Abstract
Background and Aims
Many Australian legumes have evolved in low-phosphorus (P) soils and low-rainfall areas. Therefore a study was made of the interaction of soil [P] and water availability on growth, photosynthesis, water-use efficiency (WUE) and P nutrition of two Australian native legumes with pasture potential, Cullen australasicum and C. pallidum, and the widely grown exotic pasture legume, lucerne (Medicago sativa).
Methods
Plants were grown in a glasshouse at 3, 10 and 30 mg P kg−1 dry soil for 5 months. At week 10, two drought treatments were imposed, total pot dried (all-dry) and only top soil dried (top-dry), while control pots were maintained at field capacity.
Key Results
Shoot dry weight produced by lucerne was never higher than that of C. australasicum. For C. pallidum only, shoot dry weight was reduced at 30 mg P kg−1 dry soil. The small root system of the Cullen species was quite plastic, allowing plants to access P and moisture efficiently. Lucerne always had a higher proportion of its large root system in the top soil layer compared with Cullen species. All species showed decreased photosynthesis, leaf water potential and stomatal conductance when exposed to drought, but the reductions were less for Cullen species, due to tighter stomatal control, and consequently they achieved a higher WUE. All species showed highest rhizosphere carboxylate concentrations in the all-dry treatment. For lucerne only, carboxylates decreased as P supply increased. Citrate was the main carboxylate in the control and top-dry treatments, and malate in the all-dry treatment.
Conclusions
Multiple adaptive responses of Cullen species and lucerne favoured exploitation of low-P soils under drought. The performance of undomesticated Cullen species, relative to that of lucerne, shows their promise as pasture species for environments such as in south-western Australia where water and P are limiting, especially in view of a predicted drying and warming climate.
Keywords: Australian native legumes, carboxylates, climate change, Cullen spp., drought, Medicago sativa, novel crops, perennial pastures, phosphorus, photosynthesis, root distribution, water-use efficiency
INTRODUCTION
Drought negatively affects the physiological and biochemical processes of many plants and thus reduces growth. In Australia, half of the total arable land area is regularly affected by drought (Smithson and Sanchez, 2001) and this is expected to expand under predicted climate-change scenarios (Mpelasoka et al., 2008). Plant adaptations to dry-climate scenarios include: (a) escaping the water deficit by growing in wetter times of the year; (b) avoiding the water deficit, for instance by reducing transpiration or increasing water uptake; and (c) maintaining growth under water deficit whilst tolerating severe deficit through survival mechanisms (Tardieu, 2005). When the water availability to roots decreases, plants tend to reduce transpiration by two means. In the short-term a reduction in stomatal conductance reduces the water flux through the plant. In the longer-term a reduction in leaf area may occur. When stomata partially close, thereby decreasing transpiration, the decrease in leaf water potential tends to be compensated, i.e. leaves become more hydrated. This mechanism allows leaves to maintain their water status within a narrow range. However, there is a constitutive link between high transpiration rate (a ‘favourable’ trait linked to photosynthesis) and low leaf water potential (an ‘unfavourable’ trait associated with tissue dehydration) (Tardieu, 2005).
After nitrogen (N), phosphorus (P) is usually the most limiting nutrient for crop production (Schachtman et al., 1998). Phosphorus fertilization improves tolerance to drought stress in white clover (Trifolium repens; Singh and Sale, 2000), moth bean (Vigna aconitifolia; Garg et al., 2004), barley (Hordeum vulgare; Jones et al., 2005) and soybean (Glycine max; Jin et al., 2006). Application of P under drought increases water-use efficiency (WUE – the ratio of carbon assimilation rate to transpiration rate) and shoot [N], shoot dry weight (d. wt) and decreases dry matter allocated to roots in several crop and tree species (Graciano et al., 2005; Jin et al., 2006). Also, selection for drought resistance improves yield of common bean (Phaseolus vulgaris) in P-limited environments (Beebe et al., 2008). These results highlight the significant interactions between moisture availability and P nutrition for plant performance. However, shoot-related parameters have received more attention than those of roots, which are harder to investigate. Consequently, there is much less information available on root characteristics. In particular, the response of the rhizosphere interactions between P and drought are not well understood (Wittenmyer and Merbach, 2005).
Since P in soils is relatively immobile and often unavailable, morphological, physiological and biochemical mechanisms have evolved in plants to allow them to respond to P deficiency (Vance et al., 2003; Raghothama and Karthikeyan, 2005). However, P movement in soil is further restricted under drought (Sardans and Peñuelas, 2007). Typical morphological responses to low-P availability include increased root development, higher root : shoot ratios, finer roots, longer root hairs and the formation of arbuscular mycorrhizas, all of which facilitate exploration of a greater soil volume (Raghothama, 1999; Smith and Read, 2008). In addition, the supply of P in soils is typically heterogeneous and most roots grow preferentially in regions that contain favourably high concentrations of P (Lynch and Brown, 2001; Hodge, 2004). Plants can also enhance P acquisition by altering their root physiology (Neumann and Martinoia, 2002), such as by enhanced exudation of carboxylates (e.g. malate and citrate) and phosphohydrolases (Richardson et al., 2000; Wouterlood et al., 2005). Root exudates are also important in the maintenance of root–soil contact, which is especially important to the plant under drought and drying conditions, when hydraulic continuity is lost (Walker et al., 2003). In shoots, P deficiency can reduce photosynthetic rate (A) and stomatal conductance (gs) (Jacob and Lawlor, 1991; Ghannoum and Conroy, 2007) and thereby restrict plant growth. Leaf area, mesophyll conductance (gm), gs and electron transport are the main determinants of carbon assimilation (Tardieu, 2005; Flexas et al., 2008; Galle et al., 2009) and leaf area and gs also determine transpiration by plants (Tardieu, 2005). Therefore, there is an inherent link between moisture and P availability, and plant physiological processes and growth. However, there is a lack of information on these physiological responses.
In Australia, dryland salinity has developed due to a hydrological imbalance that occurs when native, deep-rooted perennial vegetation is removed and replaced with shallow-rooted annual crops and pastures (Cocks, 2001). In response to this problem, Australian perennial legumes are now considered to have potential for development as pasture legumes (Denton et al., 2006; Dear et al., 2007; Robinson et al., 2007; Pang et al., 2009a). These species are likely to have evolved in local, P-impoverished environments (Handreck, 1997). P uptake is poorly regulated at high P availability in some Australian species (Shane et al., 2004; Ryan et al., 2009), and many native species, but by no means all, are sensitive to P toxicity (Handreck, 1997; Ryan et al., 2009). Large quantities of superphosphate have been applied to agricultural soils to improve crop yields in Australia (Bolland et al., 1997). An understanding of the morphological and physiological traits that affect P acquisition under drought is important to facilitate the development of native Australian legumes for agricultural purposes and this has been highlighted as a need for further research (Denton et al., 2006; Pang et al., 2009b). Knowledge to assist in the selection of species with enhanced P acquisition will be beneficial in low-input agroecosystems (Burkitt et al., 2007) and may improve the productivity and sustainability of high-input agroecosystems (Denton et al., 2006, Harris et al., 2008).
The genus Cullen has been identified as a genus with particular potential for development of future perennial pasture legumes (Cocks, 2001; Dear et al., 2007; Hayes et al., 2009). In particular, Cullen australasicum appears well adapted to both the alkaline soils of the South Australian wheatbelt and the acidic soils of the Western Australian wheatbelt (Bennett et al., 2006; Li et al., 2008). Its natural habitat ranges from deep, sandy soils to sandy clays, and it is often found in grasslands (Cocks, 2001). The genus Cullen is also found in Africa, Spain, Portugal, Italy, and southern Asia, with 25 of the 32 species being native to Australia (Hayes, 2009). It is known for its medicinal, aromatic and pharmaceutical properties as well as its resistance to spotted alfalfa aphids (Therioaphis trifolii) (Cocks, 2001; Dear et al., 2007; Hayes, 2009). At present, a germplasm bank of Cullen is maintained by the South Australian Research and Development Institute and microsatellite markers have been developed (Kroiss et al., 2009). Incorporation of this native pasture species into agricultural systems would result in greater plant diversity and, presumably, better adaptation to diverse climatic and soil conditions (Cocks, 2001). However, the root and shoot responses (e.g. root growth, plasticity, concentration and composition of rhizosphere carboxylates, photosynthetic capacity and WUE) of Cullen species under the interaction of drought and P have not been studied.
The objective of this study was, therefore, to compare the effects of the interaction of soil [P] and water availability on growth, photosynthesis and P nutrition of the native legumes Cullen australasicum and Cullen pallidum. The responses were compared with those of a widely cultivated exotic perennial legume, Medicago sativa (lucerne or alfalfa). The following hypothesizes were made: (i) that root mass ratios (RMR – ratio between root d. wt and total plant d. wt) will be higher at low P supply and under drought conditions; (ii) that root distribution will shift towards the top layer of soil at a lower P supply, and under drought condition plants will produce more deep roots (plasticity of root distribution to variation in soil [P] and moisture); (iii) that rhizosphere carboxylate concentration will increase at low P supply and under drought conditions, with Cullen species being more responsive than M. sativa in regards to hypotheses (i), (ii) and (iii); (iv) that photosynthetic rate, gs and photosynthetic nitrogen-use efficiency (PNUE – the ratio of carbon assimilation rate to leaf N concentration) will be reduced and WUE will be increased under lower P supply and further reduction of A, gs and PNUE (increase of WUE) will occur under drought; and (v) that the native legumes will be more productive in soils with low availability of P and moisture than M. sativa, and that with increasing availability of soil P and moisture M. sativa will become more productive.
MATERIALS AND METHODS
Growth conditions
Cullen australasicum (Schltdl.) J.W. Grime accessions SA4966 and SA42762 (SA4966 late flowering and SA42762 early flowering), C. pallidum (N.T.Burb.) J.W.Grimes accession SA44387 and Medicago sativa L. ‘SARDI-10’ were grown in 1-m-tall, 10-cm-diameter vertically split pots. Seeds of C. australasicum and C. pallidum were collected from seed-multiplication plots established at the Shenton Park field station of the University of Western Australia and lucerne seeds from the Genetic Resource Centre at the South Australian Research and Development Institute. The experiment consisted of three P treatments: P3 (3 mg P kg−1 dry soil), P10 (10 mg P kg−1 dry soil) and P30 (30 mg P kg−1 dry soil) and three moisture treatments: control (watered from both top and bottom using a wick system throughout the experiment); top-dry (watered from both top and bottom using a wick system until 10 weeks, and thereafter the top compartment was allowed to dry out, while the wick from the bottom remained active and transferred water up to 30 cm from the bottom of the pot) and all-dry (watered from both top and bottom using a wick system until 10 weeks, after which the entire pot was allowed to dry out by removing the water sources until harvesting). Wicks were made up of 5-mm-diameter jute ropes. Two wicks per pot were placed along the inner wall of a pot from opposite directions, and the upper and lower ends were put into a cup of water at the top and a bucket of water with a 5-cm layer of water at the bottom, respectively. Therefore, until the drought treatments began, wicks transferred the water throughout the soil profile uniformly. Even though average [P] of a treatment was as given above, due to the stratified manner of P application within a pot (3 : 1 : 0 at top 20 cm; middle 40 cm; bottom 40 cm, respectively), the exact [P] within a given layer of a pot differed (Table 1). Three replicate pots of each species × phosphorus × moisture combination were established. Pots were filled with 12 kg pot−1 of thoroughly washed, steam-sterilized river sand in a three-step process, in order to fill each layer with an exact [P], together with other nutrients. Basic soil [P] was 1–2 mg P kg−1 dry sand with a pH (CaCl2) of 6·5, as determined by CSBP FutureFarm analytical laboratories, Bibra Lake, Australia. All essential nutrients other than P were provided by amending the sand with 126·6 mg kg−1 Ca(NO3)2·4H2O, 42·8 mg kg−1 NH4NO3, 178 mg kg−1 K2SO4, 101 mg kg−1 MgSO4.7H2O, 11 mg kg−1 CaCl2.2H2O, 12 mg kg−1 MnSO4.H2O, 8·8 mg kg−1 ZnSO4.7H2O, 1·96 mg kg−1 CuSO4.5H2O, 0·68 mg kg−1 H3BO3, 1·01 mg kg−1 NaMoO4.2H2O and 32·9 mg kg−1 FeNaEDTA. Phosphorus was supplied as KH2PO4, with changing concentrations for different treatments, as given above. Additional potassium was supplied in the P3 and P10 treatments as KCl to balance the level of potassium. Cullen and lucerne seeds were mechanically scarified and soaked in water, to enhance germination, before being sown in seedling trays at staggered times according to their pre-determined germination time. The experiment was set out in a glasshouse at the University of Western Australia, Perth (31 °59′S, 115 °53′E) in a randomized complete block design. Three seedlings were planted in each pot and thinned to one plant at 2–3 weeks. Weekly additions of 300 mL of 2 mm NH4NO3 commenced at week 6 to ensure an adequate N supply (Denton et al., 2006). Nitrogen was supplied through both top and bottom water sources for the control treatment. For the all-dry treatment, N was supplied until the drought treatment began, and for the top-dry treatment N was applied only from the bottom after the drought treatment began. The glasshouse was unheated and had an average daytime temperature of 23 °C during the experiment, which was conducted from May to September 2008.
Table 1.
Phosphorus concentration (mg P kg−1 dry soil) in three layers of the pots in each P treatment
| Treatment |
|||
|---|---|---|---|
| Depth (cm) | P3 | P10 | P30 |
| 0–20 (top) | 10 | 30 | 90 |
| 20–60 (middle) | 3 | 10 | 30 |
| 60–100 (bottom) | 0 | 0 | 0 |
Physiological measurements
Starting from 2 d before the drought began at 10 weeks and continuing weekly until the end of the experiment, pots were weighed and net water loss of a pot determined. Leaf water potential (Ψ) was measured at midday (1200–1400 h) in a pressure chamber (Soilmoisture Equipment Corp., Santa Barbara, CA, USA) on petioles of young fully expanded leaves. Measurements of Ψ were made 2 d before the drought treatment started and 2 d before the final harvest.
Photosynthetic rate and gs were measured between 1000 and 1400 h on the youngest fully expanded leaf of all plants, using a portable gas-exchange system (LI6400 portable; LiCor Inc., Lincoln, NE, USA) equipped with a light source (6400–02B LED, LiCor). Measurements were taken 1 week before starting the drought treatment for all plants and continued for the all-dry treatment at 2- to 3-d intervals until these plants had been harvested. Further, measurements were taken 1 week before harvesting from top-dry and control treatments. Photosynthetic photon flux density at the leaf surface was maintained at 1500 µmol m−2 s−1 during the measurement of A and leaf temperature was maintained at 25 °C. Ambient humidity of the incoming air to the leaf chamber was left at that of the glasshouse environment. A was measured when the ambient CO2 concentration of the incoming gas stream was 380 µmol mol−1, a value close to that during plant growth.
Plant analyses
Plants were harvested at 12 or 16 weeks, depending on the moisture treatment. Each individual plant was harvested when it reached the permanent wilting point (PWP), i.e. the soil water potential at which a plant can no longer absorb water from the soil. Among all-dry treated plants, M. sativa reached the PWP within 7–10 d after imposing the drought (12 weeks after the experiment began). However, the Cullen species reached PWP 20–25 d after the drought treatment began. Medicago sativa plants in the top-dry moisture treatment also reached the PWP 38–43 d after the drought treatment began (16 weeks after the experiment began). At week 16 all remaining plants were harvested. Cullen plants in the top-dry and Cullen and lucerne plants in the control moisture treatment did not wilt at any time. The soil column was separated into three sections, representing layers of different [P]: 0–20, 20–60, 60–100 cm. Root systems were gently removed from the bulk soil. Roots were shaken slightly to remove the excess soil and the remaining soil was defined as the rhizosphere soil (Veneklaas et al., 2003). Root fractions from each layer were transferred to a 200-mL vial and washed in a measured amount of 0·2 mm CaCl2 solution ranging from 20 to 150 mL. The root system was gently dunked in the solution until as much rhizosphere soil as possible was removed. Care was taken to minimize root damage. However, it cannot be fully excluded that some carboxylates originated from within the roots, due to minor cellular damage (Pearse et al., 2006). A subsample of the rhizosphere extract was then filtered using a 0·2-μm syringe filter into a 1-mL HPLC vial. The vial was acidified with one drop of concentrated phosphoric acid, placed in dry ice, and transferred to a –20 °C freezer until HPLC analysis. The root system was then washed more thoroughly to remove any residual soil.
The d. wt of the three layers of the root system and that of the shoots was determined after drying at 60 °C for 1 week. To determine [P], roots, stems and leaves of each plant were ground separately in a steel ball mill. When estimating root [P] for each plant, root portions from the top, middle and bottom soil layers were combined. An approx. 100-mg subsample was taken and digested in nitric/perchloric acid and analysed using the molybdo-vanado-phosphate method (Kitson and Melon, 1944).
Leaf [N] was determined by dry combustion (Nelson and Sommers, 1996) using an elemental CN analyser (Elementar Analysensysteme GmbH, Hanau, Germany) and is expressed on an area basis ([N]area). The ratio of A and gs was used to derive the photosynthetic water-use efficiency (WUE) and the ratio of A and [N]area was used to derive the PNUE. Therefore, expression of leaf [N] on a leaf area basis was more appropriate than on a leaf weight basis.
Carboxylate analyses
Organic acids in rhizosphere samples were analysed by HPLC (600E pump, 717plus autoinjector, 996 photodiode array detector; Waters, Milford, MA, USA) (Cawthray, 2003). In brief, separation was achieved at 23 ± 0·5 °C on an Alltima C-18 column (250 × 4·6 mm internal diameter with 5 µm packing; Alltech Associates, Deerfield, IL, USA) using a mobile phase consisting of 93 % 25 mm KH2PO4 at pH 2·50 (with concentrated phosphoric acid) and 7 % methanol at 1 mL min−1. A gradient elution programme using 60 % methanol was used every fifth sample to flush the column of the more hydrophobic compounds and reduce carry-over. Samples in the autoinjector were held at 10 °C. Detection was at 210 nm, with photo diode array (PDA) acquisition from 195 to 400 nm to enable positive identification of organic acids by comparing retention time and PDA peak spectral analyses, including peak purity, of standards with the unknowns. Calibration curves for each organic acid were generated from peak area versus the mass of standard organic acids injected. Data acquisition and processing was with Empower™ 2 (Waters) software. Retention times and PDA data of organic acid standards including malic, iso-citric, malonic, shikimic, lactic, acetic, maleic, citric, succinic, fumaric, cis-aconitic and trans-aconitic acids were used to identify organic acids in rhizosphere exudate samples. Typical sample injections were 50 or 100 µL, and run-time was 15 min per sample, with a 50 min run-time for the methanol flushing step.
Oxalic acid in rhizosphere exudate samples was analysed by HPLC (600E pump, 717plus autoinjector, 996 PDA detector; Waters). In brief, separation was achieved at 25 ± 0·5 °C on a Hypersil Hypercarb (100 × 4·6 mm internal diameter with 7 µm packing; Q-Lab, Eagle Farm, QLD, Australia) using a mobile phase consisting of 0·1 % trifluoroacetic acid at 1 mL min−1. A gradient elution programme using 60 % methanol was used every fifth sample to flush the column of the more hydrophobic compounds and reduce carry-over. Samples in the auto-injector were held at 10 °C. Detection was at 210 nm, with PDA acquisition from 195 to 400 nm to enable positive identification of organic acids by comparing retention time and PDA peak spectral analyses, including peak purity, of standards with the unknowns. The calibration curve for oxalic acid was generated from peak area versus the mass of standard oxalic acid injected. Data acquisition and processing was with Empower™ 2 (Waters) software. Typical sample injections were 50 µL and run-time was 8 min per sample with a 20-min run-time for the methanol-flushing step.
Statistical analyses
Data were subjected to three-way analysis of variance in SAS/STAT software Version 9.1 (SAS, 2003) to examine the impact of species, moisture, P and their interactions on response variables. No transformations were needed to meet ANOVA assumptions. Results of the statistical analysis are summarized in Table 2. Comparisons between means were made using Tukey's Honest Significant Difference procedure. Means are presented with standard error and significance is expressed at P < 0·05. Since a significant difference of Ψ before starting the drought was observed among species, the initial values were treated as covariates to compare Ψ among species after the drought treatment. A and gs relationships were fitted to a non-rectangular hyperbola obtained through PROC NLIN in SAS to estimate maximum photosynthesis (Amax) and to compare the A and gs relationship among species. When studying A, gs, WUE and PNUE, data from the different Cullen accessions/species were pooled, since there were no significant differences among those accessions/species.
Table 2.
Significance of different sources of variability
| Source of variability |
||||||||
|---|---|---|---|---|---|---|---|---|
| Character | S | P | M | S × P | S × M | P × M | S × P × M | R2 |
| Pot weight (start of drought treatments) | n.s. | n.s. | n.s. | – | – | – | – | 0·84 |
| Pot weight (at harvest) | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. | 0·79 |
| Leaf water potential (start of drought treatments) | * | n.s. | – | n.s. | – | – | – | 0·82 |
| Leaf water potential (at harvest) | ** | n.s. | *** | n.s. | *** | n.s. | n.s. | 0·86 |
| Leaf [P] | n.s. | *** | *** | n.s. | *** | *** | * | 0·88 |
| Stem [P] | * | *** | *** | n.s. | ** | ** | n.s. | 0·75 |
| Root [P] | n.s. | *** | *** | n.s. | n.s. | n.s. | n.s. | 0·56 |
| Shoot d. wt | *** | *** | *** | * | * | ** | n.s. | 0·85 |
| Root d. wt | *** | *** | *** | ** | *** | n.s. | n.s. | 0·90 |
| RMR | *** | * | n.s. | * | n.s. | n.s. | n.s. | 0·88 |
| Total carboxylates | n.s. | n.s. | *** | * | n.s. | n.s. | *** | 0·55 |
| Carboxylate composition | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | 0·39 |
| A (start of drought treatments) | n.s. | n.s. | – | n.s. | – | – | – | 0·74 |
| A (1 week before harvest) | *** | n.s. | *** | n.s. | ** | n.s. | n.s. | 0·78 |
| gs (1 week before harvest) | ** | n.s. | *** | n.s. | * | n.s. | n.s. | 0·77 |
| WUE | *** | n.s. | * | n.s. | * | n.s. | n.s. | 0·56 |
| PNUE | *** | n.s. | *** | n.s. | ** | n.s. | n.s. | 0·84 |
| Narea | * | * | ** | * | * | n.s. | * | 0·58 |
Significant effects are indicated for species (S), phosphorus treatment (P), moisture treatment (M) and their interactions (n.s., no significant difference; *, P < 0·05; **, P < 0·01; ***, P < 0·001). For each character the R2 value for the tested full model is given.
RESULTS
Net water loss of a pot and midday leaf water potential
Pot weight did not differ among the three moisture treatments when the drought treatments were imposed at 10 weeks (13·0 ± 0·02 kg pot−1) (Table 2). However, pot weight of both all-dry and top-dry treatments gradually declined during the drought period. At harvest, pot weight of the all-dry treatment was lower (12·2 ± 0·04 kg pot−1) than that for the top-dry treatment (12·7 ± 0·03 kg pot−1), while there was no weight loss in control plants throughout the experiment. Phosphorus treatment and species had no impact on net water loss in any moisture treatment (Table 2).
Mid-day Ψ before the all-dry and top-dry treatments were imposed differed among species but was unaffected by P treatment (Table 2). It was higher than –1 MPa for all species (Fig. 1A). Cullen pallidum had the highest Ψ, –0·61 MPa, which was higher than that of M. sativa (–0·95 MPa). However, Ψ for C. australasicum (accessions SA4966 and SA42762) did not differ from that of C. pallidum and M. sativa.
Fig. 1.
Marginal mean midday leaf water potential (Ψ) (A) before the drought treatments were imposed and (B) at harvest for C. australasicum accession SA4966 (Ca1), C. australasicum accession SA42762 (Ca2), C. pallidum accession SA44387 (Cp) and M. sativa cv. SARDI-10 (Luc) under three moisture treatments; moist soil (Control), top soil dry (Top-dry) and completely dry soil (All-dry) (mean ± s.e., n ≥ 5). Note that P treatment had no effect (Table 2).
At harvest, a significant species × moisture interaction occurred for Ψ, with the P treatment again having no impact (Table 2). Control plants maintained a higher Ψ during the entire experiment and the values of Ψ at harvest did not differ among species (Fig. 1B) or from those observed before starting the drought treatment. However, in the top-dry treatment at harvest, M. sativa had Ψ values lower than –2 MPa, while Cullen species maintained a higher Ψ. Furthermore, M. sativa in this treatment wilted during midday, but recovered at night. All species in the all-dry treatment had very low Ψ values; lower than those in the control and the top-dry treatment. Medicago sativa plants started wilting and shedding leaves within 1 week of the start of the all-dry treatment, while such a response did not occur until after 2 weeks for Cullen species (data not shown).
Plant tissue phosphorus
Leaf and stem [P] had numerous interactions with moisture and P treatment and species, while root [P] varied only with moisture and P treatments (Table 2). Leaf, stem and root [P] increased with increasing soil [P] from P3 to P30 across the species and moisture treatments (Fig. 2).
Fig. 2.
Phosphorus concentrations in leaves, stems and roots (as indicated) of C. australasicum accession SA4966 (Ca1), C. australasicum accession SA42762 (Ca2), C. pallidum accession SA44387 (Cp) and M. sativa cv. SARDI-10 (Luc) grown under three moisture regimes: (A) completely dry soil (all-dry), (B) top-soil dry (top-dry) and (C) moist soil (control), treated with 3 mg P kg−1 dry soil (P3), 10 mg P kg−1 dry soil (P10) and 30 mg P kg−1 dry soil (P30) (mean ± s.e., n = 3). Note the change in y-axis scale.
When comparing moisture treatments, plants in the all-dry treatment had a higher [P] in their leaf, stem and root tissues compared with control and top-dry treatments. In particular, leaves of C. australasicum accessions SA4966 and SA42762 at P30 in the all-dry treatment accumulated very high concentrations of P.
Leaf [P] relative to stem and root [P] of Cullen species and M. sativa differed with moisture treatment. Leaf [P] of M. sativa was generally higher than, or similar to, that of stem and root [P] at all moisture treatments, whereas for Cullen species in the top-dry moisture treatment, leaf [P] was lower, and less than stem and root [P].
Shoot and root dry weight
Shoot d. wt varied among species, moisture and P treatments and there were numerous interactions between these factors (Table 2). Shoot d. wt in the all-dry treatment was considerably lower than that in the top-dry and control treatments, and it was less variable. Shoot d. wt of M. sativa increased with P supply in both control and top-dry treatments. However, shoot d. wt of C. australasicum accessions SA4966 and SA42762 increased with P supply only in the control moisture treatment (Fig. 3A). Medicago sativa did not produce more shoot d. wt than Cullen species at any moisture and P combination. Cullen australasicum accession SA42762 produced more shoot d. wt at P30 at the control moisture treatment than the other species. Contrary to the other species, shoot d. wt of C. pallidum increased only up to P10, in both the control and top-dry treatments. In the all-dry treatment, shoot d. wt of C. australasicum accession SA42762 at P10 was higher than that at P3. Apart from that, there were no differences in shoot d. wt of C. australasicum accession SA4966, C. pallidum and M. sativa among P treatments in the all-dry treatment.
Fig. 3.
(A) Shoot d. wt and (B) root d. wt of C. australasicum accession SA4966, C. australasicum accession SA42762, C. pallidum accession SA44387 and M. sativa cv. SARDI-10 (as indicated) following growth at 3 mg P kg−1 dry soil (P3), 10 mg P kg−1 dry soil (P10) and 30 mg P kg−1 dry soil (P30) and under three moisture regimes; moist soil (Control), top soil dry (Top-dry) and completely dry soil (All-dry) (mean ± s.e., n = 3).
Root d. wt also varied among species, P and moisture treatments with several interactions (Table 2). Root d. wt in the all-dry treatment was lower than that in the top-dry and control treatments for all species, and it was also less variable. Medicago sativa produced more root d. wt than the Cullen species at all moisture and P combinations (Fig. 3B). Root d. wt of M. sativa at P30 was higher than that at P3 under both control and top-dry moisture treatments, while root d. wt of Cullen species was less responsive to increasing P.
Dry matter partitioning to shoots and roots
RMR had a significant P × species interaction, while moisture treatments had no effect (Table 2). RMR of M. sativa was much higher than that of Cullen species and was consistent across P treatments (Fig. 4).
Fig. 4.
Marginal means for root mass ratio (RMR) of C. australasicum accession SA4966, C. australasicum accession SA42762, C. pallidum accession SA44387 and M. sativa cv. SARDI-10 (as indicated) following growth at 3 mg P kg−1 dry soil (P3), 10 mg P kg−1 dry soil (P10) and 30 mg P kg−1 dry soil (P30) (mean ± s.e., n = 9). Note that moisture treatment had no effect (Table 2).
Root distributions
Root distribution in a pot (expressed in terms of percentage root d. wt), separated in three layers (top, middle and bottom) was examined. A significant four-way interaction of moisture × species × P × root layer was found (F = 19·7, P < 0·001). Irrespective of the moisture and P treatments, M. sativa produced >40 % of its roots in the top layer of a pot and this decreased to approx. 20 % in the bottom layer (Fig. 5). The proportion of roots produced in the bottom layer by Cullen species was higher than that of M. sativa, across moisture and P treatments. Even though Cullen species produced smaller root systems than M. sativa (Fig. 3), roots of C. australasicum accessions SA4966 and SA42762 had a higher plasticity to applied P. For C. australasicum accession SA42762, irrespective of the moisture treatment, the proportion of roots produced in the bottom layer increased by approx. 15 % (and the proportion in the top layer decreased correspondingly) when P supply increased from P3 to P30. Only for the control and top-dry moisture treatments was a similar response found for C. australasicum accession SA4966. Furthermore, at P3 in the top-dry treatment, for both C. australasicum accessions, the proportion of roots in the bottom layer was approx. 10 % higher than for the control. Such a response was not observed for C. pallidum.
Fig. 5.
Root-distribution pattern within a 1-m profile in pots, partitioning the roots into the top 20 cm, the middle 40 cm and the bottom 40 cm (as indicated) for C. australasicum accession SA4966 (Ca1), C. australasicum accession SA42762 (Ca2), C. pallidum accession SA44387 (Cp) and M. sativa cv. SARDI-10 (Luc) grown under three moisture regimes: moist soil (Control), top-soil dry (Top-dry) and completely dry soil (All-dry), treated with 3 mg P kg−1 dry soil (P3), 10 mg P kg−1 dry soil (P10) and 30 mg P kg−1 dry soil (P30) (mean of n = 3).
Variation in rhizosphere carboxylate concentration
Rhizosphere carboxylate concentration had a significant species × moisture × P interaction (Table 2), and additionally varied among the three layers of a pot (top, middle and bottom) (F = 16·3, P < 0·0001). Carboxylate concentration in the all-dry treatment was higher than the top-dry and control treatments, irrespective of the species, P treatment and layer of a pot (Fig. 6). In most instances, for all the species, carboxylate concentration did not differ between top-dry and control treatments. For M. sativa, carboxylate concentration in the top layer of a pot was lower than the bottom layer and also the concentration in the top layer of a pot declined with the increase of soil [P], irrespective of the moisture treatment. However, for Cullen species, carboxylate concentration did not differ among the layers of a pot in most instances nor did it decline with the increase of soil [P] in the top layer of a pot. This caused Cullen species to have a higher carboxylate concentration at the top layer of a pot at P30 than did M. sativa. Only for C. pallidum, in the all-dry treatment, did carboxylate concentration increase with the increase of soil [P].
Fig. 6.
Concentration of carboxylates in the rhizosphere of C. australasicum accession SA4966 (Ca1), C. australasicum accession SA42762 (Ca2), C. pallidum accession SA44387 (Cp) and M. sativa cv. SARDI-10 (Luc; as indicated), grown under three moisture regimes: (A) completely dry soil (all-dry), (B) top-soil dry (top-dry) and (C) moist soil (control), treated with 3 mg P kg−1 dry soil (P3), 10 mg P kg−1 dry soil (P10) and 30 mg P kg−1 dry soil (P30), separated into the top 20 cm (black), middle 40 cm (white) and bottom 40 cm (grey) of a pot (mean ± s.e., n = 3). Note the change in y-axis scale.
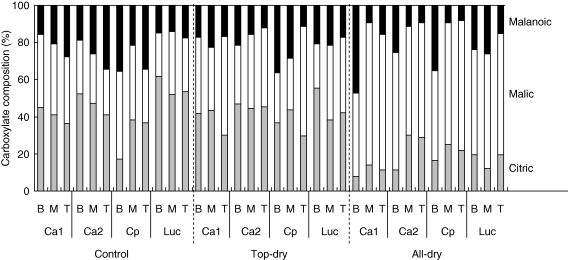
Carboxylate composition varied among moisture treatments, but did not differ among species or P treatments (Table 2 and Fig. 7). Over 98 % of the total carboxylates comprised citrate, malate and malonate. Very low concentrations of citric, cis-aconitic, trans-aconitic, fumaric and oxalic acids were also present. In the all-dry treatment, malate contributed 45–76 % to the total carboxylates for all species and this was higher than in the control and top-dry treatments (23–48 %). Conversely, in the control and top-dry treatments, there was a higher % of citrate (30–62 %) than in the all-dry treatment (7–30 %), except in the lower portion of the pot for C. pallidum in the control moisture treatment.
Fig. 7.
Percentage composition of carboxylates (malanoic, malic, and citric, as indicated) in rhizosphere soil collected from the bottom 40 cm of a pot (B), middle 40 cm of a pot (M) and top 20 cm of a pot (T) of C. australasicum accession SA4966 (Ca1), C. australasicum accession SA42762 (Ca2), C. pallidum accession SA44387 (Cp) and M. sativa cv. SARDI-10 (Luc), under three moisture regimes: moist soil (Control), top-soil dry (Top-dry) and completely dry soil (All-dry) (mean of n = 3).
Photosynthetic response
At the start of the drought treatment, A of all the species was similar (13–17 µmol m−2 s−1) and did not differ with P treatment (Table 2). Three days after imposing the all-dry treatment, A of M. sativa decreased to 2 µmol m−2 s−1 (Fig. 8). Ten days after imposing the all-dry treatment, M. sativa leaves had negative A values and had wilted and died. In contrast, Cullen species maintained a stable A for approx. 2 weeks and A then gradually declined. Control plants maintained positive A throughout the experimental period (Fig. 8) and values differed among species. The P treatments had no effect.
Fig. 8.
Marginal means for the time course of photosynthesis (A) before and after starting the drought treatment (drought began at time = 0) for plants in the all-dry treatment until harvest. Also shown are values for control plants measured at harvest (time = 42): C. australasicum accession SA4966, C. australasicum accession SA42762, C. pallidum and M. sativa cv. SARDI-10 as indicated (mean ± s.e., n = 9). Note that P treatment had no effect (Table 2).
One week before harvest, a significant species × moisture interaction was found for gs and A, while there was no effect of P treatment (Table 2). At this time both gs and A were lower for all the species in the all-dry treatment than the top-dry and control treatments (Fig. 9). Regression analysis of A against gs revealed that A of M. sativa was lower than that of Cullen species for a given level of gs for all three moisture treatments. There was no difference in A among Cullen species at any level of gs. The estimated values of maximum photosynthetic rates (Amax) of the A vs. gs relationship for Cullen species were 16·8 ± 2·3 µmol m−2 s−1, 31·2 ± 2·7 µmol m−2 s−1 and 35·3 ± 1·2 µmol m−2 s−1 for all-dry, top-dry and control treatments, respectively. Similarly, Amax values of M. sativa for top-dry and control treatments were 12·5 ± 3·2 µmol m−2 s−1 and 25·3 ± 2·4 µmol m−2 s−1, respectively. The model used for other treatments was unable to fit an A vs. gs relationship for all-dry M. sativa plants.
Fig. 9.
Relationship between photosynthesis (A) and stomatal conductance (gs) for C. australasicum accession SA4966, C. australasicum accession SA42762, C. pallidum accession SA44387 and M. sativa cv. SARDI-10 (as indicated) in the all-dry (A), top-dry (B) and control (C) moisture treatments. Measurements were made 1 week before harvest (mean of A and gs ± s.e., n ≥ 8 of M. sativa and n ≥ 22 for Cullen species). Note that P treatment had no effect (Table 2) and A and gs of Cullen species were similar.
PNUE, WUE and leaf [N]
There was a significant species × moisture interaction for both PNUE and WUE and no effect of P treatment (Table 2). In the control moisture treatment, Cullen species had a higher mean PNUE and WUE than M. sativa (Fig. 10). Once the drought treatment was imposed (in the all-dry treatment), WUE of Cullen species increased while the PNUE decreased, whereas for M. sativa PNUE decreased while WUE was unchanged.
Fig. 10.
Relationship between photosynthetic nitrogen-use efficiency (PNUE) and water-use efficiency (WUE) when Cullen species and M. sativa plants were grown under control moisture treatment and exposed to water stress (all-dry treatment). Cullen australasicum accessions SA4966 and SA42762 and C. pallidum accession SA44387 were considered together to represent one common group, against M. sativa cv. SARDI-10 as the other group. Photosynthesis data were collected 1 week before harvest and each symbol represents one observation on a single plant (mean ± s.e., of WUE and PNUE for Cullen species and M. sativa n ≥ 9). Note that P treatment had no effect (Table 2).
Leaf [N] was measured at harvest and a three-way interaction of species × moisture × P was present (Table 2 and Fig. 11). While leaf [N] of Cullen species was highly variable, leaf [N] was consistent for M. sativa across treatment combinations. Also, leaf [N] of Cullen species in the all-dry treatment was higher than in the top-dry and control moisture treatments in most instances.
Fig. 11.
Leaf nitrogen concentrations per unit area of C. australasicum accession SA4966, C. australasicum accession SA42762, C. pallidum accession SA44387 and M. sativa cv. SARDI-10 (as indicated) following growth at 3 mg P kg−1 dry soil (P3), 10 mg P kg−1 dry soil (P10) and 30 mg P kg−1 dry soil (P30) and under three moisture regimes: moist soil (Control), top soil dry (Top-dry) and completely dry soil (All-dry) (mean ± s.e., n = 3).
DISCUSSION
Changes in RMR in response to drought and P supply
An increase in RMR is a typical morphological response to low P supply (Lynch et al., 1991; Nielsen et al., 2001) and drought (Gregory et al., 1995). However, neither Cullen species nor M. sativa increased their RMR with a decrease of soil [P] or moisture and Cullen species had a lower RMR than M. sativa, irrespective of moisture and P treatments. Medicago sativa has long been grown in fertile and moist environments (Griffiths, 1949; Small, 2009) and M. sativa roots were not expected to exhibit the same plastic responses to low soil [P] and drought as Cullen species. Similar patterns of RMR values as observed here have been found for Australian Kennedia species (Fabaceae) and Ptilotus polystachyus (Amaranthaceae) when they were compared with exotics M. sativa and Cichorium intybus, respectively (Denton et al., 2006; Ryan et al., 2009). Given that the Cullen species did not have higher RMR values than M. sativa and RMR of all the species did not change with changing soil [P] or moisture, their roots are expected to have alternative mechanisms to acquire P and moisture efficiently from nutrient-impoverished dry soils.
Root system plasticity
As far as is known, this is the first report which describes the root system plasticity of any Australian native legume species under a drought × P interaction. Hypothesis (i) that plants would produce relatively more deep roots during drought to access water and relatively more shallow roots in P-deficient soils to access P was tested. Hypothesis (ii) was not supported by the responses of M. sativa and C. pallidum, but was supported by the response of the two C. australasicum accessions. Medicago sativa allocated over 40 % of its root mass in the top 20 cm of soil irrespective of the moisture and P treatments. Similar patterns of M. sativa root distribution within a pot were reported by Denton et al. (2006). In mature M. sativa stands in the field, approx. 60–70 % of total root biomass was found in the top 15 cm (Barnes and Sheaffer, 1985). We believe that the higher RMR and a higher absolute amount of roots in the top layer of a pot, irrespective of the moisture and P supply, allowed M. sativa to explore the top soil to access adequate amounts of P.
Contrary to the root distribution of M. sativa, Cullen species had a uniform root distribution across top, middle and bottom layers of a pot in most instances. In addition, both C. australasicum accessions produced a higher proportion of roots in the top layer of a pot at P3 than at P30, irrespective of the moisture treatment, whereas C. pallidum and M. sativa did not. The configuration of roots in space and time is an important adaptation to low-P environments (Lynch and Brown, 2001; Grime and Mackey, 2002; Lynch, 2005) and root foraging in top soil is considered to be an effective strategy for acquiring P from low-P soils, as [P] is usually the greatest in this horizon, owing to the contribution of decaying litter, higher organic matter and microbial activity (Lynch and Brown, 2001; Zhu et al., 2005). Since the top 20 cm of a pot was fertilized with P to varying degrees, plants were expected to respond to this surface soil [P].
When both moisture and P were limited (top-dry and P3 treatments, respectively) the proportion of roots in the bottom soil layer for C. australasicum accessions increased relative to the control, while C. pallidum and M. sativa were not responsive. Such a response can be considered as a marginal rate of substitution of investment to obtain one resource for investment to acquire another; i.e. a reflection of the relative benefit of acquiring water rather than P to support plant growth (Lynch and Ho, 2005). Therefore, the results suggest that for Cullen species, due to a smaller root system and a lower RMR than M. sativa, ideal placement of roots in the soil profile was crucial. Thus, C. australasicum positioned a higher proportion of its roots in the top soil when the P supply was low and the soil was moist, and enhanced the development of more deep roots (even at low [P]) during drought. This prevented Cullen plants from wilting (together with the benefit from tight stomatal control) under top-dry conditions, when M. sativa plants did wilt. However, such plastic root growth responses did not occur in the all-dry treatment due to the rapid death of plants after the treatment was imposed.
Rhizosphere carboxylates
As far as we are aware, this is the first report which describes the rhizosphere carboxylate dynamics (concentration and composition) under drought for any plant species. Also, literature regarding exudates of M. sativa roots, only under moist soils, is limited to a few studies (Lipton et al., 1987; Masaoka et al., 1993; Gherardi and Rengel, 2004; Pang et al., 2009a). In the current experiment, the increased carboxylate concentration (μmol g−1 root d. wt) in the rhizosphere of the all-dry moisture treatment for all species might have favoured the access of extra P from dry soil as explained by Walker et al. (2003), and supported hypothesis (iii). Only for M. sativa, did rhizosphere carboxylate concentration decrease with increasing soil [P] (from P3 to P30 as well as from the bottom layer of a pot to the top layer, where the bottom layer was not fertilized with P; Table 1) which supported hypothesis (iii). However, due to the fact that root d. wt increased with soil [P] for M. sativa, the total amount of carboxylates (μmol root system−1) in the P treatments was similar. Therefore, even though root distribution within a pot of M. sativa was not plastic in response to soil moisture and P supply (Fig. 5), a high RMR and the effect on rhizosphere carboxylates with soil [P] and moisture, probably favoured efficient uptake of soil P.
In contrast to M. sativa, carboxylate concentration in the rhizosphere of Cullen species did not decrease with increasing soil [P] (either from P3 to P30 or from the bottom layer of a pot to the top layer). Also hypothesis (iii), that Cullen species would produce more rhizosphere carboxylates than M. sativa, was not supported, except for the top layer of a pot at P30 across moisture treatments. In fact this difference was due to the greater reduction in carboxylates in M. sativa at P30 compared with P10 and P3 and was not due to the increased amount of carboxylate per unit root mass in Cullen species. Also, for Cullen species, total carboxylates in the rhizosphere did not differ among P treatments due to the production of similar root d. wt and carboxylate concentrations. These responses highlight that Cullen species do not reduce rhizosphere carboxylates (down-regulation) at high P supply as M. sativa did. A similar response has been reported for chickpea in moist sand (Wouterlood et al., 2005). The rhizosphere carboxylate concentration that was measured is the balance between the carboxylate exudation and degradation. These exudation and degradation rates were not measured separately in the present experiment and might change with soil moisture content and [P]; this needs to be investigated further.
Even though the composition of rhizosphere carboxylates changed among moisture treatments (higher proportion of citric acid in control and top-dry treatments and more malic acid in the all-dry treatment), there were no differences among species or P treatments. Citrate has been reported to be the main carboxylate anion in leguminous plants (Lipton et al., 1987; Gerke et al., 2000, and references therein) and this was the case in the control and top-dry treatments in the current study. We believe that the altered carboxylate composition in the all-dry moisture treatment for both Cullen species and M. sativa may be due to the differences in exudation rates, diffusivity and/or half-life of carboxylates in the rhizosphere (Gerke et al., 2000); it might also be associated with properties like hydraulic redistribution, as described by Lambers et al. (2006). All these changes may have affected the availability of P in the dry rhizosphere.
Photosynthesis, stomatal conductance, WUE and PNUE
Even though lower P supply was expected to reduce A and gs, only species and moisture treatments changed A and gs. Medicago sativa plants had a lower A at a given level of gs than Cullen species under all three moisture treatments. Thus M. sativa transpired more per unit of carbon assimilated (lower WUE) which supports hypothesis (iv). The more rapid decrease in A of M. sativa than in the Cullen species after imposing the drought might be due to several reasons. (a) Medicago sativa has long been grown in fertile and moist environments in Asia (Griffiths, 1949; Small, 2009) and may not be be well adapted to areas with low summer rainfall (Cocks, 2001; Dear et al., 2007). Conversely, many Australian perennial legumes have evolved in P-impoverished landscapes (Handreck, 1997) and are expected to resist drought better than M. sativa. Similar results have been obtained by Wright et al. (2003) in a comparison of perennial plant species in Australia and USA from moist and dry environments. (b) Cullen species had tighter stomatal control and hence used moisture more efficiently. When stomata close partially, transpiration is decreased and this mechanism allows leaves to maintain their water status in a narrow range (Tardieu, 2005). Thus, leaves of Cullen species tended towards being isohydric, through a feed-forward stomatal control, maintaining a high daytime leaf water status without detrimental effects due to drying soil (Lambers et al., 2008a). Such a response may have been particularly important for Cullen species due to their low RMR. In accordance with this, M. sativa plants in the top-dry treatment had lower Ψ values than Cullen species. Higher gs and lower Ψ of M. sativa was coupled with their root-distribution pattern, where the few deep roots at depth with a thicker tap-root were apparently unable to support the transpiration demand when exposed to drought.
PNUE and WUE varied among species and moisture treatments, while P treatment had no effect, which did not support hypothesis (iv). When exposed to drought, WUE of Cullen species increased, even though PNUE decreased for both Cullen species and M. sativa, supporting hypothesis (iv). This response of Cullen species can be interpreted as a trade-off between efficient use of water and N, as described by Wright et al. (2003). However, M. sativa failed to establish such an adaptive response. For Cullen species this increase in WUE was at least partly supported by the greater reduction of gs at a given level of A when exposed to drought compared with that in M. sativa. Conversely, to maintain a high A at reduced gs, an increase in photosynthetic capacity, and thus leaf N, would be required. Inexorably, PNUE would be lower for such plants, achieving higher WUE at low availability of water (Wright et al., 2003). Apart from the reduction in gs, drought stress can also decrease mesophyll conductance (gm) and impair CO2 diffusion to chloroplasts resulting in a lower A (Flexas et al., 2008); alternatively, it may increase gm which compensates for the reduction in A (Galle et al., 2009). The increase of [N]area of Cullen species in the all-dry treatment compared with the control (Fig. 11) was not invariably associated with maintaining PNUE at the level of the control, due to the very large reduction in A under drought. As Wright et al. (2003) point out, the major benefit of a high [N]area strategy in low-rainfall species is that it permits lower gs for a given A, and thus reduces transpirational water loss. Under drought, Cullen species used a similar strategy to achieve a higher WUE, whilst WUE was not increased for M. sativa. The present results for Cullen, as for those of Wright et al. (2003) on other Australian species, reflect the tendency of Australian soils, due to their age, to be severely impoverished in P, whereas N is relatively abundant, when compared with younger soils (Lambers et al., 2008b).
Shoot growth and tissue [P]
The similar growth response of C. australasicum, C. pallidum and M. sativa at low [P] was contrary to hypothesis (v) that C. australasicum and C. pallidum would grow faster than M. sativa at low [P] and moisture availability. Furthermore, M. sativa did not gain any additional shoot growth compared with Cullen species at high [P] in any moisture treatment (Fig. 2). In contrast, supporting hypothesis (v), both shoot and root d. wt of C. pallidum were reduced at P30 compared with that at P10. Recently, in a P-response study, Pang et al. (2009b) reported both M. sativa and C. australasicum achieved a maximum growth (d. wt) at 24 mg P kg−1 dry soil. Therefore, P3 and P30 used in the current study represent a low and an optimum P supply for M. sativa and C. australasicum, respectively. The reduced growth of C. pallidum at high [P] might be due to P toxicity caused by the high amount of P in the rhizosphere in the low P-buffering river sand. Even though shoot and root d. wt of C. pallidum were reduced at P30 (Fig. 2), tissue [P] was unchanged and [P] for Cullen species and M. sativa at P30 was much lower than reported for many native Australian species and crop plants during P toxicity (Shane et al., 2004, and references therein). In fact, at P30, C. pallidum maintained physiological processes such as A, gs, WUE and PNUE in a similar manner to C. australasicum. Therefore, the reduced growth of C. pallidum at P30 was unlikely to be due to P toxicity and the exact reason remains unclear. The growth response of C. pallidum suggests that compared with C. australasicum it might have evolved in environments with more severely P-impoverished soils.
Shoot d. wt for all species was lowest in the all-dry treatment. This was due to (a) early harvest of the plants, as plants were harvested once they reached the PWP; and, more importantly, (b) shedding of some of their leaves. Once plants had started to show midday wilting symptoms, these plants also started to shed mature leaves and only a few leaves were left at the time of harvest.
Conclusions
Cullen species and M. sativa showed different responses to low-P soils and the responses of Cullen species to drought are promising for their potential to be used as pasture species. Cullen species distributed their roots relatively uniformly in the 1-m soil profile, while M. sativa roots were mostly located in the P-rich layer close to the soil surface. When [P] in the top soil decreased, root proliferation in top soil increased for Cullen species and when drought was imposed, the proportion of deep roots increased, even at low P supply, while rhizosphere carboxylate concentration remained the same. Medicago sativa did not exhibit such a plastic root distribution response. However, M. sativa carboxylate concentration decreased at P30. Cullen pallidum reduced its growth at 30 mg P kg−1 dry soil. Both Cullen species and M. sativa decreased A, Ψ and gs when exposed to drought, but for Cullen species the reductions were less due to a tighter stomatal control and, consequently, they achieved a higher WUE. These multiple adaptive responses of root d. wt, root architecture, rhizosphere carboxylate dynamics, shoot d. wt, A and WUE of Cullen species and lucerne favoured the exploitation of low-P soils under drought. Since the undomesticated Cullen species produced the same amount of d. wt as that produced by M. sativa, these novel species clearly show potential as pasture legumes, especially in areas where moisture and P are limited.
ACKNOWLEDGEMENTS
We thank Greg Cawthray for the HPLC analyses, and Stuart J. Pearse and M. W. Shane for the valuable comments provided on an earlier version of this manuscript. This study was supported by the School of Plant Biology, and the Future Farm Industries Cooperative Research Centre, The University of Western Australia. L. D. B. Suriyagoda also appreciates the SIRF/UIS Scholarship awarded by the University of Western Australia and further scholarship support from the late Frank Ford.
LITERATURE CITED
- Barnes DK, Sheaffer CC. Alfalfa. In: Heath ME, Barnes RF, Metcalf DS, editors. Forages: the science of grassland agriculture. Ames, IA: Iowa State University Press; 1985. pp. 89–97. [Google Scholar]
- Beebe SE, Rao IM, Cajiao C, Grajales M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Science. 2008;48:582–592. [Google Scholar]
- Bennett R, Colmer T, Real D, Ryan M. Hardy Australians: ecogeography of Cullen suggests perennial legumes for low rainfall pastures. In: Turner NC, Acuna T, Johnson RC, editors. Ground-breaking stuff. Proceedings of the 13th Australian Agronomy Conference. Perth: Australian Society of Agronomy; 2006. pp. 10–14. [Google Scholar]
- Bolland MDA, Lewis DC, Gilkes RJ, Hamilton LJ. Review of Australian phosphate rock research. Australian Journal of Agricultural Research. 1997;37:845–859. [Google Scholar]
- Burkitt LL, Small DR, McDonald JW, Wales WJ, Jenkin ML. Comparing irrigated biodynamic and conventionally managed dairy farms. 1. Soil and pasture properties. Australian Journal of Experimental Agriculture. 2007;47:479–488. [Google Scholar]
- Cawthray GR. An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant exudates. Journal of Chromatography A. 2003;1011:233–240. doi: 10.1016/s0021-9673(03)01129-4. [DOI] [PubMed] [Google Scholar]
- Cocks PS. Ecology of herbaceous perennial legumes: a review of characteristics that may provide management options for the control of salinity and waterlogging in dryland cropping systems. Australian Journal of Agricultural Research. 2001;52:137–151. [Google Scholar]
- Dear BS, Li GD, Hayes RC, Hughes SJ, Charman N, Ballard RA. Cullen australasicum (syn. Psoralea australasica): a review and some preliminary studies related to its potential as a low rainfall perennial pasture legume. The Rangeland Journal. 2007;29:121–132. [Google Scholar]
- Denton MD, Sasse C, Tibbett M, Ryan MH. Root distributions of Australian herbaceous perennial legumes in response to phosphorus placement. Functional Plant Biology. 2006;33:1091–1102. doi: 10.1071/FP06176. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espej A, Galmes J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Galle A, Florez-Sarasa I, Tomas M, et al. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? Journal of Experimental Botany. 2009;60:2379–2390. doi: 10.1093/jxb/erp071. [DOI] [PubMed] [Google Scholar]
- Garg BK, Burman U, Kathju S. The influence of phosphorus nutrition on the physiological response of moth bean genotypes to drought. Journal of Plant Nutrition and Soil Science. 2004;167:503–508. [Google Scholar]
- Gerke J, Beißner L, Rŏmer W. The quantitative effect of chemical phosphate mobilization by carboxylate anions on P uptake by a single root. I. The basic concept and determination of soil parameters. Journal of Plant Nutrition and Soil Science. 2000;163:207–212. [Google Scholar]
- Ghannoum O, Conroy JP. Phosphorus deficiency inhibits growth in parallel with photosynthesis in a C3 (Panicum laxum) but not two C4 (P. coloratum and Cenchrus ciliaris) grasses. Functional Plant Biology. 2007;34:72–81. doi: 10.1071/FP06253. [DOI] [PubMed] [Google Scholar]
- Gherardi MK, Rengel Z. The effect of manganese supply on exudation of carboxylates by roots of lucerne (Medicago sativa) Plant and Soil. 2004;260:271–282. [Google Scholar]
- Graciano C, Guiamet JJ, Goya JF. Impact of nitrogen and phosphorus fertilization on drought responses in Eucalyptus grandis seedlings. Forest Ecology and Management. 2005;212:40–49. [Google Scholar]
- Gregory PJ, Palta JA, Batts GR. Root systems and root:mass ratio-carbon allocation under current and projected atmospheric conditions in arable crops. Plant and Soil. 1995;187:221–228. [Google Scholar]
- Griffiths FP. Production and utilization of alfalfa. Economic Botany. 1949;3:170–183. [Google Scholar]
- Grime JP, Mackey JML. The role of plasticity in resource capture by plants. Evolutionary Ecology. 2002;16:299–307. [Google Scholar]
- Handreck KA. Phosphorus requirements of Australian native plants. Australian Journal of Soil Research. 1997;35:241–289. [Google Scholar]
- Harris CA, Clark SG, Reed KFM, Nie ZN, Smith KF. Novel Festuca arundinacea Shreb. and Dactylis glomerata L. germplasm to improve adaptation for marginal environments. Australian Journal of Experimental Agriculture. 2008;48:436–448. [Google Scholar]
- Hayes RC, Li GD, Dear BS, Humphries AW, Tidd JR. Persistence, productivity, nutrient composition, and aphid tolerance of Cullen spp. Crop and Pasture Science. 2009;60:1184–1192. [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Jacob J, Lawlor DW. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. Journal of Experimental Botany. 1991;42:1003–1011. [Google Scholar]
- Jin J, Wang G, Liu X, Pan X, Herbert SJ, Tang C. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. Journal of Plant Nutrition. 2006;29:1433–1449. [Google Scholar]
- Jones CA, Jacobsen JS, Wraith JM. Response of malt barley to phosphorus fertilization under drought conditions. Journal of Plant Nutrition. 2005;28:1605–1617. [Google Scholar]
- Kitson RE, Melon MG. Colorimetric determination of phosphorus as molybdovanadophosphoric acid. Industrial and Engineering Chemistry, Analytical Edition. 1944;16:379. [Google Scholar]
- Kroiss L, Moody M, Barker S, Byrne M, Ryan M. Development, characterization and transferability of microsatellite markers for Cullen australasicum (Leguminosae) Conservation Genetics. 2009;10:1803–1805. [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany. 2006;98:693–713. doi: 10.1093/aob/mcl114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, Pons TL. Plant physiological ecology. 2nd edn. New York, NY: Springer; 2008a. [Google Scholar]
- Lambers H, Shaver G, Raven JA, Smith SE. N- and P-acquisition change as soils age. Trends in Ecology and Evolution. 2008b;23:95–103. doi: 10.1016/j.tree.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Li GD, Lodge GM, Moore GA, et al. Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia. Australian Journal of Experimental Agriculture. 2008;48:449–466. [Google Scholar]
- Lipton DS, Blanchard RW, Blevins DG. Citrate, malate, and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiology. 1987;85:315–317. doi: 10.1104/pp.85.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Root architecture and nutrient acquisition. In: BassiriRad H, editor. Nutrient acquisition by plants: an ecological perspective. Berlin: Springer-Verlag; 2005. pp. 147–183. (Ecological Studies Vol. 181) [Google Scholar]
- Lynch JP, Brown KM. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 2001;237:225–237. [Google Scholar]
- Lynch JP, Ho MD. Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil. 2005;269:45–56. [Google Scholar]
- Lynch J, Lauchli A, Epstein E. Vegetative growth of the common bean in response to phosphorus nutrition. Crop Science. 1991;31:380–387. [Google Scholar]
- Masaoka Y, Kojima M, Sugihara S, Yoshihara T, Koshino M, Ichihara A. Dissolution of ferric phosphate by alfalfa (Medicago sativa L.) root exudates. Plant and Soil. 1993;155/156:75–78. [Google Scholar]
- Mpelasoka F, Hennessy K, Jones R, Bates B. Comparison of suitable drought indices for climate change impacts assessment over Australia towards resource management. International Journal of Climatology. 2008;28:1283–1292. [Google Scholar]
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, et al., editors. Methods of soil analysis. Madison WI: Soil Science Society of America; 1996. pp. 961–1010. Part 3. Chemical methods. [Google Scholar]
- Neumann G, Martinoia E. Cluster roots: an underground adaptation for survival in extreme environments. Trends in Plant Science. 2002;7:162–167. doi: 10.1016/s1360-1385(02)02241-0. [DOI] [PubMed] [Google Scholar]
- Nielsen KL, Eshel A, Lynch JP. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. Journal of Experimental Botany. 2001;52:329–339. [PubMed] [Google Scholar]
- Pang J, Ryan MH, Tibbett M, et al. Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant and Soil. 2009a doi:10.1007/s11104-009-0249-x. [Google Scholar]
- Pang J, Tibbett M, Denton MD, et al. Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant and Soil. 2009b In press. doi:10·1007/s11104-009-0088-9. [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant and Soil. 2006;288:127–139. [Google Scholar]
- Raghothama KG. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS. Phosphate acquisition. Plant and Soil. 2005;274:37–49. [Google Scholar]
- Richardson AE, Hadobas PA, Hayes JE. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant, Cell & Environment. 2000;23:397–405. [Google Scholar]
- Robinson K, Bell LW, Bennett RG, Henry DA, Tibbett M, Ryan MH. Perennial legumes native to Australia: a preliminary investigation of nutritive value and response to cutting. Australian Journal of Experimental Agriculture. 2007;47:170–176. [Google Scholar]
- Ryan MH, Ehrenberg S, Bennett RG, Tibbett M. Putting the P in Ptilotus: a phosphorus-accumulating herb native to Australia. Annals of Botany. 2009;103:901–911. doi: 10.1093/aob/mcp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardans J, Peñuelas J. Drought changes phosphorus and potassium accumulation patterns in an evergreen Mediterranean forest. Functional Ecology. 2007;21:191–201. [Google Scholar]
- SAS. SAS/STAT User's Guide, Version 9.1. Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. Phosphorus uptake by plants: from soil to cell. Plant Physiology. 1998;116:447–453. doi: 10.1104/pp.116.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Lambers H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae) Journal of Experimental Botany. 2004;55:1033–1044. doi: 10.1093/jxb/erh111. [DOI] [PubMed] [Google Scholar]
- Singh DK, Sale PWG. Growth and potential conductivity of white clover roots in dry soil with increasing phosphorus supply and defoliation frequency. Agronomy Journal. 2000;92:868–874. [Google Scholar]
- Small E. Distribution of perennial Medicago with particular reference to agronomic potential for semiarid Mediterranean climate. In: Bennett SJ, editor. New perennial legumes for sustainable agriculture. Perth: University of Western Australia Press; 2009. pp. 57–80. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn. London: Academic Press; 2008. [Google Scholar]
- Smithson PC, Sanchez PA. Plant nutritional problems in marginal soils of developing countries. In: Ae N, Arihara J, Okada K, Srinivasan A, editors. Plant nutrient acquisition. New Perspectives. Tokyo: Springer; 2001. pp. 32–68. [Google Scholar]
- Tardieu F. Plant tolerance to water deficit: physical limits and possibilities for progress. Comptes Rendus Geosciences. 2005;337:57–67. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Stevens T, Cawthray GR, Turner NC, Grigg AM, Lambers H. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant and Soil. 2003;248:187–197. [Google Scholar]
- Walker TS, Bais HP, Grotewold E, Vivanco JM. Root exudation and rhizosphere biology. Plant Physiology. 2003;132:44–51. doi: 10.1104/pp.102.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenmyer L, Merbach W. Plant responses to drought and phosphorus deficiency: contribution of phytohormones in root-related processes. Journal of Plant Nutrition and Soil Science. 2005;168:531–540. [Google Scholar]
- Wouterlood M, Lambers H, Veneklaas EJ. Plant phosphorus status has a limited influence on the concentration of phosphorus-mobilising carboxylates in the rhizosphere of chickpea. Functional Plant Biology. 2005;32:153–159. doi: 10.1071/FP04084. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M. Least-cost input mixtures of water and nitrogen for photosynthesis. The American Naturalist. 2003;161:98–111. doi: 10.1086/344920. [DOI] [PubMed] [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP. Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays) Functional Plant Biology. 2005;32:749–762. doi: 10.1071/FP05005. [DOI] [PubMed] [Google Scholar]