Abstract
Background
A large proportion of vineyards are located in regions with seasonal drought (e.g. Mediterranean-type climates) where soil and atmospheric water deficits, together with high temperatures, exert large constraints on yield and quality. The increasing demand for vineyard irrigation requires an improvement in the efficiency of water use. Deficit irrigation has emerged as a potential strategy to allow crops to withstand mild water stress with little or no decreases of yield, and potentially a positive impact on fruit quality. Understanding the physiological and molecular bases of grapevine responses to mild to moderate water deficits is fundamental to optimize deficit irrigation management and identify the most suitable varieties to those conditions.
Scope
How the whole plant acclimatizes to water scarcity and how short- and long-distance chemical and hydraulic signals intervene are reviewed. Chemical compounds synthesized in drying roots are shown to act as long-distance signals inducing leaf stomatal closure and/or restricting leaf growth. This explains why some plants endure soil drying without significant changes in shoot water status. The control of plant water potential by stomatal aperture via feed-forward mechanisms is associated with ‘isohydric’ behaviour in contrast to ‘anysohydric’ behaviour in which lower plant water potentials are attained. This review discusses differences in this respect between grapevines varieties and experimental conditions. Mild water deficits also exert direct and/or indirect (via the light environment around grape clusters) effects on berry development and composition; a higher content of skin-based constituents (e.g. tannins and anthocyanins) has generally being reported. Regulation under water deficit of genes and proteins of the various metabolic pathways responsible for berry composition and therefore wine quality are reviewed.
Keywords: Vitis vinifera, varieties, stomatal conductance (gs), intrinsic water-use efficiency (WUEiAn/gs), isohydric, anisohydric, abscisic acid, berry composition
INTRODUCTION: VINEYARDS AND WATER SCARCITY
Most of the world's wine-producing regions experience seasonal drought. With an increase in aridity predicted in the near future according to global climate models (IPCC, 2007), water deficits may become a limiting factor in wine production and quality. Global warming is also affecting grapevine development, as indicated by changes in phenology and earlier harvests observed throughout the world (Jones and Davies, 2000; Webb et al., 2007), with some European regions coming closer to the thresholds of temperature and rainfall for optimum grapevine growth (Jones et al., 2005). In recent years, water deficit is also occurring in cool climate wine regions that exhibit special topography (van Leeuwen and Seguin, 2006; Zsófi et al., 2009a). The frequency of extreme events such as heat waves or heavy rains is also predicted to increase, with negative effects on yield and quality of grapes. Sudden supra-optimal temperatures under conditions of water scarcity may lead to massive leaf shedding, with a consequent source–sink imbalance and incomplete berry maturation due to insufficient available carbohydrates. These effects are unlikely to be uniform across varieties (Schultz, 2000; Jones et al., 2005). The constraints posed by climate change require adaptive management, namely irrigation to stabilize yield, maintaining or improving wine quality (Dry and Loveys, 1998; Medrano et al., 2003; Chaves et al., 2007) and other associated management techniques (e.g. soil cover) to minimize the effects of concentrated rainfall (Monteiro and Lopes, 2007; Schultz, 2007). The search for varieties adapted to growing seasons with altered length and displaying higher resilience to environmental stress is also critical to optimum berry ripening.
By contrast, the enhanced pressure on water resources increased the global perception of the need to reduce the ‘water footprint’ for irrigated crops (www.fao.org/nr/water/aquastat/data/query/index.html; Cominelli et al., 2009). An improvement in the productivity of water use is therefore required in vineyard management, with finely tuned deficit irrigation being able to fulfil that role.
To understand the physiological and molecular bases of plant responses to mild to moderate water deficits is therefore of utmost importance to modulate the appropriate balance between vegetative and reproductive development, to improve crop water use (Blum, 2009) and to control fruit quality under deficit irrigation (Chaves et al., 2007). Chemical signals are important players in plant adaptation to environmental stresses. Since the mid-1980s evidence has been provided on the signalling role of compounds synthesized in drying roots of different species (including grapevines); they have been associated with leaf stomatal closure and/or inhibition of meristematic development (Loveys, 1984; Davies and Zhang, 1991). Although root-sourced chemical signalling is widely accepted, the identity and regulation of these signals is still under debate (Holbrook et al., 2002; Schachtmann and Goodger, 2008). Nevertheless, such knowledge has enabled us to manipulate responses to soil water availability in some crops, so that changes in shoot water status are minimized and performance under moderate stress is improved (Davies et al., 2002; Chaves and Oliveira, 2004).
The timing and intensity of water deficits influence the extent of alterations occurring in berry metabolism and therefore in wine colour and flavour (namely aroma). Whether these effects are acting predominantly through berry size or the synthesis of berry compounds is also discussed here. The accumulated knowledge made possible by studies of transcriptomics and proteomics during different stages of berry development in different varieties and environmental conditions will also be highlighted.
THE RATIONALE FOR DEFICIT IRRIGATION – WHY MILD TO MODERATE WATER DEFICIT MAY BE FAVOURABLE TO GRAPE BERRY QUALITY
Grapevines are well adapted to semi-arid climate such as that of the Mediterranean, due to the large and deep root system and physiological drought avoidance mechanisms, such as an efficient stomatal control of transpiration and of xylem embolism (Lovisolo et al., 2002), and/or the ability to adjust osmotically (Rodrigues et al., 1993; Patakas and Noitsakis, 1999). However, the combined effect of drought, high air temperature and high evaporative demand during summer in these areas is known to limit grapevine yield and berry and wine quality (Escalona et al., 1999; Chaves et al., 2007; Costa et al., 2007). Dramatic reductions in plant carbon assimilation may occur due to severe decline in photosynthesis under supra-optimal leaf temperatures combined with water deficits, as well as to a partial loss of canopy leaf area (Flexas et al., 1998, 2002; Maroco et al., 2002; Chaves et al., 2003, 2007; Souza et al., 2003, 2005b; Santos et al., 2007). The use of irrigation in these environments arises as a solution to prevent excessive canopy temperature, maintain quality in wine production and, in more extreme cases, guarantee plant survival. Nevertheless, irrigation remains of considerable debate. On the one hand, small water supplements may increase yield and maintain or even improve berry quality (Matthews and Anderson, 1989; Santos et al., 2003, 2005). On the other hand, irrigation may promote excessive vegetative growth with a negative impact on berry pigments (colour) and sugar content, and therefore decrease wine quality (Bravdo et al., 1985; Dokoozlian and Kliewer, 1996). Larger canopy leaf area will also tend to increase the incidence of fungal diseases (Dry and Loveys, 1998).
Modern irrigation management is shifting from an emphasis on production per unit soil area towards maximizing water productivity (production per unit of consumed water) (Fereres and Soriano, 2007). Consideration must be given not only to the total seasonal water available in a region but also the timing when water deficits are likely to occur, in order to adjust water needs to the available resources, using a limited supply of water most effectively (Passioura, 2007). The use of deficit irrigation strategies, implying that water is supplied at levels below full crop evapotranspiration (ETc) throughout the growing season or in specific phenological stages, relies on observations in several crops subjected to moderate water deficits that yield is not significantly reduced and quality of production may even increase under such conditions. This has been the case for several fruit tree crops (see review by Fereres and Soriano, 2007) and grapevines (Dry et al., 2001; Chaves et al., 2007). In addition to the classic deficit irrigation (DI), which does not require specific technical control, two other deficit irrigation strategies – regulated deficit irrigation (RDI) and partial rootzone drying (PRD) – have been applied in recent years by finely tuning deficit irrigation in the scales of time (specific timing of the application) and space (alternating dry–wet zones), respectively. Although deficit irrigation is already applied to vast regions worldwide in a more or less uncontrolled/unsophisticated way, the scientific knowledge underlying its optimal functioning is still needed.
Under RDI plant water status is maintained within predefined limits of deficit (with respect to maximum water potential) during certain phases of the seasonal development, normally when fruit growth is least sensitive to water reductions (Kang and Zhang, 2004). The rationale underlying this practice is that optimization of numbers of fruits, fruit size and quality will be achieved by keeping grapevine vigour in balance with potential production. If water deficit is applied early in the season, the effects will be achieved mostly through a reduction of berry cell division (McCarthy et al., 2002); if water deficits are imposed at later stages, the major effect will be an inhibition of berry growth (Williams and Matthews, 1990).
In PRD, roots are exposed to alternate drying and wetting cycles. Theoretically, roots of the watered side of soil will maintain favourable plant water relations, whereas dehydration in the other side will induce chemical signalling that will reach the leaves via the transpiration stream, reducing stomatal conductance and/or growth (Davies et al., 1994; Santos et al., 2003; Kang and Zhang, 2004; Costa et al., 2007). This will bring about an increase in water-use efficiency (WUE). PRD irrigation may also have an impact on root growth, leading to increased root development in the deeper soil layers (Dry et al., 2000; Santos et al., 2007). Moreover, an increase in root hydraulic conductance, putatively resulting from aquaporin stimulation by abscisic acid (ABA), and the induction of new secondary roots was reported in fruit trees subjected to PRD (Kang and Zhang, 2004).
There are, however, contrasting results in the literature, several studies in grapevine reporting no significant differences between PRD and DI (Pudney and McCarthy, 2004; Bravdo et al., 2004; Gu et al., 2004; Baeza and Lissarrague, 2005). These apparent contradictions may be related to differences in the intensity of the chemical signalling under PRD irrigation that seems to be dictated by the type of soil, the prevalent rainfall and evaporative demand in the region, as well as the frequency of switching irrigation from one side of the rootzone to the other (Dry et al., 2001; Chaves et al., 2007). Genotypic differences in stomatal sensing of water deficits or the delivery of ABA by the root-stock may also explain different results (Antolín et al., 2006; De la Hera et al., 2007). Drought-sensitive varieties may respond better to PRD (Souza et al., 2005a). The type of soil will impact on the extent of soil water redistribution, which in turn will buffer dehydration in the dry rootzone. Bravdo (2005) suggests that hydraulic redistribution from deeper to shallower roots may prevent under field conditions the clear results obtained in potted plants subjected to PRD under split root systems (Davies et al., 2002). Dry (2005) also suggested that PRD may not be successful when soil porosity favours lateral spread of irrigation water, or when an insufficient volume of irrigation is applied for restoration of the wet side to field capacity at the time of the switch. In fact, when soil water status of the wet part of the root system is low, there is insufficient soil water in the dry part of the root system to maximize ABA export from the entire root system (Dodd et al., 2008a, b). There is also evidence that in low-vigour vineyards PRD is unable to induce better agronomical output than the conventional DI strategy, as the growth inhibition that is more pronounced in PRD than in DI will decrease source (leaves) to sink ratio below the optimum, resulting in yield losses without any improvement in berry quality (C. M. Lopes et al., unpubl. res.). Moreover, Sadras (2009) in a meta-analysis of a broad range of horticultural crops showed that in general there was no improvement in irrigation water productivity (yield per unit irrigation water applied) under PRD, as compared with DI.
PHYSIOLOGICAL RESPONSE TO MODERATE WATER DEFICITS IN GRAPEVINE
Under mild to moderate water deficits stomata closure is among the early plant responses, restricting water loss and carbon assimilation (Chaves et al., 2003). Direct effects on photosynthetic metabolism (Lawlor and Tezara, 2009) and on the expression of a multitude of genes (Chaves et al., 2009) may also be present at early stages. Under long-standing water deficits acclimatization responses do occur, including those related to growth inhibition and to osmoregulation; these are key elements for the maintenance of plant water status and therefore plant carbon assimilation under water scarcity.
In grapevine, it has been reported for several varieties and different experimental conditions (greenhouse and field; short- and long-term) that photosynthesis is quite resistant to water stress (Flexas et al., 2002; Souza et al., 2003, 2005a; Chaves et al., 2007). Under low to moderate water availabilities occurring under deficit irrigation, maintenance of the activity of Calvin Cycle enzymes and of the maximum rates of carboxylation (Vcmax) and electron transport (Jmax) has generally been observed (Souza et al., 2005a). However, when stress is intensified a decline in those parameters occurs, more markedly in Jmax (Maroco et al., 2002; Souza et al., 2005a), possibly a result of decreased ATP production. Lawlor and Tezara (2009) raised the hypothesis that reactive oxygen species produced under conditions of low CO2 and excess light might induce oxidative damage to chloroplastic ATPase.
Grapevine is prone to down-regulation of photosynthesis in the afternoon, a phenomenon that might also occur in well-watered vines mainly as a result of stomatal closure in response to high vapour pressure deficit (VPD) and high irradiance (Correia et al., 1995) and/or to decreased stem hydraulic conductance (Salleo and Lo Gullo, 1989; Vandeleur et al., 2009). Although several lines of evidence suggest that grapevines are resistant to photoinhibition (Correia et al., 1990; Chaumont et al., 1997; Flexas et al., 2001; Medrano et al., 2002; Souza et al., 2003), maximum efficiency of photosystem II (measured by the dark-adapted Fv/Fm fluorescence ratio) was shown to decline under intense drought (Quick et al., 1992).
Photosynthetic rates generally decline at lower pre-dawn water potentials than stomatal conductance, when grapevines are subjected to moderate water deficits. As a consequence, intrinsic water use efficiency (A/gs or WUEi) is usually higher in vines under deficit irrigation (mild to moderate water deficits) than under well-watered conditions. This is reflected in a lower water use and higher WUE by the crop, an important aim of deficit irrigation strategies in vineyards (Gaudillère et al., 2002; Chaves et al., 2004; Souza et al., 2005b).
When water supply declines, stomatal guard cells respond to leaf water potential and both respond to and control the supply and loss of water by the leaves (Leuning et al., 2003). Under these circumstances, intercellular CO2 partial pressure (pi) can control stomatal opening via the supply of CO2 to the chloroplast or via the demand for CO2 by photosynthesis. The decrease in gs in response to mild water stress usually leads to a linear decline in transpiration (under constant VPD) and of pi, because CO2 demand by the chloroplasts (photosynthetic capacity) remains the same (Chaves and Oliveira, 2004). Under low light intensity but high air humidity, as occurs in the morning or evening, grapevine stomata may be widely open at low photosynthetic rates, leading to low WUEi. By contrast, stomatal closure at midday, an important adaptation to high VPD in some species of xeric habitats (Maroco et al., 1997), may lead to an increase in WUEi when photosynthesis is maintained. This has been observed in grapevine (Souza et al., 2003). When analysing WUEi it is therefore important to study it throughout the day. Field studies using ‘Moscatel’, ‘Castelão’ and ‘Aragonez’ (syn. ‘Tempranillo’) showed that deficit irrigation strategies (e.g. PRD and conventional DI, both at 50 % ETc) promoted an increase in WUE, when compared with fully irrigated grapevines (100 % ETc), both in the short term (as expressed by the A/gs ratio) and the long term (estimated via δ13C) (Souza et al., 2005b). An increase in WUE and related water savings under deficit irrigation was also reported in studies carried out in different grapevine varieties and in different locations (Dry et al., 2000; Stoll et al., 2000; Loveys et al., 2004; Poni et al., 2007; Marsal et al., 2008).
GENOTYPE-DEPENDENT RESPONSES TO WATER DEFICITS IN VITIS VINIFERA
It is acknowledged that the timing and intensity of the response to soil and atmospheric water deficits, namely in what concerns stomatal control, depends greatly on genotype. This has profound implications in irrigation management, in particular the timing and amount of irrigation to optimize source–sink relationships, in order to achieve optimal fruit quality in each variety (Medrano et al., 2003; Chaves et al., 2007; Poni et al., 2007). Vitis vinifera L. is characterized by large genetic variability with several thousand varieties/varieties being cultivated worldwide (Alleweldt et al., 1990; Galet, 2000; Schultz, 2003). European countries like France, Spain or Portugal host a large number of native V. vinifera varieties. However, most of those genotypes remain uncharacterized, which limits their use for breeding, for example to increase WUE or improve berry quality traits.
Genotype-related differences in WUE and water stress resistance may arise from constitutive differences in leaf gas-exchange, the plant's capacity to osmoregulate and plant hydraulics. Photosynthesis, stomatal conductance and WUEi were shown to vary with grapevine variety (Chaves et al., 1987; Schultz, 1996, 2003; Bota et al., 2001; Soar et al., 2006; Palliotti et al., 2009). Yet variation in photosynthetic efficiency seems to be small (Bota et al., 2001), suggesting that genotypic variation in WUE is largely linked to diversity in stomatal conductance, under both well-watered and water-deficit conditions (Escalona et al., 1999; Gaudillère et al., 2002; Chaves and Oliveira, 2004). Under drought conditions, a close relationship was found between stomatal function and plant hydraulics (Sperry, 1986; Cochard et al., 2002; Sperry et al., 2002). Stomata keep water flow within safe limits preventing the plants from exceeding those limits at any particular water potential, therefore avoiding xylem embolism (Sperry et al., 2002). Higher stomata sensitivity to water deficits may compensate for higher vulnerability to cavitation under drought (Schultz, 2003). Vitis vinifera shows high hydraulic conductivity in the main stem axis (Lovisolo et al., 2007). However, leaf hydraulic conductance can substantially constrain water transport, being a more important hydraulic bottleneck than the stem (Sack et al., 1993). It is also known that hydraulic conductance of roots and shoots influences stomatal regulation and plant transpiration (Lovisolo and Schubert, 1998; Aasamaa et al., 2001; Rogiers et al., 2009). The distribution of vessel sizes varies with variety and the larger sizes often result in higher sensitiveness to embolism under drought conditions (Chouzouri and Schultz, 2005).
Leaf morpho-anatomy and related biochemistry (epicuticular wax composition, lipid composition, mesophyll thickness, etc.) may also play a role in explaining plant adaptation to water stress (Syvertsen et al., 1995; Boyer et al., 1997; Cameron et al., 2006). Differences among V. vinifera have been reported in these characteristics (Schultz, 1996; Moutinho-Pereira et al., 2007).
Grapevine is generally considered a ‘drought-avoiding’ species, with an efficient stomatal control over transpiration (Chaves et al., 1987; Schultz, 2003). However, some genotypes have shown a better control of stomata than others in response to water deficits and accordingly have been classified as isohydric (drought avoiders or ‘pessimistic’); the others, showing lower control over stomatal aperture under water stress, were considered anisohydric, with an ‘optimistic’ response (Schultz, 2003; Soar et al., 2006). Schultz (2003) considered ‘Grenache’ to be a nearly isohydric genotype showing a marked regulation of stomatal conductance to decreasing soil water, whereas ‘Syrah’ exhibited a response closer to an anisohydric type. The same contrasting behaviour between ‘Grenache’ and ‘Syrah’ in response to atmospheric moisture stress was found by Soar et al. (2006), who attributed the higher sensitivity of stomata in ‘Grenache’ to the higher concentration of ABA in the xylem sap as compared with ‘Syrah’. They provided evidence of a midday increment of the expression of key genes involved in the ABA biosynthetic pathway, significantly higher in the leaves of ‘Grenache’ than in ‘Syrah’. This was not observed in the roots.
However, contradictory reports appeared in the literature showing that the same variety could behave differently depending on experimental conditions (see Table 1 and the review by Lovisolo et al., 2010). For example, ‘Syrah’ and ‘Grenache’ that exhibited an anisohydric and near-isohydric behaviour, respectively, in field experiments (Schultz, 2003; Soar et al., 2006) did not display the same stomatal behaviour when experiments were performed with potted plants (Chouzouri and Schultz, 2005).
Table 1.
List of grapevine varieties categorized as a function of the response of the water potential to water deficit (iso- or anisohydric), cultivated in soil (F) or in pots (P), with the corresponding range of values of water potential measured in each experiment
| Variety | Category | Set-up | Range of ψ (MPa) | References |
|---|---|---|---|---|
| ‘Chardonnay’ | Anisohydric | F and P | −0·4 to −1·0 | Tyerman (2007), Vandeleur et al. (2009), Rogiers et al. (2009) |
| ‘Cabernet Sauvignon’ | Anisohydric | F | −0·7 to −1·5 | Williams and Baeza (2007) |
| Isohydric | F | −0·25 to −1·5 | Chalmers (2007) | |
| ‘Falanghina’ | Near-isohydric | F | −0·7 to −1·8 | Giorio et al. (2007) |
| ‘Kékfrancos’ | Near-isohydric | F | −0·1 to −1·2 | Zsófi et al. (2008, 2009a, b) |
| ‘Grenache’ | Near-isohydric | F and P | −0·2 to −1·4 | Schultz (2003), Santesteban et al. (2009) |
| (Not clear) | P | −0·2 to −0·4 | Chouzouri and Schultz (2005) | |
| ‘Lambrusco’ | Isohydric | P | −0·6 to −1·2 | Poni et al. (2009) |
| ‘Montepulciano’ | Anisohydric | F | – | Silvestroni et al. (2005) |
| ‘Manto Negro’ | Isohydric | F | −0·05 to −0·7 | Medrano et al. (2003) |
| Anisohydric | – | – | Lovisolo et al. (2010) | |
| ‘Merlot’ | Anisohydric | F | −0·8 to −1·3 | Williams and Baeza (2007), Shellie and Glenn (2008) |
| ‘Portugiesier’ | Near-isohydric | – | −0·1 to −0·9 | Zsófi et al. (2008) |
| ‘Riesling’ | Anysohydric | – | – | Lovisolo et al. (2010) |
| ‘Sangiovese’ | Isohydric | F and P | −0·2 to −1·3 | Poni et al. (2007), Silvestroni et al. (2005) |
| Anysohidric | P | −0·55 to −1·3 | Poni et al. (2007) | |
| ‘Seedless Thomson’ | Anysohidric | F | −0·7 to −1·3 | Williams and Baeza (2007) |
| ‘Semillon’ | Anisohydric | F and P | −0·4 to −1·8 | Rogiers et al. (2009) |
| ‘Soultanina’ | Isohydric | P | −0·15 to −0·8 | Paranychianakis et al. (2004) |
| ‘Syrah | Anisohydric | F and P | −0·2 to −0·8 | Schultz (2003), Chalmers (2007), Rogiers et al. (2009), Santesteban et al. (2009) |
| −0·2 to −1·4 | ||||
| (Not clear) | P | −0·2 to −0·4 | Chouzouri and Schultz (2005) | |
| ‘Tempranillo’ (syn. ‘Aragonez’) | Isohydric | F and P | −0·05 to −1·3 | Medrano et al. (2003), Antolín et al. (2006), Sousa et al. (2006) |
| Near-isohydric | F | −0·2 to −1·5 | Intrigliolo et al. (2005) | |
| Anisohydric | F and P | – | Lovisolo et al. (2010), Santesteban et al. (2009) | |
| ‘Touriga Nacional’ | Anisohydric | F | −0·2 to −1·5 | Moutinho-Pereira et al. (2004) |
| ‘Viognier’ | Near-isohydric | F | – | Shellie and Glenn (2008) |
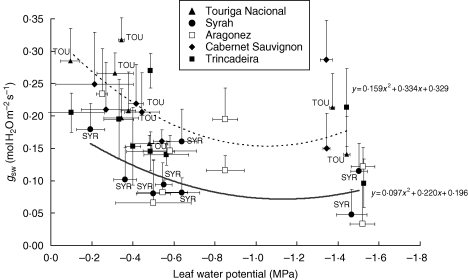
Recent studies performed in our group have also revealed differences between varieties [‘Touriga Nacional’, ‘Trincadeira’, ‘Aragonez’ (syn. ‘Tempranillo’), ‘Cabernet Sauvignon’ and ‘Syrah’, see Table 2] in the response of leaf stomatal conductance to deficit irrigation under field conditions. Stomatal conductance of ‘Touriga Nacional’ remained highest during the day (morning and afternoon) for similar leaf water potential, suggesting an anisohydric type of response (Fig. 1). In contrast, ‘Syrah’ showed the lowest conductance of the five varieties, particularly at noon, therefore exhibiting a near-isohydric response, contrary to earlier reports (Schultz, 2003; Soar et al., 2006).
Table 2.
Pre-dawn leaf water potential (ψpd), leaf temperature (Tleaf), leaf stomatal conductance to water vapour (gsw), net assimilation (An), intrinsic water use efficiency (WUEi) and δ13C measured for five Vitis vinifera varieties
| Variety | ψpd (MPa) | Tleaf (°C) | gsw (mol H2O m−2 s−1) | An (μmol CO2 m−2 s−1) | WUEi (μmol CO2 mol H2O−1) | δ13C (‰) |
|---|---|---|---|---|---|---|
| ARA | −0·25 ± 0·01 | 30·5 ± 0·2 | 0·076 ± 0·006 | 15·2 ± 0·8 | 59 ± 5 | −27·60 ± 0·47 |
| TRI | −0·10 ± 0·06 | 30·7 ± 1·6 | 0·074 ± 0·005 | 14·1 ± 0·5 | 54 ± 4 | −27·96 ± 0·75 |
| SYR | −0·19 ± 0·02 | 34·4 ± 1·5 | 0·049 ± 0·008 | 12·1 ± 0·7 | 93 ± 12 | −27·39 ± 0·66 |
| CAB | −0·21 ± 0·07 | 31·4 ± 1·6 | 0·085 ± 0·008 | 12·4 ± 0·5 | 45 ± 4 | −27·66 ± 1·07 |
| TOU | −0·10 ± 0·02 | 29·5 ± 1·5 | 0·115 ± 0·007 | 15·6 ± 0·7 | 69 ± 14 | −28·54 ± 0·69 |
ARA, ‘Aragonez’ (= ‘Tempranilho’); TRI, ‘Trincadeira’; SYR, ‘Syrah’; CAB, ‘Cabernet Sauvignon’; TOU, ‘Touriga Nacional’. Vines were grown in field conditions in southern Portugal (38 °48′N, 7 °29′W) and were 6–8 years old. Plants were grafted on the 1103-P rootstock, planted at a density of 4000 plants ha−1 and trained on a bilateral Royal Cordon system. Leaf water potential was measured with a pressure chamber (Model 1000; PMS instrument Co.). Leaf temperature was assessed by thermal imaging (IR Snapshot 525, 8–12 µm detector) at midday, and was immediately followed by measurements of leaf stomatal conductance using a portable photosynthesis system (Licor-6400, Li-COR Inc.) equipped with a transparent leaf chamber. Values of An and WUEi were determined at saturating light (1200 µmol m−2s−1), 360 p.p.m. CO2 and block temperature of 25 °C, using a Licor-6400 equipped with a 6400-02B LED light source. Measurements were carried at the beginning of August 2007. Values are means ± s.d. (n = 3–8 replicates) (J. M. Costa and M. F. Ortuño, unpubl. res.).
Fig. 1.
Relationship between leaf stomatal conductance to water vapour (gsw) and leaf water potential (ψ) measured throughout the day (pre-dawn and midday) for five different Vitis vinifera varieties, ‘Touriga Nacional’, ‘Syrah’, ‘Aragonez’ (‘Tempranilho’), ‘Cabernet Sauvignon’ and ‘Trincadeira’, as indicated.Vines were grown in field conditions in southern Portugal (38 °48′N, 7 °29′W) and were 6–8 years old. Vines were grafted on the 1103-P rootstock, planted at a density of 4000 plants ha−1 and trained on a bilateral Royal Cordon system. Measurements took place during the summer season (beginning of August) of three consecutive years: 2006, 2007 and 2008. ψ was measured with a pressure chamber (Model 1000; PMS instrument Co., Corvallis, OR, USA) and gsw was measured with a portable photosynthesis system (Licor-6400, LI-COR Inc., Lincoln, NE, USA) equipped with a transparent leaf chamber. Horizontal and vertical bars indicate the standard deviation (n = 8). Lines represent regression lines estimated for the varieties ‘Touriga’ and ‘Syrah’, as indicated.
For ‘Sangoviese’, Poni et al. (2007) pose questions regarding its classification with respect to response to water stress. The authors discuss in their paper that because the first criterion to classify genotypes as being isohydric or anysohydric is how their leaf-water status (namely midday leaf-water potential) responds to a soil-water deficit treatment, they would classify ‘Sangoviese’ as anisohydric. However, several effects posed by partial rootzone drying on these vines, such as a fast cessation of shoot growth, leaves tending to assume a vertical orientation during midday to reduce light interception, and a pronounced and steady increase of WUEi, have been reported as being more typical of an isohydric strategy.
Bearing in mind the available data, a classification of grapevine varieties as strictly iso- or anisohydric may prove inappropriate. It seems plausible that stomatal responses to water deficits in a specific variety will vary according to the particular combination of the rootstock, the climate (VPD and temperature), and the intensity and duration of water deficits. In fact, under prolonged water deficits more rigid cell walls may develop, leading to a larger decline in plant water potential at midday, characteristic of the anysohydric response. Moreover, osmotic adjustment may contribute to the maintenance of open stomata at lower water potentials, by enabling an improved turgor in response to a slowly imposed water deficits. This combination of responses will interact with scion structural factors such as water-conducting capacity of stems and petioles to dictate response to water deficits.
This is an area of research deserving further investigation in order to clarify the relative importance of the factors involved in the dynamic response of stomata to water deficits.
LONG-DISTANCE SIGNALLING OF WATER DEFICITS
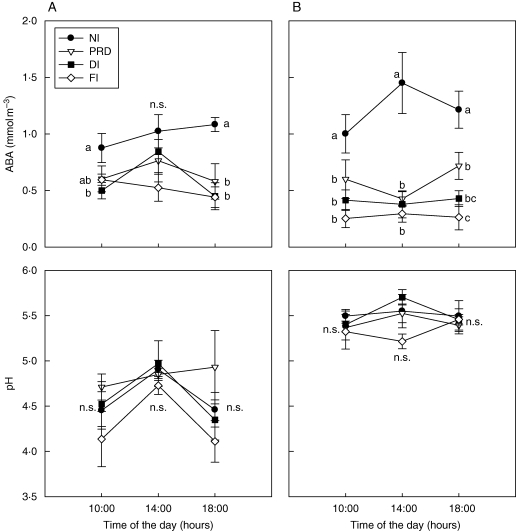
Under soil drying plants reduce water use by stomatal closure and decreased growth. Hydraulic and chemical signals sent from drying roots to the shoot are involved in the regulation of these responses (Davies et al., 1994; Dodd et al., 1996; Liu et al., 2003). However, the relative importance of the two types of signalling in the control of stomatal aperture and leaf growth is still the subject of discussion. Depending on the species and/or experimental conditions hydraulic limitation may dominate over root chemical signalling (Comstock, 2002; Voisin et al., 2006; Neumann, 2008; Ahmadi et al., 2009). This seems to be the case in some woody species, where chemical root-to-shoot signalling appears to be inefficient in controlling stomatal behaviour (Augé and Moore, 2002) or when other abiotic stresses co-occur with drought, as usually happens when plants are growing in their natural environment. Nevertheless, the primary role of a root-to-shoot hydraulic signal is generally followed by an increased ABA biosynthesis in the shoot that regulates stomata (Christmann et al., 2007) and leaf growth (Chazen et al., 1995; Neumann et al., 1997). Moreover, a great deal of evidence highlights the importance of ABA as a root-sourced signal transported via the xylem and involved in stomatal regulation of droughted plants (reviewed by Dodd et al., 1996, 2006; Wilkinson and Davies, 2002; Davies et al., 2005). Even so, other compounds such as the precursors of ABA (Sauter et al., 2002; Lee et al., 2006; Jiang and Hartung, 2008), low concentration of cytokinins (Shashidhar et al., 1996; Stoll et al., 2000; Hansen and Dorffling, 2003) and changes in mineral composition or pH of the xylem (Wilkinson and Davies, 1997; Hartung et al., 1998; Prokic et al., 2006; Jia and Davies, 2007) might also be implicated in the regulation of water use at the leaf level (reviewed by Schachtmann and Goodger, 2008). Much evidence suggests that xylem sap pH can indeed modulate stomatal and growth responses to root chemical signals produced in drying soils (Wilkinson and Davies, 1997, 2002; Wilkinson, 2004). For acidic xylem sap pH, ABAH is taken by the leaf and metabolized or partitioned into alkaline compartments in the symplast of leaf cells, away from the sites of action of the hormone on stomata. Conversely, as pH increases, the proportion of ionized ABA transported in the xylem sap rises (not taken up by mesophyll cells) and so is maintained longer in the leaf apoplast adjacent to the guard cells, having greater control on stomatal behaviour (Hartung et al., 1998; Wilkinson, 2004). This effect is particularly important in grapevines as usually they have pH values close to the pKa of ABA (pH 4·8), as shown by Stoll et al. (2000) and Rodrigues et al. (2008). Indeed, work done with the grapevine variety ‘Castelão’ (Rodrigues et al., 2008) provided evidence for a synergistic effect of increased pH and ABA, explaining stomatal closure at berry maturity, whereas earlier in the season (véraison) a low xylem pH was measured and no correlation between ABA and gs was found (Fig. 2). Sharp and Davies (2009) found that drought-induced change in pH is more common in herbaceous than in woody perennial species. In fact, among 22 woody species they observed an increase in pH in only four of them, the majority maintaining a pH similar to the well-watered plants (Sharp and Davies, 2009).
Fig. 2.
Diurnal changes in concentration of abscisic acid (ABA) in the xylem sap and pH of the xylem sap of field-grown Castelão grapevines in Pegões, Portugal, under four water treatments (NI, non-irrigated; PRD, partial rootzone drying; DI, deficit irrigated; FI, fully irrigated), measured on two days of the 2002 growing season: (A) veraison (25 July) and (B) mid-ripening (22 August). For each measurement time values are the mean of four measurements. Error bars indicate the standard error. Different letters show statistically significant differences among treatments at P < 0·05. (Rodrigues et al., 2008).
Grapevine stomata also strongly respond to plant water status, through hydraulic tensions developed in the xylem affecting leaf turgor. Positive correlations between pre-dawn water potential and maximum gs have generally been found in grapevines subjected to water deficits (Correia et al., 1995; Flexas et al., 1998; Rodrigues et al., 2008). As in other species a decrease in shoot hydraulic conductivity has been shown to occur in water-stressed grapevines (Schultz and Matthews, 1988; Lovisolo and Schubert, 1998; Lovisolo et al., 2002) and is linearly correlated with gs under mild stress levels (Lovisolo and Schubert, 1998). Moreover, it was shown that a decline in leaf water potential might enhance stomatal sensitivity to ABA. This interactive effect can explain the decrease in gs observed at midday in grapevines growing under field conditions, including well-watered ones, in spite of constant diurnal [ABA] in the xylem stream (Correia et al., 1995; Rodrigues et al., 2008)
When considering deficit irrigation, there is no clear picture of the relative importance of hydraulic and chemical signalling on plant response to water deficit. There are studies indicating a marked decrease of gs in PRD grapevines relative to conventionally irrigated vines, in spite of comparable shoot water status (Dry and Loveys, 1999; Du et al., 2006), therefore suggesting the involvement of a non-hydraulic signal in stomatal regulation. Several other studies, however, did not find evidence for a more marked stomatal closure in PRD than in DI grapevines (Souza et al., 2003; Dorji et al., 2005; De la Hera et al., 2007; Marsal et al., 2008; Rodrigues et al., 2008). The higher water status of PRD plants may be derived from the observed restriction in vegetative growth of PRD plants (Santos et al., 2003, 2005; Chaves et al., 2007), leading to lower plant water use and thus more water available in the soil near the root system. Differences in root architecture, with an increased ability to exploit deeper soil layers (Dry et al., 2000; Mingo et al., 2004; Santos et al., 2007), have also been reported as well as an increase of root hydraulic conductivity after root rewatering. In fact, recent work in an irrigated pear orchard showed that root sap flow on the wet side of PRD plants was enhanced compared with control plants equally watered on both sides of the root system (Kang et al., 2003). Also, Green et al. (1997) observed in mature apple trees that previously dehydrated roots responded to irrigation by exhibiting higher sap flow rates than usually occurs when the entire root zone is watered. This increase in root hydraulic conductivity seems to be mediated by aquaporin activity (Martre et al., 2002; Lovisolo and Schubert, 2006) as a significant part of the radial water transport takes place through the cell-to-cell pathway (Martre et al., 2002; Siefritz et al., 2002).
Considering the causes for the observed restriction of vegetative growth under similar or better water status in PRD grapevines as compared with DI, chemical signals are the likely candidates to explain these results (Chaves et al., 2007). Such chemical root-to-shoot signalling probably involves a reduction of cytokinins (CKs) (Kudoyarova et al., 2007) or an increase of ethylene (Sobeih et al., 2004). CKs are synthesized mainly in the roots (Aloni et al., 2005) and were shown to play an important role as long-distance signalling molecules (Schmulling, 2002; Werner et al., 2003; Hirose et al., 2008). Dry et al. (2001) observed shoot growth inhibition in PRD grapevines in parallel with a marked decrease in the concentration of CK in shoots and roots. This effect was reversed by exogenous application of a synthetic CK. Similarly, a marked reduction in zeatin and zeatin riboside concentrations in roots, shoot tips and buds was found in PRD grapevines (Stoll et al., 2000). Although most results in other species also point to a decrease in the delivery of CKs to the xylem sap in water-stressed plants (Bano et al., 1993; Shashidhar et al., 1996; Hansen and Dorffling, 2003), there are exceptions with the opposite effect (CK increase) (Pospísilová et al., 2005).
CHANGES IN BERRY GROWTH, METABOLISM AND COMPOSITION UNDER WATER DEFICIT
Water deficit influences berry development, metabolism and final composition, and its timing and intensity dictate the extent of alterations occurring in wine colour and flavour. Interestingly, water deficit was also shown to enhance photoprotection mechanisms in berries (Deluc et al., 2009). In general, mild water deficits were shown to have a positive impact on wine quality in red varieties (Bravdo et al., 1985). In this context, deficit irrigation can provide the means to manipulate wine sensory characteristics. However, the effects of deficit irrigation on berry and wine quality will depend on the climatic characteristics during the growing season, soil type, grapevine variety and timing of application (Dry and Loveys, 1998; Santos et al., 2003, 2005).
Transcriptional analysis of grape berries from vines subjected to moderate water deficits at the end-ripening stage showed alterations in mRNA expression patterns particularly associated with cell-wall, sugar and hormone metabolism (Deluc et al., 2007). The most profound alterations were related to ethylene, auxin and ABA, but an enhancement of the expression of several genes of the phenylpropanoid pathway was also observed.
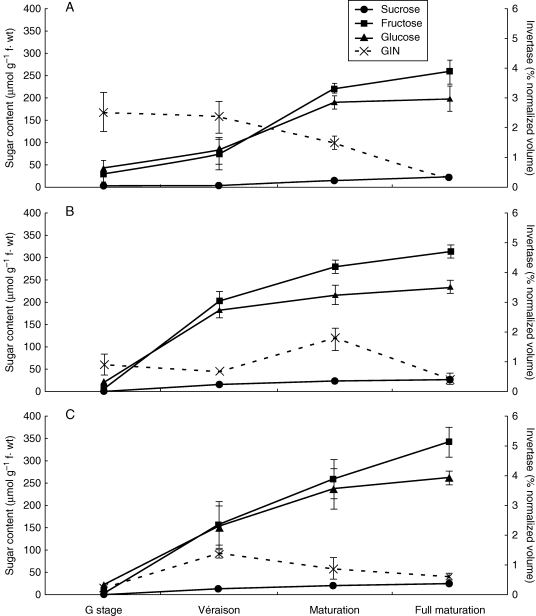
The impact of water deficit on grape berry proteomes was reported by Grimplet et al. (2009). These authors studied the alterations observed in the skin, pulp and seed proteomes of fully ripe berries when comparing water-deficit vines (no irrigation) with well-watered plants (irrigation from pre-véraison to the end of berry maturity) and showed that 7 % of pericarp proteins were water-stress responsive. Using such an approach, we are currently studying the proteome dynamics of grapevines of the variety ‘Aragonez’ (syn. ‘Tempranillo’) along berry development using three irrigation strategies. When comparing berries of fully irrigated (FI) vines with ones from deficit irrigated (RDI) and rain-fed (NI) vines, several proteins were identified as stress responsive. One such protein was vacuolar invertase (GIN1), which was significantly down-regulated under NI and RDI when compared with FI conditions (Fig. 3). These alterations were observed at green stage (pre-véraison) and véraison. Moreover, the peak of expression of this protein, which was reported to occur at véraison by others (Deluc et al., 2007; Giribaldi et al., 2007; Negri et al., 2008), was observed later in RDI than in FI berries. These results suggest that water availability modulates not only the amount but also the timing of protein expression. It suggests as well that changes taking place very early during berry development, such as at the green berry stage, may have a profound effect on the final berry maturity (R. Francisco et al., unpubl. res.).
Fig. 3.
The influence of water deficit on sugar metabolism and vacuolar invertase (GIN1) expression profile along fruit ripening. (A) Plants under full irrigation conditions; (B) plants under regulated deficit irrigation conditions and (C) plants under no irrigation but rain-fed. 2-DE spot volume is represented as the percentage of normalized volume. Symbols represent means ± s.e. (n = 3).
Vine water status is known to influence fruit composition through an indirect effect on berry size, and therefore the ratio of skin to pulp, which increases in the smaller berries of vines subjected to water deficits (Bravdo et al., 1985; Kennedy et al., 2002). There is, however, a direct, possibly greater effect on skin tannin and anthocyanin contents (Roby et al., 2004). The reported increase in skin tannin and anthocyanin that accompanies water deficits seems to result from different sensitivity of berry tissues to water deficits, with the exocarp being less affected than the inner mesocarp (Roby et al., 2004). Proteomic studies in berries from grapevines subjected to different irrigation treatments that suggest that metabolic differences in response to water status occur at early stages of berry development (R. Francisco et al., unpubl. res.) confirm that they are partly independent of the effect on berry size.
Berry growth
Grape berry is a non-climacteric fruit with a double sigmoid growth curve (Coombe, 1976). Stages I and III of growth are separated by a lag phase (stage II). During stage I, imported carbohydrates are used for seed development, cell proliferation and expansion, and synthesis of organic acids (Coombe, 1992). At this stage the berry is exclusively connected to the vine through the xylem, and the impact of water deficit on berry growth is thought to occur directly by changes in water import by the xylem, which possibly induces a decrease in mesocarp cell turgor (Thomas et al., 2006). There is consequently a reduction in the expansion of grape berries. However, it is also possible that the ABA synthesized under water stress limits cell division and consequently small berries are produced. The second hypothesis correlates well with the observed inhibition of grape development following water deficit at pre-véraison. This leads to a cascade of events culminating in earlier grape ripening (e.g. accelerating sugar and anthocyanin accumulation and malic acid breakdown) (Castellarin et al., 2007a, b). The beginning of the second phase of berry growth (stage III), known as véraison, is characterized by softening and colouring of the berry and a size increase. After véraison a reduction in berry size due to water deficit is probably the result of more than one mechanism (Thomas et al., 2006). At this stage, the berry's connectivity to the vine is via the phloem (Thomas et al., 2006). Moreover, a reduction of berry size might be only indirectly caused by water stress, through a decrease in photosynthesis (Wang et al., 2003). Post-véraison water deficit increases the proportion of whole-berry fresh mass represented by seeds and skin (Roby and Matthews, 2004) and berries present ‘thicker skins’ at harvest probably due to a decrease in the activity of pectin methylesterase enzyme (Deytieux-Belleau et al., 2008), as was shown in water-stressed tomato cherry fruit (Barbagallo et al., 2008). This results in higher content of skin-based constituents (e.g. tannins and anthocyanins) on a berry mass basis and as a consequence the must from those berries is much richer in skin-derived extractives (Chatelet et al., 2008).
Accumulation of sugars and organic acids
Grape quality largely depends on sugar/acid balance at harvest. Prior to véraison, most sucrose imported into the berries is metabolized with little if any storage. However, following véraison hexoses accumulate in the berries at high concentration (1 m or even more). Grapevine is thought to be a symplastic phloem ‘loader’ due to the presence of plasmodesmata connecting mesophyll cells with phloem-associated cells (Gamalei, 1989). It has been suggested that the symplastic connections via plasmodesmata between the sieve tubes and the mesocarp cells remain for quite a long period during berry development. Phloem unloading seems to occur via efflux into the apoplast and subsequent uptake by sink cells. Sucrose from the phloem can be imported from the apoplast via direct sucrose transporters or it can be hydrolysed to glucose and fructose by cell-wall-bound invertases and taken up by monosaccharide transporters. In grape berry, it is known that invertase expression considerably precedes the onset of sugar accumulation (Davies and Robinson, 1996). This suggests that the triggering of ripening depends on the activation of sugar transporters (for a review, see Conde et al., 2007).
Moderate water deficit promotes sugar accumulation either as a result of inhibiting lateral shoot growth, which induces a reallocation of carbohydrates to fruits, or as a direct effect of ABA signalling on fruit ripening (Coombe, 1989). Indeed, experimental evidence suggested activation of ABA-mediated uptake of hexose (Deluc et al., 2009). However, the mechanisms underlying accumulation of hexoses under water deficit have not been elucidated completely.
The effects of water deficit on sugar content of grapevine berries are variety-dependent (Gaudillère et al., 2002). For example, no significant changes were observed in ‘Merlot’ sugar content under water deficits, while a significant increase in sugar content was observed in ‘Cabernet Sauvignon’ berries (Castellarin et al., 2007a, b). Similarly, Deluc et al. (2009) observed an increase in berry sugar content under water deficits in ‘Cabernet Sauvignon’ but not in ‘Chardonnay’. This may be explained either by differences in vigour, and therefore source/sink equilibrium, between varieties, or by different mechanisms underlying the response of grape berry development to water limitation according to the timing and intensity of water stress imposition. Indeed, it was shown that water deficit has more effect on berry sugar accumulation when imposed before véraison (Keller, 2005; Keller et al., 2006).
In most cases, no titratable acidity changes have been observed in the must from moderately water-stressed vines (Matthews and Anderson, 1989; Esteban et al., 1999). However, some studies report a reduction of titratable acidity due to deficit irrigation as compared with full irrigation (Sheltie, 2006; Santos et al., 2007). Malate/tartarate ratio is in general lower due to malate breakdown in vines with low water status (Matthews and Anderson, 1989).
Polyphenols
Among the different classes of polyphenols present in grape berries the most important are flavonoids [anthocyanins, flavonols and proanthocyanidins (also called condensed tannins)] and stilbenes. They are mainly localized in exocarp and seed endocarp tissues and it is well known that vine water status affects accumulation of polyphenols in these tissues. Regulating grapevine water deficit is a powerful tool to manage the amount of these compounds and improve wine quality (Kennedy et al., 2002).
Anthocyanins are synthesized via the flavonoid pathway in the berry skin of red grapevines from the onset of ripening (véraison) but they are non-existent in white grapevine varieties due to a multi-allelic mutation (Walker et al., 2007). Water deficit has been considered to enhance accumulation of anthocyanins, through the stimulation of anthocyanin hydroxylation, probably by upregulating the gene encoding the enzyme F3′5′H (Mattivi et al., 2006; Castellarin et al., 2007b). This enzyme converts hydroxylated anthocyanins (cyanidin and delphinidin) into their methoxylated derivates (peonidin, petunidin and malvidin) (Kennedy et al., 2002; Castellarin et al., 2007b). Indeed, the major anthocyanins synthesized in the berries under water deficits are peonidin 3-O-β-glucoside and malvidin 3-O-β-glucoside, because methoxylation of delphinidin to produce its derivate petunidin rarely occurs (Castellarin et al., 2007b; Deluc et al., 2009).
Water stress seems to have a greater impact on anthocyanin composition than on its total concentration. Early imposition of water stress led to increased sugar accumulation, which accelerates anthocyanin synthesis (Castellarin et al., 2007b), probably due to ‘sucrose boxes’ in the promoters of LDOX and DFR genes (Gollop et al., 2001, 2002). Gene regulation of the anthocyanin pathway was known to be affected by the timing of imposition of water deficit (Castellarin et al., 2007a).
Flavonols play a fundamental role in grape quality, as they act as co-pigments with anthocyanins and stabilize colour in young red wines (Boulton, 2001). Flavonol biosynthesis is closely related to that of anthocyanins (Jeong et al., 2006). However, in contrast to anthocyanins, a small number of flavonols were identified and available data were limited to a few grape varieties (Mattivi et al., 2006). The main flavonols reported in grape berries are quercetin-3-glucoside and quercetin-3-O-glucuronide (Downey et al., 2003). Deficit irrigation was reported to have a moderate effect on flavonol synthesis in red grapevines (Grimplet et al., 2007). In turn, the timing of water deficit does not change flavonol content (Kennedy et al., 2002). Mattivi et al. (2006) have suggested that anthocyanins and flavonols share the same biosynthetic enzymes. This may indicate that, like anthocyanins, changes to flavonol under water deficits may occur rather in composition than in accumulation. More recently, in a white grapevine (‘Chardonnay’), flavonol concentrations were reported to increase under water deficits, which was not the case in a red grapevine (‘Cabernet Sauvignon’) in the same study (Deluc et al., 2009). This suggests a greater need for berry photoprotection in these varieties, as previously shown in apples with low levels of anthocyanins (Merzlyak et al., 2008).
Proanthocyanidins or condensed tannins are flavan-3-ol oligomers. They are important sensory components, providing wine with bitterness and astringency. However, little is known about proanthocyanidins (for reviews, see Dixon et al., 2005; Xie and Dixon, 2005) and a standardized measure of tannins has not yet been adopted (Downey et al., 2006). Besides, changes occurring in proanthocyanidins during grape development are complex, involving increases in the degree of polymerization, in the proportion of (–)-epigallocatechin extension units, and in polymer-associated anthocyanins (Kennedy et al., 2002). Proanthocyanidins appear to be only slightly affected by water deficit (Downey et al., 2006) and the increases in skin tannin that accompany water deficits appear to result more from differential growth sensitivity of the inner mesocarp and the exocarp than from direct effects on phenolic biosynthesis (Roby et al., 2004). The effect of concentration of seed tannins on wine characteristics is not known (Matthews and Nuzzo, 2007). Moreover, few works have reported whether water status influences seed proanthocyanidin content. Two studies performed with the same variety (although in different environments) did not show any significant effects of water deficit on seed proanthocyanidins (Kennedy et al., 2000; Geny et al., 2003). A gene expression study undertaken by our team (O. Zarrouk et al., unpubl. res.) demonstrated differential expression during grape berry development of the ANR gene in grape seeds and a slight downregulation under water stress.
Stilbenes belong to the non-flavonoid class of phenolic compounds. Generally, stilbenes are considered as phytoalexins, and their formation in grape leaves was correlated with disease resistance. Resveratrol is considered the most bioactive stilbene in grapevines (Bavaresco et al., 2008). In grape berries, resveratrol synthesis is catalysed by stilbene synthase (STS), which shares the same substrates used by chalcone synthase for flavonoid production (Versari et al., 2001). It accumulates mainly in the grape skin and seeds, and it has been found both in red and white grapes at a large range of concentrations, depending on biotic and abiotic conditions (Jimenez et al., 2007). Conflicting results have been found on the effects of water deficit on resveratrol synthesis. Research conducted by Vezzuli et al. (2007) observed little effect of drought on resveratrol concentrations in grape berry skin. An increase in mRNA abundance of STS was reported by Grimplet et al. (2007), which suggests an increase in resveratrol accumulation (Versari et al., 2001). Under moderate water deficit, gene expression of STS1 and STS2 in grape seeds showed an upregulation at berry maturity (O. Zarrouk et al., unpubl. res.).
Aromas
The aroma that builds up in grapes results from several compounds (terpenoids and their derivatives, esters, aldehydes and thiols) stored as non-volatile precursors mainly in exocarp vacuoles.
The influence of the irrigation strategy on grape berry aromas has not received much research. However, two major studies suggest that deficit irrigation alters several sensory attributes of the wine as well as the concentration of carotenoids and their derivatives in berries, as compared with standard irrigation grapevines (Chapman et al., 2005; Bindon et al., 2007). Chapman et al. (2005) reported that water deficits led to wine with more fruity and less vegetal aromas than those from vines with high water status, in the variety ‘Cabernet Sauvignon’. According to these authors water deficits may have led to a greater flux of carbon through alternative biosynthetic pathways leading to an increase in amino acids (precursors of esters in wines) and in carotenoids, resulting in a more fruity aroma. Bindon et al. (2007) observed that deficit irrigation led to an increase in the concentration of hydrolytically released C13-norisoprenoids (β-damascenone, β-ionone and 1,1,6- trimethyl-1,2-dihydronaphthalene) in ‘Cabernet Sauvignon’ grape berries at harvest. Furthermore, transcriptomic analysis of genes encoding enzymes involved in the biosynthesis of volatile compounds revealed an increase in the transcript abundance of one terpenoid synthase, one carotenoid cleavage dioxygenase and several lipoxygenases under conditions of water deficits (Deluc et al., 2009). However, we should emphasize that the correlation of enzyme transcript abundance with the reaction products they catalyse is not straightforward, given the complexity of gene regulation, enzyme activity modulation and differential expression of multigenic families.
CONCLUSIONS AND THE WAY FORWARD
Deficit irrigation is an efficient strategy to improve WUE and control vigour in grapevine, allowing an optimal grape maturity and therefore a high wine quality. It is now acknowledged that the efficiency of deficit irrigation (whatever the sub-type) in modulating WUE, growth and grape berry composition is dependent on the variety characteristics (namely vigour and drought avoiding traits), the type of soil and the prevailing weather (rainfall and temperature). More in-depth and wider studies of varieties in response to environmental stresses are instrumental to the understanding of grapevine adaptation to more arid climates. Further knowledge on berry development, including the timing of accumulation of various berry components, and their dependence on water availability, is critical for an optimal choice of irrigation strategy. Proteomic and transcriptomic studies are providing new avenues for that understanding. Available data suggest that water deficits interact with development to alter the expression of genes responsible for some grape berry compounds and metabolite transporters. Although some of those changes seem to be transient, it is plausible that they will have an impact on berry maturity and final wine quality.
ACKNOWLEDGEMENTS
R.F., O.Z., A.R., T.S. and J.M.C. are supported by fellowships granted by Fundação para a Ciência e Tecnologia (FCT). WATERWEB, POCI/AGR/59079/2004 and PPCDT/AGR/61980/2004 projects provided funds to support part of the research presented. We are very grateful to Olga Grant for help with the English text and for judicious comments.
LITERATURE CITED
- Aasamaa K, Sõber A, Rahi M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Australian Journal of Plant Physiology. 2001;28:765–774. [Google Scholar]
- Ahmadi SH, Andersen MN, Poulsen RT, Plauborg F, Hansen S. A quantitative approach to developing more mechanistic gas exchange models for field grown potato: a new insight into chemical and hydraulic signalling. Agricultural and Forest Meteorology. 2009;149:1541–1551. [Google Scholar]
- Alleweldt G, Spiegel-Roy P, Reisch B. Grapes (Vitis) Acta Horticulturae. 1990;290:291–327. [Google Scholar]
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany. 2005;56:1535–1544. doi: 10.1093/jxb/eri148. [DOI] [PubMed] [Google Scholar]
- Antolín MC, Ayari M, Sánchez-Díaz M. Effects of partial rootzone drying on yield, ripening and berry ABA in potted Tempranillo grapevines with split roots. Australian Journal of Grape and Wine Research. 2006;12:13–20. [Google Scholar]
- Augé RM, Moore JL. Stomatal response to nonhydraulic root-to-shoot communication of partial soil drying in relation to foliar dehydration tolerance. Environmental and Experimental Botany. 2002;47:217–229. [Google Scholar]
- Baeza P, Lissarrague JR. Agronomic and ecophysiological responses of field-grown Cabernet Sauvignon grapevines to three irrigation treatments. Acta Horticulturae. 2005;689:373–379. [Google Scholar]
- Bano A, Dorffling K, Bettin D, Hahn H. Abscisic acid and cytokinins as possible root-to-shoot signals in xylem sap of rice plants in drying soil. Australian Journal of Plant Physiology. 1993;20:109–115. [Google Scholar]
- Barbagallo RN, Chisari M, Branca F, Spagna G. Pectin methylesterase, polyphenol oxidase and physicochemical properties of typical long-storage cherry tomatoes cultivated under water stress regime. Journal of the Science of Food and Agriculture. 2008;88:389–396. [Google Scholar]
- Bavaresco L, Vezzuli S, Civardi S, et al. Effect of lime-induced leaf chlorosis on Ochratoxin A, trans-Resveratrol, and ε-Viniferin production in grapevine (Vitis vinifera L.) berries infected by Aspergillus carbonarius. Journal of Agriculture and Food Chemistry. 2008;56:2085–2089. doi: 10.1021/jf073456+. [DOI] [PubMed] [Google Scholar]
- Bindon KA, Dry PR, Loveys BR. Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. cv. Cabernet Sauvignon grape berries. Journal of Agriculture and Food Chemistry. 2007;55:4493–4500. doi: 10.1021/jf063331p. [DOI] [PubMed] [Google Scholar]
- Blum A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop Research. 2009;112:119–123. [Google Scholar]
- Bota J, Flexas J, Medrano H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Annals of Applied Biology. 2001;138:353–361. [Google Scholar]
- Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: a critical review. American Journal of Enology and Viticulture. 2001;52:67–87. [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD. CO2, and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology. 1997;114:185–191. doi: 10.1104/pp.114.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravdo B. Physiological mechanisms involved in the production of non-hydraulic root signals by partial rootzone drying – A review. Acta Horticulturae. 2005;689:267–275. [Google Scholar]
- Bravdo B, Hepner Y, Loinger C, Tabacman H. Effect of irrigation and crop level on growth, yield and wine quality of Cabernet Sauvignon. American Journal of Enology and Viticulture. 1985;36:132–139. [Google Scholar]
- Bravdo B, Naor A, Zahavi T, Gal Y. The effects of water stress applied alternatively to part of the wetting zone along the season (PRD-partial rootzone drying) on wine quality, yield, and water relations of reed wine grapes. Acta Horticulturae. 2004;664:101–109. [Google Scholar]
- Cameron KD, Teece MA, Smart LB. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiology. 2006;140:176–183. doi: 10.1104/pp.105.069724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin S, Matthews MA, Gaspero GD, Gambetta GA. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta. 2007a;227:101–112. doi: 10.1007/s00425-007-0598-8. [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruit of grapevine under seasonal water deficit. Plant, Cell and Environment. 2007b;30:1381–1399. doi: 10.1111/j.1365-3040.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- Chalmers YM. Influence of sustained deficit irrigation on physiology and phenolic compounds in winegrapes and wine. 2007. PhD thesis, Adelaide University. http://digital.library.adelaide.edu.au/dspace/bitstream/2440/50101/1/02whole.pdf .
- Chapman DM, Roby G, Ebeler SE, Guinard JX, Matthews MA. Sensory attributes of Cabernet Sauvignon wines made from vines with different water status. Australian Journal of Grape and Wine Research. 2005;11:339–347. [Google Scholar]
- Chatelet DS, Rost TL, Matthews MA, Shackel KA. The peripheral xylem of grapevine (Vitis vinifera) berries. 2. Anatomy and development. Journal of Experimental Botany. 2008;59:1997–2007. doi: 10.1093/jxb/ern061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont M, Osorio ML, Chaves MM, Vanacker H, Morot-Gaudry JF, Foyer CH. The absence of photo-inhibition during mid-morning depression of photosynthesis in Vitis vinifera grown in semi-arid and temperate climates. Journal of Plant Physiology. 1997;150:743–751. [Google Scholar]
- Chaves MM, Oliveira MM. Mechanisms underlying plant resilience to water deficits – Prospects for water-saving agriculture. Journal Experimental Botany. 2004;55:2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Tenhunen JD, Harley P, Lange OL. Gas exchange studies in two portuguese grapevine cultivars. Physiologia Plantarum. 1987;70:639–647. [Google Scholar]
- Chaves MM, Pereira JS, Maroco J. Understanding plant response to drought – from genes to the whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Santos TP, Souza CR, et al. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Annals of Applied Biology. 2007;150:237–252. [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazen O, Hartung W, Neumann PM. The different effects of PEG 6000 and NaCl on leaf development are associated with differential inhibition of root water transport. Plant, Cell and Environment. 1995;18:727–735. [Google Scholar]
- Chouzouri A, Schultz HR. Hydraulic anatomy, cavitation susceptibility and gas-exchange of several grapevine varieties. Acta Horticulturae. 2005;689:71–78. [Google Scholar]
- Christmann A, Weiler EW, Steudle E, Grill E. A hydraulic signal in root-to-shoot signalling of water shortage. The Plant Journal. 2007;52:167–174. doi: 10.1111/j.1365-313X.2007.03234.x. [DOI] [PubMed] [Google Scholar]
- Cochard H, Coll L, Le Roux X, Améglio T. Unraveling the effects of plant hydraulics on stomatal conductance during water stress in Walnut. Plant Physiology. 2002;128:282–290. [PMC free article] [PubMed] [Google Scholar]
- Cominelli E, Galbiati M, Tonelli C, Bowler C. Water: the invisible problem. EMBO Reports. 2009;10:671–676. doi: 10.1038/embor.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock JP. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. Journal of Experimental Botany. 2002;53:195–200. doi: 10.1093/jexbot/53.367.195. [DOI] [PubMed] [Google Scholar]
- Conde C, Silva P, Fontes N, et al. Biochemical changes throughout grape berry development and fruit and wine quality. Food. 2007;1:1–22. [Google Scholar]
- Coombe BG. The development of fleshy fruits. Annual Review of Plant Physiology. 1976;27:207–228. [Google Scholar]
- Coombe BG. The grape berry as a sink. Acta Horticulturae. 1989;239:149–158. [Google Scholar]
- Coombe BG. Research on the development and ripening of the grape berry. American Journal of Enology and Viticulture. 1992;43:101–110. [Google Scholar]
- Correia ML, Chaves MM, Pereira JS. Afternoon depression in photosynthesis in grapevine leaves – evidence for a high light stress effect. Journal of Experimental Botany. 1990;41:417–426. [Google Scholar]
- Correia MJ, Pereira JS, Chaves MM, Rodrigues ML, Pacheco CA. ABA xylem concentrations determine maximum daily leaf conductance of field-grown Vitis vinifera L. plants. Plant, Cell and Environment. 1995;18:511–521. [Google Scholar]
- Costa JM, Ortuño MF, Chaves MM. Deficit irrigation as strategy to save water: physiology and potential application to horticulture. Journal of Integrative Plant Biology. 2007;49:1421–1434. [Google Scholar]
- Davies C, Robinson SP. Sugar accumulation in grape berries. Cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiology. 1996;111:275–283. doi: 10.1104/pp.111.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annual Revview of Plant Physiology. 1991;42:55–76. [Google Scholar]
- Davies WJ, Tardieu F, Trejo CL. How do chemical signals work in plants that grow in drying soil. Plant Physiology. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Wilkinson S, Loveys B. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. The New Phytologist. 2002;153:449–460. doi: 10.1046/j.0028-646X.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Kudoyarova G, Hartung W. Long-distance ABA signalling and its relation to other signalling pathways in the detection of soil drying and the mediation of the plant response to drought. Journal of Plant Growth Regulation. 2005;24:285–295. [Google Scholar]
- De la Hera ML, Romero P, Gómez-Plaza E, Martinez A. Is partial rootzone drying an effective irrigation technique to improve water use efficiency and fruit quality in field-grown wine grapes under semiarid conditions? Agricultural Water Management. 2007;87:261–274. [Google Scholar]
- Deluc LG, Grimplet J, Wheatley MD, et al. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics. 2007;8:429. doi: 10.1186/1471-2164-8-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LG, Quilici DR, Decendit A, et al. Water deficit alters differentially metabolic pathways affecting important flavour and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics. 2009;10:212. doi: 10.1186/1471-2164-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deytieux-Belleau C, Vallet A, Donèche B, Geny L. Pectin methylesterase and polygalacturonase in the developing grape skin. Plant Physiology and Biochemistry. 2008;46:638–646. doi: 10.1016/j.plaphy.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB. Proanthocyanidins: a final frontier in flavonoid research? New Physiologist. 2005;165:9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Stikic R, Davies WJ. Chemical regulation of gas exchange and growth of plants in drying soil in the field. Journal of Experimental Botany. 1996;47:1475–1490. [Google Scholar]
- Dodd IC, Theobald JC, Bacon MA, Davies WJ. Alternation of wet and dry sides during partial rootzone drying irrigation alters root to shoot signalling of ABA. Functional Plant Biology. 2006;33:1081–1089. doi: 10.1071/FP06203. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. Abscisic acid signalling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits abscisic acid export to the shoots. Plant, Cell and Environment. 2008a;31:1263–1274. doi: 10.1111/j.1365-3040.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. Accounting for sap flow from different parts of the root system improves the prediction of xylem ABA concentration in plants grown with heterogeneous soil moisture. Journal of Experimental Botany. 2008b;59:4083–4093. doi: 10.1093/jxb/ern246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokoozlian NK, Kliewer WM. Influence of light on grape berry growth and composition varies during fruit development. Journal of the American Society of Horticultural Science. 1996;121:869–874. [Google Scholar]
- Dorji K, Behboudian MH, Zegbe-Domínguez JA. Water relations, growth, yield, and fruit quality of hot pepper under deficit irrigation and partial rootzone drying. Scientia Horticulturae. 2005;104:137–149. [Google Scholar]
- Downey MO, Harvey JS, Robinson SP. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Australian Journal of Grape and Wine Research. 2003;9:15–27. [Google Scholar]
- Downey MO, Dokoozlian NK, Krstic MP. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: a review of recent research. American Journal of Enology and Viticulture. 2006;57:257–268. [Google Scholar]
- Dry PR. International Symposium on Irrigation Management in Wine and Table Grape Vineyards. Santiago, Chile: INIA; 2005. Use of irrigation strategies for maximization of water use efficiency and wine quality in Australia. [Google Scholar]
- Dry P, Loveys BR. Factors influencing grapevine vigour and the potential for control with partial rootzone drying. Australian Journal of Grape and Wine Research. 1998;4:140–148. [Google Scholar]
- Dry PR, Loveys BR. Grapevine shoot growth and stomatal conductance are reduced when part of the root system is dried. Vitis. 1999;38:151–156. [Google Scholar]
- Dry PR, Loveys BR, Düring H. Partial drying of the rootzone of grape. II. Changes in the pattern of root development. Vitis. 2000;39:9–12. [Google Scholar]
- Dry PR, Loveys BR, McCarthy MG, Stoll M. Strategic irrigation management in Australian vineyards. Journal International de Science de la Vigne et du Vin. 2001;35:129–139. [Google Scholar]
- Du T, Kang S, Zhang J, Li F, Hu X. Yield and physiological responses of cotton to partial rootzone irrigation in the oasis field of northwest China. Agricultural Water Management. 2006;84:41–52. [Google Scholar]
- Escalona JM, Flexas J, Medrano H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Australian Journal of Plant Physiology. 1999;26:421–433. [Google Scholar]
- Esteban MA, Villanueva MJ, Lissarrague JR. Effect of irrigation on changes in berry composition of Tempranillo during maturation. Sugars, organic acids, and mineral elements. American Journal of Enology and Viticulture. 1999;50:418–434. [Google Scholar]
- Fereres E, Soriano MA. Deficit irrigation for reducing agricultural water use. Journal of Experimental Botany. 2007;58:147–159. doi: 10.1093/jxb/erl165. [DOI] [PubMed] [Google Scholar]
- Flexas J, Escalona JM, Medrano H. Down-regulation of photosynthesis by drought underfield conditions in grapevine leaves. Australian Journal of Plant Physiology. 1998;25:893–900. [Google Scholar]
- Flexas J, Hendrickson L, Chow WS. Photoinactivation of photosystem II in high light-acclimated grapevines. Australian Journal of Plant Physiology. 2001;28:755–764. [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology. 2002;29:461–471. doi: 10.1071/PP01119. [DOI] [PubMed] [Google Scholar]
- Galet P. Précis de viticulture. 7th edn. Montpellier: Imprimerie Déhan; 2000. [Google Scholar]
- Gamalei Y. Structure and function of leaf minor veins in trees and herbs. A taxonomic review. Trees. 1989;3:96–110. [Google Scholar]
- Gaudillère JP, Van Leeuwen C, Ollat N. Carbon isotope composition of sugars in grapevine, an integrated indicator of vineyard water status. Journal of Experimental Botany. 2002;53:757–763. doi: 10.1093/jexbot/53.369.757. [DOI] [PubMed] [Google Scholar]
- Geny L, Saucier C, Bracco S, Daviaud F, Glories Y. Composition and cellular localization of tannins in grape seeds during maturation. Journal of Agricultural and Food Chemistry. 2003;51:8051–8054. doi: 10.1021/jf030418r. [DOI] [PubMed] [Google Scholar]
- Giorio P, Basile A, Sorrentino G, Albrizio R. Physiological responses of Falanghina grapevines in soils with different water availability in Southern Italy. Acta Horticulturae. 2007;754:235–240. [Google Scholar]
- Giribaldi M, Perugini I, Sauvage FX, Shubert A. Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics. 2007;7:3154–3170. doi: 10.1002/pmic.200600974. [DOI] [PubMed] [Google Scholar]
- Gollop R, Farhi S, Perl A. Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Science. 2001;161:579–588. [Google Scholar]
- Gollop R, Even S, Colova-Tsolova V, Perl A. Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. Journal of Experimental Botany. 2002;53:1397–1409. [PubMed] [Google Scholar]
- Green SR, Clothier BE, McLeod DJ. The response of sap flow in apple roots to localised irrigation. Agricultural Water Management. 1997;33:63–78. [Google Scholar]
- Grimplet J, Deluc LG, Tillett RL, et al. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics. 2007;8:187. doi: 10.1186/1471-2164-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimplet J, Wheatley MD, Jouira HB, Deluc LG, Cramer GR, Cushman JC. Proteomic and selected metabolite analysis of grape berry tissues under well-watered and water-deficit stress conditions. Proteomics. 2009;9:2503–2528. doi: 10.1002/pmic.200800158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SL, Du GQ, Zoldoske D, et al. Effects of irrigation amount on water relations, vegetative growth, yield and fruit composition of Sauvignon Blanc grapevines under partial rootzone drying and conventional irrigation in the San Joaquin Valley of California, USA. Journal of Horticultural Science and Biotechnology. 2004;79:26–33. [Google Scholar]
- Hansen H, Dorffling K. Root-derived trans-zeatin riboside and abscisic acid in drought-stressed and rewatered sunflower plants: interaction in the control of leaf diffusive resistance? Functional Plant Biology. 2003;30:365–375. doi: 10.1071/FP02223. [DOI] [PubMed] [Google Scholar]
- Hartung W, Wilkinson S, Davies WJ. Factors that regulate abscisic acid concentrations at the primary site of action at the guard cell. Journal of Experimental Botany. 1998;51:361–367. [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. Journal of Experimental Botany. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- Holbrook NM, Shashidhar VR, James RA, Munns R. Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. Journal of Experimental Botany. 2002;53:1503–1514. [PubMed] [Google Scholar]
- Intrigliolo DS, Pérez D, Castel JR. Water relations of field grown drip irrigated ‘Tempranillo’ grapevine. Acta Horticulturae. 2005;689:317–323. [Google Scholar]
- IPCC. Climate change 2007: the physical basis summary for policy makers. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Jeong ST, Goto-Yamamoto N, Hashizume K, Esaka M. Expression of the flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase genes and flavonoid composition in grape (Vitis vinifera) Plant Science. 2006;170:61–69. [Google Scholar]
- Jia W, Davies WJ. Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiology. 2007;143:68–77. doi: 10.1104/pp.106.089110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Hartung W. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. Journal of Experimental Botany. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- Jimenez JB, Orea JM, Ureña AG, Escribano P, de la Osa PL, Guadarrama A. Short anoxic treatment to enhance trans-resveratrol content in grapes and wine. European Food Research and Technology. 2007;224:373–378. [Google Scholar]
- Jones GV, Davis RE. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. American Journal of Enology and Viticulture. 2000;51:249–261. [Google Scholar]
- Jones GV, White MA, Owen RC, Storchmann C. Climate change and global wine quality. Climate Change. 2005;73:319–343. [Google Scholar]
- Kang S, Zhang J. Controlled alternate partial rootzone irrigation: its physiological consequences and impact on water use efficiency. Journal of Experimental Botany. 2004;55:2437–2446. doi: 10.1093/jxb/erh249. [DOI] [PubMed] [Google Scholar]
- Kang S, Hu X, Jerie P, Zhang J. The effects of partial rootzone drying on root, trunk flow and water balance in an irrigated pear (Pyrus communis L.) orchard. Journal of Hydrology. 2003;280:192–206. [Google Scholar]
- Keller M. Deficit irrigation and vine mineral nutrition. American Journal of Enology and Viticulture. 2005;56:267–283. [Google Scholar]
- Keller M, Smith JP, Bondada BR. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany. 2006;57:2577–2587. doi: 10.1093/jxb/erl020. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Matthews MA, Waterhouse AL. Changes in grape seed polyphenols during fruit ripening. Phytochemistry. 2000;55:77–85. doi: 10.1016/s0031-9422(00)00196-5. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Matthews MA, Waterhouse AL. Effect of maturity and vine water status on grape skin and wine flavonoids. American Journal of Enology and Viticulture. 2002;53:268–274. [Google Scholar]
- Kudoyarova GR, Vysotskaya LB, Cherkozyanova Alla, Dodd IC. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. Journal of Experimental Botany. 2007;58:161–168. doi: 10.1093/jxb/erl116. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, et al. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126:1109–1120. doi: 10.1016/j.cell.2006.07.034. [DOI] [PubMed] [Google Scholar]
- van Leeuwen C, Seguin G. The concept of terroir in viticulture. Journal of Wine Research. 2006;17:1–10. [Google Scholar]
- Leuning R, Tuzet A, Perrier A. Stomata as part of the soil-plant atmosphere continuum. In: Mencuccini M, Grace J, Moncrieff J, McNaughto K, editors. Forests at the land–atmosphere interface. Edinburgh: CAB International; 2003. pp. 9–28. [Google Scholar]
- Liu F, Jensen CR, Andersen MN. Hydraulic and chemical signals in the control of leaf expansion and stomatal conductance in soybean exposed to drought stress. Functional Plant Biology. 2003;30:65–73. doi: 10.1071/FP02170. [DOI] [PubMed] [Google Scholar]
- Loveys BR. Abscisic acid transport and metabolism in grapevine (Vitis vinifera L.) New Phytologist. 1984;98:575–582. [Google Scholar]
- Loveys BR, Stoll M, Davies WJ. Physiological approaches to enhance water use efficiency in agriculture: exploiting plant signalling in novel irrigation practice. In: Bacon MA, editor. Water use efficiency in plant biology. Lancaster: University of Lancaster; 2004. pp. 113–141. [Google Scholar]
- Lovisolo C, Schubert A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. Journal of Experimental Botany. 1998;49:693–700. [Google Scholar]
- Lovisolo C, Schubert A. Mercury hinders recovery of shoot hydraulic conductivity during rehydration: evidence from a whole-plant approach. New Phytologist. 2006;172:469–478. doi: 10.1111/j.1469-8137.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- Lovisolo C, Hartung W, Schubert A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Functional Plant Biology. 2002;29:1349–1356. doi: 10.1071/FP02079. [DOI] [PubMed] [Google Scholar]
- Lovisolo C, Secchi F, Nardini A, Salleo S, Buffa R, Schubert A. Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance. Physiologia Plantarum. 2007;130:543–551. [Google Scholar]
- Lovisolo C, Perrone I, Carra A, et al. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non hydraulic interactions at the whole plant level: a physiological and molecular update. Functional Plant Biology. 2010 (in press) [Google Scholar]
- Maroco JP, Pereira JS, Chaves MM. Stomatal responses to leaf-to-air vapour pressure deficit in Sahelian species. Australian Journal of Plant Physiology. 1997;24:381–387. [Google Scholar]
- Maroco JP, Rodrígues ML, Lopes C, Chaves MM. Limitations to leaf photosynthesis in field-grown grapevine under drought – metabolic and modelling approaches. Functional Plant Biology. 2002;29:451–459. doi: 10.1071/PP01040. [DOI] [PubMed] [Google Scholar]
- Marsal J, Mata M, del Campo J, et al. Evaluation of partial rootzone drying for potential field use as a deficit irrigation technique in commercial vineyards according to two different pipeline layouts. Irrigation Science. 2008;26:347–356. [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. Plasma membrane aquaporins play a significant role during recovery from water deficits. Plant Physiology. 2002;130:2101–2110. doi: 10.1104/pp.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MA, Anderson MM. Reproductive development in grape (Vitis vinifera L.): responses to seasonal water deficits. American Journal of Enology and Viticulture. 1989;40:52–60. [Google Scholar]
- Matthews MA, Nuzzo V. Berry size and yield paradigms on grapes and wines quality. Acta Horticulturae. 2007;754:423–436. [Google Scholar]
- Mattivi F, Guzzon R, Vrhovsek U, Stefanini M, Velasco R. Metabolite profiling of grape: flavonols and anthocyanins. Journal of Agriculture and Food Chemistry. 2006;54:7692–7702. doi: 10.1021/jf061538c. [DOI] [PubMed] [Google Scholar]
- McCarthy MG, Loveys BR, Dry PR, Stoll M. Regulated deficit irrigation and partial rootzone drying as irrigation management techniques for grapevines. FAO Water Reports. 2002;22:79–87. [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulías J, Flexas J. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany. 2002;89:895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano H, Escalona JM, Cifre J, Bota J, Flexas J. A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: effects of water availability from leaf photosynthesis to grape yield and quality. Functional Plant Biology. 2003;30:607–619. doi: 10.1071/FP02110. [DOI] [PubMed] [Google Scholar]
- Merzlyak MN, Melø TB, Naqvi KR. Effect of anthocyanins, carotenoids, and flavonols on chlorophyll fluorescence excitation spectra in apple fruit: signature analysis, assessment, modelling, and relevance to photoprotection. Journal of Experimental Botany. 2008;59:349–359. doi: 10.1093/jxb/erm316. [DOI] [PubMed] [Google Scholar]
- Mingo DM, Theobald JC, Bacon MA, Davies WJ, Dodd IC. Biomass allocation in tomato (Lycopersicon esculentum) plants grown under partial rootzone drying: enhancement of root growth. Functional Plant Biology. 2004;31:971–978. doi: 10.1071/FP04020. [DOI] [PubMed] [Google Scholar]
- Monteiro A, Lopes CM. Influence of cover crop on water use and performance of vineyard in Mediterranean Portugal. Agriculture, Ecosystems and Environment. 2007;121:336–342. [Google Scholar]
- Moutinho-Pereira JM, Correia CM, Gonçalves B, Bacelar EA, Torres-Pereira JM. Leaf gas exchange and water relations of grapevines grown in three different conditions. Photosynthetica. 2004;42:81–86. [Google Scholar]
- Moutinho-Pereira J, Magalhães N, Gonçalves B, Bacelar E, Brito M, Correia C. Gas exchange and water relations of three Vitis vinífera L. cultivars growing under Mediterranean climate. Photosynthetica. 2007;45:202–207. [Google Scholar]
- Negri AS, Prinsi B, Rossoni M, et al. Proteome changes in the skin of the grape cultivar Barbera among different stages of ripening. BMC Genomics. 2008;9 doi: 10.1186/1471-2164-9-378. doi:10.1186/1471-2164-9-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PM. Coping mechanisms for crop plants in drought-prone environments. Annals of Botany. 2008;101:901–907. doi: 10.1093/aob/mcn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P, Chazen O, Bogoslavsky L, Hartung W. Role of root-derived ABA in regulating early leaf growth responses to water deficits. In: Altman A, Waisel Y, editors. Biology of root formation and development. New York: Plenum Press; 1997. pp. 147–154. [Google Scholar]
- Palliotti A, Silvestroni O, Petoumenou D. Photosynthetic and photoinhibition behavior of two field-grown grapevine cultivars under multiple summer stresses. American Journal of Enology and Vitiulture. 2009;60:189–198. [Google Scholar]
- Paranychianakis NV, Chartzoulakis KS, Angelakis AN. Influence of rootstock, irrigation level and recycled water on water relations and leaf gas exchange of Soultanina grapevines. Environmental Experimental Botany. 2004;52:185–198. [Google Scholar]
- Passioura J. The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany. 2007;58:113–117. doi: 10.1093/jxb/erl212. [DOI] [PubMed] [Google Scholar]
- Patakas A, Noitsakis B. Mechanisms involved in diurnal changes of osmotic potential in grapevines under drought conditions. Journal of Plant Physiology. 1999;154:767–774. [Google Scholar]
- Poni S, Bernizzoni F, Civardi S. Response of ‘Sangiovese’ grapevines to partial rootzone drying: gas-exchange, growth and grape composition. Scientia Horticulturae. 2007;114:96–103. [Google Scholar]
- Poni S, Bernizzonia F, Civardia S, Gattia M, Porro D, Caminc F. Performance and water-use efficiency (single-leaf vs. whole-canopy) of well-watered and half-stressed split-root Lambrusco grapevines grown in Po Valley (Italy) Agriculture, Ecosystems & Environment. 2009;129:97–106. [Google Scholar]
- Pospísilová J, Vágner M, Malbeck J, Trávnícková A, Batková P. Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biologia Plantarum. 2005;49:533–540. [Google Scholar]
- Prokic L, Jovanovic Z, McAinsh MR, Vucinic Z, Stikic R. Species-dependent changes in stomatal sensitivity to absicisic acid mediated by external pH. Journal of Experimental Botany. 2006;57:675–683. doi: 10.1093/jxb/erj057. [DOI] [PubMed] [Google Scholar]
- Pudney S, McCarthy MG. Water use efficiency of field grown Chardonnay grapevines subjected to partial rootzone drying and deficit irrigation. Acta Horticulturae. 2004;664:567–573. [Google Scholar]
- Quick WP, Chaves MM, Wendler R, et al. The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant, Cell and Environment. 1992;15:25–35. [Google Scholar]
- Roby G, Matthews MA. Relative proportions of seed, skin and flesh, in ripe berries from Cabernet Sauvignon grapevines grown in a vineyard either well irrigated or under water deficit. Australian Journal of Grape and Wine Research. 2004;10:74–82. [Google Scholar]
- Roby G, Harbertson JF, Adams DA, Matthews MA. Berry size and vine water deficits as factors in winegrape composition: anthocyanins and tannins. Australian Journal of Grape and Wine Research. 2004;10:100–107. [Google Scholar]
- Rodrigues ML, Chaves MM, Wendler R, et al. Osmotic adjustment in water stressed grapevine leaves in relation to carbon assimilation. Australian Journal of Plant Physiology. 1993;20:309–321. [Google Scholar]
- Rodrigues ML, Santos T, Rodrigues AP, et al. Hydraulic and chemical signalling in the regulation of stomatal conductance and plant water use of field grapevines growing under deficit irrigation. Functional Plant Biology. 2008;35:565–579. doi: 10.1071/FP08004. [DOI] [PubMed] [Google Scholar]
- Rogiers SY, Greer DH, Hutton RJ, Landsberg JJ. Does nigh-time transpiration contribute to anisohydric behaviour in a Vitis vinifera cultivar? Journal Experimental Botany. 2009;60:3751–3763. doi: 10.1093/jxb/erp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sadras VO. Does partial rootzone drying improve irrigation water productivity in the field? A meta-analysis. Irrigation Science. 2009;27:183–190. [Google Scholar]
- Salleo S, Lo Gullo MA. Different aspects of cavitation resistance in Ceratonia siliqua, a drought-avoiding Mediterranean tree. Annals of Botany. 1989;64:325–336. [Google Scholar]
- Santesteban LG, Miranda C, Royo JB. Effect of water deficit and rewatering on leaf gas exchange and transpiration decline of excised leaves of four grapevine (Vitis vinifera L.) cultivars. Scientia Horticulturae. 2009;121:434–439. [Google Scholar]
- Santos T, Lopes C, Rodrigues ML, et al. Partial rootzone drying effects on growth and fruit quality of field-grown grapevines (Vitis vinifera) Functional Plant Biology. 2003;30:663–671. doi: 10.1071/FP02180. [DOI] [PubMed] [Google Scholar]
- Santos T, Lopes C, Rodrigues ML, et al. Effects of partial rootzone drying irrigation on cluster microclimate and fruit composition of Castelão field-grown grapevines. Vitis. 2005;44:117–125. [Google Scholar]
- Santos T, Lopes CM, Rodrigues ML, et al. Partial rootzone drying irrigation affects cluster microclimate improving fruit composition of ‘Moscatel’ field-grown grapevines. Scientia Horticulturae. 2007;112:321–330. [Google Scholar]
- Sauter A, Dietz KJ, Hartung W. A possible stress physiological role of abscisic acid conjugates in root to shoot signalling. Plant, Cell and Environment. 2002;25:233–228. doi: 10.1046/j.1365-3040.2002.00747.x. [DOI] [PubMed] [Google Scholar]
- Schachtmann DP, Goodger JQD. Chemical root to shoot signalling under drought. Trends in Plant Science. 2008;13:281–287. doi: 10.1016/j.tplants.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Schmulling T. New insights into the functions of cytokinins in plant development. Journal of Plant Growth Regulation. 2002;21:40–49. doi: 10.1007/s003440010046. [DOI] [PubMed] [Google Scholar]
- Schultz HR. Water relations and photosynthetic responses of two grapevine cultivars of different geographical origin during water stress. Acta Horticulturae. 1996;427:251–266. [Google Scholar]
- Schultz HR. Climate change and viticulture: a European perspective on climatology, carbon dioxide and UV-B effects. Australian Journal of Grape and Wine Research. 2000;1:1–12. [Google Scholar]
- Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell and Environment. 2003;26:1393–1405. [Google Scholar]
- Schultz HR. Proceedings Congres on Climate and Viticulture. Saragoza: Centro Transferencia Agroalimentaria; 2007. Climate change: implications and potential adaptation of vine growth and wine composition; pp. 87–92. [Google Scholar]
- Schultz HR, Matthews MA. Resistance to water transport in shoots of Vitis vinifera L. Plant Physiology. 1988;88:718–724. doi: 10.1104/pp.88.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RG, Davies WJ. Variability among species in the apoplastic pH signalling response to drying soils. Journal of Experimental Botany. 2009;60:4363–4370. doi: 10.1093/jxb/erp273. [DOI] [PubMed] [Google Scholar]
- Shashidhar VR, Prasad TG, Sudharshan L. Hormonal signals from roots to shoots of sunflower (Helianthus annuus L.) Moderate soil drying increases delivery of absicisic acid and depresses delivery of cytokinins in the xylem sap. Annals of Botany. 1996;78:151–155. [Google Scholar]
- Shellie K, Glenn DM. Wine grape response to kaolin particle film under deficit and well-watered conditions. Acta Horticulturae. 2008;792:587–591. [Google Scholar]
- Sheltie KC. Vine and berry response of Merlot (Vitis vinifera L.) to differential water stress. American Journal of Enology and Viticulture. 2006;57:514–518. [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. PIP1 Plasma membrane aquaporins in tobacco: from cellular effects to function in plants. The Plant Cell. 2002;14:869–876. doi: 10.1105/tpc.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestroni O, Mattioli S, Neri D, Palliotti A, Cartechini A. Down-regulation of photosynthetic activity for field-grown grapevines. Acta Horticulturae. 2005;689:285–291. [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Australian Journal of Grape and Wine Research. 2006;12:2–12. [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial rootzone drying. Journal of Experimental Botany. 2004;55:2353–2363. doi: 10.1093/jxb/erh204. [DOI] [PubMed] [Google Scholar]
- Sousa TA, Oliveira MT, Pereira JM. Physiological indicators of plant water status of irrigated and non-irrigated grapevines in low rainfall area of Portugal. Plant and Soil. 2006;282:127–134. [Google Scholar]
- Souza CR, Maroco JP, Santos T, et al. Partial rootzone-drying: regulation of stomatal aperture and carbon assimilation in field grown grapevines (Vitis vinifera cv Moscatel) Functional Plant Biology. 2003;30:653–662. doi: 10.1071/FP02115. [DOI] [PubMed] [Google Scholar]
- Souza CR, Maroco J, Santos T, et al. Control of stomatal aperture and carbon uptake by deficit irrigation in two grapevine cultivars. Agriculture, Ecosystems and Environment. 2005a;106:261–274. [Google Scholar]
- Souza CR, Maroco J, Santos T, et al. Impact of deficit irrigation on water use efficiency and carbon isotope composition (δ13C) of field-grown grapevines under Mediterranean climate. Journal of Experimental Botany. 2005b;56:2163–2172. doi: 10.1093/jxb/eri216. [DOI] [PubMed] [Google Scholar]
- Sperry JS. Relationship of xylem embolism to xylem pressure potential, stomatal closure, and shoot morphology in the palm Rhapis excelsa. Plant Physiology. 1986;80:110–116. doi: 10.1104/pp.80.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Comstock JP, Oren R. Water deficits and hydraulic limits to leaf water supply. Plant, Cell and Environment. 2002;25:251–264. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Stoll M, Loveys B, Dry P. Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany. 2000;51:1627–1634. doi: 10.1093/jexbot/51.350.1627. [DOI] [PubMed] [Google Scholar]
- Syvertsen JP, Lloyd J, McConchie C, Kriedemann PE, Farquhar GD. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant, Cell and Environment. 1995;18:149–157. [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant, Cell and Environment. 2006;29:993–1001. doi: 10.1111/j.1365-3040.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Tyerman S. A novel plant-based sensor to monitor vine water stress. Final Report. Cooperative Research Centre for Viticulture. Australia. 2007. http://www.crcv.com.au/research/programs/two/2.1.7%20Final%20Report.pdf .
- Vandeleur RK, Mayo G, Shelden MC, Gillihamn M, Kaiser BN, Tyerman SD. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anysohydric cultivars of grapevine. Plant Physiology. 2009;149:445–460. doi: 10.1104/pp.108.128645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versari A, Parpinello GP, Tornielli GB, Ferrarini R, Giulivo C. Stilbene compounds and stilbene synthase expression during ripening, wilting and UV treatment in grape cv. Corvina. Journal of Agriculture and Food Chemistry. 2001;49:5531–5536. doi: 10.1021/jf010672o. [DOI] [PubMed] [Google Scholar]
- Vezzuli S, Civardi S, Ferrari F, Bavaresco L. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. American Journal of Enology and Viticulture. 2007;58:530–533. [Google Scholar]
- Voisin AS, Reidy B, Parent B, et al. Are ABA, ethylene or their interaction involved in the response of leaf growth to soil water deficit? An analysis using naturally occurring variation or genetic transformation of ABA production in maize. Plant, Cell and Environment. 2006;29:1829–1840. doi: 10.1111/j.1365-3040.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- Walker AR, Lee E, Bogs J, McDavid DAJ, Thomasand MR, Robinson SP. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant Journal. 2007;49:772–785. doi: 10.1111/j.1365-313X.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Deloire A, Carbonneau A, Federdpiel B, Lopez F. An in vivo experimental system to study sugar phloem unloading in ripening grape berries during water deficiency stress. Annals of Botany. 2003;92:523–528. doi: 10.1093/aob/mcg159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb LB, Whetton PH, Barlow EWR. Modelled impact of future climate change on the phenology of winegrapes in Australia. Australian Journal of Grape and Wine Research. 2007;13:165–175. [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. Water use efficiency and chemical signalling. In: Bacon MA, editor. Water use efficiency in plant biology. Oxford: Blackwell Publishing/CRC Press; 2004. pp. 75–112. [Google Scholar]
- Wilkinson S, Davies WJ. Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiology. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant, Cell and Environment. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- Williams LE, Baeza P. Relationships among ambient temperature and vapor pressure deficit and leaf and stem water potentials of fully irrigated, field-grown grapevines. American Journal of Enology and Viticulture. 2007;58:173–181. [Google Scholar]
- Williams LE, Matthews MA. Grapevine. In: Stewart BA, Nielsen DR, editors. Irrigation of agricultural crops (agronomy) Wisconsin: ASA-CSSA-SSSA; 1990. pp. 1019–1055. [Google Scholar]
- Xie DY, Dixon A. Proanthocyanidin biosynthesis-still more questions than answers? Phytochemistry. 2005;66:2127–2144. doi: 10.1016/j.phytochem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Zsófi Z, Tóth E, Váradi G, Rusjan D, Bálo B. The effect of progressive drought on water relations and photosynthetic performance of two grapevine cultivars (Vitis vinifera L.) Acta Biologica Szegediensis. 2008;52:321–322. [Google Scholar]
- Zsófi Z, Gál L, Szilágyi Z, et al. Use of stomatal conductance and pre-dawn water potential to classify terroir for the grape variety Kékfrankos. Australian Journal of Grape and Wine Research. 2009a;15:36–47. [Google Scholar]
- Zsófi Z, Váradi G, Bálo B, Marschall M, Nagy Z, Dulai S. Heat acclimation of grapevine leaf photosynthesis: mezo and macroclimatic aspects. Functional Plant Biology. 2009b;36:310–322. doi: 10.1071/FP08200. [DOI] [PubMed] [Google Scholar]