Abstract
Caenorhabditis elegans is an androdioecious nematode with both hermaphrodites and males. Although males can potentially play an important role in avoiding inbreeding and facilitating adaptation, their existence is evolutionarily problematic because they do not directly generate offspring in the way that hermaphrodites do. This review explores how genetic, population genomic, and experimental evolution approaches are being used to address the role of males and outcrossing within C. elegans. Although theory suggests that inbreeding depression and male mating ability should be the primary determinants of male frequency, this has yet to be convincingly confirmed experimentally. Genomic analysis of natural populations finds that outcrossing occurs at low, but not negligible levels, and that observed patterns of linkage disequilibrium consistent with strong selfing may instead be generated by natural selection against outcrossed progeny. Recent experimental evolution studies suggest that males can be maintained at fairly high levels if populations are initiated with sufficient genetic variation and/or subjected to strong natural selection via a change in the environment. For example, as reported here, populations adapting to novel laboratory rearing and temperature regimes maintain males at frequencies from 5% to 40%. Laboratory and field results still await full reconciliation, which may be facilitated by identifying the loci underlying among-strain differences in mating system dynamics.
Keywords: androdioecy, experimental evolution, male mating, outbreeding depression, outcrossing, self-fertilization
What role might males play in a population of hermaphrodites since the hermaphrodites are already capable of reproducing on their own? Males are “costly” because they require significant resources to be produced but do not directly produce offspring themselves (Maynard Smith 1971, 1978; Bell 1982; Uyenoyama 1984; Lively and Lloyd 1990). All other things being equal, a population that has a mixture of hermaphrodites and males will have a lower growth rate than a population that is purely hermaphroditic. At an individual level, this means that a mutation that increases the frequency of males will be selected against unless males confer some other advantage that is linked to their mode of reproduction (Lively and Lloyd 1990; Barton 1995a; Otto and Barton 1997; Howard and Lively 1998). Making matters worse, mating with males (or other hermaphrodites) can itself be deleterious because of direct physical damage, behavioral interference, physiological manipulation, and/or transmission of sexual parasites (Fowler and Partridge 1989; Clutton-Brock and Parker 1995; Gems and Riddle 1996). Thus, unless males convey some substantial benefit—unless the offspring of matings that result from outcrossing with males have higher fitness than those resulting from self-fertilization (selfing) by hermaphrodites—then we would expect males to be eliminated from these populations.
This is the conundrum of androdioecy, the rare sexual mating system composed of males and hermaphrodites (Charlesworth B and Charlesworth D 1978; Charlesworth 1984; Otto et al. 1993; Pannell 1997, 2002; Weeks et al. 2006) that is exhibited by the model nematode Caenorhabditis elegans (Brenner 1974; Stewart and Phillips 2002; Cutter and Payseur 2003). In C. elegans, hermaphrodites cannot cross with other hermaphrodites, and so any outcrossing that does occur must do so via males. This mating system can have a profound effect on the evolutionary fate of new mutations, whether they are deleterious or advantageous. In the absence of males, any new deleterious mutations that arise within a selfing lineage have a good chance of becoming fixed because selfing promotes homozygosity (Figure 1). Continual accumulation and fixation of deleterious mutations should steadily erode fitness within a given lineage (Heller and Maynard Smith 1979; Lande and Schemske 1985; Charlesworth D and Charlesworth B 1987; Charlesworth et al. 1993; Schultz and Lynch 1997). This force is counterbalanced by the fact that selfing increases the probability that recessive mutations will be exposed to natural selection and thereby become “purged” from the population (reviewed by Byers and Waller 1999; Crnokrak and Barrett 2002; also see Lande and Schemske 1985; Charlesworth D and Charlesworth B 1987; Charlesworth et al. 1993). The presence of males slows the march toward homozygosity and can therefore mitigate the negative consequences of inbreeding depression, but by the same turn, males allow deleterious recessive mutations to continue to segregate within populations. The balance between fixation, loss, and segregation generated by the presence or absence of males helps to determine the level of potential inbreeding depression present within the population, whereas, at the same time, standing levels of inbreeding depression play a critical role in structuring the frequency of males that we expect to see in any given population (Hill and Robertson 1966; Heller and Maynard Smith 1979; Charlesworth 1984; Lande and Schemske 1985; Charlesworth D and Charlesworth B 1987; Charlesworth et al. 1990, 1993; Otto et al. 1993; Stewart and Phillips 2002; Cutter and Payseur 2003).
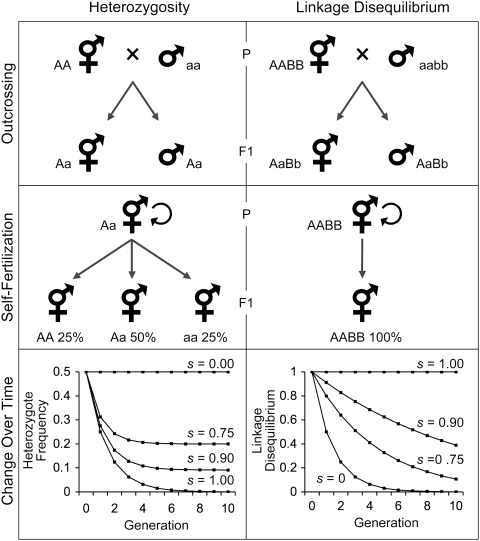
Figure 1.
Some genetic consequences of outcrossing and selfing. Left: outcrossing maintains heterozygosity, whereas self-fertilization creates an excess of homozygotes. Right: outcrossing can break linkage between alleles at different loci, whereas self-fertilization maintains linkage disequilibria over time. These patterns are generated by the coancestry within and between loci generated by selfing (middle) and are manifest within populations by changing levels of heterozygosity and linkage disequilibrium (bottom). Here, heterozygosity is determined by 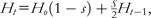 , where s is the rate of self-fertilization and H0 is the initial heterozygosity before selfing (scaled here to its maximum value of 0.5; Crow and Kimura 1970). Linkage disequilibria at generation t are given by , where D0 is the amount of linkage disequilibrium at generation zero (scaled to 1.0 for convenience) and is the effective amount of recombination scaled by the rate of selfing within the population: (Nordborg 1997; Barrière and Félix 2005). The actual recombination rate, r, was set to 0.5 for this example.
, where s is the rate of self-fertilization and H0 is the initial heterozygosity before selfing (scaled here to its maximum value of 0.5; Crow and Kimura 1970). Linkage disequilibria at generation t are given by , where D0 is the amount of linkage disequilibrium at generation zero (scaled to 1.0 for convenience) and is the effective amount of recombination scaled by the rate of selfing within the population: (Nordborg 1997; Barrière and Félix 2005). The actual recombination rate, r, was set to 0.5 for this example.
Because selfing reduces the effectiveness of recombination, as evidenced by the long-term maintenance of linkage disequilibrium (Figure 1), new adaptive mutations can effectively become trapped within selfing lineages, potentially impeding their capacity for adaptation (Hill and Robertson 1966; Felsenstein 1974; Barton 1995b). Males allow genetic exchange and could therefore have an important role to play during a population's response to changing environments (Stebbins 1957; Maynard Smith 1978; Bell 1982; Crow 1992). Overall, then, a number of critical evolutionary processes could be influenced by enhanced outcrossing and the presence of males. We would therefore predict that there should be circumstances in which males are able to overcome the cost of their own existence and be maintained within androdioecious populations, such as those seen in C. elegans (see also Otto et al. 1993; Weeks et al. 2000, 2002). The important questions then become: what are the central features of these circumstances and what patterns do we actually observe in nature?
Making and Maintaining Males in C. elegans
Androdioecious populations are especially useful for examining questions regarding the evolution of breeding systems because they have the potential to span the range of possible matings from complete selfing to complete outcrossing. C elegans is particularly valuable in this respect for 2 reasons. First, because hermaphrodites cannot cross with one another, the questions of the evolution of outcrossing and the maintenance of males are inexorably tied to one another. Second, because C. elegans has been such a powerful genetic model system, we know a great deal about its sex determination, which in turn allows us to directly manipulate its mating system using well-defined mutants.
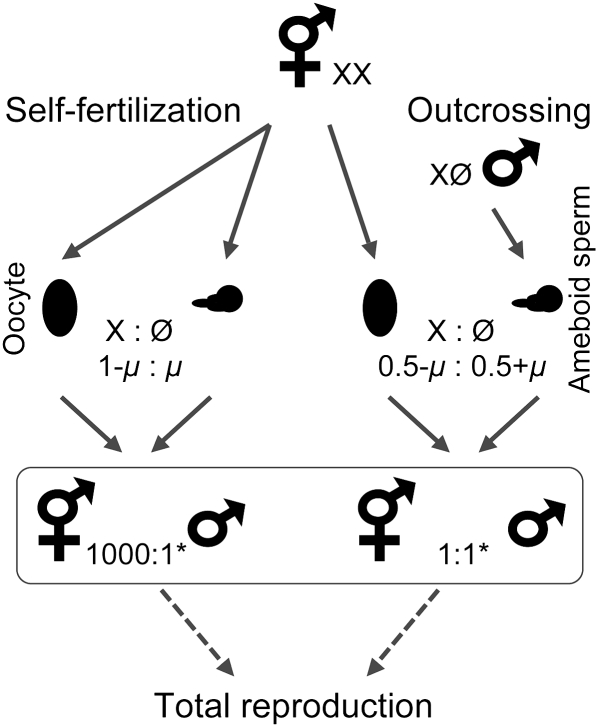
Hermaphrodites in C. elegans are protandrous, with their gonads first producing sperm, which are stored in their spermatheca and later used to self-fertilize eggs, before shifting to produce eggs themselves (L'Hernault 1997; Schedl 1997). Recent work on the evolution of sex determination mechanisms within the genus Caenorhabditis has demonstrated that the transition from dioecy to androdioecy has occurred at least twice independently within this group (Kiontke et al. 2004; Haag and Doty 2005; Nayak et al. 2005). Thus, C. elegans hermaphrodites can be viewed as being morphologically equivalent to females that produce their own sperm early during sexual maturity (Baldi et al. 2009). This helps to explain why hermaphrodites cannot cross with one another: they have no direct means of transferring sperm (in this case via the male tail). Hermaphrodites are XX, whereas males are XØ. Males can therefore be produced by 2 distinct processes. First, mating between a hermaphrodite and male results in normal segregation of the sex chromosomes, leading to 50% male and 50% hermaphroditic offspring (Figure 2). Second, spontaneous nondisjunction of the X chromosome leads to an aneuploid sperm or egg that will, when fused with an X-bearing partner, result in an XØ male (Figure 2).
Figure 2.
Sex ratios resulting from self-fertilization and outcrossing in Caenorhabditis elegans. Hermaphrodite eggs (X) can be fertilized by self (X) or male (X or Ø) sperm. Offspring resulting from self-fertilization are nearly all hermaphrodites, with a small fraction of males produced by nondisjunction of the X chromosome (μ). Outcrossed progeny are approximately 50% male and 50% hermaphrodite with a correction for the nondisjunction rate. Individual hermaphrodites can produce both self and male-sired offspring; thus, sex ratio in the progeny depends on how many embryos were sired by each parent. *, Approximate ratios based on N2.
The evolutionary history of this group of nematodes makes it an ideal system to study transitions between dioecy to androdioecy and outcrossing to selfing. The genetic tools that are available to manipulate sex determination, sex ratios, and mating system dynamics provide an opportunity to critically test evolutionary theories that are not available in any other system (Table 1). For example, the fog-2 gene (Schedl and Kimble 1988) plays an important role in the transition between male and female function in the hermaphrodite gonad and appears to have uniquely arisen within C. elegans (Clifford et al. 2000; Nayak et al. 2005). The FOG-2 protein binds RNA from the sex determination factor tra-2 during sexual maturation, shutting down the regular feminizing effects of tra-2 and thereby generating sperm-producing gonads (Clifford et al. 2000). Thus, fog-2 mutant males produce sperm, whereas fog-2 mutant hermaphrodites do not, making fog-2 mutant hermaphrodites functionally equivalent to females and changing the mating system from androdioecious to dioecious (or gonochoristic). Similar mutations generate X-specific nondisjunction, disrupt XØ dosage compensation, and alter the timing of the shift between sperm and egg production in hermaphrodites (Table 1). Each of these mutations allows different aspects of the mating system to be probed in a systematic way. Easy laboratory maintenance and short (3–4 days at 20 °C) generation times further allow these genetic tools to be exploited using approaches from experimental evolution. The combination of all these factors generates a powerful system in which both the specific biology of C. elegans and more general questions regarding the evolution of mating systems and the role of males can be explored.
Table 1.
Mutations with effects on mating system dynamics in Caenorhabditis elegans
| Gene | Function | Mating system effect | Functional background | Evolutionary applications |
| fog-2 | F-box protein that binds tra-2 mRNA before sexual maturity, thereby initiating germ-line production of sperm in hermaphrodites. | Mutants do not undergo protandrous production of sperm, effectively feminizing hermaphrodites, leading to dioecy and obligate outcrossing. | Schedl and Kimble (1988); Clifford et al. (2000); Nayak et al. (2005) | Stewart and Phillips (2002); Katju et al. (2008); Morran, Parmenter, et al. (2009) |
| him-5 | Influences the number and distribution of X chromosome pairing events; molecular function currently unknown. | Causes X-specific nondisjunction and so leads to high frequency of males. | Hodgkin et al. (1979); Broverman and Meneely (1994) | Chasnov and Chow (2002); Cutter and Payseur (2003) |
| spe-8 | Protein tyrosine kinase involved in the sperm activation signaling pathway. | Produces nonfunctional sperm in hermaphrodites, leading to dioecy. | L'Hernault et al. (1988); Muhlrad and Ward (2002) | LaMunyon and Ward (2002); LaMunyon et al. (2007) |
| spe-26 | Actin-binding protein needed for normal spermatogenesis. | Effects as in spe-8. | Varkey et al. (1995); Minniti et al. (1996) | Cutter (2005) |
| tra-2 | Membrane-bound protein that controls trafficking of the sex determination signaling pathway. | Mutants transform hermaphrodites into males. A temperature-sensitive version can be used to mimic temperature-dependent sex determination, such as that found in turtles. | Hodgkin and Brenner (1977); Kuwabara et al. (1992); Kuwabara and Kimble (1995); Hodgkin (2002) | Janzen and Phillips (2006); Chandler et al. (2009) |
| tra-3 | Regulatory protease that cleaves tra-2. | Can delay the transition between sperm and egg production in hermaphrodites, thereby shifting the pattern of sex allocation. | Hodgkin and Brenner (1977); Sokol and Kuwabara (2000) | Hodgkin and Barnes (1991) |
| xol-1 | Metabolic kinase that helps assess the X/autosome ratio and regulates X chromosome dosage compensation. | Mutants disrupt dosage compensation, which leads to male (XØ) lethality and results in obligate selfing. | Miller et al. (1988); Rhind et al. (1995) | Morran, Parmenter, et al. (2009) |
In this review, we first examine models that articulate the evolutionary forces that should be controlling the maintenance of males within C. elegans populations and highlight some of the laboratory experiments that have tested these models. We then review evidence for the prevalence of males and outcrossing within natural populations of C. elegans, noting that although males appear to be quite rare, there is still a strong genetic signal of their influence. This observation is in stark contrast with more recent results that show males have the potential to be maintained at surprisingly high levels in the laboratory. We conclude with a discussion of the information needed to resolve these discrepancies and possible directions this research may take in the future.
Males—In Theory (and Experimental Tests)
In the most common laboratory strain of C. elegans, N2 Bristol, males are found at a frequency that is essentially indistinguishable from the rate at which they are produced by nondisjunction (male frequency ≤ 0.002; Hodgkin 1983; Chasnov and Chow 2002; Teotónio et al. 2006; nondisjunction 0.001–0.004; Hodgkin et al. 1979; Rose and Baillie 1979; Cutter and Payseur 2003; Teotónio et al. 2006). The similarity between these frequencies may suggest that males are not generally the product of successful outcrossing between males and hermaphrodites but are instead primarily produced by nondisjunction events. This observation calls into question the role of males and, because males facilitate outcrossing, the value of outcrossing in populations of C. elegans. Thus, to understand the role of males in C. elegans, we must first consider whether males are maintained only because they can be produced by nondisjunction and are therefore maintained at a kind of mutation–selection balance or whether males are playing a more active role in this species and are therefore maintained by natural selection.
Are males more than meiotic mistakes? Although the presence or absence of a sex chromosome required to drive male development in C. elegans can result from nondisjunction, sex chromosome number is merely a signal that leads to a wide suite of sex-specific developmental and neurological responses (reviewed by Wolff and Zarkower 2008). If outcrossing is rare in C. elegans, then the genes underlying male development, morphology, and behavior, which make up a large fraction of the C. elegans genome (Jiang et al. 2001), should be subject to relaxed selection. Given sufficient time under relaxed selection, mutation accumulation in male-specific genes should result in the erosion of male function (Chasnov and Chow 2002; Cutter and Ward 2005). However, Cutter and Ward (2005) found that genes expressed exclusively in males, not associated with sperm, are among the most conserved in the C. elegans genome. Thus, male-specific genes appear either to be maintained by selection or relaxed selection has not occurred for a sufficient amount of time for the resultant mutation accumulation to be detectable (Loewe and Cutter 2008).
Although it seems obvious that male-specific genes would degrade in the absence of males, in fact the entire genome would be at risk of accumulating deleterious mutations within obligately selfing lineages. The genome-wide accumulation of slightly deleterious mutations, known as Muller's Ratchet, is predicted to lead to extinction in either asexual (Muller 1964) or strictly selfing (Heller and Maynard Smith 1979) lineages. By calculating the expected time to extinction of C. elegans under strict selfing, Loewe and Cutter (2008) demonstrated that C. elegans either only recently became predominantly selfing (see also Cutter et al. 2008) or that outcrossing occurs with sufficient frequency to allow the species to persist. Because outcrossing, which is only achieved by successful mating between males and hermaphrodites, occurs in nature at low but detectable levels (see below; Barrière and Félix 2005, 2007; Haber et al. 2005; Sivasundar and Hey 2005; Cutter 2006), the latter explanation for the persistence of the species is at least plausible. Although it is clear that male function is maintained in C. elegans, it remains to be conclusively determined whether this is because selfing is of such recent origin that male function has not had time to decay or because males and male function are under direct selection.
Presuming that C. elegans males are maintained by selection, the low frequency of males in C. elegans populations seems somewhat surprising. If males have an important role, why are they so rare? According to the model of Stewart and Phillips (2002), given the 2-fold cost of sex and negligible rates of spontaneous male production (see above), the following must be true for males to be maintained at stable frequencies higher than their rate of spontaneous production:
| (1) |
where α is the fertilization success of males, σ is a measure of sex-biased viability, β is the portion of eggs fertilized by hermaphrodites, and δ is inbreeding depression (the relative reduction in the fitness of self-fertilized vs. outcrossed progeny) (Stewart and Phillips 2002, cf. Eq. 2; see also Otto et al. 1993). Chasnov and Chow (2002) and Cutter and Payseur (2003) have constructed similar models that highlight essentially the same factors as being central for the determination of male frequency. Because sex-specific differences in egg to adult viability have not been observed under standard laboratory conditions (Hodgkin 1987) and survivorship from the final larval stage through the first 5 days of adulthood appears to be sex neutral (Gems and Riddle 1996, 2000), σ is assumed to be very small. Although we might expect deleterious recessive mutations to affect males more than hermaphrodites because of their hemizygous X, if selfing is very prevalent, then the X will frequently be homozygous in hermaphrodites thus mitigating the effects of zygosity on sex-specific fitness It is possible that the importance of σ may be environment specific and more prevalent in natural populations. For instance, males survive dauer (the resting stage induced by starvation and/or stress) better than hermaphrodites (Morran, Cappy, et al. 2009), but even in this case, the difference is relatively small.
Thus, if sex-specific viability differences are small, then for male frequencies to exceed the nondisjunction rate, 1) males must successfully sire 2 times more offspring than hermaphrodites and/or 2) male-sired (noninbred) progeny must have a fitness advantage over selfed progeny (Stewart and Phillips 2002; see also Chasnov and Chow 2002; Cutter and Payseur 2003). However, inbreeding depression has not been observed in this species (Johnson and Wood 1982; Johnson and Hutchinson 1993; Chasnov and Chow 2002; Dolgin et al. 2007). Repeated generations of self-fertilization appear to have purged deleterious mutations (Ohta and Cockerham 1974; Lande and Schemske 1985; Husband and Schemske 1996; Charlesworth B and Charlesworth D 1998) from C. elegans lineages, possibly rendering outcrossing, and thus males, obsolete (Chasnov and Chow 2002; Weeks et al. 2006). Indeed, loss of males from male-enriched inbred populations is well documented (Chasnov and Chow 2002; Stewart and Phillips 2002; Teotónio et al. 2006; Manoel et al. 2007; Wegewitz et al. 2008), indicating that outcrossing is detrimental to hermaphrodite fitness and thus strongly selected against in those populations.
Attempts to experimentally induce inbreeding depression by increasing the input of deleterious mutations beyond the abilities of C. elegans to purge them have met with some success. When mutant alleles that interfere with mismatch repair mechanisms (Cutter 2005) or extrinsic mutagens such as UV radiation and ethyl methanesulfonate (Manoel et al. 2007) are used as sources of mutation, males can achieve frequencies higher than their nonmutated controls. Thus, we see that inbreeding depression/mutation-based factors can influence male frequency in C. elegans (Cutter 2005; Manoel et al. 2007). It is questionable, however, whether persistent mutation pressure actually generates significant inbreeding depression. Because the mutations are generated at the same time that males are being driven from the population, most of the mutations may be being purged from the populations via residual selfing. It is known that even fairly small selfing populations of C. elegans can purge mutations in the short term (Estes et al. 2004; but see Morran, Parmenter, et al. 2009). Additionally, the gains in male frequency observed under these conditions are modest—males are still driven out of populations over time (Cutter 2005; Manoel et al. 2007)—and the increases in male frequency due to mutational input are strain specific (Manoel et al. 2007). Thus, to date, inbreeding depression avoidance and mutation-based models alone fail to satisfactorily explain why males would be maintained by selection in this species.
In general, then, males must sire at least 2 times more offspring than hermaphrodites produce by self-fertilization to overcome the cost of males and be maintained in a population. In C. elegans, male siring success must exceed this standard because hermaphrodites can produce self-fertilized offspring both before and after mating (Figure 2), biasing total reproductive output toward hermaphrodites (Otto et al. 1993). Although C. elegans males are thought to have poor reproductive success, direct estimates of male mating ability suggest that males in the N2 laboratory strain can be responsible for 50–90% of the offspring produced in mixed populations (Stewart and Phillips 2002; Cutter and Payseur 2003). Even at these rates, however, males are predicted to be lost from these populations relatively quickly (Cutter and Payseur 2003), as is the observed outcome (Stewart and Phillips 2002; Cutter 2005). Relative to males in their dioecious sister species C. remanei, C. elegans males appear ill equipped to outcross at higher frequencies. In C. remanei, males induce a paralytic response in their mating partner that is coupled with a widening of the vulval slit (Garcia et al. 2007). In contrast, C. elegans males must mate mobile hermaphrodites, unassisted by a factor that would facilitate spicule insertion, the most tenuous aspect of mating (Liu and Sternberg 1995). Additionally, it appears that C. elegans hermaphrodites are less attractive to potential mates than are C. remanei females, possibly due to differences in mating pheromone production (Chasnov and Chow 2002; Chasnov et al. 2007; but see White et al. 2007; Srinivasan et al. 2008). These challenges, coupled with the propensity for C. elegans hermaphrodites to eject male ejaculate (Barker 1994; Kleemann and Basolo 2007) and sprint away from males until after their self-sperm is depleted (Kleemann and Basolo 2007), may in part account for the inability of C. elegans males to mate effectively enough to maintain themselves at higher frequencies.
Overall, then, theory predicts that the maintenance of males in C. elegans is favored when either inbreeding depression is high or males can mate effectively enough to overcome the cost of males. However, C. elegans neither exhibits inbreeding depression nor do C. elegans males appear to mate particularly effectively. Males are maintained in some strains, and yet, nondisjunction alone is insufficient to explain this fact. The theory is therefore incompatible with the data, which suggests that some other factor must be missing from these equilibrium models.
Males—In Nature
It is no secret that the conditions generally experienced by C. elegans in the laboratory are nothing like the conditions encountered by populations in nature (Fitch 2005; Barrière and Félix 2007). The laboratory environment provides worms with an abundant nonpathogenic bacterial food source, consistent and moderate temperatures, and, assuming diligence on the part of researchers, no threat of desiccation. This is in stark contrast to the limited, spatially and temporally patchy, and potentially pathogenic food sources encountered by natural populations (Kurz and Ewbank 2000; Sifri et al. 2005) that must also contend with fluctuating temperatures (Anderson and Coleman 1982; Feder 1997) and the constant threat of desiccation. If, as theory predicts, males are important for preventing inbreeding depression or increasing rates of adaptation, they may be obsolete under the relaxed selection encountered in laboratory conditions but still be quite valuable under natural conditions.
Are males and outcrossing essential for natural populations? Perhaps, the most direct approach to answering this question is to measure male frequency in worms collected in the field (Table 2). Although this type of sampling is rare, collections by Barrière and Félix (2005, 2007) have yielded only 4 males after the isolation of thousands of individuals from 10 different locales throughout France sampled multiple times. Thus, current male frequency data from natural populations support the laboratory observation that males are rare.
Table 2.
Estimation of outcrossing rates in natural populations
| Method | Timescale | Outcrossing rate calculation | Rationale | Caveats |
| Male frequency | One generation, male frequency above the nondisjunction rate only maintained through continual outcrossing | Outcrossing = 2 × male frequency − μ, where μ is the rate of X chromosome nondisjunction (Stewart and Phillips 2002) | Males are the product of outcrossing events (Figure 2). Male frequency can be used to infer outcrossing rates because outcrossing rates dictate male frequency. | Males are directly observed. As long as they are not the product of nondisjunction, this is a direct measure of outcrossing. |
| Heterozygote frequency | Several generations, signal diminishes over time with subsequent selfing | Outcrossing = 1 − 2F/(1 − F), where F = 1 − Hobs/Hexp and H is the proportion of heterozygous individuals (Sivasundar and Hey 2005) | Outcrossing can generate heterozygosity, whereas selfing generates homozygosity (Figure 1). Barring mutation, the presence of heterozygous loci, is indicative of recent outcrossing events. | Assumes that heterozygotes are ultimately derived from outcrossing under Hardy–Weinberg conditions. Estimates could be perturbed by overdominance. |
| Linkage disequilibrium | Many generations, outcrossing breaks linkage and then subsequent selfing fixes recombinant alleles within a lineage. | Outcrossing = 1 − 2F/(1 + F), where F = (cth − cob)/cth and cth is the theoretical recombination rate and cob is calculated from correlation data between loci and Ne value (Barrière and Félix 2005). | Selfing maintains linkage, whereas recombination of alleles from a different lineage, facilitated by outcrossing, breaks linkage. | Must make assumptions about effective population size and simulate the effects of recombination under a coalescent process, assuming a constant recombination rate across the genome. |
Direct measures of male frequency from natural collections are limited in that they only reflect outcrossing or nondisjunction events that occurred in the preceding generation (Table 2). With such a limited temporal range, male frequency may by itself be a poor estimator of outcrossing rates in natural populations. However, the contrasting genetic consequences of outcrossing and selfing (Figure 1) enable the detection of outcrossing events in the genomes of individuals from predominantly selfing populations. This allows outcrossing rates to be estimated from field collections of hermaphrodites through the presence of heterozygous loci or the absence of linkage disequilibrium in a population (Table 2). These genomic approaches have the advantage of detecting past outcrossing events at different temporal scales.
Despite sampling populations from different geographic regions and utilizing different methods for estimating outcrossing rates (Table 2), all studies that have sampled natural populations agree that self-fertilization is the primary mode of reproduction in C. elegans (Barrière and Félix 2005, 2007; Haber et al. 2005; Sivasundar and Hey 2005; Rockman and Kruglyak 2009). Although each study finds evidence of outcrossing to some degree, specific estimates vary from essentially 0 to as high as 22%. For example, Haber et al. (2005) did not isolate any heterozygotes when sampling natural populations from Germany, although they caution that this may be an artifact of collection procedures. However, they did not detect significant levels of linkage disequilibrium between 2 pairs of microsatellite loci within the German populations, indicating that rare outcrossing had generated genomic mixing between lineages.
Similarly, Barrière and Félix (2005, 2007) periodically sampled a set of populations from France and estimated mean outcrossing rates of 1.3–1.7% based on heterozygosity data. However, estimates based on linkage disequilibrium are approximately 100-fold lower in these populations. Cutter (2006) and Rockman and Kruglyak (2009) also find pervasive linkage disequilibrium in the worldwide collection of natural isolates. Taken together, these studies suggest that outcrossing occurs rarely in European populations but at frequencies that are still higher than those observed in the standard laboratory strain. In contrast, samples from several populations in California (Sivasundar and Hey 2005) suggest that natural outcrossing rates can be much higher than those estimated in Europe. Using heterozygote frequencies, Sivasundar and Hey (2005) estimated an average outcrossing rate of 22%, with some populations containing substantial numbers of heterozygotes, whereas others were completely homozygous. However, much like the French populations, measurements of linkage disequilibrium in the Californian populations yielded significantly lower outcrossing rate estimates than those obtained with heterozygosity data (Sivasundar 2005). Therefore, in general, outcrossing rates seem to vary widely across populations, but the overall genomic impact of outcrossing appears to be minimal at large timescales.
What might be the source of the large difference in estimates based on heterozygosity and linkage disequilibrium in natural populations? Barrière and Félix (2007) suggest that this result is consistent with a pattern of strong selection against recombinant offspring. Under this hypothesis, outcrossing is regularly occurring, but recombinant offspring are selected out of the population and therefore fail to make the lasting genetic contribution expected to be revealed in the breakdown of linkage disequilibrium (Barrière and Félix 2007). This interpretation is supported by the observation that outbreeding depression, the fitness of outcrossed offspring being lower than that of inbred progeny, is widespread among natural isolates. Dolgin et al. (2007) found that outcrossing between 5 different mating pairs of unrelated C. elegans strains altered several life-history traits, effectively reducing the fitness of outcrossed offspring relative to selfed offspring. Similarly, Seidel et al. (2008) identified a widely distributed 2-locus gene interaction system that is lethal to offspring generated by a cross between incompatible strains.
These results suggest that outcrossing may actually be selected against within inbred populations, which in turn lead us to expect that males would be consistently driven out of natural populations. However, these observations seem at odds with the outcrossing rate estimates from natural populations (Barrière and Félix 2005, 2007; Haber et al. 2005), particularly the Californian populations (Sivasundar and Hey 2005), and with the evidence suggesting that males are maintained in C. elegans populations by selection. Rather, the outcrossing rate estimates obtained from both the French (Barrière and Félix 2005, 2007) and Californian (Sivasundar and Hey 2005) populations indicate that outcrossing occurs more often than what would be expected if males were only maintained at X chromosome nondisjunction frequencies. Thus, outcrossing is occurring in nature, but its evolutionary role is unclear.
Males—In the Laboratory
The lens through which C. elegans is generally viewed is strongly shaped by observations and experiments in the canonical laboratory strain N2. However, it is no longer clear that extrapolation from N2 to C. elegans as a species is universally appropriate because N2 appears to have undergone laboratory adaptation leading to genetic and behavioral changes (e.g., Anderson et al. 2007; McGrath et al. 2009). In terms of outcrossing rates and male frequency, this N2 bias has led to the broad acceptance of male frequency in C. elegans laboratory populations as ∼0.1% and the idea that C. elegans males are ineffective at outcrossing (Chasnov and Chow 2002). However, male maintenance and male mating ability in N2 are not representative of C. elegans as a whole. In fact, it has been known for more than a decade that N2 males do not mate as effectively as males of other strains (Hodgkin and Doniach 1997; Wegewitz et al. 2008). Furthermore, there is substantial variation among natural isolates for outcrossing and male mating–related phenotypes including male sperm size (LaMunyon and Ward 2002), the rate of male loss from enriched populations, male frequency, mating ability (Teotónio et al. 2006; Wegewitz et al. 2008), rates of male production by nondisjunction (Teotónio et al. 2006), and copulatory plug formation (Hodgkin and Doniach 1997; Palopoli et al. 2008).
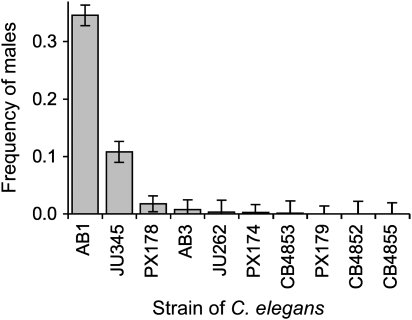
In our own survey of 10 natural isolates from France, United Kingdom, Australia, and the United States (reported for the first time here), we find that populations initiated by single, unmated hermaphrodites can achieve male frequencies as high as 35% after only 10 generations (Figure 3). Importantly, these differences in male frequency are both heritable and substantial, spanning approximately 2 orders of magnitude. Whereas some strains maintain males at frequencies similar to N2 (Figure 3), others show surprisingly high male frequencies and even exceed levels predicted by theory for male maintenance in this species (Stewart and Phillips 2002). These data probably reveal only a fraction of the variation that exists in nature. Therefore, the genetic context under which we evaluate the role of males may greatly influence our results. For instance, such variation in male maintenance may explain the disparity in outcrossing rate estimates between the Californian and European populations. Clearly, the role of males in C. elegans must be evaluated in the context of a broader sample of natural isolates.
Figure 3.
Variation in male frequency among natural isolates of Caenorhabditis elegans. A survey of male frequency in 10 natural isolates of C. elegans reveals variation among strains that spans nearly 2 orders of magnitude (F9,54 = 43.6, P < 0.0001). Strains were allowed to recover from freezing for 2 generations and then maintained for 10 generations under conditions similar to Manoel et al. (2007) before male frequency was determined. Male frequency was determined for 6 replicate populations per line (each initiated by single, unmated hermaphrodites) by sexing a total of ∼400 worms per replicate. Although differences among replicates were also significant (F47,54 = 8.2, P < 0.0001), they accounted for only 10.2% of the total variance, whereas strain accounted for 87.1%. Values are least square means ± 1 standard error of the mean.
Regardless of the factors that dictate male frequency, if selection maintains males, then outcrossing must be at least conditionally favored over selfing. Outcrossing is thought to both reduce the risk of fixing deleterious mutations (Heller and Maynard Smith 1979; Lande and Schemske 1985; Charlesworth et al. 1993; Lynch et al. 1995; Schultz and Lynch 1997) and facilitate more rapid adaptation to novel environmental conditions (Stebbins 1957; Maynard Smith 1978; Crow 1992) compared with selfing. Using experimental evolution, Morran, Parmenter, et al. (2009) directly tested the fitness effects of these selective pressures on outcrossing and selfing populations of C. elegans. They utilized the genetic tools of the C. elegans system to alter the mating system of populations (Table 1), incorporating the xol-1 mutation to generate obligate selfing populations and the fog-2 mutation to generate obligate outcrossing populations while also using wild-type mating populations capable of both outcrossing and selfing. Obligate outcrossing populations maintained fitness despite exposure to elevated mutation rates in a selective environment, whereas obligate selfing populations were unable to purge their mutation load and suffered significant fitness loss over time. Additionally, obligate outcrossing populations rapidly adapted to a virulent pathogen, but obligate selfing populations failed to exhibit an adaptive response. Though less fit than the obligate outcrossing populations, wild-type mating populations generally exhibited greater fitness than the obligate selfing populations. Moreover, exposure to these conditions led to the evolution of greater outcrossing rates in the wild-type mating populations, which consequently elevated male frequency over time. Therefore, as predicted, outcrossing confers fitness benefits under specific conditions, even in populations capable of both selfing and outcrossing.
In addition to being conditionally advantageous in C. elegans, outcrossing may also be essential for maintaining fitness in some C. elegans strains. When Morran, Parmenter, et al. (2009) imposed obligate selfing on a strain that naturally maintains males at frequencies up to 25%, populations gradually lost fitness over time even under benign conditions. Therefore, both environmental conditions and genetic background may provide the context needed to favor outcrossing over selfing.
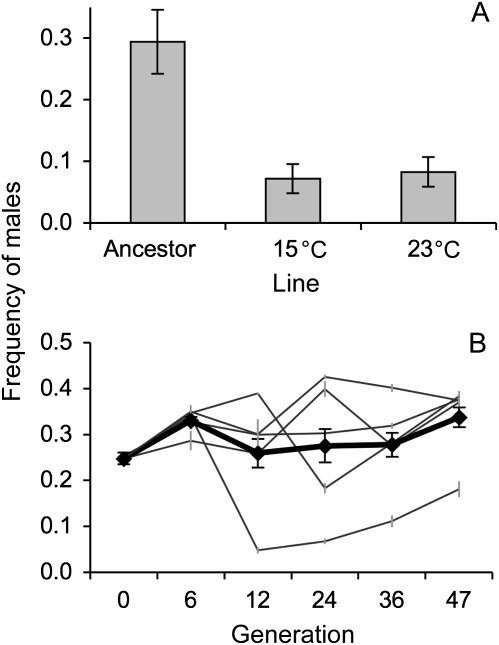
Emerging results from other experimental evolution studies also support this general picture. For example, treatment of genetically heterogeneous populations of C. elegans with the pesticide Levamisole initially renders C. elegans males physically incapable of mating, and thus, males are rapidly driven out of populations (Lopes et al. 2008). However, males significantly increase in frequency in populations that became resistant to the pesticide, suggesting that outcrossing is favored during adaptation to the novel environment. Similarly, we have observed the long-term maintenance of males in 2 ongoing experimental evolution studies, which we report for the first time here. First, in a study designed to test the evolution of temperature-dependent fitness, a hybrid strain reared at either 15 °C or 23 °C maintained males at relatively high frequency (∼8%) after more than 55 generations of adaptation (Figure 4A). Second, genetically heterogeneous populations undergoing laboratory adaptation in conditions not previously shown to favor male maintenance (Manoel et al. 2007) maintained males at average frequencies ranging from 26% to 34% over 47 generations of experimental evolution (Figure 4B). Similar maintenance of males at high frequencies in genetically heterogeneous populations has also been observed in other experimental evolution studies (Lopes et al. 2008; Teotónio H, personal communication).
Figure 4.
Evidence of male maintenance in 2 experimental evolution studies. (A) A hybrid population derived by 10 generations of random mating between 2 divergent Caenorhabditis elegans strains, CB4856 and CB4857, at 18.5 °C (the ancestral population) was then reared in 10 replicates at 15 °C or 23 °C for 57 and 69 generations, respectively, before freezing. All 3 lines were thawed simultaneously, allowed to recover at the correct temperature, and scored for male frequency (based on a minimum of 200 worms per replicate). Although male frequency declines from near 30% in the ancestor (likely a by-product of the crosses performed to generate the ancestor), males are maintained at high frequency (∼8%) at both temperatures after more than 57 generations. (B) A genetically heterogeneous ancestral population was derived by systematically crossing 16 strains of C. elegans (AB1, AB3, CB4852, CB4853, CB4855, CB4857, CB4858, N2, PB303, PB306, RC30, JU262, JU345, PX174, PX178, and PX179). Five replicate populations of the ancestral population were evolved independently for 47 generations under conditions similar to Manoel et al. (2007). Male frequency was measured by synchronously thawing stocks of each replicate population from generations 6,12, 24, 36, 47, and the ancestral population. Worms were allowed to recover from freezing for 2 generations and then hatched-off, plated in triplicate, and allowed to develop to adulthood. The frequency of males in each population was determined from a minimum of 700 individuals per line per generation. Generation (F4,50 = 30.04, P = < 0.0001), line (F4,50 = 174.33, P < 0.001), and the interaction between generation and line (F16,50 = 27.22, P < 0.0001) were all significant. The 5 replicate populations are in gray, and average male frequency is in black. Values are least square means ± 1 standard error of the mean.
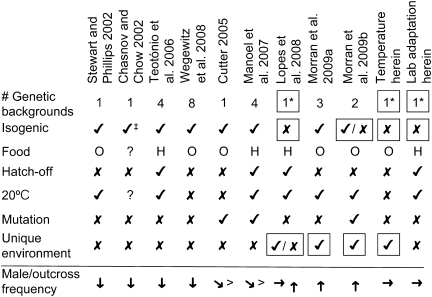
A survey of the experimental work on male maintenance in C. elegans reveals a pattern that correlates with the long-term success of males. Populations that exhibit increases in male frequency, or simply maintain a significant number of males over time, share at least 1 of 2 characteristics: genetic diversity and exposure to novel environmental conditions (Figure 5). Genetically heterogeneous populations can maintain males at levels higher than most isogenic strains (Figure 5). Additionally, populations under selection in novel environments exhibit increases in male frequency. The importance of novel environmental conditions is highlighted by the contrasting results of Cutter (2005) and Manoel et al. (2007) with those of Morran, Parmenter, et al. (2009). All 3 studies evaluate male frequencies over time in populations exposed to elevated mutation rates. The experiments by Cutter (2005) and Manoel et al. (2007) were performed under previously established laboratory conditions, and males declined in frequency (Figure 5). However, the populations mutated by Morran, Parmenter, et al. (2009) were subject to strong selection in novel environments and exhibit significant increases in male frequency over time (Figure 5). Thus, selection imposed by a novel environment may be necessary to fully offset the cost of males, even under elevated mutation rates.
Figure 5.
Summary of male maintenance experiments in Caenorhabditis elegans. Males/outcrossing can be maintained (horizontal arrow) or increase (up arrow) in frequency in experiments initiated with a heterogeneous populations or when isogenic lines are exposed to unique selective laboratory environments. When mutation rates are artificially increased, populations can have more males (>) than nonmutated controls; however, males are still lost from populations over time (diagonal down arrow). Males are otherwise lost (down arrow) from isogenic populations regardless of bacterial food type (Escherichia coli OP50 or HT115), maintenance regime (i.e., hatch-off), and temperature. Factors uniquely observed in concert with maintenance or increased male/outcrossing frequency are highlighted with boxes. Relative to factors listed at left, ✓, applicable; χ, not applicable; *, Starting strain derived by crossing up to 16 unique isogenic lines; ‡, Included wild-type and him-5 alleles in the N2 background; ?, unspecified.
Conclusions and Future Directions
Given genetic heterogeneity or selection pressure in a novel environment, male frequencies in C. elegans populations can be elevated to as high as 50%, which is indicative of a fully outcrossing population (Figure 2; Morran, Parmenter, et al. 2009). Populations appear to be outcrossing at a variety of rates in nature, but overall, the rates estimated from natural populations are considerably lower than those observed under selection in the laboratory. It would seem that natural populations, which should be under strong selection, could benefit from elevated outcrossing rates. Yet, males have proved difficult to find in natural populations (Barrière and Félix 2005, 2007), and genetic signatures of the mating system imply selection against outcrossing (Dolgin et al. 2007; Seidel et al. 2008).
If the value of outcrossing is conditional, then the role of males in C. elegans may also be conditional. Rather than continually paying the cost of producing males, it may be more beneficial to outcross facultatively as conditions dictate. Morran, Cappy, et al. (2009) found that in response to exposure to the dauer lifestage, an environmentally induced, stress resistant, and migratory developmental phase, C. elegans populations outcrossed much more readily than populations maintained under standard laboratory conditions. Exposure to dauer permitted males to sweep into populations, despite initially low male frequencies (Figure 5). The dauer phase is suspected to play a prominent role in natural populations because a large proportion of the worms isolated from nature are in dauer (Barrière and Félix 2005, 2007) and dauer induction is a signal of current or impending environmental change. Therefore, facultative outcrossing driven by dauer exposure may be selectively beneficial in natural populations. The intermittent nature of dauer and thus dauer-induced facultative outcrossing would likely cause outcrossing rates to fluctuate over time. This scenario may contribute to the variation observed among outcrossing rate estimates from natural populations (Barrière and Félix 2005, 2007; Haber et al. 2005; Sivasundar and Hey 2005). The population genetic effects of periodic outcrossing and their influence on patterns of molecular variation require further examination. To accurately assess the role of outcrossing and the potential role of facultative outcrossing in natural populations, we must sample more thoroughly and across different timescales. The most recent study by Barrière and Félix (2007) sampled multiple populations at several time points. Conducting studies of this kind over a broad geographical range and on finer timescales may provide key insights into the role of males in natural populations.
The contrast between standing levels of heterozygosity and strong, whole-genome linkage disequilibrium within natural populations of C. elegans (Barrière and Félix 2005, 2007; Sivasundar 2005; Sivasundar and Hey 2005; Cutter 2006; Rockman and Kruglyak 2009) suggests that what appear to be “selfing” lineages may in fact be maintained by natural selection (Barrière and Félix 2007). This hypothesis is strongly supported by the existence of outbreeding depression among most natural isolates (Dolgin et al. 2007). Continuous selfing generates strong linkage disequilibrium across the genome, which in turn allows epistatic gene combinations to always be found on a consistent genetic background. Breaking up these combinations via outcrossing is one potential source of outbreeding depression. Such an interaction system has been discovered, for instance, by Seidel et al. (2008). Similar outbreeding depression effects have also been observed among selfing lineages of the nematode Pristioncus pacificus, which appears to have weakly performing males (Click et al. 2009). The influences of linkage, epistasis, and outbreeding depression need to be more fully incorporated into models of C. elegans mating systems (e.g., Equation 1).
Many of the recent experimental evolution studies that report the maintenance of high, sustained frequencies of males were generated from base populations that were initiated by systematically crossing many existing natural isolates. Thus, the forced crossing scheme used in these studies is likely to overcome any initial outbreeding depression barrier and move the populations into a much different genomic state in which previously linked genetic combinations are now mixed together. This has the potential to generate novel inbreeding depression via associative overdominance (Charlesworth D and Charlesworth B 1987) but may also simply serve to allow male-specific alleles to segregate in new beneficial genetic backgrounds.
Theory predicts that increasing levels of inbreeding should allow males to be maintained within C. elegans populations; yet, experiments that have bombarded populations with increased mutation rates have for the most part only observed modest increases in male frequency (Cutter 2005; Manoel et al. 2007), even though males can play a critical role in responding to the deleterious effects of mutations (Morran, Parmenter, et al. 2009). However, each of these experiments introduced mutations into partially selfing backgrounds that may have served to purge the mutations as soon as they entered the population. A more effective test of the inbreeding hypothesis could be achieved by investigating the capacity for selfing to invade a population with a preexisting standing level of inbreeding depression (for instance, against a fog-2 outcrossing background). Thorough analyses of crosses between natural isolates, such as those conducted by Seidel et al. (2008), are needed to explore the genetic basis of the potential for inbreeding and outbreeding depression and will help to illuminate the delicate dance between genetic effects that are enhanced by the linkage generated by repeated selfing and the mixing of lineages generated by occasional outcrossing.
Given that we observe variation in outcrossing rates and the maintenance of males among natural isolates, what are the mechanisms underlying these differences in male frequency? Theory predicts (e.g., Equation 1) that male mating ability is likely to strongly influence male frequency. To mate successfully, males must first locate a mate and then execute a complex series of actions culminating in the transfer of sperm to the hermaphrodite (see reviews by Barr and Garcia 2006; Emmons 2006). Each step in this process requires coordination of chemosensory and mechanosensory input (Liu and Sternberg 1995) and specific muscle contractions (e.g., Garcia et al. 2001) that may vary among strains. Variation among strains for relevant phenotypes such as copulatory rate (Wegewitz et al. 2008) and ability to produce copulatory plugs (Hodgkin and Doniach 1997; Palopoli et al. 2008) have already been observed. It is not obvious, however, which aspects of male mating actually underlie differences in realized male frequencies. Furthermore, it is becoming clear that male mating ability is, at least in part, determined by hermaphrodites.
In contrast to the view of C. elegans hermaphrodites as ambivalent mating partners (e.g., Emmons 2006), there is growing evidence that hermaphrodites play an active role in determining the outcome of attempts to be mated by males. It appears that hermaphrodites can exert some control over the mating process by swimming away from males when they still have self-sperm available for fertilization (Kleemann and Basolo 2007) and by ejecting male ejaculate after insemination (Barker 1994; Kleemann and Basolo 2007). Additionally, male siring success varies among hermaphrodites from different strains (Wegewitz et al. 2008; Morran, Cappy, et al. 2009; Anderson JL, Martha S, Rockman M, Kruglyak L, Phillips PC, unpublished data), indicating a genetic basis for this variation. Because C. elegans is such a powerful genetic model system, complete with a full arsenal of molecular, genetic, and genomic tools, it should be possible to identify and functionally characterize the genes underlying variation in both male and hermaphrodite-specific contributions to male frequency in this species. With this type of information, we will be able to specifically test whether or not loci influencing outcrossing frequency are under selection in C. elegans.
Identifying the genetic basis of natural variation in mating success, inbreeding and outbreeding depression and other features of the mating system will allow us to close the circle and to more effectively bring experimental hypothesis-testing approaches from the laboratory to bear on the extensive population genomic information emerging from studies of natural isolates (e.g., Rockman and Kruglyak 2009). Although we are beginning to get a few glimpses of the evolutionary role of males in this species, enough puzzles still remain to make C. elegans a powerful system for understanding the evolution of mating systems.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (5F32HD055057 to J.L.A.); National Institutes of Health (Genetics Fellowship to L.T.M.); National Science Foundation (DEB-0236180 and DEB-0641066 to P.C.P., DEB-0710386 to L.T.M. and P.C.P.); National Institutes of Health National Center for Research Resources.
Acknowledgments
We are grateful to Lori Albergotti, Tina Tague, and Sarah Martha for technical support. We thank Jessie Chiem for generating and performing the temperature adaptation study, Henrique Teotónio for helpful discussion, Julie Tolman-Thompson for developing the ancestor of the laboratory adaptation experimental evolution study, and 3 anonymous reviewers for providing valuable feedback on this work. We also thank the organizers of the conference “Evolution of Sex & Recombination: In Theory and in Practice, 2009” for their efforts. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center.
References
- Anderson JL, Albergotti L, Proulx S, Peden C, Huey RB, Phillips PC. Thermal preference of Caenorhabditis elegans: a null model and empirical tests. J Exp Biol. 2007;210:3107–3116. doi: 10.1242/jeb.007351. [DOI] [PubMed] [Google Scholar]
- Anderson RV, Coleman DC. Nematode temperature responses—a niche dimension in populations of bacterial-feeding nematodes. J Nematol. 1982;14:69–76. [PMC free article] [PubMed] [Google Scholar]
- Baldi C, Cho S, Ellis RE. Mutations in two independent pathways are sufficient to create hermaphroditic nematodes. Science. 2009;326:1002–1005. doi: 10.1126/science.1176013. [DOI] [PubMed] [Google Scholar]
- Barker DM. Copulatory plugs and paternity assurance in the nematode Caenorhabditis elegans. Anim Behav. 1994;48:147–156. [Google Scholar]
- Barr MM, Garcia LR. 2006. Male mating behavior [Internet] In: The C. elegans Research Community, editor. WormBook. doi: 10.1895/wormbook.1.78.1. [cited 2006 Jun 19]. Available from: http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière A, Félix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol. 2005;15:1176–1184. doi: 10.1016/j.cub.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Barrière A, Félix MA. Temporal dynamics and linkage disequilibrium in natural Caenorhabditis elegans populations. Genetics. 2007;176:999–1011. doi: 10.1534/genetics.106.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH. A general model for the evolution of recombination. Genet Res. 1995a;65:123–144. doi: 10.1017/s0016672300033140. [DOI] [PubMed] [Google Scholar]
- Barton NH. Linkage and the limits to natural selection. Genetics. 1995b;140:821–841. doi: 10.1093/genetics/140.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley (CA): University of California Press; 1982. [Google Scholar]
- Brenner S. Genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broverman S, Meneely P. Meiotic mutants that cause a polar decrease in recombination on the X chromosome in Caenorhabditis elegans. Genetics. 1994;136:119–127. doi: 10.1093/genetics/136.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers DL, Waller DM. Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annu Rev Ecol Syst. 1999;30:479–513. [Google Scholar]
- Chandler C, Phillips PC, Janzen F. The evolution of sex-determining mechanisms: lessons from temperature-sensitive mutations in sex determination genes in Caenorhabditis elegans. J Evol Biol. 2009;22:192–200. doi: 10.1111/j.1420-9101.2008.01639.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. Model for evolution of dioecy and gynodioecy. Am Nat. 1978;112:975–997. [Google Scholar]
- Charlesworth B, Charlesworth D. Some evolutionary consequences of deleterious mutations. Genetica. 1998;101(s1):62–74. [PubMed] [Google Scholar]
- Charlesworth D. Androdioecy and the evolution of dioecy. Biol J Linn Soc. 1984;22:333–348. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst. 1987;18:237–268. [Google Scholar]
- Charlesworth D, Morgan MT, Charlesworth B. Mutation accumulation in finite outbreeding and inbreeding populations. Genet Res. 1993;61:39–56. [Google Scholar]
- Charlesworth D, Morgan T, Charlesworth B. Inbreeding depression, genetic load, and the evolution of outcrossing rates in a multilocus system with no linkage. Evolution. 1990;44:1469–1489. doi: 10.1111/j.1558-5646.1990.tb03839.x. [DOI] [PubMed] [Google Scholar]
- Chasnov JR, Chow KL. Why are there males in the hermaphroditic species Caenorhabditis elegans? Genetics. 2002;160:983–994. doi: 10.1093/genetics/160.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci USA. 2007;104:6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click A, Savaliya CH, Kienle S, Herrmann M, Pires-daSilva A. Natural variation of outcrossing in the hermaphroditic nematode Pristionchus pacificus. BMC Evol Biol. 2009;9:75. doi: 10.1186/1471-2148-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford R, Lee MH, Nayak S, Ohmachi M, Giorgini F, Schedl T. FOG-2, a novel f-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development. 2000;127:5265–5276. doi: 10.1242/dev.127.24.5265. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Parker GA. Sexual coercion in animal societies. Anim Behav. 1995;49:1345–1365. [Google Scholar]
- Crnokrak P, Barrett SCH. Perspective: purging the genetic load: a review of the experimental evidence. Evolution. 2002;56:2347–2358. doi: 10.1111/j.0014-3820.2002.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Crow JF. An advantage of sexual reproduction in a rapidly changing environment. J Hered. 1992;83:169–173. doi: 10.1093/oxfordjournals.jhered.a111187. [DOI] [PubMed] [Google Scholar]
- Crow JK, Kimura M. An introduction to population genetics theory. New York: Harper and Row; 1970. [Google Scholar]
- Cutter AD. Mutation and the experimental evolution of outcrossing in Caenorhabditis elegans. J Evol Biol. 2005;18(1):27–34. doi: 10.1111/j.1420-9101.2004.00804.x. [DOI] [PubMed] [Google Scholar]
- Cutter AD. Nucleotide polymorphism and linkage disequilibrium in wild populations of the partial selfer Caenorhabditis elegans. Genetics. 2006;172:171–184. doi: 10.1534/genetics.105.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter AD, Payseur BA. Rates of deleterious mutation and the evolution of sex in Caenorhabditis. J Evol Biol. 2003;16:812–822. doi: 10.1046/j.1420-9101.2003.00596.x. [DOI] [PubMed] [Google Scholar]
- Cutter AD, Ward S. Sexual and temporal dynamics of molecular evolution in C. elegans development. Mol Biol Evol. 2005;22:178–188. doi: 10.1093/molbev/msh267. [DOI] [PubMed] [Google Scholar]
- Cutter AD, Wasmuth JD, Washington NL. Patterns of molecular evolution in Caenorhabditis preclude ancient origins of selfing. Genetics. 2008;178:2093–2104. doi: 10.1534/genetics.107.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin ES, Charlesworth B, Baird SE, Cutter AD. Inbreeding and outbreeding depression in Caenorhabditis nematodes. Evolution. 2007;61:1339–1352. doi: 10.1111/j.1558-5646.2007.00118.x. [DOI] [PubMed] [Google Scholar]
- Emmons SW. Sexual behavior of the Caenorhabditis elegans male. Int Rev Neurobiol. 2006;69:99–123. doi: 10.1016/S0074-7742(05)69004-6. [DOI] [PubMed] [Google Scholar]
- Estes S, Phillips PC, Denver DR, Thomas WK, Lynch M. Mutation accumulation in populations of varying size: the distribution of mutational effects for fitness correlates in Caenorhabditis elegans. Genetics. 2004;166:1269–1279. doi: 10.1534/genetics.166.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME. Necrotic fruit: a novel model system for thermal ecologists. J Therm Biol. 1997;22:1–9. [Google Scholar]
- Felsenstein J. Evolutionary advantage of recombination. Genetics. 1974;78:737–756. doi: 10.1093/genetics/78.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch DHA. Evolution: an ecological context for C. elegans. Curr Biol. 2005;15:R655–R658. doi: 10.1016/j.cub.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruit flies. Nature. 1989;338:760–761. [Google Scholar]
- Garcia LR, LeBoeuf B, Koo P. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics. 2007;175:1761–1771. doi: 10.1534/genetics.106.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell. 2001;107:777–788. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- Gems D, Riddle DL. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature. 1996;379:723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- Gems D, Riddle DL. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics. 2000;154:1597–1610. doi: 10.1093/genetics/154.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag ES, Doty AV. Sex determination across evolution: connecting the dots. PLoS Biol. 2005;3:21–24. doi: 10.1371/journal.pbio.0030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Schüngel M, Putz A, Müller S, Hasert B, Schulenburg H. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Mol Biol Evol. 2005;22:160–173. doi: 10.1093/molbev/msh264. [DOI] [PubMed] [Google Scholar]
- Heller R, Maynard Smith J. Does Muller's ratchet work with selfing? Genet Res. 1979;32:289–293. [Google Scholar]
- Hill WG, Robertson A. Effect of linkage on limits to artificial selection. Genet Res. 1966;8:269–294. [PubMed] [Google Scholar]
- Hodgkin J. Male phenotypes and mating efficiency in Caenorhabditis elegans. Genetics. 1983;103:43–64. doi: 10.1093/genetics/103.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. Primary sex determination in the nematode C. elegans. Development. 1987;101:5–16. doi: 10.1242/dev.101.Supplement.5. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Exploring the envelope. Systematic alteration in the sex-determination system of the nematode Caenorhabditis elegans. Genetics. 2002;162:767–780. doi: 10.1093/genetics/162.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Barnes T. More is not better: brood size and population growth in a self-fertilizing nematode. Proc Biol Sci. 1991;246:19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977;86:275–287. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RS, Lively CM. The maintenance of sex by parasitism and mutation accumulation under epistatic fitness functions. Evolution. 1998;52:604–610. doi: 10.1111/j.1558-5646.1998.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Janzen F, Phillips PC. Exploring the evolution of environmental sex determination, especially in reptiles. J Evol Biol. 2006;19:1775–1784. doi: 10.1111/j.1420-9101.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Ryu J, Kiraly M, Duke K, Reinke V, Kim SK. Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2001;98:218–223. doi: 10.1073/pnas.011520898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Hutchinson EW. Absence of strong heterosis for life-span and other life-history traits in Caenorhabditis elegans. Genetics. 1993;134:465–474. doi: 10.1093/genetics/134.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Wood WB. Genetic analysis of life-span in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1982;79:6603–6607. doi: 10.1073/pnas.79.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katju V, LaBeau E, Lipinski K, Bergthorsson U. Sex change by gene conversion in a Caenorhabditis elegans fog-2 mutant. Genetics. 2008;180:669–672. doi: 10.1534/genetics.108.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DHA. Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci USA. 2004;101:9003–9008. doi: 10.1073/pnas.0403094101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann GA, Basolo AL. Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Anim Behav. 2007;74:1339–1347. [Google Scholar]
- Kurz CL, Ewbank JJ. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 2000;8(3):142–144. doi: 10.1016/s0966-842x(99)01691-1. [DOI] [PubMed] [Google Scholar]
- Kuwabara P, Kimble J. A predicted membrane protein, TRA-2A, directs hermaphrodite development in Caenorhabditis elegans. Development. 1995;121:2995–3004. doi: 10.1242/dev.121.9.2995. [DOI] [PubMed] [Google Scholar]
- Kuwabara P, Okkema P, Kimble J. tra-2 encodes a membrane protein and may mediate cell communication in the Caenorhabditis elegans sex determination pathway. Mol Biol Cell. 1992;3:461–473. doi: 10.1091/mbc.3.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMunyon C, Bouban O, Cutter A. Postcopulatory sexual selection reduces genetic diversity in experimental populations of Caenorhabditis elegans. J Hered. 2007;98:67–72. doi: 10.1093/jhered/esl052. [DOI] [PubMed] [Google Scholar]
- LaMunyon CW, Ward S. Evolution of larger sperm in response to experimentally increased sperm competition in Caenorhabditis elegans. Proc R Soc Lond B Biol Sci. 2002;269:1125–1128. doi: 10.1098/rspb.2002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. 1. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- L'Hernault S, Shakes D, Ward S. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics. 1988;120:435–452. doi: 10.1093/genetics/120.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault SW. Spermatogenesis. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Plainview (NY): Cold Spring Harbor Laboratory Press; 1997. pp. 271–294. [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Lively CM, Lloyd DG. The cost of biparental sex under individual selection. Am Nat. 1990;135:489–500. [Google Scholar]
- Loewe L, Cutter AD. On the potential for extinction by Muller's ratchet in Caenorhabditis elegans. BMC Evol Biol. 2008;8:125. doi: 10.1186/1471-2148-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes PC, Sucena É, Santos ME, Magalhães S. Rapid experimental evolution of pesticide resistance in C. elegans entails no costs and affects the mating system. PLoS ONE. 2008;3:e3741. doi: 10.1371/journal.pone.0003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Conery J, Burger R. Mutational meltdowns in sexual populations. Evolution. 1995;49:1067–1080. doi: 10.1111/j.1558-5646.1995.tb04434.x. [DOI] [PubMed] [Google Scholar]
- Manoel D, Carvalho S, Phillips PC, Teotónio H. Selection against males in Caenorhabditis elegans under two mutational treatments. Proc R Soc Lond B Biol Sci. 2007;274:417–424. doi: 10.1098/rspb.2006.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J. The origin and maintenance of sex. In: Williams GC, editor. Group selection. Chicago (IL): Aldine-Atherton; 1971. pp. 163–175. [Google Scholar]
- Maynard Smith J. The evolution of sex. Cambridge (UK): Cambridge University Press; 1978. [Google Scholar]
- McGrath PT, Rockman MV, Zimmer M, Jang H, Macosko EZ, Kruglyak L, Bargmann CI. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Plenefisch J, Casson L, Meyer B. xol-1: a gene that controls the male modes of both sex determination and X chromosome dosage compensation in C. elegans. Cell. 1988;55:167–183. doi: 10.1016/0092-8674(88)90019-0. [DOI] [PubMed] [Google Scholar]
- Minniti A, Sadler C, Ward S. Genetic and molecular analysis of spe-27, a gene required for spermiogenesis in Caenorhabditis elegans hermaphrodites. Genetics. 1996;143:213–223. doi: 10.1093/genetics/143.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran LT, Cappy BJ, Anderson JL, Phillips PC. Sexual partners for the stressed: facultative outcrossing in the self-fertilizing nematode Caenorhabditis elegans. Evolution. 2009a;63:1473–1482. doi: 10.1111/j.1558-5646.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morran LT, Parmenter MD, Phillips PC, Forthcoming Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature. 2009b;462:350–352. doi: 10.1038/nature08496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad P, Ward S. Spermiogenesis initiation in Caenorhabditis elegans involves a casein kinase 1 encoded by the spe-6 gene. Genetics. 2002;161:143–155. doi: 10.1093/genetics/161.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. The relation of recombination to mutational advance. Mutat Res. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Nayak S, Goree J, Schedl T. fog-2 and the evolution of self-fertile hermaphroditism in Caenorhabditis. PLoS Biol. 2005;3:57–71. doi: 10.1371/journal.pbio.0030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg M. Structured coalescent processes on different time scales. Genetics. 1997;146:1501–1514. doi: 10.1093/genetics/146.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Cockerham CC. Detrimental genes with partial selfing and effects on a neutral locus. Genet Res. 1974;23:191–200. doi: 10.1017/s0016672300014816. [DOI] [PubMed] [Google Scholar]
- Otto SP, Barton NH. The evolution of recombination: removing the limits to natural selection. Genetics. 1997;147:879–906. doi: 10.1093/genetics/147.2.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Sassaman C, Feldman MW. Evolution of sex determination in the Conchostracan shrimp Eulimnadia texana. Am Nat. 1993;141:329–337. doi: 10.1086/285476. [DOI] [PubMed] [Google Scholar]
- Palopoli MF, Rockman MV, TinMaung A, Ramsay C, Curwen S, Aduna A, Laurita J, Kruglyak L. Molecular basis of the copulatory plug polymorphism in Caenorhabditis elegans. Nature. 2008;454:1019–1022. doi: 10.1038/nature07171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell J. The maintenance of gynodioecy and androdioecy in a metapopulation. Evolution. 1997;51:10–20. doi: 10.1111/j.1558-5646.1997.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Pannell JR. The evolution and maintenance of androdioecy. Annu Rev Ecol Syst. 2002;33:397–425. [Google Scholar]
- Rhind N, Miller L, Kopczynski J, Meyer B. xol-1 acts as an early switch in the C. elegans male/hermaphrodite decision. Cell. 1995;80:71–82. doi: 10.1016/0092-8674(95)90452-2. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Kruglyak L. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000419. doi: 10.1371/journal.pgen.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Baillie DL. Effect of temperature and parental age on recombination and nondisjunction in Caenorhabditis elegans. Genetics. 1979;92:409–418. doi: 10.1093/genetics/92.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T. Developmental genetics of the germ line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Plainview (NY): Cold Spring Harbor Laboratory Press; 1997. pp. 241–269. [PubMed] [Google Scholar]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz ST, Lynch M. Mutation and extinction: the role of variable mutational effects, synergistic epistasis, beneficial mutations, and degree of outcrossing. Evolution. 1997;51:1363–1371. doi: 10.1111/j.1558-5646.1997.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Seidel HS, Rockman MV, Kruglyak L. Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science. 2008;319:589–594. doi: 10.1126/science.1151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri CD, Begun J, Ausubel FM. The worm has turned—microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 2005;13(3):119–127. doi: 10.1016/j.tim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Sivasundar A. Genetic variation, population structure and outcrossing in Caenorhabditis elegans. [dissertation] [Piscataway (NJ)]: Rutgers University; 2005. p. 98. [Google Scholar]
- Sivasundar A, Hey J. Sampling from natural populations with RNAi reveals high outcrossing and population structure in Caenorhabditis elegans. Curr Biol. 2005;15:1598–1602. doi: 10.1016/j.cub.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Sokol S, Kuwabara P. Proteolysis in Caenorhabditis elegans sex determination: cleavage of TRA-2A by TRA-3. Genes Dev. 2000;14:901–906. [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in. Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Self-fertilization and population variation in higher plants. Am Nat. 1957;91:337–354. [Google Scholar]
- Stewart AD, Phillips PC. Selection and maintenance of androdioecy in Caenorhabditis elegans. Genetics. 2002;160:975–982. doi: 10.1093/genetics/160.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotónio H, Manoel D, Phillips PC. Genetic variation for outcrossing among Caenorhabditis elegans isolates. Evolution. 2006;60:1300–1305. [PubMed] [Google Scholar]
- Uyenoyama MK. On the evolution of parthenogenesis—a genetic representation of the cost of meiosis. Evolution. 1984;38:87–102. doi: 10.1111/j.1558-5646.1984.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Varkey J, Muhlrad P, Minniti A, Do B, Ward S. The Caenorhabditis elegans spe-26 gene is necessary to form spermatids and encodes a protein similar to the actin-associated proteins kelch and scruin. Genes Dev. 1995;9:1074–1086. doi: 10.1101/gad.9.9.1074. [DOI] [PubMed] [Google Scholar]
- Weeks SC, Benvenuto C, Reed SK. When males and hermaphrodites coexist: a review of androdioecy in animals. Integr Comp Biol. 2006;46:449–464. doi: 10.1093/icb/icj048. [DOI] [PubMed] [Google Scholar]
- Weeks SC, Crosser BR, Bennett R, Gray M, Zucker N. Maintenance of androdioecy in the freshwater shrimp, Eulimnadia texana: estimates of inbreeding depression in two populations. Evolution. 2000;54:878–887. doi: 10.1111/j.0014-3820.2000.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Wegewitz V, Schulenburg H, Streit A. Experimental insight into the proximate causes of male persistence variation among two strains of the androdioecious Caenorhabditis elegans (Nematoda) BMC Ecol. 2008;8:12. doi: 10.1186/1472-6785-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JQ, Nicholas TJ, Gritton J, Tuong L, Davidson ER, Jorgensen EM. The sensory circuitry for sexual attraction in C. elegans males. Curr Biol. 2007;17:1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Wolff JR, Zarkower D. Somatic sexual differentiation in Caenorhabditis elegans. Curr Top Dev Biol. 2008;83:1–39. doi: 10.1016/S0070-2153(08)00401-8. [DOI] [PubMed] [Google Scholar]