of children, during the same period of time.
Previously, we reported the incidence of research-identified autism from 1976–1997 among residents of Olmsted County, Minnesota, age ≤ 21 years (Barbaresi, Katusic, Colligan, Weaver, & Jacobsen, 2005). In the current study, we compare the incidence of clinical diagnoses of ASD to the incidence of research-identified autism in the same population, during the same timeframe.
Methods
Study Setting and Subjects
More than 95% of all medical care in Olmsted County, Minnesota is provided locally by Mayo Clinic and Olmsted Medical Center. Through the Rochester Epidemiology Project, all inpatient and outpatient diagnoses are indexed for computerized retrieval (Medical Diagnostic Index) (Melton, 1996). Medical records contain complete, detailed information from providers of all care to county residents, including developmental, psychiatric, neurologic and psychologic assessments. All public and private school records are available though a contractual agreement. School records include notations from teachers, parents, and school psychologists related to any developmental or learning problem, cognitive assessments, and information related to special education services.
Subjects included all residents of Olmsted County, age ≤ 21 years, in each year from 1976 to 1997 (e.g., n=34,944 in 1976 and 37,726 in 1997). The protocol was approved by the IRB. The population of Rochester was approximately 98% white during 1976–1997(Katusic, Colligan, Barbaresi, Schaid, & Jacobsen, 1998).
Ascertainment of Research-Identified Autism Incident Cases
A detailed description of the ascertainment of research-identified autism cases was provided in our previous study (Barbaresi et al., 2005), and is summarized below.
Phase I: Development of Screening Tool
We first compiled a list of all developmental, psychiatric or neurologic diagnoses (n=80) ever applied to a group of 182 children with autistic disorder or pervasive developmental disorder, not otherwise specified, consistent with DSM-IV criteria, evaluated at Mayo Clinic from 1994 to 1998 (Challman, Barbaresi, Katusic, & Weaver, 2003). In addition to ASD, other common diagnoses included mental retardation, developmental delay, and language disorders. We reviewed the medical records of these 182 subjects, transcribing every reference to symptoms of autism, and created a 20-page glossary of phrases (our screening tool) consistent with the symptoms of autistic disorder as specified in DSM-IV (American Psychiatric Association, 1994).
Phase II: Identification of Autism Incident Cases Using Research Criteria
We employed the Medical Diagnostic Index to identify 3109 residents of Olmsted County, age ≤21 years, during 1976–1997, who had ever been given any one or more of the 80 clinical diagnoses described above. We then reviewed the medical records of these 3109 subjects, employing the glossary of autism symptoms to flag potential incident cases.
We identified 257 subjects whose records included at least 2 symptoms of “impaired reciprocal social interaction”. For each of these 257 subjects, we reviewed the medical and school records, recording every symptom from the glossary of DSM-IV symptoms. To be considered an incident case of research-identified autism, the subject had to fulfill the following criteria: 1) No clinical diagnosis of Rett’s Disorder or Childhood Disintegrative Disorder; 2) Symptoms required for a DSM-IV diagnosis of autism documented between January 1, 1976 and December 31, 1997 (i.e., at least 2 symptoms of impaired reciprocal social interaction, at least one symptom of impaired communication and of restricted, repetitive and stereotyped behavior/interests/activities, and a total of at least 6 symptoms), 3) Age ≤ 21 years and a resident of Olmsted County when DSM-IV criteria were fulfilled; 4) No clinical diagnosis of Schizophrenia preceding the date at which DSM-IV criteria were fulfilled, 5) At least one IQ score ≥ 35. We excluded children with severe to profound mental retardation (n=9). We did not require that symptoms were documented before age 3 years, since we could not precisely ascertain the age at which symptoms first occurred. For each potential case, the incidence date was defined as the date when the subject had documented information that fulfilled our research-diagnostic criteria. One hundred twenty-four subjects fulfilled our criteria for research-identified autism.
Ascertainment of Clinically Diagnosed Autism Incident Cases
As described above, we employed the Medical Diagnostic Index to identify all residents of Olmsted County, age ≤ 21 years, during 1976–1997, who had ever received an explicit diagnosis in the autism spectrum (autistic disorder, infantile autism, pervasive developmental disorder—not otherwise specified, atypical autism, Asperger Disorder). For each potential case, the incidence date was defined as the date when the subject had one of these diagnoses documented in their medical records.
Statistics
Age- and sex-specific incidence rates were calculated by time intervals, assuming all residents ≤21 years of age in Olmsted County, Minnesota during 1976–1997 were at risk. The denominator was estimated using census data for 1970, 1980, 1990, and 2000, with linear interpolation for the intercensus years. Rates were age- and sex-adjusted to the population structure of United States whites in 2000. Ninety-five percent confidence intervals (95% CIs) were constructed about the incidence rates and the ratio of incidence rates, assuming a Poisson error distribution.
Results
Incidence of Research-Identified Autism
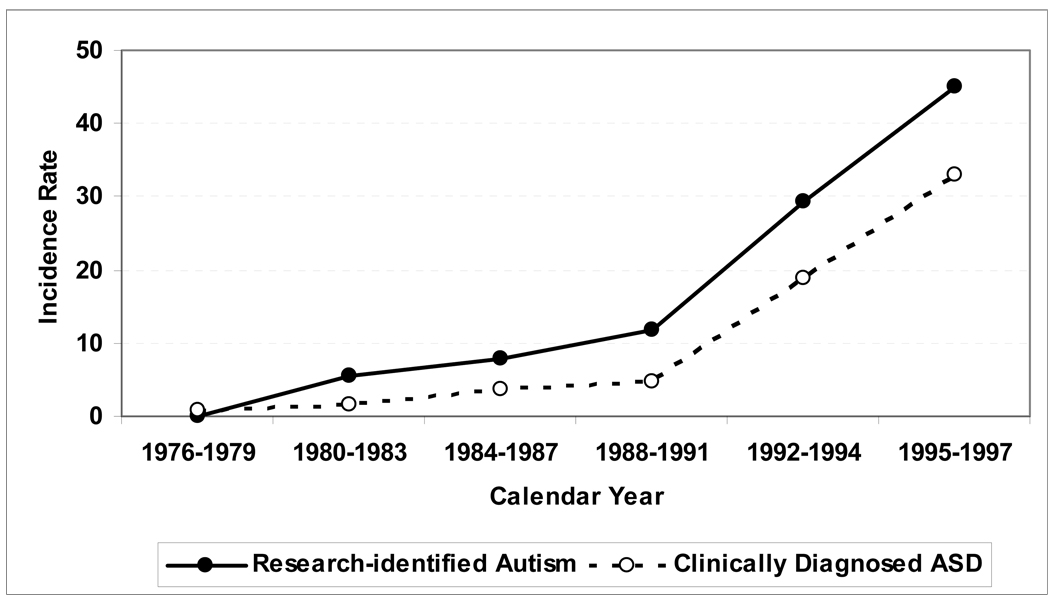
Among the 124 subjects with research-identified autism, males (n=95) outnumbered females (n=29) by 3.3 to1. The age and sex-adjusted incidence of research-identified autism increased from 5.5 per 100,000 in 1980–1983 to 44.9 per 100,000 in 1995–1997, an 8.2-fold (95% CI 3.9–19.0) increase (Table 1). Incidence was relatively stable from 1976 to 1988, but increased thereafter (Figure 1).
Table 1.
Age- and Sex-Adjusted Incidence of Research-Identified Autism and Clinical Diagnoses of Autism Spectrum Disorders
| Research-Identified Autism | Clinical Diagnoses of Autism Spectrum Disorders |
|||
|---|---|---|---|---|
| No. of Incident cases |
Incidence rate per 100,000 † (95% CI) |
No. of Incident cases |
Incidence rate per 100,000 † (95% CI) |
|
| 1976–1979 | 0 | 0 | 1 | 0.8 (0, 2.2) |
| 1980–1983 | 7 | 5.5 (1.4, 9.5) | 2 | 1.5 (0, 3.7) |
| 1984–1987 | 11 | 7.9 (3.2, 12.6) | 5 | 3.6 (0.4, 6.7) |
| 1988–1991 | 18 | 11.8 (6.3, 17.3) | 7 | 4.8 (1.2, 8.3) |
| 1992–1994 | 34 | 29.4 (19.4, 39.3) | 22 | 18.8 (10.9, 26.7) |
| 1995–1997 | 54 | 44.9 (32.9, 56.9) | 40 | 33.1 (22.8, 43.3) |
| Total | 124 | 77 | ||
Age- and sex-adjusted to the population structure of US whites, age ≤ 21 years, in 2000.
Figure 1.
Overall age- and sex- adjusted incidence, by time period, of research-identified autism and clinical diagnoses of autism spectrum disorders (AS), among residents of Olmsted County, Minnesota, age ≤ 21 years, during 1976–1997.
Incidence of Clinical Diagnoses of Autism
Among the 257 children with at least 2 symptoms of impaired social interaction, 77 received an ASD clinical diagnosis (Table 1). Among these 77 subjects, males (n=66) outnumbered females (n=11) by 6 to 1. The age and sex-adjusted incidence of clinical diagnoses of autism spectrum disorders increased, from 1.5 per 100,000 in 1980–1983 to 33.1 per 100,000 in 1995–1997, a 22.1-fold increase (95% CI 6.6 – 108.9; Table 1, Figure 1). All 77 subjects with a clinical diagnosis of an ASD ultimately fulfilled research criteria for autism, but not all did so within the 1976–1997 timeframe and/or while resident in the county.
Overlap of Research-Identified and Clinically Diagnosed Autism
Among the 124 subjects with research-identified autism, 58 (46.8%) received an ASD clinical diagnosis, any time before or within 2 years after fulfilling research criteria (9 autism/autistic disorder, 2 both autism and Asperger Disorder, 47 pervasive developmental disorder, not otherwise specified). In the period 1980–1983, 29% of research-identified children received a clinical diagnosis in the autism spectrum. In contrast, during the period 1995–1997, 74% of the children with research-identified autism also received clinical diagnosis of ASD.
Sixty-six subjects (53.2%) with research-identified autism did not receive an ASD clinical diagnosis. These subjects received a total of 188 other developmental, psychiatric or neurologic diagnoses during this four-year window (i.e., the years before or after the subject fulfilled research criteria for autism). Among these, the most frequent diagnoses were developmental delay (n=44), delayed speech and language (n=42), attention-deficit/hyperactivity disorder (n=24) and mental retardation (n=22).
Discussion
Previously, we studied the incidence of autism from 1976–1997, by retrospectively applying DSM-IV criteria to residents of Olmsted County, age ≤ 21 years, who had a diagnosis of any developmental, neurologic or psychiatric disorder during these years (Barbaresi et al., 2005). The availability of medical and school records of all Olmsted County residents allowed us to ascertain autism cases among all subjects (Melton, 1996). Further, the unique resources available through the Rochester Epidemiolgy Project allowed us to compare incidence rates of children with research-identified autism versus clinically diagnosed children with autism. Incidence rates, defined as the number of new cases of a condition arising in a population over a period of time, are necessary to study historical trends in disease frequency (Mausner & Dramer, 1985). In contrast, prevalence rates reported by previous studies, reflecting existing diagnostic criteria at a specific point in time, may not be appropriate to assess changes in the rate of occurrence of autism over time. We found the incidence of research-identified autism increased 8.2 fold, from 5.5 per 100,000 in 1980–1983 to 44.9 per 100,000 in 1995–1997. In the current study, we examined the incidence of clinical diagnoses of ASD in the same population, during the same years. We found that the incidence of clinical diagnoses in the autism spectrum increased 22.1 fold, from 1.5 per 100,000 in 1980–1983 to 33.1 per 100,000 in 1995–1997. Thus, had we relied solely on clinical diagnoses, we would have reported lower incidence rates from 1976–1997. Furthermore, the apparent, relative increase in the incidence of autism would have been exaggerated (22.1 fold for clinical diagnoses versus 8.2 fold for research identified cases).
Importantly, only 46.8% of our 124 research-identified incident cases of autism had also received a clinical diagnosis of ASD within 2 years of meeting research criteria. In contrast, all 77 subjects with a clinical diagnosis of ASD between 1976 and 1997 ultimately had symptoms consistent with our research criteria for autism documented in their records. During the early years included in this study, the majority of research-identified cases (71%) did not receive a clinical diagnosis of ASD. However, by the end of this timeframe, the majority of research identified cases (74%) did receive a clinical diagnosis of ASD. Overall, had we relied on clinical diagnoses as a case-finding strategy, we would have inaccurately described the epidemiology of autism in this population over a 20 year period from 1976–1997.
In Minnesota, the prevalence of children receiving special education services under the ASD category increased from 3 per 10,000 children (aged 6 to 11 years) in 1991–1992 to 52 per 10,000 in 2001–2002 (Gurney et al., 2003). Similarly, on a national basis, the prevalence of 10-year-old children receiving special education services in the autism disability category increased from 3.5 per 10,000 in 1992 to 18.3 per 10,000 in 2001 (Newschaffer et al., 2005). Our incidence study findings imply that previous comparisons of prevalence over time, with prevalence determined on the basis of clinical diagnoses, special education enrollment or school-identification, lead to an overestimate of the apparent increase in autism. Prevalence studies also fail to demonstrate the time trend in relation to publication of broader diagnostic criteria, increased autism awareness and changes in the special education laws.
In the recent, Centers for Disease Control (CDC), multisite study, the prevalence of autism was relatively stable among 8-year-old children from 2000 (6.7 per 1,000) to 2002 (6.6 per 1,000) (Centers for Disease Control and Prevention, 2007a, 2007b). In the CDC studies, children were considered to have “previously documented ASD classification” if they had either received special education services under the autism eligibility category or had a documented ASD clinical diagnosis. In the year 2000 study, only 481 of 1252 research-identified ASD cases (38.4%) were receiving services under the autism category. Overall, the prevalence of ASD based on “previously documented ASD” ranged from 3.1 to 6.8, compared to a range of 4.5 to 9.9 based on more rigorous, comprehensive research identification of cases that included a review of multiple sources of information for documentation of symptoms consistent with current diagnostic criteria for ASD (Centers for Disease Control and Prevention, 2007b). Similarly, in the year 2002 study, 938 of 2072 research-identified ASD cases (45.3%) were receiving services under the autism category. Prevalence estimates based on children previously classified as ASD remained lower than estimates based on research-identified cases, although specific numbers were not provided (Centers for Disease Control and Prevention, 2007a). Our study employed a case-identification strategy similar to the CDC study. However, while the CDC study identified prevalent cases, our study identified incident cases of autism in the same population over time. Nevertheless, we too found that reliance on previously identified cases (clinical diagnoses of ASD), would fail to identify many children who fulfilled research diagnostic criteria based on DSM-IV. The incidence of clinically diagnosed ASD (with 95 percent CI) was 1.5 per 100,000 (0.0–3.7) 1980–1983 and 33.1 (22.8–43.3) in 1995–1997. The age-adjusted incidence of research-identified autism during the same time periods was 5.5 (1.4–9.5) per 100,000 and 44.9 (32.9–56.9), respectively. Reliance solely on clinical diagnosis would also have led to an increase in our estimate of the apparent change in the incidence of autism over time (22.1 fold increase), compared to the estimate derived from a comprehensive epidemiologic approach, based on information from all school and healthcare sites for the entire population (8.2 fold increase). Our study and the recent CDC studies both suggest that future studies based on clinically and/or on educationally identified autism will continue to report increases in prevalence when, in fact, such findings may be due primarily to improvement in the identification of children with ASD rather than a true increase in the number of affected children by (Gernsbacher et al., 2005).
In conjunction with the changing diagnostic criteria for autism, combined with increased awareness of autism, one possible explanation for the apparent increase in clinically or educationally identified cases is the phenomenon of “diagnostic shifting”. In the past children who would fulfill broader, contemporary diagnostic criteria for an ASD may have been given other diagnoses, such as mental retardation. Newschaffer et. al, in their study of the prevalence of children receiving special education services under the autism category, argued that the phenomenon of “diagnostic shifting” was not supported by their findings (Newschaffer et al., 2005). Specifically, they reported that there was no decrease in other special education categories concomitant with the increase seen in the autism category. In contrast, Shattuck completed a similar study of administrative prevalence of autism among children ages 6 to 11 years in US special education from 1984 to 2003 (Shattuck, 2006). Shattuck’s study differed from that of Newschaffer et al, in that he evaluated not only the overall national trends for prevalence of students receiving special education in various categories, but also completed analyses that reflect the fact that these trends varied considerably from state to state. Shattuck found that changes in autism prevalence were part of a more widespread shift characterized by decreases in the number of children receiving services for mental retardation or learning disabilities, with simultaneous increases in the number receiving services for autism, other health impairments, traumatic brain injury and developmental delay {Shattuck, 2006). In our previous study, we reported that there was an increase in both the incidence of research-identified autism as well as the collective incidence of all other developmental disorders (Barbaresi et al., 2005). We found, however, that the apparent increase in the incidence of other developmental disorders was less dramatic (1.8 fold) than the change in the incidence of autism (8.2 fold). The first federal special education laws in 1975 preceded the increase in the identification of children with any developmental disorder, including autism (Palfrey & Rodman, 1999). Subsequent to this legislation, families and health-care providers had a valid reason to bring children with developmental disorders to medical attention; namely, to obtain a formal diagnosis that would allow the child to receive appropriate special education services. In contrast to the steady increase in the incidence of other developmental disorders from 1976 to 1997, the incidence of research-identified autism was steady until 1988–1991, increasing thereafter. The publication of DSM-III-R in 1987 and the amendments to the special education laws in 1991 preceded this change (American Psychiatric Association, 1987; Palfrey & Rodman, 1999). Since the incidence of both autism and other developmental disorders increased in Olmsted County between 1976–1997, and since the number of children with research-identified autism(124) was small compared to the number of children with other developmental disorders (3080), it was not possible for us to directly address the issue of possible “diagnostic shifting” in this population. Nevertheless, the percentage of children with research-identified autism who received an ASD clinical diagnosis increased significantly over time (29% in 1980–1983 versus 74% in 1995–1997). In earlier years, children with research-identified autism were more likely to be given other diagnoses, such as mental retardation. This finding would be consistent with “diagnostic shifting”, even though we cannot provide conclusive evidence of this phenomenon in our study.
In our previously published study, we discussed possible explanations for the apparent eight-fold increase in the incidence of research-identified autism among children in Olmsted County, Minnesota from 1976 to 1997. {Barbaresi, 2005) We were not able to exclude the possibility that some environmental factors have caused an increase in autism; additional studies are needed to address this possibility. However, we described that the MMR vaccine was introduced in Minnesota almost 20 years before the increase in the incidence of autism, suggesting that MMR did not contribute to this phenomenon. The timing of the change in autism incidence in Olmsted County was coincident with the introduction of broader diagnostic criteria, increased availability of education services and increased awareness of autism, supporting the hypothesis that these factors were major contributors to the apparent increase in the incidence of research-identified autism in Olmsted County during these years.
Several potential limitations should be noted. We did not directly re-assess our subjects, so we could not directly verify the information from medical and school records. However, our study design enabled us to apply DSM-IV criteria to every subject, regardless of the year in which the subject was seen clinically. We ascertained autism cases among children who had received at least one diagnosis of a developmental problem. However, the broad list of clinical diagnoses employed in the initial phase of the study, the completeness of the data throughout the timeframe of this study, and the availability of school and medical records for virtually all residents of Olmsted County minimize the possibility that cases of autism were missed. The racial demographics of our population suggest caution in generalizing our findings. Nevertheless, a recent study demonstrated that the prevalence of autism is comparable for black and white children (Yeargin-Allsopp et al., 2003). Given the retrospective nature of this study, we could not determine the age at which a child manifested their first symptom. We therefore defined the incidence date for each case as the date at which were able to demonstrate that the child fulfilled our research-diagnostic criteria (for research-identified cases) or the age of clinical diagnosis (for clinically-identified cases). Nevertheless, we were able to determine the median age at which the first symptom of autism was documented (2.4 years for cases who were residents of Olmsted County at birth and 3.0 years for those who were not ). Of course, in the absence of biologic markers, the “incidence “ of any developmental disorder can only be the age at which symptoms emerge to a degree sufficient to reach a diagnostic threshold
This population-based study shows there has been an increase in the incidence of research-identified autism among children in Olmsted County, Minnesota from 1976 to 1997. Had we relied on clinically diagnosed cases to determine the incidence of autism, we would have failed to identify many children who fulfill DSM-IV criteria and hence we would have underestimated the incidence of autism in this population. Furthermore, had we relied solely on clinically diagnosed cases to determine incidence, the apparent magnitude of the change in incidence would have been greatly exaggerated. Our findings are consistent with the hypothesis that children who fulfill contemporary diagnostic criteria for autism were often diagnosed with other developmental disorders in earlier years. These findings highlight the importance of comprehensive epidemiologic approaches, with rigorous case-identification criteria, in order to obtain accurate findings on the occurrence of autism. They also suggest that reliance on clinically and/or educationally identified cases provides inaccurate and misleading estimates of the true number of children affected by autism.
Acknowledgements
Supported by a research grant from Mr. David S. and Mrs. Elaine Dana. The authors gratefully acknowledge the contributions of Ms. Diane Siems, Study Coordinator, Ms. Candice Klein and Ms. Jeaneen Alcorn for data collection, Ms. Sondra Buehler for assistance in manuscript preparation, Ms. Katherine Clement-Brown for assistance with data management, and Independent School District #535 for their cooperation and collaboration.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Revised Third ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition ed. Washington: American Psychiatric Association; 1994. [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. The incidence of autism in Olmsted County, Minnesota, 1976–1997: results from a population-based study. Archives of Pediatrics & Adolescent Medicine. 2005;159(1):37–44. doi: 10.1001/archpedi.159.1.37. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P. Prevalence of autism in a United States population: The Brick Township, New Jersey, Investigation. Pediatrics. 2001;108(5):1155–1161. doi: 10.1542/peds.108.5.1155. [DOI] [PubMed] [Google Scholar]
- Burd L, Fisher W, Kerbeshian J. A prevalence study of pervasive developmental disorders in North Dakota. J Am Acad Child Adolesc Psychiatry. 1987;26:704–710. doi: 10.1097/00004583-198709000-00014. [DOI] [PubMed] [Google Scholar]
- Caronna EB. Dipping deeper into the reservoir of autistic spectrum disorder. Arch Pediatr Adolesc Med. 2003;157:612–621. doi: 10.1001/archpedi.157.7.619. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, 14 sites, United States, 2002. Morbidity & Mortality Weekly Report. Surveillance Summaries. 2007a;56(1):12–28. [PubMed]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, six sites, United States, 2000. Morbidity & Mortality Weekly Report. Surveillance Summaries. 2007b;56(1):1–11. [PubMed]
- Challman TD, Barbaresi WJ, Katusic SK, Weaver AL. The yield of the medical evaluation of children with pervasive developmental disorders. J Autism Dev Disord. 2003;33(2):187–192. doi: 10.1023/a:1022995611730. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The prevalence of autism. JAMA. 2003;289(1):87–89. doi: 10.1001/jama.289.1.87. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Du Mazaubrun C. Prevalence of infantile autism in four French regions. Soc Psychiatry Psychiatr Epidem. 1992;27:203–209. doi: 10.1007/BF00789007. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Simmons H, Ford T. Prevlance of pervasive developmental disorder in the British Nationwide Survey of Child Mental Health. J Am Acad Child Adolesc Psychiatry. 2001;40:820–827. doi: 10.1097/00004583-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Dawson M, Goldsmith HH. Three reasons not to believe in an autism epidemic. Current Directions in Psychological Science. 2005;14(2):55–58. doi: 10.1111/j.0963-7214.2005.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C. Infantile autism and other childhood psychoses in a Swedish urpan region: Epidemiological aspects. J Child Psychol Psychiatry. 1984;25:35–43. doi: 10.1111/j.1469-7610.1984.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Fritz MS, Ness KK, Sievers P, Newschaffer CJ, Shapiro EG. Analysis of prevalance trends in autsm spectrum disorder in Minnesota. Arch Pediatr Adolesc Med. 2003;157:622–627. doi: 10.1001/archpedi.157.7.622. [DOI] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc. 1998;73:1053–1061. doi: 10.4065/73.11.1053. [DOI] [PubMed] [Google Scholar]
- Mausner JS, Dramer S. Epidemiology: An Introductory Text. Philadelphia: W.B. Saunders Co; 1985. [Google Scholar]
- Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Falb MD, Gurney JG. National autism prevalence trends from United States special education data. Pediatrics. 2005;115(3):e277–e282. doi: 10.1542/peds.2004-1958. [DOI] [PubMed] [Google Scholar]
- Palfrey JS, Rodman JS. Legislation for the Education of Children with Disabilities. In: Levine MD, Carey WB, Crocker AC, editors. Developmental-Behavioral Pediatrics, Third Edition. Philadelphia, PA: W.B. Saunders Company; 1999. pp. 869–872. [Google Scholar]
- Ritvo ER, Freeman BJ, Pingree C. The UCLA-University of Utah epidemiological study of autism: prevalence. Am J Psychiatry. 1989;146:194–245. doi: 10.1176/ajp.146.2.194. [DOI] [PubMed] [Google Scholar]
- Shattuck PT. Diagnostic substitution and changing autism prevalence. Pediatrics. 2006;117(4):1438–1439. doi: 10.1542/peds.2005-2911. [comment] [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C. Autism and autistic-like conditions in Swedish rural and urban areas: a population study. Br J Psychiatry. 1986;149:81–87. doi: 10.1192/bjp.149.1.81. [DOI] [PubMed] [Google Scholar]
- Wing L, Potter D. The epidemiology of Autistic Spectrum Disorders: is the prevalence rising? MRDD Research Reviews. 2002;8:151–161. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]