Abstract
Background:
The role of decompressing the intradural space through a durotomy as a treatment option for acute traumatic cervical spinal cord injury has not been explored in an animal model, to our knowledge. We sought to determine the role of durotomy and duraplasty in the treatment of acute cervical spinal cord injury and its effects on inflammation, scar formation, and functional recovery.
Methods:
Seventy-two adult female Sprague-Dawley rats were assigned to three groups: contusion injury alone, contusion injury with a decompressive durotomy, and contusion injury with a decompressive durotomy followed by placement of a dural allograft. A mild (200-kdyn [2-N]) contusive injury was delivered to the exposed spinal cord at C5. The injured segment was reexposed four hours after injury, and a durotomy with decompression was performed. When a dural allograft was used it was affixed to the surrounding intact dura with use of a fibrin sealant. The Grip Strength Meter was used to assess forelimb function. Animals were killed at two and four weeks, and immunohistochemical analysis was performed to assess scar formation, inflammatory cell infiltration, and lesional volume.
Results:
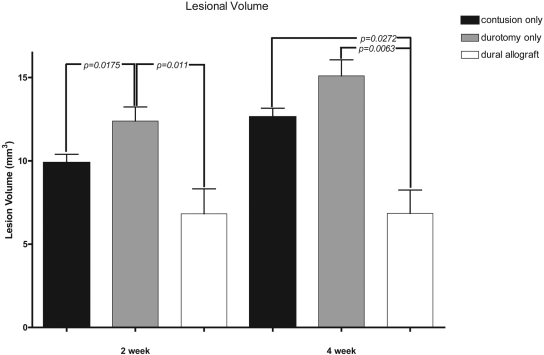
Immunohistochemical analysis revealed increased scar formation, cavitation, and inflammatory response in the animals treated only with a decompressive durotomy. Relative to the group with a contusion injury alone, the animals treated with a durotomy followed by a dural allograft had decreased cavitation and scar formation. Lesional volume measurements showed a significantly increased cavitation size at four weeks in both the contusion-only (mean and standard deviation, 12.6 ± 0.5 mm3) and durotomy-only (15.1 ± 1 mm3) groups relative to the animals that had received a dural allograft following durotomy (6.8 ± 1.4 mm3).
Conclusions:
Functional recovery after acute cervical spinal cord injury was better in animals treated with decompression of the intradural space and placement of a dural allograft than it was in animals treated with decompression alone. These functional data correlated directly with histological evidence of a decrease in spinal cord cavitation, inflammation, and scar formation.
Clinical Relevance:
Surgical decompression of the intradural space followed by dural allografting after an acute traumatic cervical spinal cord injury may be an important approach to reducing the deficits resulting from the secondary injury and warrants further investigation.
Although there have been multiple basic-science and clinical advances in the management of spinal cord injury, there remains no single efficacious therapeutic regimen to prevent the devastating paralysis associated with this injury. Over the past twenty years, the survival rate and long-term outcomes of patients with spinal cord injury have improved with advances in both their medical and surgical management. Decompression of the spinal cord and maintenance of adequate vascular perfusion to ensure physiologic spinal cord blood flow remain two very important strategies that are believed to have a substantial benefit in terms of neurological outcome1,2.
While decompression of the extradural elements is the primary focus in the management of patients with a clinical spinal cord injury, little attention has been given to the potential deleterious secondary events that occur in the spinal cord as a result of an intact dura in the face of primary contusive trauma3-6. Following contusive spinal cord injury initiated by displaced osseous and soft-tissue elements, the ensuing edema and hemorrhage within the spinal cord and nerve roots may result in an expanding volume and increased intradural pressures against a relatively noncompliant dura7. It has been proposed that increased swelling alters normal cerebrospinal fluid pressure gradients and thereby promotes extravasation of fluid into the extracellular parenchyma of the spinal cord. The end result is a decreased spinal cord perfusion pressure and ischemia. This ischemia may cause further secondary injury.
Surgical decompression of the dura in patients with acute spinal cord injury has been explored clinically in the past with impressive but guarded results8. To our knowledge, no animal studies have been performed to examine the histological and functional response to decompression of the intradural elements following contusion injury. We are aware of no standard treatment algorithm for decompression of the subarachnoid space following spinal cord injury. We sought to determine the role of surgical decompression with either durotomy alone or durotomy and duraplasty in the treatment of acute cervical spinal cord injury and to evaluate its effects on inflammation, scar formation, and functional recovery.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (Charles River Laboratories, Wilmington, Massachusetts), weighing 220 to 240 g, were used for all surgical procedures involving the production of a spinal cord injury. The institutional review board of the University of California, Irvine, approved all surgical protocols. Seventy-two rats were randomly assigned to three groups of twenty-four rats each: (1) contusion injury alone, (2) contusion injury with decompressive durotomy alone, and (3) contusion injury with decompressive durotomy followed by placement of a dural allograft. For the surgery, the animals were anesthetized with an intraperitoneal injection of a mixture of 50 mg/kg of ketamine and 2.6 mg/kg of xylazine. Testing of the corneal reflex and whether there was withdrawal from a painful stimulus of the hindlimb was used to ensure that the anesthesia was adequate.
Creation of Spinal Cord Injury Model
A mild contusive injury was delivered to the cervical spinal cord of all of the rats. Prior to surgery, the animals were prepared and anesthetized as described above. A dorsal midline incision was made, and the paravertebral musculature was separated from the vertebra at the C4-C6 levels. A laminectomy was performed under microscope magnification at C5 with use of fine scissors and a rongeur. Once the spinal cord was exposed, the spinal column was rigidly immobilized with a stereotactic frame fixed cranially and caudally to the exposed segment. A 200-kdyn (2-N) injury was delivered to the center of the exposed spinal cord with use of a commercially available force-based impactor (Infinite Horizon Impactor; Precision Systems and Instrumentation, Lexington, Kentucky)9,10 (see Appendix). This device is equipped with a 2.5-mm stainless-steel impactor tip attached to a calibrated load cell and is designed to deliver consistent force11. Following the injury, the animals were carefully released from the stereotactic frame and a layered closure was performed with use of absorbable sutures (4-0 chromic gut).
The forty-eight animals that were to receive a decompressive durotomy were anesthetized again four hours following the initial injury, and the segment was reexposed. A durotomy with a diameter of approximately 4 mm was carried out with use of microsurgical instruments. The exposed intradural spinal cord was examined, and any residual hemorrhage and hematoma were carefully evacuated. The twenty-four animals randomized to receive only a durotomy underwent wound closure with use of the techniques described above. The animals that were to receive the dural allograft were prepared for the transplantation.
Dural Allograft Harvest and Transplantation
Adult female Sprague-Dawley rats (Charles River Laboratories), weighing 280 to 300 g, were used for the dural harvest. After they were killed, large sections of dura mater were dissected from around the spinal cord. The dura was placed in a Petri dish with careful attention to maintaining proper surface orientation prior to grafting. The cadaveric dura mater was stored in Hanks balanced salt solution at 37°C overnight prior to grafting.
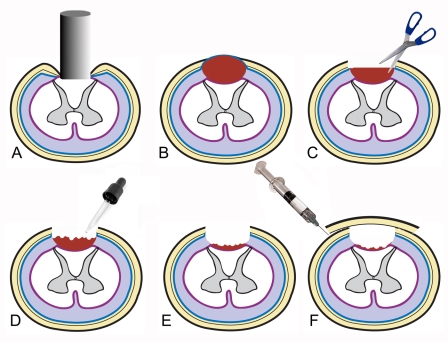
With care taken to maintain the surface orientation of the cadaveric dura, the allograft was overlaid onto the edges of the durotomy site, ensuring contact between the graft and host dura with the use of fibrin sealant (Tisseel; Baxter BioScience, Beltsville, Maryland) (Fig. 1).
Fig. 1.
Schematic representation of the surgical technique. A = spinal cord impaction, B = subdural edema and hematoma formation, C = durotomy, D = evacuation of hematoma and expansion of intradural space, E = decompressed subarachnoid space, and F = duraplasty.
Behavioral Assessment: Grip Strength Meter
The Grip Strength Meter (designed by TSE Systems [Chesterfield, Missouri] and distributed by SciPro [Sanborn, New York]) was used to assess forelimb function according to a previously described protocol12. The grip strength of each forepaw was measured with the Grip Strength Meter five times per session for a total of five sessions prior to the spinal cord injury and then every other day following the surgery until the animal was killed. The Grip Strength Meter measured the maximum force (in grams) before the animal released the bar. In order to enhance reliability, a single experimenter (J.S.S.) obtained the measurements from all of the animals.
Tissue Preparation
Animals were killed at fourteen and twenty-eight days following the spinal cord injury with pentobarbital (Nembutal; 100 mg/kg) and perfused transcardially with cold saline solution (0.9%) followed by cold 4% paraformaldehyde. The spinal cords were carefully dissected and were postfixed in 4% paraformaldehyde at 4°C overnight. The tissues were cryoprotected in sucrose gradient and stored overnight at 4°C. The spinal cords were then submerged in Tissue-Tek (VWR International, West Chester, Pennsylvania) and frozen over dry ice. A cryostat was used to cut 20-μm sections spanning beyond the visibly injured level. The sections were mounted onto polylysine-treated slides and stored at –80°C until they were ready for staining.
Immunohistochemical Analysis
Sections were incubated in monoclonal mouse anti-rat ED-1 antibody (1:300; AbD Serotec, Raleigh, North Carolina), monoclonal rat anti-mouse OX-42 antibody (1:400; Serotec), monoclonal mouse anti-glial fibrillary acidic protein (GFAP) (1:400; Sigma, St. Louis, Missouri), and monoclonal mouse anti-chondroitin sulfate proteoglycan (CSPG) (1:300; Sigma) overnight at 4°C. On the following day, the sections were reacted with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG secondary antibodies (1:200; Sigma) for one hour. Sections were then mounted with medium containing DAPI (4',6-diamidino-2-phenylindole) (Sigma), visualized under an Olympus DP7x fluorescence microscope (Center Valley, Pennsylvania), and photographed with a Digital CAMera Application Programming Interface (DCAM-API; Hamamatsu Photonics, Hamamatsu City, Japan). The primary antibodies were used to assess scar formation and inflammatory infiltration, with ED-1 staining for systemic macrophages; OX-42, for activated macrophages and microglia; GFAP, for astrocytes; and CSPG, for potent inhibitors of central nervous system regeneration.
Measurement of Lesional Volume
Tissue samples intended for lesion volume analysis were prepared with use of hematoxylin and eosin stain, and sections were stained serially. Lesion volumes were then calculated with use of ImageJ 1.41 (National Institutes of Health, Bethesda, Maryland), an image processing and analysis program13. The experimenter (R.A.) was blinded to the treatment group when analyzing the specimens. The lesion areas were outlined in three separate trials with use of a freehand technique. The lesion areas for each section were then averaged over the three trials and multiplied by the section length to obtain the lesion volume.
Statistical Analysis
Grip Strength Meter
At each time point, the average force exerted by the left and right forepaws was determined from the measurements gathered. A repeated-measures two-way analysis of variance was conducted to identify differences between groups and between time points in the postinjury period. The Bonferroni test was used for post hoc analysis to correct for multiple comparisons.
Lesional Volume
The significance of the differences in lesional volume between treatment groups was initially determined with use of one-way analysis of variance. The unpaired t test was utilized to determine the significance of differences between treatment groups at both time points. Differences were considered significant if p was <0.05. Data were analyzed and histograms were created with use of GraphPad Prism (version 4.0; GraphPad Software, La Jolla, California).
Source of Funding
Funding for this study was provided by both the NIH-NINDS (National Institutes of Health/National Institute of Neurological Disorders and Stroke) and the Roman Reed Foundation.
Results
Six of the seventy-two animals died within a day after the injury (overall survival rate, 92%). Five other animals were removed from the study secondary to forepaw injuries that they had sustained in the postinjury period (see Appendix).
Gross Examination
Gross examination of the spinal cords at the time of reexposure (four hours following injury) revealed swelling and the formation of a subdural hematoma. After the durotomy was performed, the spinal cord appeared to herniate from the underlying intradural space.
Gross examination of the spinal cord following harvest showed that the animals that had received the durotomy only had more notable scarring and cavitation than the animals that had received a durotomy followed by placement of a dural allograft (see Appendix).
Histological Analysis
Morphometric analysis revealed consistently increased cellular infiltration around the lesion site, on both ED-1 and OX-42 stained sections, in the animals that had received the durotomy without allograft transplantation (Figs. 2, 3, and 4). This finding was consistent between the specimens analyzed at the two-week time point and those evaluated at the four-week time point. The group that had received contusion only and the group that had received a durotomy followed by placement of a dural allograft also had increased cellular density in the areas surrounding the lesion, although it was not as marked as that seen in the animals treated with a durotomy alone. These differences between the groups were not verified quantitatively. Antibodies staining for glial scar formation (CSPG and GFAP) showed similar increases in astrocyte proliferation and fibrosis surrounding the cavitation and extending more diffusely throughout the perilesional areas in the animals that had received only a durotomy as compared with the other two groups. This scarring was more pronounced at the four-week time point than at the two-week time point. Slight differences in immunoreactivity for glial cell markers were observed between the contusion-alone and dural allograft groups, with slightly less scar formation observed in the latter.
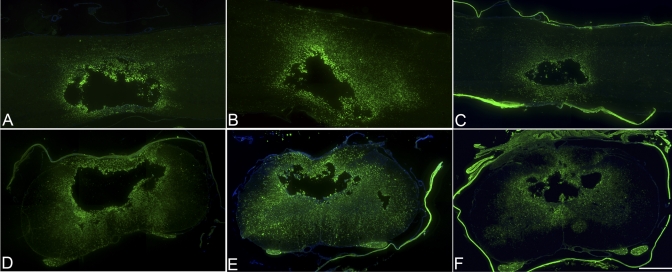
Fig. 2.
Immunohistochemical analysis with ED-1 (×4). At four weeks, the animals that had received a dural allograft following decompression (C and F) showed a decreased inflammatory response relative to that in the durotomy-only group (B and E) and that in the contusion-only group (A and D). The animals that had received a durotomy alone displayed the greatest inflammatory response. Scale bar = 500 μm.
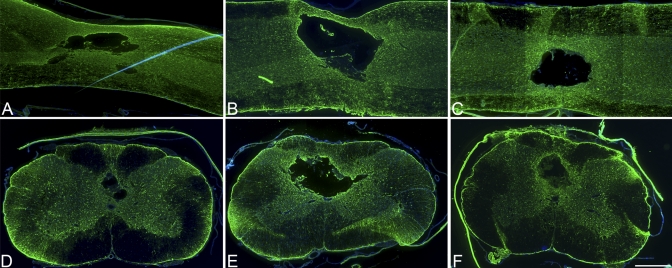
Fig. 3.
Immunohistochemical analysis with anti-glial fibrillary acidic protein (GFAP) (×4). At four weeks, the animals that had received a decompressive durotomy alone (B and E) displayed the most extensive astrocyte proliferation, indicating greater scarring relative to that in the contusion-only group (A and D) and that in the dural allograft group (C and F). Scale bar = 500 μm.
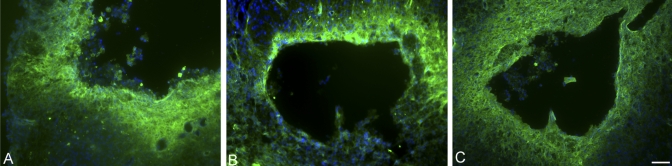
Fig. 4.
Immunohistochemical analysis with OX-42 (×10). The animals that had received a dural allograft following decompression (C) showed the least amount of perilesional infiltration of macrophages and activated microglia, as compared with the amount in the contusion-only group (A) and that in the durotomy-only group (B). Scale bar = 50 μm.
Lesional volume measurements showed a significant decrease in cavitation size at four weeks in the group that had received decompression followed by placement of a dural allograft (mean and standard deviation, 6.8 ± 1.4 mm3) relative to the sizes in the contusion-only (12.6 ± 0.5 mm3; p = 0.0272) and durotomy-only (15.1 ± 1 mm3; p = 0.0063) groups. At two weeks, there was no significant difference in cavitation size between the contusion-only (9.9 ± 0.5 mm3) and dural allograft (6.8 ± 1.5 mm3) groups (p = 0.101), but the durotomy-only group showed a significantly increased cavitation size (12.39 ± 0.8 mm3) relative to the cavitation sizes in the contusion-only (p = 0.0175) and dural allograft (p = 0.011) groups at this time point (Fig. 5).
Fig. 5.
Lesional volume measurements (shown as the mean and standard deviation) demonstrated significantly increased (p < 0.05) cavitation size at four weeks in both the contusion-only and the durotomy-only groups relative to the size in the animals that had received a dural allograft following decompression. At two weeks, the animals that had received a durotomy alone showed significantly increased (p < 0.05) cavitation size relative to that in the other treatment groups.
Functional Recovery
The Grip Strength Meter has been shown to provide sensitive, quantifiable, and reproducible measurements of forepaw strength following cervical spinal cord injury in the rat12,14,15. Pathways involved in the grip strength task and their role in recovery are currently being elucidated14.
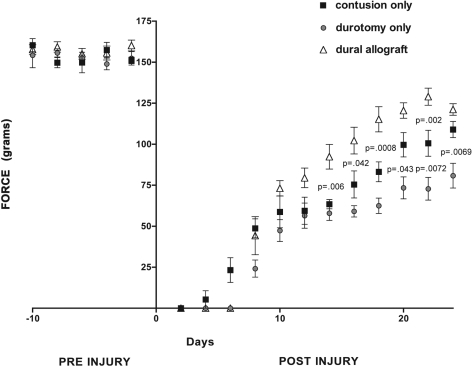
The preinjury Grip Strength Meter measurements showed no significant differences among the groups. The Grip Strength Meter measurements performed ten days following the spinal cord injury showed that the animals that had received a dural allograft had better (but not significantly better) strength than the animals that had received a contusion injury alone. The difference between these groups was significant at fourteen days (mean and standard deviation, 92.4 ± 18.4 g compared with 63.4 ± 6.9 g, p = 0.006), sixteen days (102.2 ± 19.9 g compared with 75.4 ± 20.2 g, p = 0.042), eighteen days (115.2 ± 18.8 g compared with 83.2 ± 14.9 g, p = 0.0008), and twenty-two days (129.0 ± 12.8 g compared with 100.6 ± 19.4 g, p = 0.002). Of note, the grip strength values measured at the final time point (at twenty-four days) revealed no significant difference (p = 0.0775), with an apparent downward trend in functional recovery in the dural allograft group (121.2 ± 8.6 g) relative to the contusion-only animals (108.9 ± 12 g). Although grip strength measurements were not obtained at later time points, this may indicate a possible end to a trend in functional recovery.
Animals treated with a decompressive durotomy alone (without dural allograft transplantation) consistently had the worst functional outcome after two weeks postinjury. Relative to the contusion-only group, this group showed a trend toward poorer grip strength recovery, and this trend was significant at twenty days (mean and standard deviation, 99.7 ± 18.1 g compared with 75.8 ± 16.4 g, p = 0.043), twenty-two days (100.6 ± 19.4 g compared with 77.2 ± 16.9 g, p = 0.0072), and twenty-four days (108.9 ± 12 g compared with 80.8 ± 18.4 g, p = 0.0069). No animals in any group achieved complete recovery to baseline levels (Fig. 6).
Fig. 6.
Following the injury, the animals that had received a dural allograft following decompression demonstrated significantly improved Grip Strength Meter values (shown as the mean and standard deviation) relative to those in the contusion-only and durotomy-only groups (p < 0.05). The durotomy-only group displayed a significantly worse functional outcome relative to that in the other groups (p < 0.05). No significant differences in grip strength were found among the groups in the preinjury period.
Interpreting the functional relevance of these discrepancies in forelimb motor recovery is difficult, although some general observations can be made. The animals that displayed the worst functional outcome at four weeks (the durotomy-only group [range, 57 to 83 g]) required more frequent hand feedings and bladder expressions and never regained the appearance of normally functioning forelimbs. The animals that displayed the best forelimb grip-strength recovery (the dural allograft recipients [range, 124 to 144 g]) displayed nearly baseline forelimb locomotor activity and feeding habits.
Discussion
Many strategies to reduce secondary injury in the setting of acute spinal cord injury have been proposed with an attempt to address the many involved pathways. However, there have been few strategies to surgically address the intradural pathoanatomical changes that contribute to the secondary injury process, including edema, ischemia, and alterations in the cerebrospinal fluid flow. Decompression of the osseous and soft-tissue elements (extradural) in the setting of acute spinal cord injury has long been the focus of early surgical intervention. Whether it is preferable to perform acute or delayed surgical decompression remains a topic of intense controversy, as a result in part of equivocal outcomes in both treatment groups2,16. It is possible that “traditional” methods of decompression are not addressing intradural compressive changes that contribute to secondary spinal cord injury. Our data suggest that duraplasty following a mild contusion injury results in improved functional recovery and limits secondary inflammatory infiltration, glial scar formation, and cystic cavitation. We are not aware of any previous animal study of the role of decompression of the spinal cord by means of a durotomy and duraplasty.
Perkins and Deane performed a durotomy decompression in six patients with an acute spinal cord injury (Frankel grades A and B)8. They described a distended and pulseless thecal sac that, following the durotomy, displayed evidence of vascular decongestion and reperfusion. All patients showed neurological improvement, and three had complete recovery.
Edema is a well-recognized cause of secondary spinal cord injury in animals and humans17-23. It has been suggested that, following spinal cord injury, the swollen spinal cord compresses the dural elements, resulting in increased intraparenchymal cord pressure23. This increase in pressure can have a tamponade effect on the spinal vasculature, initially limiting venous outflow from the spinal cord and ultimately exacerbating an already hostile ischemic environment. It is probable that this environment existed in our model. In the animals that received a decompressive durotomy, the spinal cord appeared to herniate partially through the durotomy site following the decompression, indicating substantial parenchymal edema.
Alterations in cerebrospinal fluid flow and pressure hydrodynamics and their contribution to ischemia and parenchymal edema in the face of acute spinal cord injury are of great interest. Following spinal cord injury, the resulting spinal cord swelling and any sustained external pressure may block normal cerebrospinal fluid flow24. The resulting rise in intradural pressure may further perpetuate spinal cord edema because of altered pressure gradients and limitations in regulating fluid balance secondary to altered vascular flow. Decreasing intrathecal pressures by cerebrospinal fluid drainage in the setting of acute spinal cord injury has been explored. Animal studies have shown potential tissue protective qualities following acute spinal cord injury although the effects on spinal cord perfusion and functional recovery are yet to be clearly defined25. Recently, a prospective randomized clinical trial was conducted to evaluate the role of cerebrospinal fluid drainage during the acute phase of spinal cord injury26. Our model of decompression encompasses these principles of reducing intrathecal pressures and restoring normal gradients across the spinal cord in order to improve spinal cord perfusion and limit secondary ischemia.
The role of maintaining the integrity of the dura in patients with spinal cord injury has been explored previously. Iannotti et al. studied the histological response to a laceration-type spinal cord injury in a rodent model27. They reported that, relative to animals left with a large dural defect, animals that had received a dural allograft had decreased lesional macrophage accumulation, cystic cavitation, and scarring as well as improved cerebrospinal fluid flow. They hypothesized that maintaining the continuity of the dura following a laceration injury maintained a more physiologic pattern of cerebrospinal fluid flow and prevented extradural factors from inhibiting neuroregeneration and promoting inflammation. In a rodent clip-compression spinal cord injury model, Fernandez and Pallini also reported the importance of maintaining dural continuity to prevent epidural and spinal cord fibroblast proliferation and scar formation28.
There are limitations to our study design. The spinal cord injury protocol required a decompressive laminectomy prior to the production of the contusive spinal cord injury. This does not mimic a true clinical spinal cord injury scenario, in which a displaced spinal column may cause the primary injury and result in residual cord compression. In our model, fixation of the transplanted dural allograft was provided by a fibrin sealant. Its effects on the spinal cord and potential neuroprotective and anti-inflammatory properties need to be investigated further. The functional benefits observed in the animals that had received a duraplasty began at fourteen days and continued through the twenty-second day postinjury. This functional recovery appeared to trend downward as the study period ended. Further investigation is required to determine if these effects are sustained beyond the four-week time point. Although we believed that twenty-four animals allocated to each study group would be adequate to achieve meaningful data, this assumption was based on previous small-animal spinal cord injury models; no formal power analysis was performed.
Our data demonstrate that acute decompression of the spinal cord combined with a duraplasty may limit the degree of secondary injury in a small-animal model. Both functional and histological evidence of neuroprotection were evident in the animals in which dural decompression had been performed and yet the integrity of the overlying dura had been maintained. Although these results are compelling, they are only a starting point and do not elucidate the mechanism of neuroprotection. Further definition of the role of durotomy and duraplasty in patients with spinal cord injury will require a more comprehensive investigation of the secondary injury events involved with these treatment strategies.
Cervical spinal cord injury continues to result in devastating long-term disability. Regaining even modest control of upper-extremity function can have a profound effect on quality of life. Surgical decompression combined with duraplasty after an acute traumatic cervical spinal cord injury may be an important new way to attenuate secondary injury and thus warrants further investigation.
Appendix
Figures showing the surgical setup and spinal cord impactor, a flowchart of the study, and gross spinal cord specimens are available with the electronic version of this article on our web site at jbjs.org (go to the article citation and click on “Supporting Data”).
Supplementary Material
Acknowledgments
Note: The authors thank Kelli Sharp for her technical advice and expertise.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the NIH/NINDS (National Institutes of Health/National Institute of Neurological Disorders and Stroke) and the Roman Reed Foundation. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26 [DOI] [PubMed] [Google Scholar]
- 2.Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(11 Suppl):S28-36 [DOI] [PubMed] [Google Scholar]
- 3.Carlson D, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85:86-94 [PubMed] [Google Scholar]
- 4.Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042-9 [DOI] [PubMed] [Google Scholar]
- 5.Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, Herbison GJ, Northrup BE. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-13 [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld JF, Vaccaro AR, Albert TJ, Klein GR, Cotler JM. The benefits of early decompression in cervical spinal cord injury. Am J Orthop. 1998;27:23-8 [PubMed] [Google Scholar]
- 7.Maikos JT, Elias RA, Shreiber DI. Mechanical properties of dura mater from the rat brain and spinal cord. J Neurotrauma. 2008;25:38-51 [DOI] [PubMed] [Google Scholar]
- 8.Perkins PG, Deane RH. Long-term follow-up of six patients with acute spinal injury following dural decompression. Injury. 1988;19:397-401 [DOI] [PubMed] [Google Scholar]
- 9.Anderson KD, Sharp KG, Hofstadter M, Irvine KA, Murray M, Steward O. Forelimb locomotor assessment scale (FLAS): novel assessment of forelimb dysfunction after cervical spinal cord injury. Exp Neurol. 2009;220:23-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp Neurol. 2009;220:9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179-93 [DOI] [PubMed] [Google Scholar]
- 12.Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2007;206:318-31 [DOI] [PubMed] [Google Scholar]
- 13.Rasband WS. ImageJ. Image processing and analysis in Java. US National Institutes of Health. http://rsbweb.nih.gov/ij/. Accessed 2009 Nov 12.
- 14.Anderson KD, Gunawan A, Steward O. Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp Neurol. 2005;194:161-74 [DOI] [PubMed] [Google Scholar]
- 15.Anderson KD, Abdul M, Steward O. Quantitative assessment of deficits and recovery of forelimb motor function after cervical spinal cord injury in mice. Exp Neurol. 2004;190:184-91 [DOI] [PubMed] [Google Scholar]
- 16.Levi L, Wolf A, Rigamonti D, Ragheb J, Mirvis S, Robinson WL. Anterior decompression in cervical spine trauma: does the timing of surgery affect the outcome? Neurosurgery. 1991;29:216-22 [PubMed] [Google Scholar]
- 17.Boldin C, Raith J, Fankhauser F, Haunschmid C, Schwantzer G, Schweighofer F. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine (Phila Pa 1976). 2006;31:554-9 [DOI] [PubMed] [Google Scholar]
- 18.Flanders AE, Spettell CM, Friedman DP, Marino RJ, Herbison GJ. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol. 1999;20:926-34 [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii H, Yone K, Sakou T. Magnetic resonance imaging study of experimental acute spinal cord injury. Spine (Phila Pa 1976). 1993;18:2030-4 [DOI] [PubMed] [Google Scholar]
- 20.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451-64 [DOI] [PubMed] [Google Scholar]
- 21.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417-25 [DOI] [PubMed] [Google Scholar]
- 22.Miyanji F, Furlan JC, Aarabi B, Arnold PM, Fehlings MG. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome—prospective study with 100 consecutive patients. Radiology. 2007;243:820-7 [DOI] [PubMed] [Google Scholar]
- 23.Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain. 2008;131(Pt 4):1087-98 [DOI] [PubMed] [Google Scholar]
- 24.Klekamp J, Völkel K, Bartels CJ, Samii M. Disturbances of cerebrospinal fluid flow attributable to arachnoid scarring cause interstitial edema of the cat spinal cord. Neurosurgery. 2001;48:174-86 [DOI] [PubMed] [Google Scholar]
- 25.Horn TS, Yablon SA, Stokic DS. Effect of intrathecal baclofen bolus injection on temporospatial gait characteristics in patients with acquired brain injury. Arch Phys Med Rehabil. 2005;86:1127-33 [DOI] [PubMed] [Google Scholar]
- 26.Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA, Gorelik S, Slobogean GP, Umedaly H, Giffin M, Nikolakis MA, Street J, Boyd MC, Paquette S, Fisher CG, Dvorak MF. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine. 2009;10:181-93 [DOI] [PubMed] [Google Scholar]
- 27.Iannotti C, Zhang YP, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J Neurotrauma. 2006;23:853-65 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez E, Pallini R. Connective tissue scarring in experimental spinal cord lesions: significance of dural continuity and role of epidural tissues. Acta Neurochir (Wien). 1985;76:145-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.