Abstract
Background and Aims
Experiments show that inbred progenies are frequently more damaged by herbivores than outcrossed progenies, suggesting that selfing is costly when herbivores are present and can increase the magnitude of inbreeding depression in survival and reproductive components of fitness. The present study assesses whether inbreeding increases herbivory and estimates the magnitude of inbreeding depression on reproductive components of fitness in the annual plant Datura stramonium.
Methods
Two experiments were performed under natural conditions of herbivory to assess the effect of inbreeding on plant damage in D. stramonium. In the first experiment, outcrossed progeny was generated using foreign pollen donors, whereas inbred progeny was produced by self-pollination. In both groups, survival, herbivore damage and reproductive components of fitness were measured. In the second experiment, inbred and outcrossed progenies were produced using only local pollen donors, and only damage by herbivores was measured.
Key Results
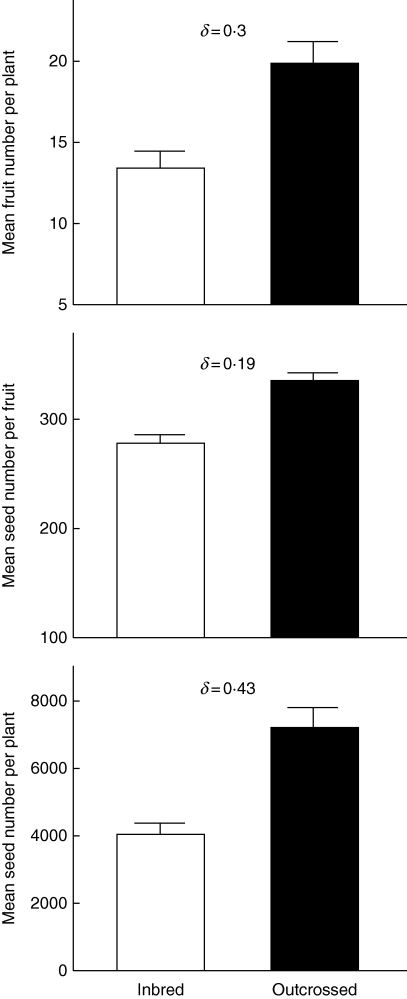
Despite yearly variation in damage caused by the same specialist herbivores, inbred progeny suffered consistently more damage than outcrossed progeny. There was a significant inbreeding depression for fruit number (δ = 0·3), seed number per fruit (δ = 0·19) and seed number per plant (δ = 0·43). Furthermore, significant genetic variation amongst families in the magnitude of inbreeding depression was observed.
Discussion
The results suggest that the plant's mating system modified the pattern of herbivory by specialist insects in D. stramonium. Inbred plants suffer not only from the genetic cost of low vigour but also from greater damage by herbivores. The mechanism by which inbreeding reduces plant resistance to herbivores remains unknown but is an interesting area for future research.
Keywords: Inbreeding depression, mating system, plant defence, total resistance, herbivores, Datura stramonium
INTRODUCTION
Selfing, the ability of an individual to fertilize ovaries with its own pollen, is common in many cultivated and wild flowering plant species (Bronstein et al., 2009; Karron et al., 2009), and inbred progeny can often exhibit very different phenotypic characteristics from outcrossed conspecifics from the same population (Armbruster and Reed, 2005; Kouonon et al., 2009; Vallejo-Marin and Barrett, 2009). Inbreeding frequently reduces individual fitness of the progeny, a phenomenon known as inbreeding depression, and there are two non-mutually exclusive genetic mechanisms whereby increased homozygosity can lower plant fitness (Charlesworth and Charlesworth, 1987). Under the dominance hypothesis, inbreeding depression arises when a progeny is homozygous for partially deleterious recessive alleles. Alternatively, the over-dominance hypothesis states that inbreeding can reduce heterozygosis, resulting in a loss of hybrid vigour (Charlesworth and Charlesworth, 1987). However, outcrossing can also have negative effects on fitness if it decouples complexes of co-adapted genes for the local environment (i.e. outbreeding depression; Maynard-Smith, 1978; Lynch, 1991). Thus, the mating system adopted by the population can be of relevance because inbreeding might be disadvantageous by lowering both the defensive capabilities of plants and their fitness components in relation to outcrossing-derived offspring.
Inbreeding can affect interactions with pollinators (Ivey and Carr, 2005; Ferrari et al., 2006), mycorrizha (Botham et al., 2009) or a plant's natural enemies; inbred offspring suffer more damage by insect herbivores than outcrossed individuals (Núñez-Farfán et al., 2007). This suggests that inbreeding can alter the genetic background of plant resistance to, or tolerance of, herbivore attack (Strauss and Karban, 1994; Carr and Eubanks, 2002; Hayes et al., 2004; Leimu et al., 2008). By limiting genetic variation, inbreeding can affect the chemical or physical qualities of plants that prevent herbivore damage, reducing further their reproductive potential. Outcrossing, by contrast, can maintain defensive traits that lower the fitness cost of herbivory (Carr and Eubanks, 2002; Ivey et al., 2004; Hull-Sanders and Eubanks, 2005). Consequently, in natural ecosystems inbred and outcrossed plants should experience different rates of herbivore attack and thus vary greatly in their potential recruitment success. As seedling herbivory is one of the key environmental factors dictating plant recruitment (Hanley and Sykes, 2009), it is vital that ecologists estimate the overall cost of inbreeding depression on plant defence.
It is also thought that severe environmental filters to plant recruitment favour outcrossing because they amplify the expression of recessive deleterious alleles responsible for inbreeding depression (Armbruster and Reed, 2005); therefore, selfing should impose higher fitness costs in the presence of herbivores if inbred offspring have a reduced anti-herbivore defence in comparison with outcrossed conspecifics. Indeed, theoretical work suggests that the interaction between selection imposed by natural enemies and inbreeding depression can prevent the adoption of selfing as a reproductive strategy (Lively and Howard, 1994). Thus, inbreeding might incur an additional cost because herbivory reduces the leaf area needed to carry out photosynthesis and can lower resource allocation to reproduction (Levri and Real, 1998).
In order to assess whether inbreeding affects damage by herbivores, inbred and outcrossed progenies in Datura stramonium (Solanaceae) were generated. Previous work on this species has suggested that plant traits are selected for their resistance to herbivores, and, in addition, negative effects of inbreeding on plant fitness have also been detected (Valverde et al., 2001; Sosenski, 2004). Plant damage by herbivores of inbred and outcrossed progenies was measured over 2 years in field conditions where plants were exposed to natural herbivores. In addition, inbreeding depression on plant survival was estimated together with three reproductive components of fitness: fruit number, seeds per fruit and seeds per plant.
METHODS
Plant species
Datura stramonium L. is a self-compatible annual plant native to Mexico but with a worldwide distribution (Weaver and Warwick, 1984; van Kleunen et al., 2007). This species produces hermaphroditic flowers with a variation in the anther–stigma distance that has been shown to influence the outcrossing rate (Motten and Antonovics, 1992; Motten and Stone, 2000). Although population outcrossing rates reported for D. stramonium are low (0·01–0·08; Motten and Antonovics, 1992), extensive amongst-family variation in outcrossing rates has been reported (range 0–0·80; Cuevas, 1996). Inbreeding depression has been reported as ranging from δ = 0·15 for fruit production up to δ = 0·39 for seed production (Núñez-Farfán et al., 1996; Stone and Motten, 2002).
In central Mexico, leaves of D. stramonium are consumed by the specialist herbivores Epitrix parvula (Coleoptera: Chrysomelidae) and the three-lined potato beetle Lema trilineata (Coleoptera: Chrysomelidae) and by the generalist Sphenarium purpurascens (Orthoptera: Pyrgomorphidae) (Núñez-Farfán and Dirzo, 1994). The foliar damage caused by these herbivores imposes selection on resistance and tolerance (Núñez-Farfán and Dirzo, 1994; Valverde et al., 2001; Fornoni et al., 2004). Tropane alkaloids and leaf trichomes are putative components of resistance to herbivory in D. stramonium (Shonle and Bergelson, 2000; Valverde et al., 2001), whereas leaf production is associated with the ability to tolerate damage by herbivores (Fornoni and Núñez-Farfán, 2000;Valverde et al., 2003).
Experimental design
Seeds used for this experiment were collected in 1999 from a population of D. stramonium in the locality of Teotihuacan (State of Mexico; 19°41′N, 98°51′E) and were stored in the laboratory until hand pollination was carried out in a greenhouse. To produce selfed and outcrossed progenies, seeds from 20 randomly chosen plants (hereafter families) were sown in plastic pots filled with a commercial soil mixture and were watered daily until germination. One seedling per family was randomly selected and transplanted individually to a 3-L plastic pot. During flowering, flowers were hand pollinated at the same position on four separate branches of each plant to minimize competition between fruits. Prior to manual pollination, flowers were emasculated. Self-pollination was accomplished by rubbing the pollen of three anthers against the stigma of the same flower. Cross-pollination was achieved by rubbing one anther of each of three different pollen donors, randomly selected, onto the stigma of a flower. Flowers were tagged and bagged after pollination. Pollen donors used for cross-pollinations were derived from a population from Ritland (MO, USA), thus ensuring that outcrossed progeny had an inbreeding coefficient f = 0 (Falconer and Mackay, 1997, p. 58). Fruits were collected in paper bags and tagged before they started to open.
In 2006, a second experiment was performed following the crossing protocol described above to generate inbred and outcrossed seeds; however, cross-pollinations were achieved using local pollen donors (i.e. from Teotihuacan). The aim was to assess if the differences in damage by herbivores between inbred and outcrossed progenies observed in 2004 were due to the ‘introduction of new resistance alleles’ from Missouri to Teotihuacan instead of inbreeding depression in plant defence.
Fieldwork
In June 2004, in an effort to obtain ten inbred seedlings and ten outcrossed seedlings for each of the 20 families (n = 400), seeds produced by the two pollination types were sowed in plastic trays in a greenhouse. In total, 169 inbred and 160 outcrossed seedlings from 17 families were obtained (total sample size = 329). In July 2004, the seedlings were transplanted to a 400-m2 experimental plot where plants were arranged in a completely randomized design (Cochran and Cox, 1957) and spaced 1 m apart to prevent competition effects on inbreeding depression (Cheptou et al., 2000).
In August 2006, inbred and outcrossed seedlings of 19 families (total sample size = 293) were planted in the same experimental plot to ensure that plants experienced similar environmental conditions, following the same procedure described above.
Herbivory
To estimate the individual proportion of damage consumed by herbivores, a random sample of 20 leaves per plant was collected, encompassing leaves along the stem and branches to prevent a biased estimation of resistance caused by possible variation in chemical defence due to leaf age. The total and damaged area of a leaf was measured using a digital image analyser (WinDias Basic; Delta-T Devices Ltd, Cambridge, UK). The intact leaf area of the damaged leaves was estimated using a regression model obtained from a sample of undamaged leaves for which lengths, widths and area were known [Leaf area = 8·2080 (Leaf length) + 0·5704 (Leaf length – 8·4861)2 – 35·9162; R2 = 0·92, P < 0·0001, n = 26). The proportion of damage (Di) of an individual plant was calculated as:
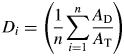 |
where AD and AT are the damaged and total area of a leaf, respectively, and n is the sample size of the leaves (after Núñez-Farfán and Dirzo, 1994). The inverse of the proportional damage, Ri = 1 – Di, is commonly related to ‘total resistance’ to herbivores (cf. Leimu and Koricheva, 2006).
Fitness estimates
Plant survival was censused from planting through reproduction and was recorded as a binary variable (alive = 1; dead = 0). At the end of the reproductive season, all mature fruits of each plant were collected individually in paper bags. The numbers of fruits and seeds are good estimators of individual fitness in this species (Núñez-Farfán and Dirzo, 1994; Núñez-Farfán et al., 1996; Stone and Motten, 2002). Survival, fruit number and seed number were estimated only in 2004.
Statistical analyses
Effects of inbreeding on herbivory
In order to compare the variation in damage between inbred and outcrossed progenies and between years (i.e. 2004 and 2006 experiments), an ANOVA was performed, including the effects of pollination type, year and their interaction.
Using the data obtained in the 2004 experiment, we assessed the effect of pollination type (fixed), family (random), and the family × pollination type interaction (random) on plant damage by herbivores and on three reproductive components of fitness by means of a mixed-model ANOVA. In order to improve normality and meet ANOVA assumptions, plant damage was arcsine-root transformed and reproductive components of fitness were log transformed, prior to statistical analyses (Zar, 1996). All analyses were run in JMP 5·01 (SAS Institute, Cary, NC, USA).
Plant survival
Plant survival amongst families and between pollination types was analysed by means of a survival analysis, assuming the Cox proportional hazards model (Vittinghoff et al., 2005).
Inbreeding depression
Inbreeding depression (δ) estimates for total resistance, fruit and seed number, and seed per fruit were computed as:
where ϖinbred and ϖoutcrossed are the mean values for each character of inbred and outcrossed progenies, respectively (Charlesworth and Charlesworth, 1987).
RESULTS
Effects of inbreeding herbivory
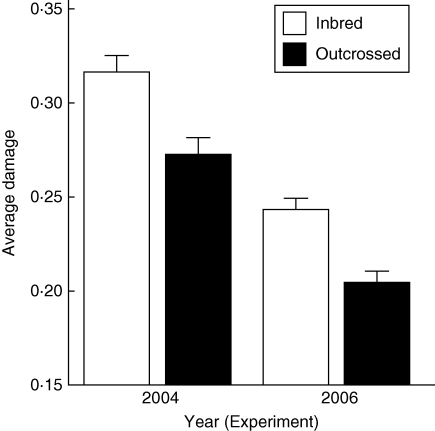
Plants were damaged by their specialist herbivores L. trilineata and E. parvula in both experiments. The ANOVA detected a significant annual effect in average foliar damage. Average damage (Di) was significantly higher in 2004 when pollen donors were from Missouri than in 2006 when outcrossing was performed with local pollen donors (Di,2004 = 0·29, s.e. = 0·005 vs. Di,2006 = 0·22, s.e. = 0·004; F = 115·24, d.f. = 1; P < 0·0001; n = 483). In both years, outcrossed progeny received lower damage than inbred progeny (2004 Di,inbred = 0·32, Di,outcross = 0·27 vs. 2006 Di,inbred = 0·24, Di,outcross = 0·20). The interaction of pollination type × year was not significant (Fig. 1), suggesting an inbreeding depression of total resistance of a similar magnitude between years (δ2004 = 0·068 vs. δ2006 = 0·05; Fig. 1).
Fig. 1.
Average proportion of damage (Di) by herbivores (+ s.e.) of inbred and outcrossed progenies of Datura stramonium under natural conditions in the locality of Teotihuacan, Mexico. In 2004, cross-pollinations were performed using pollen donors from Missouri (n = 189). In 2006, cross-pollinations were performed using pollen donors from Teotihuacan. Self-pollination was performed using pollen of the same flower in a plant (ninbred = 143, noutcrossed = 150).
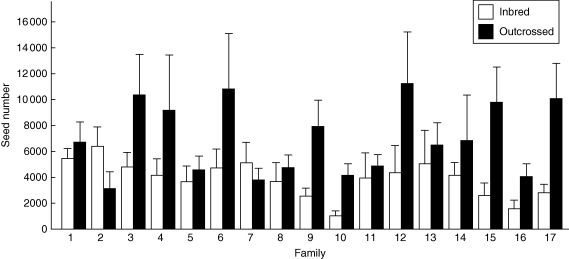
In the 2004 experiment, plant damage by herbivores was significantly affected by pollination type, whereas the effects of family and family × pollination type were not significant (Table 1a). Similarly, the three reproductive components of fitness were significantly affected by pollination type (Table 1b–d). The family × pollination type interaction was significant only for total seed number (F = 1·70, d.f. = 16; P = 0·051; n = 204; Table 1, Fig. 2), indicating the existence of genetic variation for this fitness component. The family effect was not significant for the three reproductive characters.
Table 1.
Mixed model analysis of variance of damage, number of fruits, seeds per fruit and total seed number per plant in relation to family, pollination type and interaction
| Trait | Source of variation | d.f. | SS | F |
|---|---|---|---|---|
| (a) Damage 2004 | Family | 16 | 0·15 | 0·78 n.s. |
| Pollination type | 1 | 0·07 | 6·57* | |
| Family × Pollination type | 16 | 0·19 | 1·52 n.s. | |
| Error | 156 | 1·21 | ||
| r = 0·33* | ||||
| (b) Number of fruits per plant | Family | 16 | 1·86 | 1·13 n.s. |
| Pollination type | 1 | 0·83 | 7·91* | |
| Family × Pollination type | 16 | 2·62 | 1·57 n.s. | |
| Error | 171 | 17·92 | ||
| r = 0·32* | ||||
| (c) Number of seeds per fruit | Family | 16 | 0·31 | 1·35 n.s. |
| Pollination type | 1 | 0·44 | 30·58*** | |
| Family × Pollination type | 16 | 0·28 | 1·20 n.s. | |
| Error | 171 | 2·48 | ||
| r = 0·41*** | ||||
| (d) Number of seeds per plant | Family | 16 | 3·44 | 0·13 n.s. |
| Pollination type | 1 | 2·75 | 14·52** | |
| Family × Pollination type | 16 | 5·14 | 1·70† | |
| Error | 171 | 33·45 | ||
| r = 0·36* |
Results correspond to the experiment carried out in 2004. r and error of each model is provided. ninbreeding = 96; noutcrossing = 109.
†P = 0·05; *P < 0·05; **P < 0·01; ***P < 0·001; n.s., not significant.
Fig. 2.
Mean seed number (+ s.e.) of inbred and outcrossed progenies from 17 families of Datura stramonium corresponding to the experiment carried out in 2004.
Plant survival
The probability of survival was 16 % lower for inbred than for outcrossed progeny (outcrossing = 0·71, inbreeding = 0·60), but the analysis failed to detect significant differences amongst families (χ2 = 14·53, d.f. = 16; P = 0·55; n = 329) or pollination type (χ2 = 1·89, d.f. = 1; P = 0·16; n = 329).
Inbreeding depression
As indicated by the mixed-model ANOVA, total resistance and the three reproductive components of fitness showed inbreeding depression (Table 1), with coefficients ranging from δ = 0·068 for total resistance up to δ = 0·43 for total seed number per plant (Fig. 3).
Fig. 3.
Average values (+ s.e.) of the three reproductive components (n = 204) of inbred and outcrossed progenies of Datura stramonium grown in 2004 and their corresponding inbreeding depression coefficient (δ).
DISCUSSION
Inbreeding affects the expression of plant resistance and tolerance against various natural enemies (reviewed in Núñez-Farfán et al., 2007). Because resistance to herbivory has evolved to maximize individual fitness in the presence of herbivores, variation in resistance generated by inbreeding could give rise to different selective pressures on inbred and outcrossed plants. In the present study, inbred progenies were more damaged by herbivores in 2 years regardless of the origin of parental pollen donors. The reduction in leaf area was higher in inbred than in outcrossed plants, suggesting that the plant mating system altered the pattern of herbivory. Moreover, fitness components of D. stramonium were affected by inbreeding. With the exception of plant survival, the experiment revealed the existence of genetic variations in three reproductive components of D. stramonium, as indicated by the inbreeding depression coefficients. Finally, the heterogeneous effect of inbreeding on seed production amongst families suggests the existence of genetic variation in inbreeding depression, possibly caused by a different number or type of recessive deleterious alleles carried by different lineages. Altogether, the results showed that inbreeding is costly in terms of resistance to herbivores and fitness.
Inbreeding increases homozygosis at all loci and is likely to have important consequences for complex polygenic traits of plant defence, such as resistance, tolerance and vigour (Darwin, 1876; Núñez-Farfán et al., 1996, 2007). Most studies to date have shown that one generation of inbreeding can reduce herbivore resistance (Núñez-Farfán et al., 2007); other studies, however, have failed to detect any such effect. This contrasting evidence suggests that a negative effect of inbreeding in resistance is not a rule (Strauss and Karban, 1994; Núñez-Farfán et al., 1996). Although the level of herbivory was variable amongst years, the present study showed that inbred plants received consistently more leaf damage than outcrossed plants in both years, suggesting a cost of selfing in ‘total resistance’ for this population. In contrast, there can be geographical variation in the cost of inbreeding for herbivory in D. stramonium. Núñez-Farfán et al. (1996) failed to detect differences in herbivory between inbred and outcrossed progenies in a different population even though plants were damaged by the same herbivore guild. As plants of both populations were subject to attack by the same herbivores, it seems possible that inbreeding depression in resistance can be a result of a different history of inbreeding amongst populations (Carr and Eubanks, 2002) or geographical variation in the intensity of the interaction between plants and enemies (Ouborg et al., 2000; Thompson, 2005).
The different levels of damage observed between inbred and outcrossed plants may be related to changes in leaf trichome density and/or tropane alkaloid concentration, putative components of defence in D. stramonium (Shonle and Bergelson, 2000; Valverde et al., 2001; Fornoni et al., 2003). Genetic variation and population differentiation in leaf trichome density has been detected in D. stramonium (Valverde et al., 2001), and an increase in homozygosity could have affected the expression of this character in our experimental plants. Likewise, alkaloids or other secondary compounds may be altered by inbreeding, explaining the greater amount of damage on inbred plants (Kennedy and Barbour, 1992). Other studies have found that inbreeding altered the amount of plant volatile compounds in Cucurbita peppo (Cucurbitaceae) and Solanum carolinense (Solanaceae), and that these alterations were likely to modify antagonistic and mutualistic interactions with their herbivores and pollinators (Ferrari et al., 2006; Delphia et al., 2009b). On the other hand, inbreeding could change plant nutritional value (i.e. nitrogen content), influencing herbivores to consume more leaf area in inbred than in outcrossed plants. Previous studies suggested that herbivores might compensate for a lower nutritional quality by consuming more leaf tissue (Leimu et al., 2008; Delphia et al., 2009a). How inbreeding modifies the defensive or nutritional plant characters that produce differences in herbivory in D. stramonium remains to be determined.
In plants, stressful factors, such as competition or herbivory, can affect resource allocation to growth and reproduction (Stephenson, 1981). Inbreeding can generate offspring with reduced competitive ability or lowered resistance to natural enemies under stressful environmental conditions than under a benign environment such as a greenhouse (Armbruster and Reed, 2005). The present study detected an eight-fold increase in the magnitude of inbreeding depression in seed production compared with estimates obtained for the same population in the greenhouse (i.e. δ = 0·43 in the current study vs. δ = 0·05 in the greenhouse; Sosenski, 2004). Several studies have reported intense inbreeding depression coefficients in field experiments where plants can experience different types of stress, such as nutrient and water limitation or herbivory and pathogen attack, whereas greenhouse conditions minimize those stressful conditions (Armbruster and Reed, 2005). In the present study, D. stramonium plants were exposed to natural conditions of herbivory that commonly reduce plant fitness (Valverde et al., 2001; Fornoni et al., 2004), whereas plants in the greenhouse did not receive any damage. It seems that herbivory, amongst other stressing factors, intensified selfing costs by reducing the capability of allocating resources to seed production in inbred plants of D. stramonium (Núñez-Farfán et al., 1996; Levri and Real, 1998).
Genetic variation for inbreeding depression in seed number was found here, as suggested by the different effects of inbreeding amongst families (i.e. family × pollination type interaction). In most families, outcrossed progeny outperformed inbred progeny. However, inbred progeny of two families produced more seeds than outcrossed progeny, suggesting a loss of the selfing cost in those lineages (i.e. Families 2 and 7). Amongst-family variation in inbreeding depression can be explained by a difference in the amount and/or type of recessive deleterious alleles that could be accumulated due to a random accumulation of mutations, which results in inbred lineages with varying fitness reduction as compared with their outcrossed relatives (Schultz and Willis, 1995). On the other hand, a variation in selfing rates can also produce a variation in inbreeding depression because highly self-fertilizing lineages may purge deleterious alleles, leading to low inbreeding, whereas outcrossing lineages can maintain deleterious alleles in the population (Byers and Waller, 1999). Seed production in D. stramonium depends on self-fertilization, but outcrossing still occurs within populations (Stone and Motten, 2002; van Kleunen et al., 2007). Amongst-family variation in inbreeding depression in D. stramonium has been related to the individual anther–stigma distance (i.e. herkogamy), a heritable floral character implicated in the plant mating system, suggesting that different levels of inbreeding depression might be associated with inbreeding history (Motten and Antonovics, 1992; Motten and Stone, 2000; Stone and Motten, 2002). The present study population also had individual variations in anther–stigma distance (Sosenski, 2004), suggesting that there might be some lineages with different inbreeding histories. Thus, families that experienced low inbreeding depression may have purged some deleterious mutations, whereas outcrossing families might maintain deleterious recessives alleles in the population that were exposed by self-fertilization and resulted in strong inbreeding depression.
One caveat is that our outcrossing design could have combined resistance genes from the Teotihuacan and Missouri populations in 2004, and the relative difference in damage by herbivores between inbred and outcrossed progenies might have resulted because of the potential introduction of ‘new resistance alleles’ rather than a reduction in resistance brought about by inbreeding. However, when both progenies were generated using only local pollen donors and were exposed to herbivores (i.e. Teotihuacan in 2006), outcrossed plants were again more resistant than inbred ones. Thus, the hypothesis of the introduction of new resistance alleles has no support. In addition, the combination of genes from two distant populations could produce outbreeding depression (i.e. the negative effects of outcrossing). Because outcrossed progenies were superior to inbred progenies in defence and reproductive components, this explanation can also be ruled out. Thus, the most parsimonious explanation for the different amounts of damage found between progenies in this experiment is the existence of inbreeding depression in components of resistance or nutritional value.
This study detected costs of selfing in terms of increased herbivory and reduced seed production, suggesting that herbivores can be a factor in the evolution of the plant mating system. The finding of genetic variations in inbreeding depression for seed production suggests that this population has different types and amounts of detrimental alleles not removed by selection (Byers and Waller, 1999). Thus, variation in the cost of selfing could possibly influence the existence of a mixed plant mating system due to a low ability of ‘outcrossing lineages’ to remove mildly deleterious mutations. The mating system altered the interaction of D. stramonium with its specialist herbivores given that inbred plants received more damage. However, it is necessary to disentangle the relative contribution of herbivores to inbreeding depression. Whether the differential damage by herbivores augmented the magnitude of inbreeding depression in D. stramonium remains to be addressed (Steets et al., 2007). Yet, as pointed out by Strauss et al. (2002), it is necessary to measure inbreeding depression for components of defence to enhance our knowledge about how inbreeding alters the pattern of selection exerted by herbivores.
ACKNOWLEDGMENTS
We are grateful to D. E. Carr, B. Traw, E. De La Barrera, A. Cordoba and L. E. Eguiarte for their valuable comments on an earlier version of this manuscript. C. A. Domínguez offered valuable advice during the study. We thank Mick Hanley and two anonymous reviewers for their valuable criticisms and suggested improvements. We thank A. López, L. L. Cruz and M. Moreno for their help with fieldwork. Brian Traw kindly collected the seeds from Missouri. R.B.B. is grateful to the graduate programme Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de Mexico (UNAM), and to UNAM and CONACyT (number: 181510) for the scholarships granted for doctoral studies. J.N.F was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT projects 42031-A and 81490).
LITERATURE CITED
- Armbruster P, Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Botham R, Collin CL, Ashman TL. Plant–mycorrhizal fungus interactions affect the expression of inbreeding depression in wild strawberry. International Journal of Plant Sciences. 2009;170:143–150. [Google Scholar]
- Bronstein JL, Huxman T, Horvath B, Farabee M, Davidowitz G. Reproductive biology of Datura wrightii: the benefits of a herbivorous pollinator. Annals of Botany. 2009;103:1435–1444. doi: 10.1093/aob/mcp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D, Waller DM. Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annual Review of Ecology and Systematics. 1999;30:479–513. [Google Scholar]
- Carr DE, Eubanks MD. Inbreeding alters resistance to insect herbivory and host plant quality in Mimulus guttatus (Scrophulariaceae) Evolution. 2002;56:22–30. doi: 10.1111/j.0014-3820.2002.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Cheptou PO, Imbert E, Lepart J, Escarre J. Effects of competition on lifetime estimates of inbreeding depression in the outcrossing plant Crepis sancta (Asteraceae) Journal of Evolutionary Biology. 2000;13:522–531. [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. London: John Wiley and Sons; 1957. [Google Scholar]
- Cuevas GE. 1996. Tasa de entrecruzamiento, vecindario genético y tamaño efectivo de la población de Datura stramonium L. (Solanaceae) BSc thesis, Universidad Nacional Autónoma de México, México. [Google Scholar]
- Darwin C. The effects of cross and self-fertilisation in the vegetable kingdom. London: Appleton Inc; 1876. [Google Scholar]
- Delphia CM, De Moraes CM, Stephenson AG, Mescher MC. Inbreeding in horsenettle influences herbivore resistance. Ecological Entomology. 2009a;34:513–519. [Google Scholar]
- Delphia CM, Rohr JR, Stephenson AG, De Moraes CM, Mescher MC. Effects of genetic variation and inbreeding on volatile production in a field population of horsenettle. International Journal of Plant Sciences. 2009b;170:12–20. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. Edinburgh: Longman Group Ltd; 1997. [Google Scholar]
- Ferrari MJ, Stephenson AG, Mescher MC, De Moraes CM. Inbreeding effects on blossom volatiles in Cucurbita pepo subsp texana (Cucurbitaceae) American Journal of Botany. 2006;93:1768–1774. doi: 10.3732/ajb.93.12.1768. [DOI] [PubMed] [Google Scholar]
- Fornoni J, Núñez-Farfán J. Evolutionary ecology of Datura stramonium: genetic variation and costs for tolerance to defoliation. Evolution. 2000;54:789–797. [PubMed] [Google Scholar]
- Fornoni J, Valverde PL, Núñez-Farfán J. Quantitative genetics of plant tolerance and resistance against natural enemies of two natural populations of Datura stramonium. Evolutionary Ecology Research. 2003;5:1049–1065. [Google Scholar]
- Fornoni J, Valverde PL, Núñez-Farfán J. Population variation in the cost and benefit of tolerance and resistance against herbivory in Datura stramonium. Evolution. 2004;58:1696–1704. [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. Impacts of seedling herbivory on plant coexistence and implications for species coexistence. Annals of Botany. 2009;103:1347–1353. doi: 10.1093/aob/mcp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CN, Winsor JA, Stephenson AG. Inbreeding influences herbivory in Cucurbita pepo ssp texana (Cucurbitaceae) Oecologia. 2004;140:601–608. doi: 10.1007/s00442-004-1623-2. [DOI] [PubMed] [Google Scholar]
- Hull-Sanders, Eubanks MD. Plant defense theory provides insight into interactions involving inbred plants and insect herbivores. Ecology. 2005;86:897–904. [Google Scholar]
- Ivey CT, Carr DE. Effects of herbivory and inbreeding on the pollinators and mating system of Mimulus guttatus (Phrymaceae) American Journal of Botany. 2005;92:1641–1649. doi: 10.3732/ajb.92.10.1641. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Carr DE, Eubanks MD. Effects of inbreeding in Mimulus guttatus on tolerance to herbivory in natural environments. Ecology. 2004;85:567–574. [Google Scholar]
- Karron JD, Holmquist KG, Flanagan RJ, Mitchell RJ. Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Annals of Botany. 2009;103:1379–1384. doi: 10.1093/aob/mcp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GG, Barbour JD. Resistance variation in natural and managed systems. In: Fritz RS, Simms EL, editors. Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. Chicago: University of Chicago Press; 1992. pp. 13–41. [Google Scholar]
- van Kleunen M, Fischer M, Johnson SD. Reproductive assurance through self-fertilization does not vary with population size in the alien invasive plant Datura stramonium. Oikos. 2007;116:1400–1412. [Google Scholar]
- Kouonon LC, Jacquemart AL, Zoro-Bi AI, Bertin P, Baudoin J-P, Dje Y. Reproductive biology of the andromonoecious Cucumis melo subsp. agrestis (Cucurbitaceae) Annals of Botany. 2009;104:1129–1139. doi: 10.1093/aob/mcp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimu R, Koricheva J. A meta-analysis of tradeoffs between plant tolerance and resistance to herbivores: combining the evidence from ecological and agricultural studies. Oikos. 2006;112:1–9. [Google Scholar]
- Leimu R, Kloss L, Fischer M. Effects of experimental inbreeding on herbivore resistance and plant fitness: the role of history of inbreeding, herbivory and abiotic factors. Ecology Letters. 2008;11:1101–1110. doi: 10.1111/j.1461-0248.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Levri MA, Real LA. The role of resources and pathogens in mediating the mating system of Kalmia latifolia. Ecology. 1998;79:1602–1609. [Google Scholar]
- Lively CM, Howard RS. Selection by parasites for clonal diversity and mixed mating. Philosophical Transactions of the Royal Society. 1994;346:271–281. doi: 10.1098/rstb.1994.0144. [DOI] [PubMed] [Google Scholar]
- Lynch M. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution. 1991;45:622–629. doi: 10.1111/j.1558-5646.1991.tb04333.x. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J. The evolution of sex. London: Cambridge University Press; 1978. [Google Scholar]
- Motten AF, Antonovics J. Determinants of outcrossing rate in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae) American Journal of Botany. 1992;79:419–427. [PubMed] [Google Scholar]
- Motten AF, Stone JL. Heritability of stigma position and the effect of stigma–anther separation on outcrossing in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae) American Journal of Botany. 2000;87:339–347. [PubMed] [Google Scholar]
- Núñez-Farfán J, Dirzo R. Evolutionary ecology of Datura stramonium L. in Central Mexico: natural selection for resistance to herbivorous insects. Evolution. 1994;48:423–436. doi: 10.1111/j.1558-5646.1994.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Núñez-Farfán J, Cabrales-Vargas RA, Dirzo R. Mating system consequences on resistance to herbivory and life history traits in Datura stramonium. American Journal of Botany. 1996;83:1041–1049. [Google Scholar]
- Núñez-Farfán J, Fornoni J, Valverde PL. The evolution of resistance and tolerance to herbivores. Annual Review of Ecology, Evolution and Systematics. 2007;38:541–566. [Google Scholar]
- Ouborg NJ, Biere A, Mudde CL. Inbreeding effects on resistance and transmission-related traits in the Silene–Microbotryum pathosystem. Ecology. 2000;81:520–531. [Google Scholar]
- Schultz ST, Willis JH. Individual variation in inbreeding depression: the role of inbreeding history and mutation. Genetics. 1995;141:1209–1223. doi: 10.1093/genetics/141.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonle I, Bergelson J. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (Solanaceae) Evolution. 2000;54:778–788. doi: 10.1111/j.0014-3820.2000.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Sosenski P. Variación geográfica del sistema de apareamiento en Datura stramonium en el centro de México. 2004. BSc thesis, Universidad Nacional Autónoma de México, México. [Google Scholar]
- Steets JA, Auld JR, Wolf DE, Ashman TL. The role of natural enemies in the expression and evolution of mixed mating in hermaphroditic plants and animals. Evolution. 2007;61:2043–2055. doi: 10.1111/j.1558-5646.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- Stephenson AG. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics. 1981;12:253–279. [Google Scholar]
- Stone JL, Motten AF. Anther–stigma separation is associated with inbreeding depression in Datura stramonium, a predominantly self-fertilizing annual. Evolution. 2002;56:2187–2195. doi: 10.1111/j.0014-3820.2002.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Karban R. The significance of outcrossing in an intimate plant–herbivore relationship. 1. Does outcrossing provide an escape from herbivores adapted to the parent plant. Evolution. 1994;48:454–464. doi: 10.1111/j.1558-5646.1994.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends in Ecology & Evolution. 2002;17:278–285. [Google Scholar]
- Thompson JN. The geographic mosaic of coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Vallejo-Marin M, Barrett SCH. Modification of flower architecture during early stages during the evolution of self-fertilization. Annals of Botany. 2009;103:951–962. doi: 10.1093/aob/mcp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde PL, Fornoni J, Núñez-Farfán J. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. Journal of Evolutionary Biology. 2001;14:424–432. [Google Scholar]
- Valverde PL, Fornoni J, Núñez-Farfán J. Evolutionary ecology of Datura stramonium: equal plant fitness benefits of growth and resistance against herbivory. Journal of Evolutionary Biology. 2003;16:127–137. doi: 10.1046/j.1420-9101.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. New York: Springer; 2005. [Google Scholar]
- Weaver SE, Warwick SI. The biology of Canadian weeds. 64. Datura stramonium L. Canadian Journal of Plant Science. 1984;64:979–961. [Google Scholar]
- Zar JH. Biostatistical analysis. 4th edn. NJ: Prentice Hall: Upper Saddle River; 1996. [Google Scholar]