Abstract
Objectives
Opioid-dependent patients treated with methadone have subjective sleep complaints and disrupted sleep on polysomnography (PSG). Previous studies of sleep-disordered breathing (SDB) in this population have focused on central sleep apnea (CSA). Our objectives were to: (1) characterize obstructive sleep apnea (OSA) and CSA in patients in methadone maintenance treatment (MMT) for opioid dependence; (2) examine factors associated with SDB in this population; and (3) investigate whether SDB was related to severity of subjective sleep complaints in MMT patients with subjective sleep disturbances.
Methods
We analyzed OSA and CSA from one night of home PSG in 71 patients who were in MMT for at least 3 months and had a Pittsburgh Sleep Quality Inventory (PSQI) score > 5.
Results
OSA (defined as obstructive apnea-hypoponea index (OAHI) ≥ 5) was observed in 35.2% of our sample. OSA was associated with higher body mass index, longer duration in MMT, and non-Caucasian race. CSA (defined as central apnea index (CAI) ≥ 5) was observed in 14.1% of the sample. CSA was not associated with methadone dose or concomitant drug use. Subjective sleep disturbance measured with the PSQI was not related to OSA or CSA.
Conclusions
SDB was common in this sample of MMT patients and OSA was more common than CSA. Given the lack of association between presence of SDB and severity of subjective sleep difficulties, factors other than sleep apnea must account for complaints of disturbed sleep in this population.
Keywords: methadone, opiate dependence, sleep, sleep apnea, central sleep apnea, obstructive sleep apnea, sleep-disordered breathing
1. Introduction
Dependence on heroin and prescription opiates is a serious public health problem. The cost of opiate dependence including medical care, lost productivity, crime, and social welfare has been calculated at $21.9 billion in the United States (Mark et al., 2001), and the number of Americans dependent on opiates is estimated between 800,000 and 1 million (O'Brien, 2008). One effective treatment for opioid dependence is methadone maintenance therapy (MMT). MMT has been shown to reduce mortality, HIV risk behavior, and levels of crime (Connock et al., 2007). Nevertheless, MMT patients are more likely to continue to abuse other substances and have higher mortality rates than the general population (Corkery et al., 2004).
Subjective sleep complaints occur in 75-84% of patients in MMT for opioid dependence (Peles et al., 2006; Stein et al., 2004; Wang et al., 2008). In one study, difficulties with prolonged sleep latency and poor sleep efficiency were the most common symptoms, and more than 50% of MMT patients reported use of medications to help with sleep (Peles et al., 2006). Severity of sleep symptoms has been linked to psychiatric symptoms, concomitant drug use, and pain (Peles et al., 2006; Stein et al., 2004). Whether sleep disturbances contribute to relapse in MMT patients is not known. Subjective sleep complaints in MMT patients have been corroborated by polysomnographic studies by our group (Sharkey et al., 2009) and others (Wang and Teichtahl, 2007) demonstrating sleep abnormalities such as decreased REM and decreased slow wave sleep.
Sleep-disordered breathing (SDB) is another possible factor contributing to disrupted sleep in MMT patients. SDB, including obstructive and central sleep apnea, results in oxygen desaturations and sleep fragmentation and is associated with increased risk of coronary artery disease (Peker et al., 2006), hypertension (Peppard et al., 2000), stroke (Arzt et al., 2005), depression (Peppard et al., 2006), and death (Young et al., 2008). Central sleep apnea (CSA) – mediated by hypoventilation and reduced hypercapnic and hypoxic ventilatory responsiveness (Teichtahl et al., 2005) – has been reported to occur in 30-60% of MMT patients (Teichtahl et al., 2001; Wang et al., 2005). CSA has been associated with methadone dose and concomitant benzodiazepine use in a study of chronic pain patients taking methadone (Webster et al., 2008) and with methadone blood concentration in a sample of Australian MMT patients (Wang et al., 2005).
Despite evidence of OSA in MMT patients (Teichtahl et al., 2001; Wang et al., 2005) and in patients taking methadone for chronic pain (Mogri et al., 2009; Webster et al., 2008), the major focus of most investigations of opioids and sleep-disordered breathing has been CSA. In one study of 140 chronic pain patients taking opioids, OSA (defined as AHI ≥ 5 and CAI < 5) was observed in 39% (Webster et al., 2008), whereas the prevalence of OSA in the general population is estimated at 9% in women and 24% in men (Young et al., 1993). The prevalence of OSA in patients taking methadone for opioid dependence has not been reported. The aims of the present study therefore were to: (1) characterize the extent of OSA and CSA in MMT patients, (2) examine factors associated with SDB in this population, and (3) investigate whether SDB is related to severity of subjective reports of sleep disturbance in patients enrolled in MMT for opioid addiction.
2. Methods
2.1 Study Participants
Participants were recruited from 8 MMT clinics in the Providence, Rhode Island metropolitan area from January 2006 to October 2008 as part of an ongoing clinical trial to test a pharmacological treatment for insomnia.
MMT patients were included if they had Pittsburgh Sleep Quality Index score > 5 (Buysse et al., 1989), plans to continue MMT for at least 6 months (rather than taper rapidly), proficiency in written and spoken English, stable housing, and the ability to identify two contact persons. Research assistants interviewed patients to exclude potential participants who were currently experiencing psychotic symptoms or being treated for bipolar disorder, schizophrenia, schizoaffective disorder, or schizophreniform disorder, had used trazodone in the previous 30 days, or were pregnant. Participants were also deemed ineligible if they had known chronic medical illness (e.g., poorly controlled diabetes mellitus). Known obstructive sleep apnea was an exclusion criterion, though no potential participants were excluded for sleep apnea.
In our participants, methadone was virtually always administered in a single morning dose. We measured depressive symptoms with the Beck Depression Inventory II (BDI-II) (Beck and Steer, 1993; Beck et al., 1996). The study was approved by the Rhode Island Hospital and Butler Hospital Institutional Review Boards, and participants provided informed consent and were paid for their participation.
2.2 Polysomnography
Polysomnographic recordings were made using portable Siesta or Safiro units (Compumedics, Charlotte, NC, USA) on two consecutive nights, Monday-Thursday. On the evening of a home study, two researchers went to the participant's home 1 to 3 hours before the reported usual bedtime. Participants gave a urine sample for toxicology screening and performed a breathalyzer test. The research assistants did not proceed with any participant whose behavior suggested acute intoxication that would preclude completion of the protocol.
Sleep was measured using standard techniques including electroencephalography recorded from C3 and C4 referenced to the contralateral mastoids, electrooculography from the right and left outer canthi, and electromyography from submental surface electrodes. Respiration was monitored with nasal/oral thermocouples or thermistors, nasal pressure transducers, pulse oximetry, and surface intercostal and abdominal piezo crystal respiration belts. EKG was monitored with surface electrodes on the chest and side. Research assistants started the recordings and viewed polysomnographic signals for good quality before leaving the participants' homes; they returned to the homes to collect the equipment the following morning or arranged to meet the participants at the MMT clinic.
PSG was scored in 30-second epochs according to Rechtschaffen and Kales criteria (Rechtschaffen and Kales, 1968) by a trained scorer who maintained > 90% concordance with a second trained scorer. Sleep period time was defined as the interval between the first epoch scored as sleep and the last epoch scored as sleep. Sleep efficiency was calculated by dividing total sleep time by sleep period time × 100. Sleep latency was not calculated because participants' estimates of lights out time were considered unreliable. Therefore, sleep latency is not included in the sleep efficiency calculations.
Respiratory data were scored using consensus criteria (1999). Thus, an apnea was defined as an absence of airflow lasting 10 seconds or longer. Apneas were classified as central in the absence of any respiratory effort and obstructive if respiratory effort persisted despite absent airflow. Obstructive hypopneas were scored when there was a decrease in the nasal pressure transducer signal of over 50% from baseline amplitude that lasted at least 10 seconds with presence of respiratory effort and either an oxygen desaturation greater than 3% or an EEG arousal. The obstructive apnea-hypopnea index (OAHI) was defined as the total number of obstructive and mixed apneas and hypopneas per hour of sleep. The central apnea index (CAI) was defined as the total number of central apneas divided by the total number of hours of sleep. Participants with an OAHI and/or a CAI ≥ 5 events/hour were considered to have sleep-disordered breathing (SDB) (1999; White, 1985; White, 2005).
2.3 Statistical Analysis
Data were analyzed using Stata software version 10.1 (StataCorp, College Station, TX, USA). Means, counts, and percentages are reported to summarize participants' background characteristics and to describe SDB in this sample. We report t-tests for differences in means and the Pearson χ2-test for differences in proportions when comparing participants with and without SDB. Because we were concerned that the assumptions underlying the use of standard t-tests were not always tenable with these data, we also calculated the Wilcoxon rank-sum test when comparing continuous distributions. In all cases the nonparametric rank sum test generated statistical inferences consistent with those we report. Additionally we report Fisher's exact p-values when the expected cell frequencies were not sufficient to justify use of the Pearson χ2-test. Means are listed ± standard deviation unless otherwise noted. Because OAHI and CAI scores were very skewed we used Somers Dy,x to summarize associations with selected predictors. Somers Dy,x is a nonparametric asymmetric measure of association that gives the proportionate reduction of error in predicting rank-order on y given knowledge of rank order on x (Newson, 2002; Somers, 1962).
3. Results
3.1 Demographics
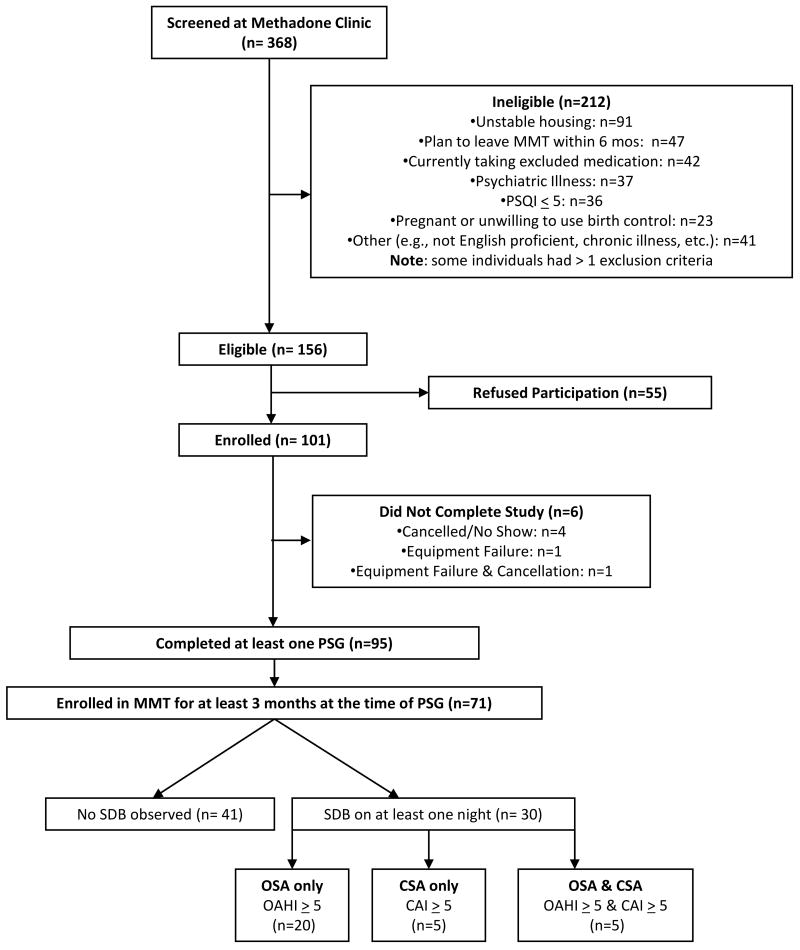
At the time of these analyses, 101 participants had enrolled in the ongoing study and valid PSG data were available for a total of 95 participants. In order to investigate SDB only in participants who were stabilized in MMT, we included data from the 71 participants who had been in MMT for at least 3 months. The 24 participants who were excluded for shorter duration of MMT did not differ from the included patients on age, gender, race/ethnicity, BMI, methadone dose, positive urine drug tests, OAHI, or CAI. Figure 1 illustrates participant outcomes.
Figure 1. Participant Outcomes.
The 71 participants ranged in age from 21 to 52 years (mean ± SD = 37.7 ± 8.1) and included 29 men (40.9%) and 42 women (59.2%). The majority of participants (80.3%) were non-Hispanic Caucasian; 11.3% were Hispanic, 7.0% were African American, and 1.4% were of other ethnic origins. The mean body mass index (BMI) was 28.4 ± 6.0 kg/m2. Average BMI was in the overweight range for men (mean = 27.7±5.1 kg/m2) and women (mean = 28.6±6.4 kg/m2). Duration of methadone maintenance treatment ranged from 3 months to 10.5 years with an average MMT enrollment period of 22.3 ± 24.2 months (median = 12.2 months). Methadone dose ranged from 25 mg to 310 mg with a mean of 108.3 ±53.9 mg (median = 100.0 mg); 28 participants (43.1%) had methadone dosages < 100 mg and 12 (18.5%) had methadone dosages of 200 mg or higher. Average methadone dose was not significantly different between men (mean =102±9 mg) and women (mean = 112±9 mg).
On the evening of their PSG, 17 (25.8%) participants tested positive for cocaine, 22 (33.3%) tested positive for tetrahydrocannabinoids (THC), and 22 (33.3%) for benzodiazepines on urine toxicology. Only 1 (1.4%) participant had a blood alcohol concentration > 0.0% but < 0.08% on the night of a sleep study. The average number of cigarettes smoked per day was 13.6 ±7.5 and only 4 participants reported no cigarette smoking.
3. 2 Obstructive Sleep Apnea and Central Sleep Apnea
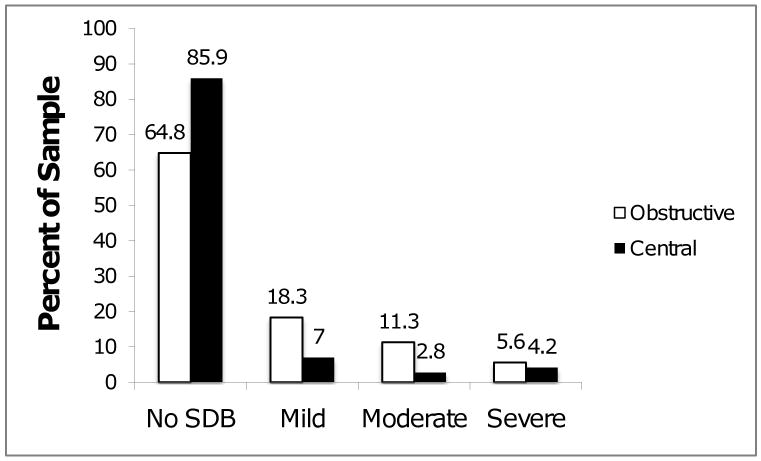
The percentage of participants meeting criteria for mild, moderate, and severe OSA and CSA are shown in Figure 2. Forty-one participants (57.7%) had no evidence of any SDB. We observed OSA (OAHI ≥ 5) in 25 participants (35.2%) and CSA (CAI ≥ 5) in 10 participants (14.1%). Gender differences in rates of OSA and CSA were not statistically significant.
Figure 2. Distribution of Apnea by Severity.
Percentage of MMT patients with no sleep-disordered breathing (SDB; OAHI < 5 and CAI < 5) or mild (5 ≤ OAHI < 15 or 5 ≤ CAI < 15), moderate (15 ≤ OAHI < 30 or 15 ≤ CAI < 30), or severe (OAHI ≥ 30 or CAI ≥ 30) sleep apnea. N=71.
There was overlap in the diagnosis of OSA and CSA. Of the 30 patients with SDB, 5 met criteria for CSA only, 20 for OSA only, and 5 met criteria for both disorders. Table 1 displays descriptive data for participants with and without SDB. Those with SDB had been on methadone maintenance for a significantly longer period of time. Participants with and without SDB did not differ significantly with respect to other background characteristics as summarized in Table 1.
Table 1.
Participant characteristics by sleep-disordered breathing status.
| No SDB (n = 41) |
CSA and/or OSA (n = 30) |
t (p =) | |
|---|---|---|---|
| Age (mean ± SD) years | 37.4 ± 8.2 | 39.3 ± 7.9 | -0.98 (.329) |
| Body Mass Index (mean ± SD) kg/m2 | 27.3 ± 5.6 | 29.9 ± 6.2 | -1.89 (.063) |
| Methadone Dosea (mean ± SD) mg | 109.7 ± 56.2 | 106.2 ± 51.4 | 0.40 (.802) |
| Months on MMTb (mean ± SD) | 17.1 ± 21.2 | 29.8 ± 26.5 | -2.18 (.036) |
| PSQI Global Score (mean ± SD) | 12.4 ± 3.4 | 13.0 ± 2.9 | -0.68 (.498) |
| BDI-II score (mean ± SD) | 20.4 ± 11.0 | 20.8 ± 13.8 | -0.16 (.874) |
| Cigarettes / Day (mean ± SD) | 14.5 ± 7.8 | 12.4 ± 9.8 | 1.17 (0.247) |
| χ2 (p =) | |||
| Male, n (%) | 15 (36.6%) | 14 (46.7%) | 0.73 (.393) |
| Race | |||
| - Caucasian, n (%) | 37 (90.2%) | 21 (70.0%) | |
| - Hispanic | 2 (4.9%) | 8 (26.7%) | 7.49 (.058) |
| - African-American | 1 (2.4%) | 1 (3.3%) | |
| - Other Ethnic Origin | 1 (2.4%) | 0 (0.0%) | |
| Opioid + on PSG nightc, n (%) | 7 (18.4%) | 2 (7.1%) | 1.74 (.187) |
| Cocaine + on PSG nightc, n (%) | 11 (29.0%) | 6 (21.4%) | 0.48 (.490) |
| THC + on PSG nightc, n (%) | 13 (34.2%) | 9 (32.1%) | 0.03 (.860) |
| Benzodiazepine + on PSG nightc, n (%) | 13 (34.6%) | 9 (32.1%) | 0.03 (.860) |
65 Valid observations;
68 valid observations;
69 valid observations.
3.3 Correlates of Sleep-Disordered Breathing
We examined several participant characteristics to determine whether any of these factors increased likelihood of OSA or CSA (Table 2). Patients with OSA had significantly higher BMI and a longer duration of MMT than those without OSA. Additionally, non-Hispanic Caucasians had significantly lower rates of OSA than racial and ethnic minorities. OSA was not associated significantly with age, methadone dose, PSQI global score, BDI-II score, number of cigarettes smoked per day, sex, or positive urine toxicology screens for cocaine, THC, or benzodiazepines.
Table 2.
Bivariate Correlates of Obstructive Sleep Apnea and Central Sleep Apnea Index Indices. Associations Estimated using Somers Dy.x (n = 71).
| OHAI | CAI | |
|---|---|---|
| Predictor | Somers Dy.x (95%CI) |
Somers Dy.x (95%CI) |
| Age | .13 (-.03; .30) |
-.07 (-.22; .08) |
| Body Mass Index | .34* (.21; .47) |
.04 (-.12; .21) |
| mg Methadone Dose | .10 (-.06; .25) |
.06 (-.13; .25) |
| Mos. Methadone Treatment | .24* (.11; .37) |
-.05 (-.20; .09) |
| Global PSQI Score | .13 (-.00; .32) |
-.06 (-.24; .12) |
| Cigarettes / Day | -.18 (-.37; .02) |
.05 (-.13; .23) |
| Gender (Male = 1) | .15 (-.13; .44) |
.11 (-.17; .38) |
| Ethnicity (Caucasian = 1) | -.47* (-.77; -.17) |
-.08 (-.44; .27) |
| Beck Depression Inventory | .05 (-.12; .23) |
.03 (-.16; .21) |
| Opioid+ (Yes = 1) | -.56 (-.88; -.23) |
.15 (-.23; .54) |
| Cocaine+ (Yes = 1) | .07 (-.26; .40) |
.03 (-.28; .35) |
| THC+ (Yes = 1) | .05 (-.26; .37) |
.03 (-.26; .32) |
| Benzodiazepine+ (Yes = 1) | .04 (-.26; .34) |
.06 (-.23; .35) |
p value < .05
CSA was not associated significantly with any of the selected correlates including methadone dose or benzodiazepine use (Table 2).
Sleep parameters for participants with and without sleep-disordered breathing (either central or obstructive apnea) are reported in Table 3. As expected based on scoring criteria for hypopneas, participants diagnosed with clinically significant SDB had significantly higher arousal index scores than those without breathing disorders. Participants with and without SDB did not differ significantly with respect to any of the other sleep measures.
Table 3.
Sleep Parameters for participants with and without SDB.
| SBDa | |||
|---|---|---|---|
| Sleep Parameter | No (n = 41) | Yes (n = 30) | t69 (p value) |
| Sleep Period Time (Minutes) | 399 (± 165) | 381 (± 126) | 0.47 (.634) |
| Total Sleep Time (Minutes) | 335 (± 145) | 315 (± 120) | 0.64 (.523) |
| Sleep Efficiency (%) | 85.1 (± 10.6) | 80.5 (± 12.7) | 1.65 (.103) |
| Wake after Sleep Onset (Minutes) | 14.9 (± 10.6) | 19.5 (± 12.7) | -1.65 (.103) |
| Stage 1 (Minutes) | 7.4 (± 4.5) | 7.2 (± 6.7) | 0.16 (.870) |
| Stage 2 (Minutes) | 219 (± 109) | 203 (± 80) | 0.72 (.476) |
| Slow Wave Sleep (Minutes) | 44.9 (± 28.4) | 46.5 (± 30.6) | -0.23 (.819) |
| REM (Minutes) | 63.2 (± 40.1) | 57.7 (± 40.1) | 0.56 (.574) |
| REM Latency (Minutes) | 104 (± 91) | 88.4 (± 92.7) | 0.71 (.480) |
| Stage 1 (%) | 2.5 (± 1.7) | 2.5 (± 2.1) | 0.01 (.995) |
| Stage 2 (%) | 64.7 (± 11.2) | 66.1 (± 13.9) | -0.47 (.640) |
| Slow Wave Sleep (%) | 15.4 (± 9.6) | 14.9 (± 9.4) | 0.41 (.811) |
| REM (%) | 17.4 (± 8.4) | 16.5 (± 11.5) | 0.37 (.715) |
| % Wake | 5.4 (± 5.8) | 12.9 (± 25.0) | -1.85 (.068) |
| Arousal Index | 6.7 (± 4.8) | 17.6 (±13.8) | -4.59 (.000) |
SDB defined as OHAI ≥ 5 or CAI ≥ 5.
4. Discussion
These data show that SDB is common in patients enrolled in methadone maintenance therapy for opioid dependence. In our sample, 42.2% of participants had at least one form of SDB. This figure is higher than has been observed in adults in the same age range recruited from the general population, where 9% of women and 24% of men were found to have OSA with an AHI ≥ 5 events per hour (Young et al., 1993) and clinically significant CSA is rarely observed (White, 2005).
4.1 Obstructive Sleep Apnea
More than one-third of the MMT patients in our study had OSA. Previous studies of patients in MMT for opioid dependence have emphasized CSA despite evidence that obstructive apneas and hypopneas are also present in this population (Teichtahl et al., 2001; Wang et al., 2005). The prevalence of OSA in our sample is higher than observed in the general population where the prevalence of OAHI ≥ 5 is 24% in men and 9% in women (Young et al., 1993). Given the increased risk of OSA in men, it is notable that male sex did not correlate with presence of OSA in our sample. Indeed, our female participants comprised a higher percentage of participants with SDB. This is consistent with findings that women experience more significant respiratory depression from opiates than men (Dahan et al., 2008), a phenomenon attributed to slower responses in the peripheral chemoreflex loop in women (Sarton, 1999). This result should be replicated in other samples of MMT patients to address the question of whether women taking chronic methadone have an increased risk of OSA.
Our observation of associations between obstructive sleep apnea and higher BMI and ethnicity are consistent with previous studies (Redline et al., 1997; Young et al., 1993). We were intrigued by the finding that duration of MMT was related to presence of OSA. We speculate that in some patients MMT may lead to weight gain, and that body mass index is, in turn, associated with development of OSA. Given the unexpected high prevalence of OSA in the female MMT patients in our study, we also examined BMI and methadone dose with respect to gender. There were no significant gender differences in BMI or methadone dose in the sample as a whole or in participants with and without SDB.
4.2 Central Sleep Apnea
In our sample, 14.1% of patients had CSA which is a lower prevalence than has been observed in other studies of patients with MMT. In the first study to examine SDB in MMT patients, 6 of 10 Australian patients had central sleep apnea (Teichtahl et al., 2001) and a follow-up study conducted by the same research group observed CSA in 30% of their sample of 50 MMT patients (Wang et al., 2005). Our sample also had a lower prevalence of CSA than was observed in a population of chronic pain patients taking opioids, in which 24% of patients had central sleep apnea (Webster et al., 2008). This difference in observed rates of CSA does not appear to be related to methadone dose, as our patients had a higher average methadone dose than the mean dose in studies where dose was reported (Teichtahl et al., 2001; Webster et al., 2008). Our lower rate of CSA may be attributable to methodological differences. Because the present study is part of a clinical trial for pharmacologic treatment of insomnia in patients with opioid dependence, we recruited MMT patients with Pittsburgh Sleep Quality Inventory scores > 5, and these patients may differ from MMT patients in general. In the study of Webster et al. (2008), patients took a variety of opioids, not just methadone, as well as other central nervous system depressant medications such as muscle relaxants and anticonvulsants. In addition to patient selection factors, other procedural differences such as studying participants at home or use of unattended ambulatory polysomnography may account for the differences in rates of CSA in our sample. On the other hand, our use of nasal pressure transducers to measure airflow would be expected to increase, not decrease, detection of respiratory events (BaHammam, 2004).
None of the variables we measured was associated with the presence of CSA in our sample. For instance, CSA was not associated with methadone dose as was shown by Webster et al. in patients taking methadone for chronic pain (Webster et al., 2008). Wang and colleagues (Wang et al., 2005) found that blood concentration of methadone explained 12% of the variance associated with central sleep apnea in 50 patients in MMT for opioid dependence. We did not measure methadone blood concentration in our study, but wide individual differences have been shown between a given methadone dose and blood level, suggesting that blood concentration may be a better measure of biologically active opioids (Eap et al., 2002). On the other hand, interindividual variability in pharmacokinetics, pharmacodynamics, and genetic polymorphisms related to methadone metabolism (Crettol et al., 2005; Eap et al., 2002) also contribute to the relationship between methadone dose and its effects on pain (Fredheim et al., 2008) and respiratory depression (Crettol et al., 2007). Thus, given the complicated relationship between methadone dose and its effects in various systems, a direct relationship between dose and sleep apnea is not necessarily expected.
We were surprised that use of benzodiazepines was not associated with CSA in our sample as has been previously shown (Webster et al., 2008). Urine toxicology collected during the evening of the sleep study may not reflect drugs consumed later during that night, however, and several participants were unwilling or unable to provide a urine sample. Thus, we may have observed a more robust effect of drugs other than methadone on central and obstructive sleep apnea if blood levels had been obtained or if we had also collected urine samples the morning following PSG. Differences in dosing interval between patients in MMT (who typically take methadone in one dose in the morning) versus those taking methadone for chronic pain (who may take several methadone doses throughout the day and closer to sleep) may also explain why we failed to show a relationship between CSA and benzodiazepines as was observed by Webster and colleagues. Further research is needed to identify factors predisposing individuals in MMT to CSA.
4.3 Relationship of SDB to Severity of Sleep Complaints
Neither obstructive nor central sleep apnea was related to the severity of subjective sleep complaints in our participants, all of whom had PSQI scores > 5. Using the standard cutoff of 5, the PSQI has been shown to have a sensitivity of 89.6% and a specificity of 86.5% for identifying individuals with sleep disorders (Buysse et al., 1989). Indeed, our participants did show polysomnographic sleep abnormalities compared to previously published normative data (Ohayon et al., 2004) including decreased sleep efficiency, decreased REM sleep, and increased Stage 2 sleep, but these sleep disturbances occurred in patients with and without SDB. We may have shown a relationship between subjective sleep complaints and SDB if we had included participants with PSQI scores ≤ 5.
As others have shown that depressive symptoms may play a role in sleep complaints in MMT patients (Wang et al., 2008), we were interested in examining the effects of depressive symptoms in our sample. Overall, our participants reported significant depressive symptoms. Nevertheless, depressive symptoms were not related to sleep-disordered breathing in this sample. Depressive symptoms were positively correlated with PSQI score (data not shown), consistent with a recent study of non-substance using middle-aged adults that showed that severity of subjective sleep complaints was not related to objective sleep measures (PSG and actigraphy), but rather to measures of anxiety and stress (Buysse et al., 2008). In the future, a higher PSQI cutoff value may be useful when assessing patients with substance dependence as has been utilized in other populations with a high prevalence of subjective sleep complaints (Jomeen and Martin, 2007). Our findings suggest that factors other than SDB account for complaints of disturbed sleep in our sample. For example, Peles and colleagues (Peles et al., 2006) have suggested that opioid use (including methadone) rather than MMT per se —may account for subjective sleep complaints in this population.
4.4 Limitations
One methodological concern with our study is our selection of participants and lack of a control group. Because these data are from an ongoing placebo-controlled trial of treatment for insomnia in MMT patients, all participants had clinically significant sleep complaints (defined as PSQI > 5). One might expect this participant selection to increase the prevalence of SDB compared to MMT patients in general. On the other hand, we also excluded patients with chronic illness such as diabetes and hypertension that are associated with sleep apnea, which would be expected to decrease the observation of clinically significant SDB in our sample. Interestingly, although known obstructive sleep apnea was one of our exclusion criterion, no potential participants were excluded for sleep apnea.
The participants in this study were of similar age and had similar methadone doses to participants in a larger study that we performed among MMT patients in Rhode Island, although the current study included a higher percentage of women (Stein et al., 2006). Compared to a recent study of MMT patients in Washington State (Banta-Green et al., 2009), our participants were of similar age and sex distribution. Thus, although our sample may not generalize to all patients in MMT, the participants are similar to other groups of MMT patients in other recent studies. Another limitation of our study is lack of blood samples to determine methadone blood concentrations, which may have helped us decipher the role of methadone dose and levels for predicting SDB. Blood samples also would have been valuable for determination of daytime PCO2 concentrations, which would have been interesting to examine in light of the blunted hypercapnic response observed in patients taking opiates (Teichtahl et al., 2005). Third, we did not report sleep latency in this cohort because research staff believe that lights out time, needed to calculate latency, was unreliable. Fourth, while we performed urine toxicology in the hours prior to the PSG, it might have been informative if we had also collected a morning after urine sample, which could have shed light on the use of illicit drugs during the PSG night. Although the use of drugs other than methadone was high in our sample, the rates were similar to those reported in other studies of MMT patients (Banta-Green et al., 2009). Given the significant rates of co-addiction in methadone patients, the role that concomitant substance use plays in the sleep complaints of MMT patients deserves further study.
Finally, it is important to know whether disrupted sleep in MMT patients is related to daytime functioning, and future studies should include measures of daytime sleepiness and/or performance to determine whether disrupted sleep and/or SDB is associated with outcome measures.
4.5 Conclusions
The use of chronic methadone for treatment of opioid dependence is associated with subjective sleep disturbance and abnormal findings on PSG. A high proportion of patients in MMT have SDB, and OSA was more common than CSA in our sample. Factors other than SDB must contribute to complaints of disturbed sleep in MMT patients. Further research is needed to determine the nature of these factors and how clinicians can ameliorate complaints of disturbed sleep in this population.
Acknowledgments
The authors thank Raynald Joseph, Jill MacCormack, Roberta Fish, Braulio Lopez, Laura DiMaio, Celeste Caviness, Meredith Sims, John Murray, and Carol Carlisle for assistance with this project, and Mary A. Carskadon, PhD for helpful comments on the manuscript.
Role of Funding Source: This work was funded by NIH R01 DA 020479 to MDS. Dr. Stein is the recipient of a NIDA Mid-Career Award K24-DA00512. NIH/NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors: Michael D. Stein designed the study. Michael D. Stein and Richard P. Millman wrote the protocol. Megan E. Kurth managed participant recruitment and data collection. Richard P. Corso recruited participants and collected data. Authors Stein, Millman, Kurth, Anderson, and Sharkey planned the data analyses. Bradley J. Anderson performed the statistical analyses. Katherine M. Sharkey wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of the American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BaHammam A. Comparison of nasal prong pressure and thermistor measurements for detecting respiratory events during sleep. Respiration. 2004;71:385–390. doi: 10.1159/000079644. [DOI] [PubMed] [Google Scholar]
- Banta-Green CJ, Maynard C, Koepsell TD, Wells EA, Donovan DM. Retention in methadone maintenance drug treatment for prescription-type opioid primary users compared to heroin users. Addiction. 2009;104:775–783. doi: 10.1111/j.1360-0443.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R. Manual for the Beck Depression Inventory. Psychological Corporation; San Antonio: 1993. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Benkert RE, Ingham RH. Factors associated with nightly variability in sleep-disordered breathing in the elderly. Chest. 1991;100:973–976. doi: 10.1378/chest.100.4.973. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chediak AD, Acevedo-Crespo JC, Seiden DJ, Kim HH, Kiel MH. Nightly variability in the indices of sleep-disordered breathing in men being evaluated for impotence with consecutive night polysomnograms. Sleep. 1996;19:589–592. doi: 10.1093/sleep/19.7.589. [DOI] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Corkery JM, Schifano F, Ghodse AH, Oyefeso A. The effects of methadone and its role in fatalities. Hum Psychopharmacol. 2004;19:565–576. doi: 10.1002/hup.630. [DOI] [PubMed] [Google Scholar]
- Crettol S, Deglon JJ, Besson J, Croquette-Krokkar M, Gothuey I, Hammig R, Monnat M, Huttemann H, Baumann P, Eap CB. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Crettol S, Monnat M, Eap CB. Could pharmacogenetic data explain part of the interindividual sensitivity to methadone-induced respiratory depression? Crit Care. 2007;11:119. doi: 10.1186/cc5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976;19:782–794. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 1975;17:458–466. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- Fredheim OM, Moksnes K, Borchgrevink PC, Kaasa S, Dale O. Clinical pharmacology of methadone for pain. Acta Anaesthesiol Scand. 2008;52:879–889. doi: 10.1111/j.1399-6576.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Drummond SPA, Clark CP, Moore P. Medication and substance abuse. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier Saunders; Philadelphia: 2005. pp. 1345–1358. [Google Scholar]
- Jomeen J, Martin CR. Assessment and relationship of sleep quality to depression in early pregnancy. Journal of Reproductive and Infant Psychology. 2007;25:87–99. [Google Scholar]
- Le Bon O, Hoffmann G, Tecco J, Staner L, Noseda A, Pelc I, Linkowski P. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118:353–359. doi: 10.1378/chest.118.2.353. [DOI] [PubMed] [Google Scholar]
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Eveloff SE, Kline LR, Millman RP. One negative polysomnogram does not exclude obstructive sleep apnea. Chest. 1993;103:756–760. doi: 10.1378/chest.103.3.756. [DOI] [PubMed] [Google Scholar]
- Mogri M, Desai H, Webster L, Grant BJ, Mador MJ. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13:49–57. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- Newson R. Parameters behind “nonparametric” statistics: Kendall's tau, Somers' D and median differences. The Stata Journal. 2002;2:45–64. [Google Scholar]
- O'Brien CP. A 50-year-old woman addicted to heroin: review of treatment of heroin addiction. JAMA. 2008;300:314–321. doi: 10.1001/jama.300.1.jrr80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend. 2006;82:103–110. doi: 10.1016/j.drugalcdep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages in Human Subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Corso RM, Brower KJ, Anderson BJ, Millman RP, Stein MD. Home Polysomnography in Methadone Maintenance Patients with Subjective Sleep Complaints. The American Journal of Drug and Alcohol Abuse. 2009;35:178–182. doi: 10.1080/00952990902839786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers RH. A new asymmetric measure of association for ordinal variables. American Sociological Review. 1962;27:799–811. [Google Scholar]
- Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, Anderson BJ. Sleep disturbances among methadone maintained patients. J Subst Abuse Treat. 2004;26:175–180. doi: 10.1016/S0740-5472(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Teichtahl H, Prodromidis A, Miller B, Cherry G, Kronborg I. Sleep-disordered breathing in stable methadone programme patients: a pilot study. Addiction. 2001;96:395–403. doi: 10.1046/j.1360-0443.2001.9633954.x. [DOI] [PubMed] [Google Scholar]
- Teichtahl H, Wang D, Cunnington D, Quinnell T, Tran H, Kronborg I, Drummer OH. Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest. 2005;128:1339–1347. doi: 10.1378/chest.128.3.1339. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11:35–46. doi: 10.1016/j.smrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, Kronborg I. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–1356. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, Goodman C, Drummer O, Grunstein RR, Kronborg I. Subjective daytime sleepiness and daytime function in patients on stable methadone maintenance treatment: possible mechanisms. J Clin Sleep Med. 2008;4:557–562. [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9:425–432. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–2224. [PubMed] [Google Scholar]
- White DP. Central sleep apnea. Med Clin North Am. 1985;69:1205–1219. doi: 10.1016/s0025-7125(16)30983-x. [DOI] [PubMed] [Google Scholar]
- White DP. Central Sleep Apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier; Philadelphia: 2005. pp. 969–982. [Google Scholar]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]