Abstract
Previous studies have established the distribution, biochemistry and functional attributes of human CD22, a B cell-restricted glycoprotein. Recently, molecular cloning of the murine CD22 equivalent revealed this molecule to be the same as the previously described Lyb8 alloantigen. Using the antl-Lyb8 mAb Cy34.1.2, the present report documents the expression patterns of CD22 within the murine B cell compartment. The results demonstrate that in the bone marrow, murine CD22 is absent on the surface of pro-B cells, pre-B cells and newly emerging lgM+ B cells. CD22 is present at a low density on immature IgMhi B cells and fully expressed on mature recirculatlng B cells. In the periphery, murine CD22 is expressed at mature levels on all B cell subsets Including follicular, marginal zone, B1 and switched B cells. Further studies showed CD22 to be retained on activated murine B cells for extended periods. Finally, In combination with CD23 and heat stable antigen, CD22 can be used to delineate the immature splenic B cells, and distinguish them from follicular and marginal zone cells. Together, the results demonstrate murine CD22 to be a useful pan marker for all mature B cell subsets.
Keywords: B cell, B lymphocytes, heat stable antigen
Introduction
The human B lymphocyte-restricted antigen CD22 has been well characterized in terms of structure, distribution and function (reviewed in 1,2). Human CD22 (hCD22) is a 130–140 kDa glycoprotein (3–6) expressed primarily on mature lgD+ B cells (3,7–9). cDNA cloning of hCD22 has identified two distinct species, hCD22α and hCD22β encoded by five and seven Ig-like domains respectively (10–12). hCD22 is present in the cytoplasm of early and pre-B cells, and first appears on the cell surface after acquisition of IgM (3,7,9,13). After activation of B cells, hCD22 has been shown to be lost and is not present on terminally differentiated cells (3).
hCD22 shows homology to a number of adhesion molecules including carcinoembryonic antigen, myelin-associated glycoprotein, N-CAM and CD33 (10–12). Functional analyses have supported a role for hCD22 in adhesion, as several groups have reported hCD22 to mediate B cell homotypic aggregation, and binding to T cells, monocytes, erythrocytes and neutrophils (10,11,14–16). Although not fully defined, the ligand for hCD22 appears to consist of a sialylated carbohydrate moiety present on a number of glycoproteins (17,18). hCD22 has also been reported to participate in signal transduction. Upon Ig ligation, only B cells expressing hCD22 will exhibit an intracellular Ca2+ flux (19) and addition of anti-CD22 antibodies will enhance anti-Ig-mediated proliferation (3,20). Further studies have implicated hCD22 as part of the B cell receptor complex (21,22) and have demonstrated hCD22 to be tyrosine phosphorylated upon surface Ig engagement (22,23). Taken together, functional studies support a strong role for CD22 in cell interactions and B cell activation.
Recently, the murine CD22 equivalent has been identified. Whereas two forms exist in the human, cloning studies have only isolated the β form in the mouse, with 62% amino acid homology to the human protein (24,25). Murine CD22 is ~150 kDa and is expressed in two allelic forms (24–26). It is encoded on chromosome 7 and has been further demonstrated to be equivalent to the Lyb8 antigen (25). Although a great deal is known about the distribution and function of human CD22, little information is available regarding the mouse counterpart.
Since our laboratory is interested in murine B cell subsets, we sought to determine the developmental expression and distribution of murine CD22 using the anti-Lyb8 mAb Cy34.1.2. In the bone marrow, CD22 was found to be present in low levels on lgMbright immature B cells, and absent on the surface of pro-B cells, pre-B cells and emerging lgM+ B cells. Circulating mature B cells in the bone marrow displayed high levels of the antigen. In the periphery, CD22 is fully expressed on all mature B cell subsets, including follicular B cells, marginal zone B cells and peritoneal B1 cells. It is also present on switched lgA+ B cells residing in Peyer’s patches. Further studies revealed that upon activation, high CD22 levels are maintained on the responding B cells. Based upon the differential expression of CD22 on immature and mature B cells in the bone marrow, experiments were also performed to test the ability of CD22 to resolve immature B cells in the periphery. In combination with B220, CD23 and heat stable antigen (HSA), CD22 was found to distinguish immature B cells from marginal zone and follicular B cells in the spleen. This observation was confirmed in mice depleted of immature B cells by long-term anti-IL-7 treatment. Together, these observations demonstrate that murine CD22 is a pan marker for all mature B cells, and can be further utilized to distinguish immature from mature stages. The results also indicate a greater range of CD22 expression on murine B cells than that reported in the human.
Methods
Mice
BALB/c mice, 8–10 weeks old, were purchased from Harlan Sprague (Indianapolis, IN) and maintained in the specific pathogen-free facility at the University of Iowa.
Flow cytometric reagents
The following mAb were utilized for flow cytometric analysis: 6B2, a rat lgG2a anti-mouse B220; Cy34.1.2, a mouse lgG1 anti-mouse CD22 [CD22b (26)]; B3B4, a rat lgG2a anti-mouse FcεRII; BP-1, a mouse lgG2a anti-mouse aminopeptidase A (kindly provided by Dr Max Cooper, University of Alabama, Birmingham, AL); M1/69, a rat lgG2b anti-mouse HSA; and 11–26, a rat IgG anti-mouse IgD (kindly provided by Dr Fred Finkelman). Semi-purified mAb were prepared by ammonium sulfate precipitation from serum-free (HB101) culture supernatants. Chromatographically purified rat IgG (Jackson ImmunoResearch, West Grove, PA.) was used for controls. The various antibodies were biotin and fluorescein conjugated using standard protocols. Antibodies were derivatized with Cyanine 5.18 using a procedure modified from Mujumdar et al. (27). Briefly, Cyanine 5.18 was reacted with antibody at a ratio of 150 µg Cyanine to 1 mg protein in 0.1 M bicarbonate buffer, pH 9.5. Goat antibodies specific for IgM (µ chain) and IgA (α chain) were purchased from Southern Biotechnology Associates (Birmingham, AL) and phycoerythrin (PE)-conjugated rat anti-mouse CD5 was purchased from PharMingen (San Diego, CA). PE-, FITC- and Texas Red-avidin was purchased from Leinco Technologies (St Louis, MO).
Flow cytometric analysis
Spleen, Peyer’s patch and bone marrow cells were teased into single cell suspensions and washed in balanced salt solution (BSS). Peritoneal cells were obtained by injecting BSS into the peritoneal cavity followed by rigorous massaging of the abdomen and extraction of the cell suspension. All cell suspensions were spun through Fico-Lite-LM (Atlanta Biologicals, Norcross, GA) followed by washing in BSS. Cells (5×105) were resuspended in staining buffer consisting of 5% calf serum (Hyclone, Logan, UT) and 0.1% NaN3 in BSS. The cells were incubated with fluoresceinated, biotinylated, PE-conjugated or Cyanine 5.18-conjugated antibodies for 20 min at 4°C, followed by washing and incubation with the appropriate avidin reagent. Cells were then washed, resuspended in fixative (1% formaldehyde in 1.25×PBS) and analyzed by flow cytometry. In all experiments, 15 µg of the anti-FcγRII antibody 2.4G2 and 10 µl of rat serum were added in the first incubation to minimize non-specific staining. Stained cells were run on a Becton Dickinson FACS 440 equipped with a primary argon-ion laser, and a rhodamine 6G CR599 dye head laser (Coherent, Palo Alto, CA) pumped by a second argon ion laser. Fluorescent signals were collected using four-decade logarithmic amplification. A minimum of 30,000 cells was collected per sample. The FACS 440 data were analyzed using a VAX station 3200 computer equipped with DESK software (kindly supplied by Wayne Moore, Stanford University, Stanford, CA). Final graphic output was performed with Macintosh Canvas software. Two-color contours are represented as 5% probability plots. The flow cytometer was calibrated prior to each experiment using 1.9 µm calibration beads (SPHERO Rainbow Fluorescent Particles, Spherotech, Libertyville, IL) and CaliBryte software. This calibration entailed the standardization of all scatter and fluorescent detectors to predetermined channel numbers. In some experiments (Figs 2–4), the average fluorescence intensity from each of the various B cell subpopulations was determined to assess the relative density of CD22 expression. Fluorescence intensity values from each experiment were first normalized prior to determining means for each population This was done in order to eliminate experiment to experiment variation in fluorescence intensity due to different fluoro-chrome combinations when staining for CD22. Specifically, the fluorescence intensity of the B cell subset with highest levels of CD22 was set at 100%, with the intensity of the remaining populations normalized as a percent of this reference population. The reference B cell subset is indicated in the appropriate figure legends, along with its average raw scale value. The 0–4 marks on the x- and y-axes of each plot signify the four-decade logarithmic scale, with 0 indicating a 0.1 scale value and 4 indicating a 1000 scale value.
Fig. 2.
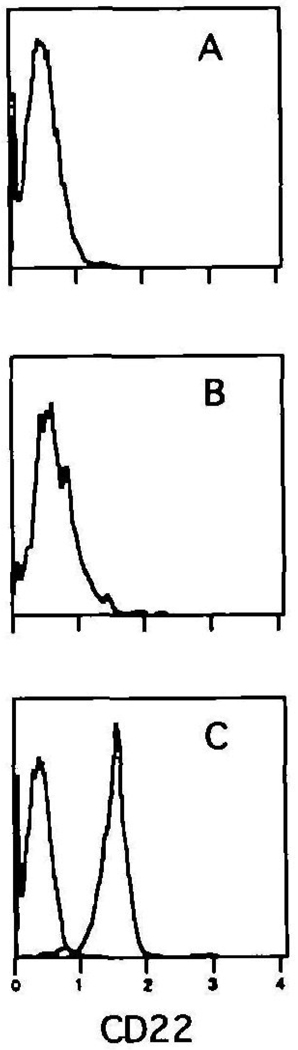
CD22 is restricted to the lgM+ B cell fraction in the bone marrow. Bone marrow cells were spun through Fico-Lite-LM and stained with FITC–B220, Texas Red–anti-IgM and biotin–anti-CD22 plus PE–avidin. The histograms in the right side of the figure illustrate the expression of CD22 on the pro- and pre- (A), immature (B), and mature (C) B cell populations The gates for each of these populations are shown in the left hand panel The plots are derived from the small light scatter gated cells The isotype control for CD22 staining is shown in (C). Mean normalized fluorescence intensity values demonstrated that populations A and B expressed 8.3 ± 1 5% (SD) and 12.5 ± 2 8% CD22 levels respectively when compared with population C [(100%) average raw scale value of 5 1 ± 0 9] One of eight experiments
Fig. 4.
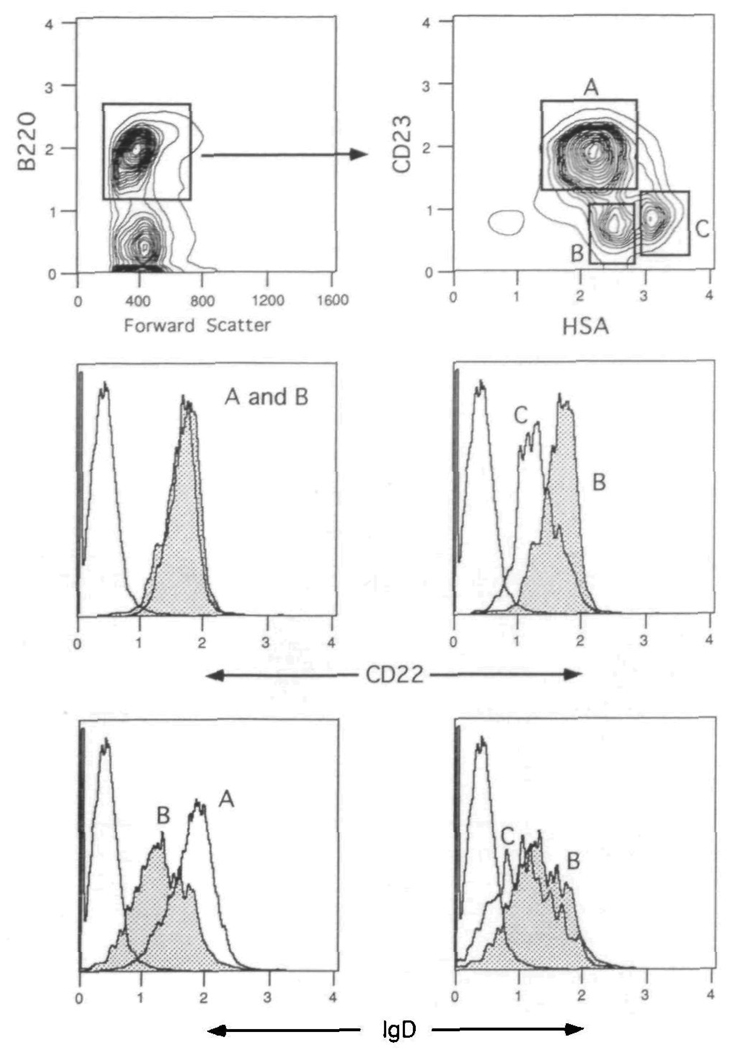
CD22 and IgD are differentially expressed on splenic B cell populations Spleen cells were spun through Fico-Lite-LM and stained with Cyanine 5 18–anti-B220, PE–anti-CD23, FITC–anti-HSA and either biotin–anti-CD22 or biotin–anti-lgD plus Texas Red–avidn The upper two contour plots show the gating strategies for the total B220+ compartment (left panel) and CD23 and HSA defined B cell populations (right panel) The lower left panels compare the CD22 and IgD expression levels on follicular (CD23+) and marginal zone (CD23−, HSA++) B cells The lower right panels compare the CD22 and IgD expression levels on marginal zone and immature (CD23−, HSA+++) B cells The isotype control is shown for comparison in the CD22 and IgD histograms. Mean normalized fluorescence intensity values demonstrated that populations B and C expressed 98 6 ± 3 4% (SD) and 57 7 ± 0 8% CD22 levels respectively when compared with population A [(100%) average raw scale value of 3 5 ± 0 9]. One of six experiments.
Cell activation
Spleen cells were harvested, washed and resuspended in medium consisting of RPMI 1640 with 10% calf serum, penicillin, streptomycin, l-glutamine and 2-mercaptoethanol. T cells were depleted using anti-Thy-1.2 antibody (HO13.4) and complement. T cell-depleted spleen cells were spun through Fico-Lite-LM, washed and resuspended in culture medium. Cells were incubated at 1×106 cells in 1 ml using 24-well plates. The following reagents alone or in combination were added for activation: lipopolysaccharide at 40 µg/ml (Difco, Detroit, Ml), recombinant IL-4 at 1000 U/ml (kindly provided by Dr Charles Maliszewski, Immunex, Seattle, WA) and Cos cell supernatant containing recombinant soluble CD40 ligand trimer (kindly provided by Dr William Fanslow, Immunex, Seattle, WA) at 10% v/v. The plates were incubated for 3–5 days followed by harvesting and FACS analysis.
Anti-IL-7 treatment
M25 (28), a mouse lgG2b anti-human IL-7 mAb which cross-reacts with murine IL-7, and Flag-M1 (28), an isotype-matched control, were purified from culture supernatants on Protein A affinity columns. Mice were injected i.p. with 1 mg of either M25 or Flag-M1 antibody in a 100 µl volume three times per week for a total of 4 weeks.
Results
Expression of CD22 on bone marrow B cells
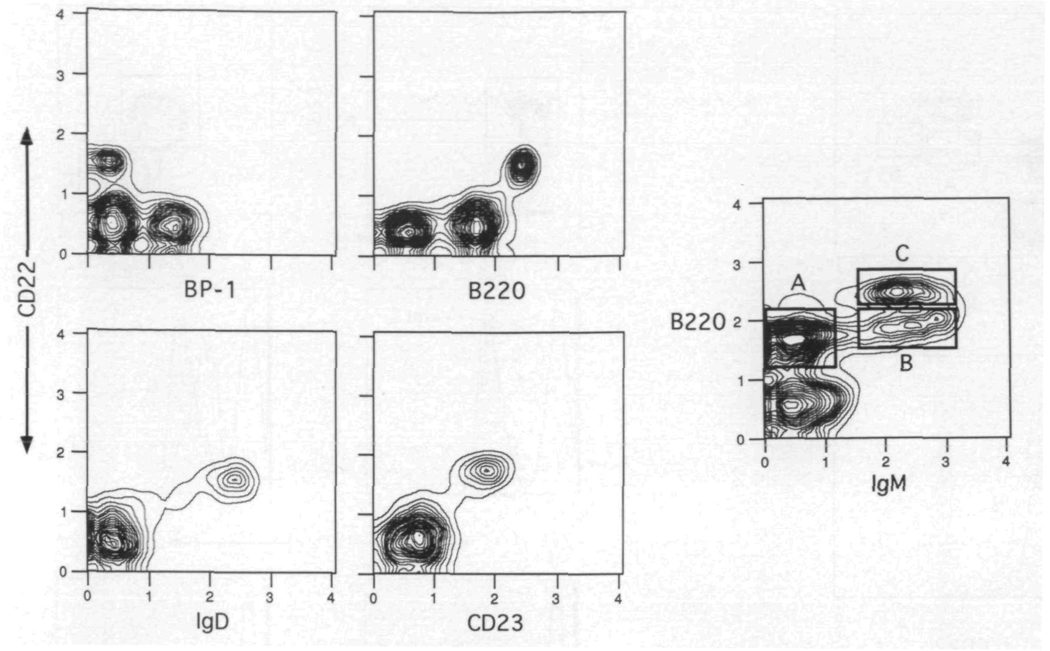
Early studies examining the expression of the Lyb8 alloantigen demonstrated its presence on the majority of mature splenic lgM+ B cells, as well as a progressive increase of Lyb8+ cells in the bone marrow and spleen of maturing mice (26). More recent studies with the rat anti-mouse CD22 antibody NIM-R6 likewise showed a concordant expression of CD22 and IgM on splenic B cells (24,25) Since no further information on the ontogeny and subset distribution of this marker has been reported, we used multi-color flow cytometry to clearly establish the expression patterns of murine CD22. In the first series of experiments, the anti-CD22b antibody Cy34.1.2 (26) was used in combination with stage-specific markers to explore the appearance of CD22 on maturing B cells present in the bone marrow of adult BALB/c mice. Figure 1 shows a series of two-color contours in which Cy34.1.2 was used with antibodies to BP-1, B220, IgD and CD23. The plots clearly demonstrate that the majority of CD22 expression is limited to B cells of a mature phenotype and not those belonging to the pre-B cell pool. Hence, CD22 is present on the B220hi, lgD+, CD23+ cells and is absent on the BP-1+ population.
Fig. 1.
CD22 is expressed on mature B cells in the bone marrow. Bone marrow cells were spun through Fico-Lite-LM and stained with anti-CD22, and either anti-B220, BP-1, anti-lgD or anti-CD23. The contour plots are derived from the small light scatter gated cells One of three experiments
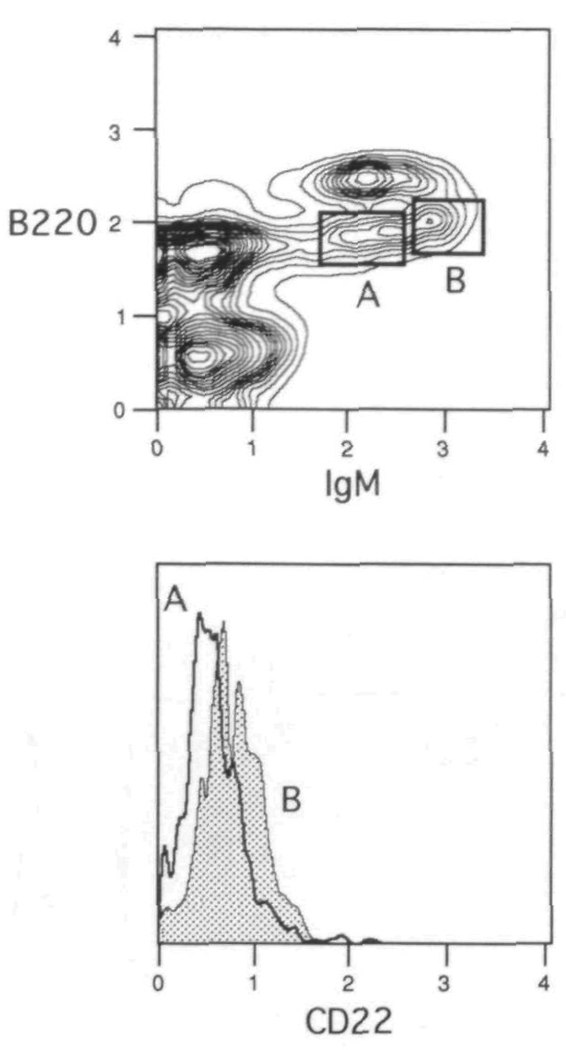
In order to assess better the appearance of CD22 during maturation, Cy34.1.2 was used to stain bone marrow cells in combination with antibodies to B220 and IgM. Figure 2 shows that as expected, the pro- and pre- B cells (B22010, IgM−; population A) are negative for CD22, whereas the mature B cells in the bone marrow (B220hi, lgM+; population C) exhibit uniform positive staining. When gating on the immature B22010, lgM+ B cells (population B), the CD22 histogram reveals most of the cells to be negative, with the suggestion of a dull staining subset. Further gating of this population, as illustrated in Fig. 3, shows the dull staining cells to be the more developed B22010, lgMhi B cells (gate B). The B22010, IgM10 B cells (gate A) or those just emerging from the pre-B cell pool (29) have yet to display CD22. Testing population B with an isotype control indicates the modest shift of CD22 expression to represent true dull positive staining, as the control antibody profile overlaps with the anti-CD22 staining of population A (data not shown). Taken together, the results suggest that CD22 first appears at low levels on the surface of immature lgMhi B cells, and is only fully expressed once final maturation occurs.
Fig. 3.
Surface expression of CD22 first appears on lgMhi immature B cells Bone marrow cells were spun through Fico-Lite-LM and stained with FITC–B220, Texas Red–anti-IgM and biotin–anti-CD22 plus PE–avidm The histogram overlays in the bottom panel demonstrate the staining pattern of CD22 on the IgM10 emerging B cells (A) and the lgMhi immature B cells (B) The gates for each of these populations are shown in the upper panel The plots are derived from the small light scatter gated cells Mean normalized fluorescence intensity values demonstrated that population A expressed 66 2 ± 4 7% (SD) when compared with population B [(100%) average raw scale value of 0.8 ± 0 3]. One of eight experiments.
Expression of CD22 on splenic B cell subsets
In a non-stimulated spleen, the B cell compartment consists of follicular mantle B cells, marginal zone B cells and a minor subset of immature B cells recently derived from the bone marrow. These populations can be defined with a number of cell surface markers. Using immunohistology, our laboratory has previously shown that murine CD23 is useful in distinguishing follicular and marginal zone B cells, with the former population expressing CD23 and latter being negative (30). Additionally, flow cytometric analysis demonstrated the lack of CD23 on immature B cells in the bone marrow and neonatal spleen (31,32). Recent works from Cancro et al. have demonstrated the utility of the HSA in separating immature from mature B cells in the spleen (33,34). Those B cells expressing the highest levels of HSA were shown to be recent bone marrow émigrés, with mature B cells displaying lower levels. We therefore utilized a four-color protocol with Cy34.1.2 and antibodies to B220, CD23, and HSA to assess expression patterns of CD22 on follicular, marginal zone and immature B cells. IgD levels were also determined on the three populations for comparison. As shown in the upper panels of Fig. 4, CD23 and HSA define three distinct subsets within the splenic B220+ compartment. These subsets are assigned as follicular (CD23+, HSA+; gate A), marginal zone (CD23−, HSA++; gate B), and immature (CD23−, HSA+++; gate C) B cells. The lower panels compare the levels of CD22 and IgD on the three populations. CD22 is present in equally high amounts on follicular and marginal zone B cells, with a lower density on the immature B cells (~40% lower as measured by mean fluorescence intensity). The higher levels of CD22 on populations A (follicular) and B (marginal zone) are consistent with the mature status of these cells. The lower expression level on population C is consistent with this subset representing immature B cells recently arrived from the bone marrow. Figure 4 further shows that IgD has a high expression level on follicular B cells, and is present at low levels on marginal zone and immature B cells, as previously demonstrated (30,35).
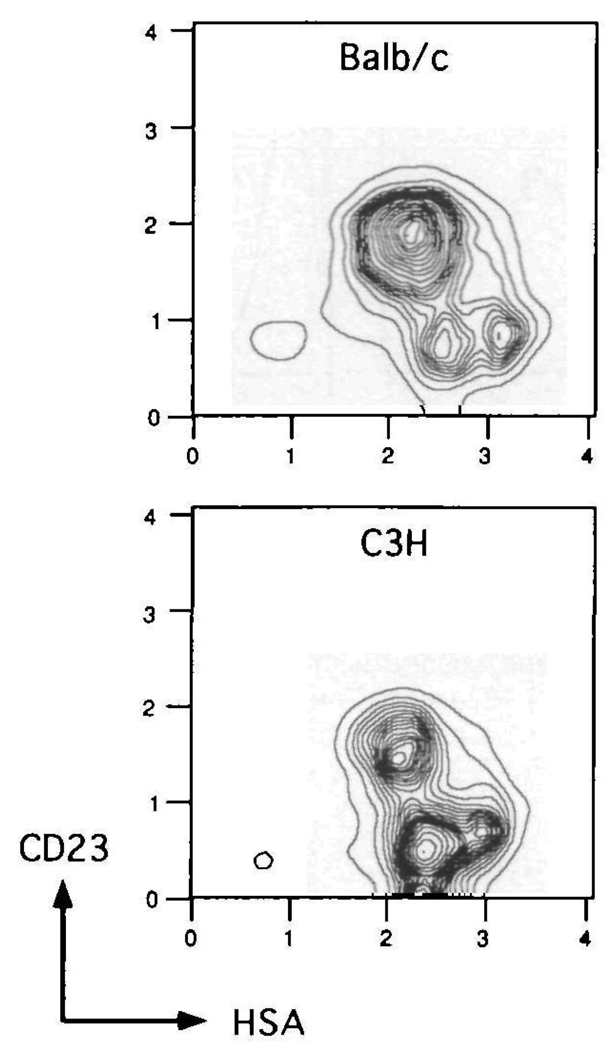
It is well established that CD23+ B cells represent the follicular mantle population in the spleen (30). In addition to the expression patterns of CD22, CD23 and HSA, we sought to solidify the assignment of the marginal zone and immature B cell populations. If the CD23−, HSA++ cells do indeed represent marginal zone B cells, then strains of mice which normally exhibit high marginal zones should have a proportional increase in cells with this phenotype. Whereas BALB/c mice typically have small splenic marginal zones, C3H mice have high or expanded marginal zones (30). When comparing the CD23 and HSA defined subsets within the B cell (B220+) compartment of these two strains, as shown in Fig. 5, it is clear that C3H mice have a greatly expanded CD23−, HSA++ population. This is confirmed by calculating the percentages of the three CD23- and HSA-defined B cell populations for BALB/c and C3H mice. Within the B220+ gated population, the follicular, marginal zone, and immature distribution is 74, 15 and 11% respectively for BALB/c (average of five adult mice) and 50, 42 and 8% respectively for C3H (average of five adult mice). This result is consistent with assignment of the CD23−, HSA++ population as marginal zone B cells. Four-color staining of C3H spleen cells revealed a pattern of CD22 and IgD expression on the three CD23- and HSA-defined subsets similar to that found for BALB/c (data not shown).
Fig. 5.
Comparison of CD23 and HSA defined splenic B cell populations in BALB/c and C3H mice. Spleen cells from two strains of mice were spun through Fico-Lite-LM and stained with Cyanine 518–anti-B220, PE–anti-CD23 and FITC-anti-HSA The CD23 and HSA contours are derived from the B220+ gated cells. One of five experiments
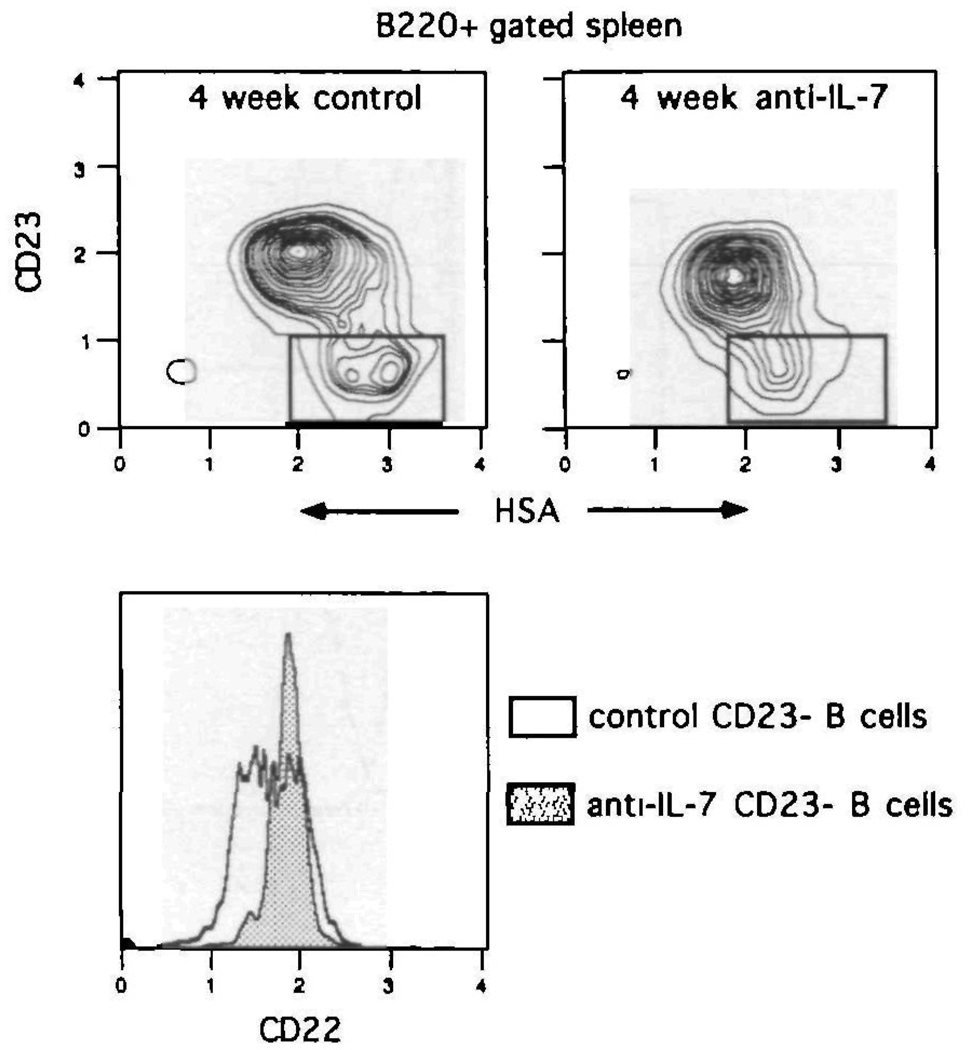
In an attempt to confirm the splenic CD23−, HSA+++ subset as immature B cells, mice were given an anti-IL-7 antibody in order to block B cell maturation. Mice were treated with the mAb M25, a mouse anti-human IL-7 antibody which cross-reacts with and neutralizes murine IL-7 (28). Treatment of mice with this antibody has been previously shown to block B cell maturation at the S7+, HSA10 pro-B cell stage and hence prevent the emergence of immature B cells from the bone marrow (28). Since M25 is an autologous (BALB/c derived) protein, it can be given to mice in high doses for extended periods of time. BALB/c mice were therefore treated for 4 weeks with M25 or an isotype-matched control, with each mouse receiving 1 mg of protein i.p. three times a week. At the end of the treatment period, splenic B cell content was examined using antibodies to B220, CD22, CD23 and HSA. Figure 6 demonstrates that after 4 weeks of in vivo IL-7 deprivation, the splenic CD23−, HSA+++ population is depleted. Control-treated mice display a normal B cell subset pattern In addition, examination of CD22 expression in the CD23− gate (lower panel, Fig. 6) reveals the specific loss of the CD2210 B cell subset in anti-IL-7-treated mice. Thus in animals where B cell maturation is blocked, the splenic CD23−, HSA+++, CD2210 B cell population is lost. This confirms the assignment of this population as immature B cells, and demonstrates the utility of CD22 in combination with CD23 and HSA to fractionate the peripheral B cell compartment.
Fig. 6.
Splenic CD23−, HSA+++ B cells are eliminated in IL-7 deprived mice. BALB/c mice were treated with anti-IL-7 (M25) or control (Flag-M1) antibody for 4 weeks Subsequent to treatment, spleen cells were spun through Fico-Lite-LM and stained with Cyanine 5.18–anti-B220, PE–anti-CD23, FITC–anti-HSA and biotin–anti-CD22 plus Texas Red–avidin The upper panels illustrate the CD23- and HSA-defined B220+ B cell subsets from control treated and IL-7 deprived mice. The lower panel shows the CD22 expression patterns on the CD23− gated subset from each mouse. One of six experiments.
Expression of CD22 on peritoneal B cell subsets
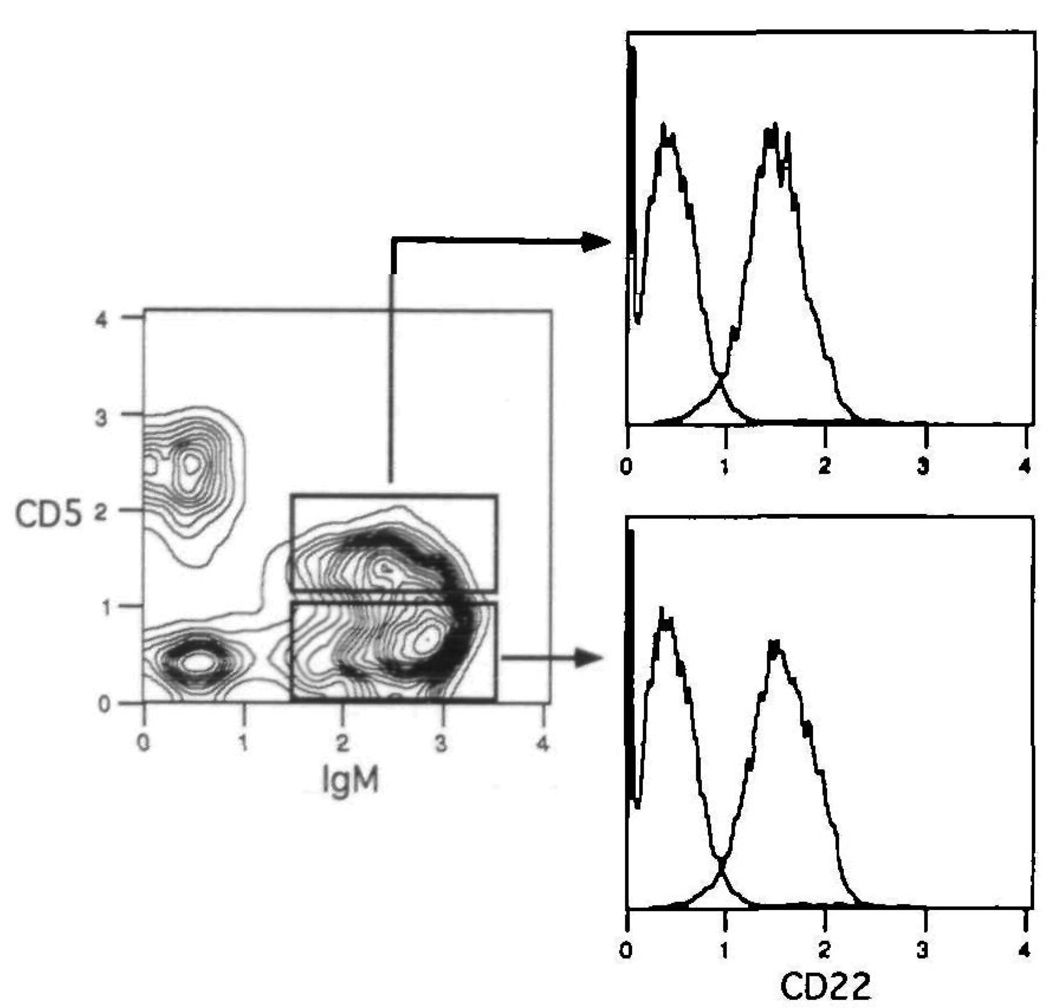
Examination of bone marrow and splenic B cells revealed that CD22 is fully expressed on mature B cells, regardless of their location. Thus, recirculating lgD+ B cells in the bone marrow, and follicular and marginal zone B cells in the spleen display the highest levels of CD22. Since B1 cells (CD5+/sister B cells) in the peritoneum represent mature B cells, one would predict this subset also to display high levels of CD22. This is verified in Fig. 7, in which peritoneal lavage cells were stained with Cy34.1.2, and antibodies to CD5 and IgM. The results show that both CD5+ (B1a) and CD5− (B1b) lgM+ B cells display mature levels of CD22. Examination of the rare population of CD5+ B cells in the spleen produced the same result (data not shown). Regardless of anatomic location therefore, mature B cells can be identified by high levels of CD22.
Fig. 7.
Peritoneal B1 cells display mature levels of CD22. Peritoneal lavage cells were spun through Fico-Lite-LM and stained with PE–anti-CD5, Texas Red–anti-IgM and biotin–anti-CD22 plus FITC–avidin The level of CD22 expression is shown for the B1a (CD5+, lgM+) and B1b (CD5−, lgM+) subsets Each histogram also contains the isotype control for comparison. One of three experiments
Assessment of CD22 expression during B cell activation
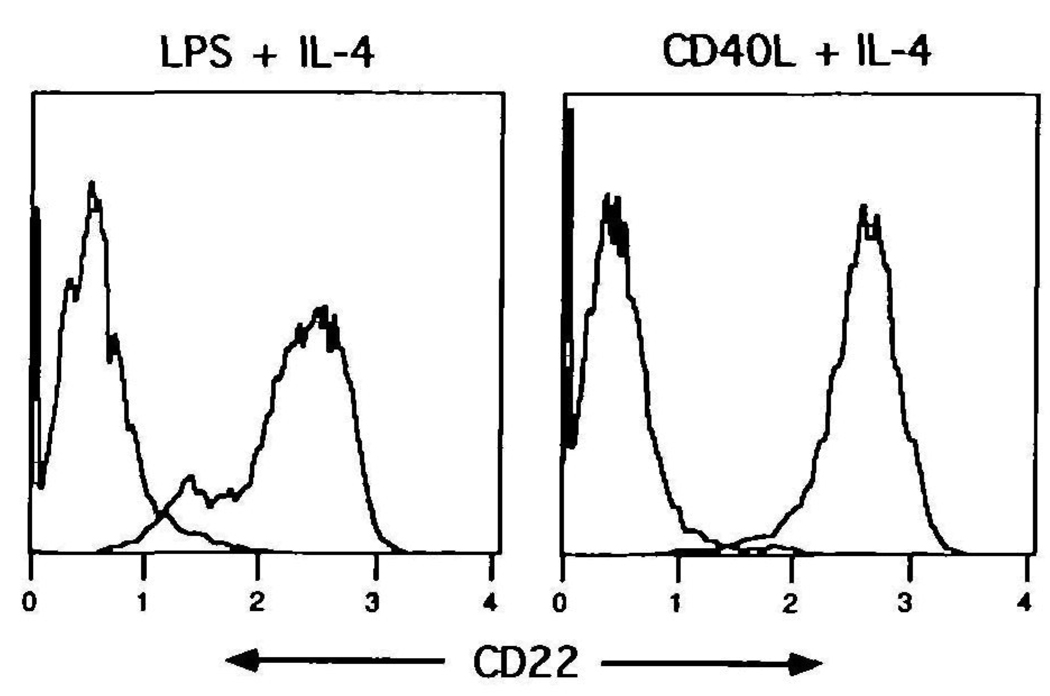
Upon activation of human B cells, hCD22 is reported to increase during the first 2 days of culture, followed by a down-regulation and marked loss of the antigen by day 3 or 4 (3). Accordingly, murine splenic B cells were stimulated with polyclonal activators, and assessed for CD22 expression after 4 days of culture. B cells were incubated with either lipopolysaccharide or recombinant CD40 ligand (trimeric form) both in the presence of IL–4 (1000 U/ml). These stimuli typically induce a peak proliferative response at 48 h of culture, as measured by thymidine uptake (data not shown). As illustrated in Fig. 8, virtually all of the stimulated B cells still retained high levels of CD22 after 4 days of stimulation. In agreement with previous studies (24), levels of CD22 on activated B cells are generally higher than on resting cells (relative increase of 5-fold over freshly isolated splenic B cells). Figure 8 also reveals a small population of B cells in the lipopolysaccharide-stimulated group with lower levels of CD22. This dull-staining subset was consistently seen with lipopolysaccharide, and was determined to consist of a subset of blasted cells. Taken together, the results demonstrate activated murine B cells to retain high levels of CD22, even after the peak of the proliferative response.
Fig. 8.
CD22 expression is maintained on activated B cells T cell-depleted spleen cells were incubated for 4 days with 40 µg/ml of lipopolysaccharide with IL-4 (1000 U/ml) or recombinant CD40 ligand (trimeric form) plus IL-4 At the end of the culture period, cells were harvested, washed and stained with FITC–anti-IgM and biotin–anti-CD22 plus Cyanine 5 18–avidin The CD22 histograms are derived from the lgM+ gated cells The isotype control for each group is shown for comparison One of three experiments.
CD22 expression on switched B cells
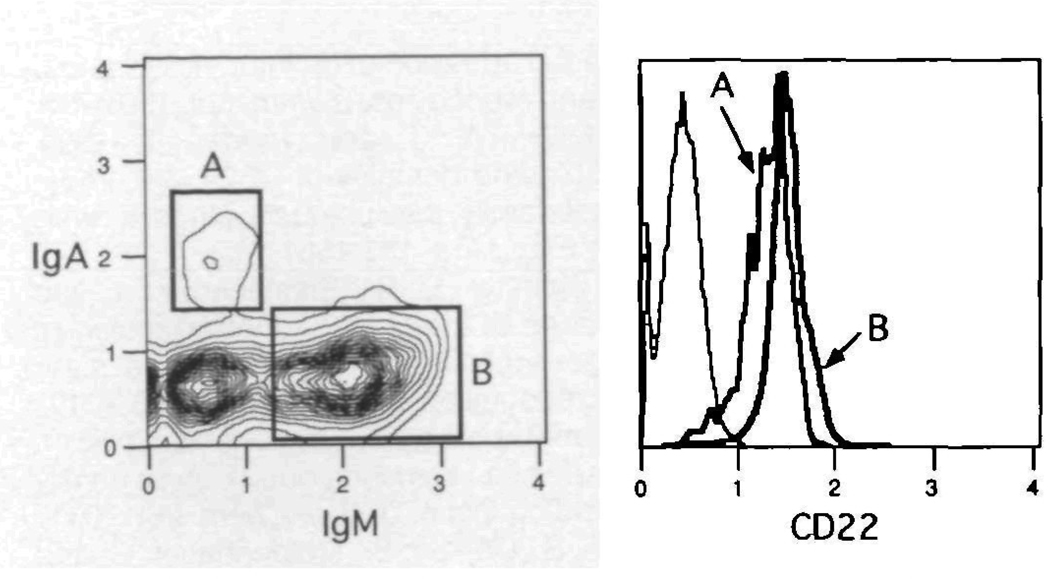
Subsequent to activation, a number of B cells can be induced to undergo switch recombination, depending upon the mix of signals. Some of these switched B cells, which now express downstream isotypes on their membrane, can be found in low numbers in peripheral tissues. In order to test the expression of CD22 on switched B cells in the mouse, Peyer's Patch cells were obtained and stained with Cy34.1.2 in combination with antibodies specific for IgA and IgM. Figure 9 demonstrates the result of this experiment and shows the presence of CD22 on IgA as well as IgM bearing B cells. Thus in the mouse, CD22 is retained on cells after activation and isotype switching.
Fig. 9.
CD22 is expressed on lgA+ Peyer’s patch B cells Cells from multiple Peyer's patches were pooled, spun through Fico-Lite-LM, and stained with FITC-anti-IgA, Texas Red-anti-IgM and biotin–anti–CD22 plus PE-avidin The histogram overlays represent the expression levels of CD22 on the resident lgA+ and lgM+ B cells. The isotype control is shown for comparison
Discussion
The human CD22 antigen has been well described in terms of its ontogenic expression and tissue distribution (reviewed in 1,2). Although its role in B cell physiology is still being defined, CD22 is likely to function as both an adhesion molecule (10,11,14–18) and a modulator of activation (2,19–23). Until recently, similar studies were not possible in the mouse due to a lack of biochemical and molecular data. With the cloning of the mouse CD22 homolog and its description as the previously defined Lyb8 antigen, more detailed studies have now become possible (24,25). The goal of the present study was to use an anti-Lyb8 alloantibody in conjunction with multi-parameter flow cytometry to assess the distribution of murine CD22 within the B cell compartment. The results show CD22 to be a late developmental marker first expressed at the late immature B cell stage. In the periphery, CD22 is found to be broadly expressed on all mature B cell subsets, including switched B cells. Finally, CD22 was demonstrated to increase upon activation and be maintained for an extended period.
The expression patterns detailed for murine CD22 bear strong similarities to those previously reported for humans. Both human and mouse CD22 appears on the surface of B cells late in development, at a stage when IgD and CD23 are being expressed (3,7–9). Previous histologic analysis of human lymphoid tissue demonstrated CD22 to be expressed on both follicular mantle and marginal zone B cells (9), similar to that found for murine splenic B cells using flow cytometry. Although both human and mouse CD22 initially increase subsequent to polyclonal activation, hCD22 appears to undergo a rapid decay such that by 4 days post-stimulation, most of the hCD22 is gone (3). This contrasts with the data herein (Fig. 8), in which murine CD22 was found to be strongly retained on activated B cells, especially those stimulated with recombinant CD40 ligand. The present study is also in good agreement with experiments in the mouse reported by Symington et al. (26) and Clark et al. (24,25). These studies demonstrated murine CD22 to be a late appearing developmental antigen (26) and to be present on the majority of lgM+, lgD+ peripheral B cells (24,25). Our results further these observations by clearly identifying the ontogenic stage at which CD22 appears, and documenting mature expression levels on splenic marginal zone, peritoneal cavity B1a and B1b B cells, and Peyer's patch switched B cells.
The present results also demonstrate the utility of CD22, when used with other markers, to delineate peripheral immature B cells. It is clear that the spleen is composed of a number of B cell subsets, some of which have redundant phenotypes (36). Within the B220 moderate to high expressing population, three B cell subsets can be defined using antibodies to CD23 and HSA. Previous work by Alman et al. demonstrated splenic immature B cells to reside within the HSAbright compartment (33,34), whereas our group showed the absence of CD23 on immature (31,32) and marginal zone B cells (30). When combining these markers, as shown in Fig. 5, one can clearly discriminate the CD23+ follicular B cells from the CD23−, HSAhigh marginal zone B cells and the CD23−, HSAbrightimmature B cells. The identity of the CD23−, HSAbright cells as immature B cells is supported by their low levels of CD22, and by their specific elimination in anti-IL-7-treated mice. Administration of anti-IL-7 to adult mice has previously been demonstrated to block B cell maturation in the bone marrow and deprive the periphery of bone marrow-derived replacements (28). Our results are thus in agreement with Alman et al., and strongly suggest that experiments testing the functional capacity of HSA10 and HSAhi B cells (37,38) may be examining the differentiative potential of follicular and immature B cells respectively.
A number of previous experiments in humans have suggested a role for CD22 in antigen receptor-mediated stimulation. Engagement of CD22 clearly augments the activating capacity of anti-IgM antibodies (3,20). CD22 is rapidly phos-phorylated upon IgM cross-linking (22,23) and is also found associated with the antigen receptor complex in detergent extracts (21,22). Analysis of the cytoplasmic sequence reveals tyrosine containing AHR1-like motifs (22), with recent work suggesting CD22 to bind the protein tyrosine phosphatase SHP when phosphorylated at these sites (39). The binding of SHP by CD22 is thought to enhance signaling through surface Ig by sequestering SHP from the activation complex, thereby diminishing its negative effects (39). When considering the appearance of CD22 during ontogeny, it is of interest that CD22−, lgM+ immature B cells fail to proliferate upon surface IgM cross-linking (40–42). Whether this defect is due to the lack of CD22 is unclear; however, CD22+ marginal zone and B1 B cells similarly fail to respond to anti-IgM stimulation (43–46). It is thus difficult to directly attribute an anti-IgM responsive versus non-responsive phenotype to CD22 expression, and suggests a more subtle role for CD22 in antigen receptor-mediated activation.
In addition to its role in activation, CD22 also serves as an adhesion molecule. Numerous studies have revealed the ability of CD22 to bind a number of glycoproteins, an interaction mediated by sialic acid (10,11,14–18). This capacity of CD22 has been speculated to aid the B cell in homotypic or heterotypic cellular interactions. Given the presence of CD22 on all mature B cells subsets (follicular, marginal zone, B1 and switched), and its absence on early B cells, the functional significance of CD22-mediated adhesion events is unclear. Since both CD22− immature B cells (33,34,41) and all CD22+ mature B cells [marginal zone and B1 cells included (36,44,47)] are competent to interact with T helper cells, a prominent role for CD22 in T cell-B cell interactions is difficult to justify. CD22 may thus play only a modifying role in T cell-dependent activation of mature B cells. One could alternatively suggest that the lack of CD22 expression is related to the localization of B cells to the bone marrow. In addition to early and immature B cells, late stage plasma cells also lack CD22 (3,9). In both the human and the mouse, the bone marrow is known to be a major repository of plasma cells (48,49).
Acknowledgements
The authors wish to thank Teresa Duling and Lisa Bogh for expert operation of the flow cytometer, Drs Max Cooper, William Fanslow, Fred Finkelman and Charles Maliszewski for reagents, and Tina Swartzendruber for preparation of the manuscript. This work was supported by NIH AI31265 and a grant from The Council for Tobacco Research.
Abbreviations
- BSS
balanced salt solution
- hCD22
human CD22
- HSA
heat stable antigen
- PE
phycoerythrin
References
- 1.Clark EA. CD22, a B cell-specific receptor, mediates adhesion and signal transduction. J Immunol. 1993;150:4715. [PubMed] [Google Scholar]
- 2.Law CL, Sidorenko SP, Clark EA. Regulation of lymphocyte activation by the cell-surface molecule CD22. Immunol. Today. 1994;15:442. doi: 10.1016/0167-5699(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 3.Dörken B, Moldenhauer G, Pezzutto A, Schwartz R, Feller A, Kiesel S, Nadler LM. HD39 (B3), a B lineage-restricted antigen whole cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986;136:4470. [PubMed] [Google Scholar]
- 4.Moldenhauer G, Schwartz R, Dǒrken B, Hämmerling GJ. Biochemical characterization and epitope analysis of B-lymphocyte-specific surface antigens defined by clustering workshop monoclonal antibodies. In: McMichael AJ, editor. Leucocyte Typing III White Cell Differentiation Antigens. Oxford: Oxford University Press; 1987. p. 378. [Google Scholar]
- 5.Boué DR, Lebien TW. Structural characterization of the human B lymphocyte-restricted differentiation antigen CD22 Comparison with CD21 (complement receptor type 2/Epstein-Barr virus receptor) J. Immunol. 1988;140:192. [PubMed] [Google Scholar]
- 6.Schwartz-Albiez R, Dörken B, Monner DA, Moldenhauer G. CD22 antigen-biosynthesis, glycosylation and surface expression of a B lymphocyte protein involved in B cell activation and adhesion. Int Immunol. 1991;3:623. doi: 10.1093/intimm/3.7.623. [DOI] [PubMed] [Google Scholar]
- 7.Campana D, Janossy G, Bofill M, Trejdosiewicz LK, Ma D, Hoffbrand AV, Mason DY, Lebacq A, Forster HK. Human B cell development. I. Phenotypic differences of B lymphocytes in the bone marrow and peripheral lymphoid tissue. J Immunol. 1985;134:1524. [PubMed] [Google Scholar]
- 8.Bofill M, Janossy G, Janossa M, Burford GD, Seymour GJ, Wernet P, Kelemen E. Human B cell development II. Subpopulations in the human fetus. J. Immunol. 1985;134:1531. [PubMed] [Google Scholar]
- 9.Ling NR, Maclennan ICM, Mason DY. B–cell and plasma cell antigens new and previously defined clusters. In: McMichael AJ, editor. Leucocyte Typing III White Cell Differentiation Antigens. Vol. 302. Oxford: Oxford University Press; 1987. [Google Scholar]
- 10.Stamenkovic I, Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;345:74. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- 11.Wilson GL, Fox CH, Fauci AS, Kehrl JH. cDNA cloning of the B cell membrane protein CD22 a mediator of B–B cell interactions. J. Exp. Med. 1991;173:137. doi: 10.1084/jem.173.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson GL, Najfeld V, Kozlow E, Menniger J, Ward D, Kehrl JH. Genomic structure and chromosomal mapping of the human CD22 gene. J. Immunol. 1993;150:5013. [PubMed] [Google Scholar]
- 13.Dörken B, Pezzutto A, Köhler M, Thiel E, Hunstein W. Expression of cytoplasmic CD22 in B-cell ontogeny. In: McMichael AJ, editor. Leucocyte Typing III. White Cell Differentiation Antigens. Oxford: Oxford University Press; 1987. p. 474. [Google Scholar]
- 14.Stamenkovic I, Sgroi D, Aruffo A, Sy MS, Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and α2,6 sialyltransferase, CD75, on B cells. Cell. 1991;66:1133. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 15.Engel P, Nojima Y, Rothstein D, Zhou L-J, Wilson GL, Kehrl JH, Tedder TF. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993;150:4719. [PubMed] [Google Scholar]
- 16.Engel P, Wagner N, Miller AS, Tedder T. Identification of the ligand-binding domains of CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J Exp. Med. 1995;181:1581. doi: 10.1084/jem.181.4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, varki A. Natural ligands of the B cell adhesion molecule CD22β carry N-linked oligosaccharides with α-2,6-linked sialic acids that are required for recognition. J Biol. Chem. 1993;268:7019. [PubMed] [Google Scholar]
- 18.Sgroi D, Varki A, Braesch-Andersen S, Stamenkovic I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J. Biol. Chem. 1993;268:7011. [PubMed] [Google Scholar]
- 19.Pezzutto A, Rabinovitch PS, Dörken B, Moldenhauer G, Clark EA. Role of the CD22 human B cell antigen in B cell triggering by anti-immunoglobulin. J. Immunol. 1988;140:1791. [PubMed] [Google Scholar]
- 20.Pezzutto A, Dörken B, Moldenhauer G, Clark EA. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp 130/140. J Immunol. 1987;138:98. [PubMed] [Google Scholar]
- 21.Peaker CJG, Neuberger MS. Association of CD22 with the B cell antigen receptor. Eur J. Immunol. 1993;23:1358. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
- 22.Leprince C, Draves KE, Geahlen RL, Ledbetter JA, Clark EA. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc Natl Acad Sci. USA. 1993;90:3236. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte RJ, Campbell M-A, Fischer WH, Sefton BM. Tyrosine phosphorylation of CD22 during B cell activation. Science. 1992;258:1001. doi: 10.1126/science.1279802. [DOI] [PubMed] [Google Scholar]
- 24.Torres RM, Law C-L, Santos-Argumedo L, Kirkham PA, Grabstein K, Parkhouse RME, Clark EA. Identification and characterization of the murine homologue of CD22, a B lymphocyte-restricted adhesion molecule. J Immunol. 1992;149:2641. [PubMed] [Google Scholar]
- 25.Law C-L, Torres RM, Sundberg HA, Parkhouse RME, Brannan CI, Copeland NG, Jenkins NA, Clark EA. Organization of the murine CD22 locus. J Immunol. 1993;151:175. [PubMed] [Google Scholar]
- 26.Symington FW, Subbarao B, Mosier DE, Sprent J. Lyb8 2 a new B cell antigen defined and characterized with a monoclonal antibody. Immunogenetics. 1982;16:381. doi: 10.1007/BF00372098. [DOI] [PubMed] [Google Scholar]
- 27.Mujumdar RB, Ernst LA, Mujumdar SR, Waggoner AS. Cyanine dye labeling reagents containing isothiocyanate groups. Cytometry. 1989;10:11. doi: 10.1002/cyto.990100104. [DOI] [PubMed] [Google Scholar]
- 28.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoctonal antibody. J Exp Med. 1993;178:257. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scher I, Sharrow SO, Wistar R, Jr, Asofsky R, Paul WE. B-lymphocyte heterogeneity: Ontogenic development and organ distribution of B-lymphocyte populations defined by their density of surface immunoglobulin. J. Exp Med. 1976;144:494. doi: 10.1084/jem.144.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldschmidt TJ, Kroese FGM, Tygrett LT, Conrad DH, Lynch RG. The expression of B cell surface receptors. III. The murine low-affinity IgE Fc receptor is not expressed on Ly 1 or 'Ly 1-like' B cells Int. Immunol. 1991;3:305. doi: 10.1093/intimm/3.4.305. [DOI] [PubMed] [Google Scholar]
- 31.Waldschmidt TJ, Conrad DH, Lynch RG. The expression of B cell surface receptors. I. The ontogeny and distribution of the murine B cell IgE Fc receptor. J. Immunol. 1988;140:2148. [PubMed] [Google Scholar]
- 32.Waldschmidt TJ, Conrad DH, Lynch RG. The expression of B cell surface receptors. II IL-4 can accelerate the developmental expression of the murine B cell IgE Fc receptor. J. Immunol. 1989;143:2820. [PubMed] [Google Scholar]
- 33.Allman DM, Ferguson SE, Cancro MP. Peripheral B cell maturation I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J Immunol. 1992;149:2533. [PubMed] [Google Scholar]
- 34.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation II. Heat-stable antigenhi splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431. [PubMed] [Google Scholar]
- 35.Kearney JF, Cooper MD, Klein J, Abney ER, Parkhouse RME, Lawton AR. Ontogeny of la and IgD on IgM-bearing B lymphocytes in mice. Exp Med. 1977;146:297. doi: 10.1084/jem.146.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Best CG, Kemp JD, Waldschmidt TJ. Murine B cell subsets defined by CD23. Methods: Companion Methods in Enzymology. 1995;8:3. [Google Scholar]
- 37.Linton P-J, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59:1049. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 38.Linton P-J, Lo D, Lai L, Thorbecke GJ, Klinman NR. Among naive precursor cell subpopulations only progenitors of memory B cells originate germinal centers. Eur. J Immunol. 1992;22:1293. doi: 10.1002/eji.1830220526. [DOI] [PubMed] [Google Scholar]
- 39.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 40.Brines RD, Klaus GGB. Effects of anti-immunoglobulin antibodies, interleukin-4 and second messenger agonists on B cells from neonatal mice. Int Immunol. 1991;3:461. doi: 10.1093/intimm/3.5.461. [DOI] [PubMed] [Google Scholar]
- 41.Chang T-L, Capraro G, Kleinman RE, Abbas AK. Anergy in immature B lymphocytes Differential responses to receptor-mediated stimulation and T helper cells. J Immunol. 1991;147:750. [PubMed] [Google Scholar]
- 42.Yellen AJ, Glenn W, Sukhatme VP, Cao X, Monroe JG. Signaling through surface IgM in tolerance-susceptible immature murine B lymphocytes Developmentally regulated differences in transmembrane signaling in splenic B cells from adult and neonatal mice. J. Immunol. 1991;146:1446. [PubMed] [Google Scholar]
- 43.Waldschmidt T, Snapp K, Foy T, Tygrett L, Carpenter C. B-cell subsets defined by the FcεR. Ann. NY Acad Sci. 1992;651:84. doi: 10.1111/j.1749-6632.1992.tb24599.x. [DOI] [PubMed] [Google Scholar]
- 44.Snapper CM, Yamada H, Smoot D, Sneed R, Lees A, Mond JJ. Comparative in vitro analysis of proliferation, Ig secretion, and Ig class switching by murine marginal zone and follicular B cells. J. Immunol. 1993;150:2737. [PubMed] [Google Scholar]
- 45.Rothstein TL, Kolber DL. Anti-Ig antibody inhibits the phorbol ester-induced stimulation of peritoneal B cells. J Immunol. 1988;141:4089. [PubMed] [Google Scholar]
- 46.Morris DL, Rothstein TL. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J Exp Med. 1993;177:857. doi: 10.1084/jem.177.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel LA, Sercarz EE, Metzger DW. Antibody response of murine B1 cells to hen eggwhite lysozyme Cell. Immunol. 1995;161:88. doi: 10.1006/cimm.1995.1012. [DOI] [PubMed] [Google Scholar]
- 48.Tew JG, DiLosa RM, Burton GF, Kosco MH, Kupp LI, Masuda A, Szakal AK. Germinal centers and antibody production in bone marrow. Immunol. Rev. 1992;126:99. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 49.Bachman MF, Kundig TM, Odermatt B, Hengartner H, Zinkernagel RM. Free recirculation of memory B cells versus antigen-dependent differentiation to antibody-forming cells. J Immunol. 1994;153:3386. [PubMed] [Google Scholar]