Abstract
Background and Aims
Clonal growth is a common phenomenon in plants and allows them to persist when sexual life-cycle completion is impeded. Very low levels of recruitment from seed will ultimately result in low levels of genotypic diversity. The situation can be expected to be exacerbated in spatially isolated populations of obligated allogamous species, as low genotypic diversities will result in low availability of compatible genotypes and low reproductive success. Populations of the self-incompatible forest herb lily-of-the-valley (Convallaria majalis) were studied with the aim of inferring the relative importance of sexual and asexual recruitment. Then the aim was to establish a relationship between genotypic diversity, sexual reproduction and the local forest environment.
Methods
Highly polymorphic microsatellite markers were used to investigate clonal diversities and population genetic structure of 20 populations of C. majalis in central Belgium.
Key Results
Most of the populations studied consisted of a single genotype and linkage disequilibrium within populations was high, manifesting clonal growth as the main mode of reproduction. A population consisting of multiple genotypes mainly occurred in locations with a thin litter layer and high soil phosphorus levels, suggesting environment-mediated sporadic recruitment from seed. Highly significant genetic differentiation indicated that populations are reproductively isolated. In agreement with the self-incompatibility of C. majalis, monoclonal populations showed very low or even absent fruit set.
Conclusions
Lack of sexual recruitment in spatially isolated C. majalis populations has resulted in almost monoclonal populations with reduced or absent sexual reproduction, potentially constraining their long-term persistence. The local forest environment may play an important role in mediating sexual recruitment in clonal forest plant species.
Keywords: Convallaria majalis, clonal, genotypic diversity, population genetics, remnant populations, SSR, forest herb, rhizomatous, self-incompatible, reproductive success
INTRODUCTION
Clonal growth, the proliferation through genetically identical individuals, is a common phenomenon in plants (Klimes et al., 1997). The capability of populations of clonal plants to persist when the completion of the sexual life-cycle is temporally inhibited due to, for example, unsuitable environmental conditions has been advocated as an important advantage of clonality (Honnay and Bossuyt, 2005; Silvertown, 2008). However, the long-term persistence of clonal plant populations depends to some extent on sexual recruitment (Eriksson, 1993; Watkinson and Powell, 1993), as monoclonal plant assemblages are more prone to infectious diseases (Schmid, 1994; Zhu et al., 2000) and may be less able to respond to changing environmental conditions (Reusch et al., 2005) compared with multi-clone assemblages. Although relatively low levels of seedling recruitment may be sufficient to maintain considerable levels of genotypic diversity (Stehlik and Holderegger, 2000; Richards et al., 2004), too sporadic or completely absent sexual recruitment may ultimately result in a large quantity of genetically identical ramets (Watkinson and Powell, 1993).
In forest herbs, substantial differences in genotypic diversity have been recorded, depending on factors such as canopy closure (Kudoh et al., 1999; Vandepitte et al., 2009) or vegetation type (Jacquemyn et al., 2005), which can strongly affect flowering, germination and/or the establishment of sexual offspring. In addition, it can be anticipated that soil characteristics such as the thickness of the litter layer, soil acidity and nutrient content shape the level of genotypic diversity as they can have a direct effect on the level of germination and seedling recruitment (Facelli and Pickett, 1991; Eriksson, 1995; Ehrlen et al., 2006; Dupuy and Chazdon, 2008). Low rates of sexual recruitment and the associated low genotypic diversity can further affect the population's long-term ability to reproduce sexually (Eckert, 2002). In self-incompatible plants species, low genotypic diversity may have a negative effect on reproductive success, due to a shortage of compatible mates (Handel, 1985; Charpentier, 2002; Honnay et al., 2006). This will in turn further lower the rate of sexual reproduction and seedling recruitment, which may become particularly evident when populations are spatially isolated. In this case, gene flow among populations will be too low to restore genotypic diversity through inflow of pollen or seeds. In the most extreme case, this process may cause local sexual extinction (Eckert, 2000; Honnay and Bossuyt, 2005). Empirical evidence of limited sexual production in clonal populations of allogamous species is, however, scarce so far (Jacquemyn and Honnay, 2008).
Here, an investigation is presented of the genetic structure of 20 isolated populations of the allogamous European lily-of-the-valley (Convallaria majalis), one of the best-known forest perennials due to its ornamental and pharmaceutical value (Tutin et al., 1993). As many herbs carpeting the floor of temperate forests, C. majalis is a rhizomatous long-lived perennial combining clonal proliferation with seed production. As it is notoriously difficult to infer the contribution of infrequent sexual reproduction to clonal diversity from observational data in rhizomatous herbs, shoots were genotyped using microsatellite markers. Levels of clonal diversity in the populations studied were then related to light penetration through the canopy, local vegetation, soil chemical characteristics and reproductive success. The specific objectives were to: (a) infer the relative importance of sexual and asexual reproduction in populations of C. majalis using different genetic marker-based criteria; (b) determine the influence of environmental variation on the level of clonal diversity; and (c) relate genotypic diversity levels with reproductive success.
MATERIALS AND METHODS
Species characteristics
The genus Convallaria (Liliaceae) consists of three very similar rhizomatous herbaceous species: the Asian (C. keiskei), American (C. majuscula) and European lily-of-the-valley (C. majalis). The two-leaf generative shoots produce white, nodding, bell-shaped flowers spreading a sweet fragrance. Non-flowering shoots bear one or two leaves (Tutin et al., 1993). Usually five to seven shoots are connected by rhizomes (Araki and Ohara, 2008).
Convallaria majalis occurs throughout most of Europe in deciduous forests on acid soils (Tutin et al., 1993). The species typically inhabits ancient forests (Bossuyt et al., 1999). Flowers bloom from April to May and are visited by insects (mostly Bombus spp. in the populations studied). Each flower can set one fruit which develops into a red berry usually containing two to four seeds. Flowering and fruit set are often limited and vary strongly among populations (Eriksson, 1999; Kosinski, 2001). Germination in the laboratory exceeds 90 % (K. Vandepitte et al., unpubl. res.). Seedlings emerge after 2–3 years (Eriksson, 1999).
In agreement with the findings of Araki et al. (2005) for the Asian lily-of-the-valley, a preliminary pollination experiment confirmed almost complete self-incompatibility of C. majalis in the present study area. Fruit and seed set were significantly higher in a natural population (fruits/flowers = 40/68; 91 seeds) than in ten manually self-pollinated (fruits/flowers: 5/65; 4 seeds) and ten untreated flowering shoots (fruits/flowers = 3/68; 2 seeds) grown in an insect-proof greenhouse.
Data collection
Twenty populations were sampled in different forest stands of 15 isolated fragments from two regions in the central part of Belgium (region A = populations 1–10 and region B = populations 11a–15b in Table 1). The largest distance between populations within a region is 10 km, and the two regions are separated by approx. 50 km [see Jacquemyn et al. (2003) and Honnay et al. (2006) for a detailed description of regions A and B, respectively]. In both regions, C. majalis typically occurred as discrete patches in unproductive ancient forest parcels belonging to the Quercion alliance on moderately moist to dry sandy loam soils. Convallaria majalis most often co-occurred with Maianthemum bifolium, Polygonatum multiflorum and Anemone nemorosa.
Table 1.
Reproductive and clonal characteristics and multilocus substructure estimators of 20 Convallaria majalis populations in Belgium
| Population | x | y | Fruit set | MLG | G : N | G | rD |
|---|---|---|---|---|---|---|---|
| 1 | 190691 | 174101 | 0·01 | 1 | 0·05 (1/20) | 0 | |
| 2 | 191771 | 175036 | 0·06 | 1 | 0·05 (1/20) | 0 | |
| 3a | 189049 | 175028 | – | 1 | 0·05 (1/19 | 0 | |
| 3b | 188909 | 175038 | – | 3 | 0·15 (3/20) | 0·42* | 0·91* |
| 4 | 190557 | 175730 | 0·04 | 1 | 0·05 (1/20) | 0 | |
| 5 | 187403 | 175152 | – | 1 | 0·05 (1/20) | 0 | |
| 6 | 188427 | 174519 | 0·11 | 3 | 0·15 (3/20) | 0·80* | 0·85* |
| 7 | 191815 | 172724 | 0·00 | 1 | 0·05 (1/20) | 0 | |
| 8 | 192934 | 172345 | 0·08 | 1 | 0·05 (1/20) | 0 | |
| 9 | 190149 | 176228 | 0·00 | 1 | 0·05 (1/20) | 0 | |
| 10 | 193618 | 171402 | 0·06 | 2 + 2† | 0·10 (4/40) | 0·90* | 0·53* |
| 11a | 162368 | 195660 | 0·11 | 4 | 0·21 (4/19) | 0·59* | 0·43* |
| 11b | 162327 | 195836 | 0·00 | 1 | 0·05 (1/20) | 0 | |
| 12a | 161543 | 196601 | 0·06 | 1 | 0·10 (2/20) | 0 | |
| 12b | 161434 | 196587 | 0·00 | 1 | 0·05 (1/20) | 0 | |
| 13a | 164639 | 196721 | 0·23 | 3 | 0·15 (3/20) | 0·51* | 0·74* |
| 13b | 164535 | 196647 | 0·39 | 2 | 0·10 (2/20) | 0·44* | 1·00* |
| 14 | 166062 | 197242 | 0·28 | 2 + 1† | 0·08 (3/40) | 0·31* | 0·97* |
| 15a | 163852 | 192246 | 0·08 | 2 + 1† | 0·08 (3/40) | 0·32* | 0·85* |
| 15b | 164356 | 192440 | 0·20 | 3‡ | 0·10 (2/19) | 0·51* | 0·99* |
Populations 1–10, region A; populations 11a–15b, region B. Populations with identical numbers were sampled in different parcels of the same forest fragment.
Lambert 72 co-ordinates were approximated from forest maps (http://geo-vlaanderen.gisvlaanderen.be/geo-vlaanderen/bossen/).
Fruit set is average number of fruits per flower.
For the population G : N ratio, the number of genotypes over the sample size, and G, genotypic diversity, see Materials and methods.
rD, the coefficient of multilocus linkage disequilibrium (Agapow and Burt 2001), is calculated for each population.
*P < 0·001 based on 1000 permutations.
†Twenty additional shoots were sampled in this large population.
‡The number of putative genets is MLG minus one as it is likely that a somatic mutation occurred.
In each the 20 C. majalis populations studied, a 6 × 6 m plot was established in May 2008. All data reported were collected within these plots. All plant species were identified and their abundance was estimated using the modified Braun-Blanquet scale (Westhof and van der Maarel, 1973). The number of flowering and non-flowering C. majalis shoots was counted in five 1-m2 quadrats laid at random within the plot, and for 20 flowering shoots the number of flowers and developed fruits were counted. The mean LAI (leaf area index, decreases with increasing light penetration) value of ten measurements taken just above the herbaceous layer was contrasted with the mean of two measurements in a nearby open field under overcast conditions. An LAI-2000 canopy analyser (LI-COR Biosciences, Lincoln, NE, USA) measuring diffuse radiation by means of a fisheye light sensor was used. Litter depth was measured at ten randomly chosen points using a ruler (0·1 cm accuracy), and mineral soil samples (5–15 cm depth) were collected for chemical analyses using an auger. Ten soil samples were taken per plot, thoroughly mixed and transferred to the laboratory in closed plastic bags. Soil samples were dried at 70 °C. Total nitrogen content was determined using Kjedahl distillation. The pH was determined in 0·1 m calcium chloride. The free inorganic phosphate content, i.e. phosphate that is readily available to plants, was estimated using the ascorbic acid molybdenum blue method and the absorbance was measured using a Technicon autoanalyser (Technicon corporation, New York, NY, USA). Total soil organic matter was estimated using loss-on-ignition (ashing temperature: 630 °C). All protocols were as described in Page et al. (1982).
Microsatellite analysis
For microsatellite analysis, young leaf material was collected from 20 randomly chosen shoots in each of the 20 plots studied, stored in liquid nitrogen and transferred to a −80 °C freezer. In the three largest populations (10, 14 and 15a), spreading over 50 m2, 20 additional shoots were sampled outside the studied plot to get more reliable estimates of the genetic variation present within these populations. In one population (12a), 20 additional shoots were sampled every 20 cm along a 4-m axis throughout the population to test the accuracy of the sampling effort.
Microsatellite markers developed for the closely related C. keiskei by Araki et al. (2006) were used. Protocols were optimized for eight primer combinations. Multiplex PCR amplification and conditions were as described in Vandepitte et al. (2007). Fluorescent labels, multiplex combinations and annealing temperatures are summarized in Table 2. Fragment separation and detection were performed on an ABI prism 3130 × l capillary sequencer using GeneScan 500 Liz-labelled size standard (Applied Biosystems). Microsatellite patterns were scored using Genemapper version 3.7 (Applied Biosystems).
Table 2.
Locus performance
| Locus_label | Ta (°C) | PCR multiplex | Range (bp) | N | n |
|---|---|---|---|---|---|
| CK19_PET | 58 | 1 | 161–175 | 6 | 479 |
| CK24_VIC | 58 | 1 | 107–139 | 11 | 478 |
| CK20_NED | 58 | 1 | 175–198 | 9 | 479 |
| CK3_FAM | 60 | 2 | 166–187 | 10 | 477 |
| CK38_FAM | 60 | 2 | 107–111 | 4 | 477 |
| CK48_VIC | 60 | 3 | 122–173 | 19 | 477 |
| CK12_NED | 55 | 4 | 172–190 | 11 | 477 |
| CK41_PET | 55 | 4 | 109–111 | 2 | 271 |
Ta = annealing temperature; N = number of alleles; n = number of individuals genotyped.
To assess the reproducibility of the protocol, two runs from independent DNA extractions were carried out for 20 samples. No errors were detected. The discriminative power of the used set of polymorphic markers to differentiate the genotypes was ascertained by plotting the number of multi-locus genotypes (MLG) versus the number of loci used for all possible combinations: a plateau was reached using four or five markers (Fig. 1). As locus 41 (two alleles) was prone to failure and did not distinguish additional MLG, it was removed from the dataset for further analysis.
Fig. 1.
Discriminative power of the set of eight loci to distinguish between genotypes. Squares represent the mean number of multi-locus genotypes (MLG) found using all possible combinations of a given number of loci, and the bars are the minimum and maximum number of MLG. If the discriminative power is sufficient a plateau should be reached; as is the case using four or five loci.
Data analysis
Apart from the replication of individuals genotyped at a set of loci, also the degree of linkage disequilibrium among loci and the apportionment of genetic diversity within populations shows the signature of strong clonal reproduction (Balloux et al., 2003; de Meeûs and Balloux, 2004). Vegetative propagation mimics complete physical linkage over the entire genome and heterozygosity can increase due to the accumulation of somatic mutations in plant species that essentially rely on clonal reproduction (Weir, 1979; Rasmussen and Kollmann, 2008). Therefore, the rate of asexual and sexual reproduction was evaluated using the multicriteria approach proposed by Halkett et al. (2005), which is based on clonal replication, degree of linkage disequilibrium (LD) and the apportionment of genetic diversity within populations. As recommended (Balloux et al., 2003; de Meeûs and Balloux, 2004), all samples per population, including replicates, were used in these calculations.
For each population, G : N (the ratio of the number of MLG found over the number of sampled individuals) and G (multilocus genotypic diversity, i.e. the probability that two individuals taken at random from the population have different genotypes), were calculated. G is equivalent to:
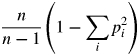 |
where pi is the frequency of the ith genotype and n is the number of individuals sampled. This value ranges between 0, if the population consists of one MLG, and 1, if all samples belong to different MLG.
To assess the degree of LD, the multilocus standardized linkage disequilibrium measurement rD was calculated for each population (de Meeûs and Balloux, 2004; Halkett et al., 2005). This estimate is based on the association index (IA) (Brown et al., 1980) and was modified by Agapow and Burt (2001) to make it independent of the sample size. Genotypic diversity and multilocus linkage disequilibrium were calculated using the Multilocus 1·3 software (Agapow and Burt, 2001). Significance of G and rD values was tested by comparing the observed dataset with 1000 simulated datasets in which sexual recombination was imposed by randomly reshuffling the alleles among individuals, independently for each locus.
F-statistics were calculated for each locus according to Nei (1978) and Weir and Cockerham (1984). Significant deviations from zero were tested by randomizing alleles between individuals within populations using 1000 randomizations (Goudet, 1995).
As most populations consisted of a single genotype (Table 1) and preliminary inspection of environmental variables and sexual recruitment showed little variation between populations consisting of two genotypes and those consisting of more than two genotypes, the effect of environmental variability on sexual recruitment was assessed using a logistic regression model comparing mono- with multiclonal populations. Independent variables were leaf area index (LAI), scores of the sample plots on the two first axes of a detrended correspondence analysis on the recorded vegetation data (DCA1, DCA2), plot scores on the axes of a factor reduction analysis on soil variables (PCA1, PCA 2 and PCA3) and region (two-level factor). Vegetation gradients based on converted Braun-Blanquet scores (1–9; van der Maarel, 2007) were determined using detrended correspondence analysis (DCA) by means of the PC-ORD software (McCune and Mefford, 2006). The first two DCA-axes represented 24·7 % and 23·7 % of the variance. Soil characteristics (N-content, P-content (mg 100 g−1), percentage organic matter, pH, C : N ratio and leaf litter depth; Table 3) were orthogonalized by PCA extraction of axes with an eigenvalue >1, applying a VARIMAX rotation and Kaiser normalization in SPSS 16·0 (SPSS Inc.). Axes explained 34·2, 26·7 and 19·93 % of the variance (Table 3).
Table 3.
Descriptive statistics of habitat variables plus factors loadings of the measured soil variables on the extracted PCA axis (all eigenvalues >1)
| Habitat variable | Range | Mean | s.d. | PCA1 | PCA2 | PCA3 |
|---|---|---|---|---|---|---|
| Soil percentage nitrogen | 0·18–0·74 | 0·44 | 0·16 | 0·89 | 0·13 | 0·11 |
| Soil soluble phosphor content (mg 100−1 g) | 0·53–1·16 | 1·00 | 0·30 | 0·05 | 0·90 | −0·10 |
| Soil percentage organic matter | 6·48–32·20 | 15·52 | 7·44 | 0·96 | 0·09 | −0·09 |
| Soil pH | 2·56–3·30 | 2·86 | 0·19 | 0·06 | −0·01 | 0·92 |
| Soil C : N ratio | 9·06–35·35 | 20·74 | 5·39 | 0·56 | 0·05 | −0·53 |
| Leaf litter depth | 2·50–9·00 | 5·25 | 1·81 | −0·14 | −0·88 | −0·06 |
| Leaf area index of the canopy (LAI) | 0·58–6·03 | 3·97 | 1·21 | |||
| Biotic composition (DCA1) | 0·00–327·34 | 135·01 | 78·79 | |||
| Biotic composition (DCA2) | 0·00–235·45 | 120·00 | 59·19 |
Significant values are highlighted in bold (P < 0·05).
Finally, fruit set and the density of flowering and non-flowering individuals was compared between mono- and multiclonal populations using two-sample independent t-tests. Statistical analyses were performed using SPSS 16·0 (SPSS Inc.).
RESULTS
Multilocus genotypes
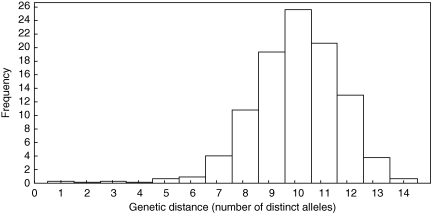
Thirty-nine different MLG were characterized among the 477 analysed C. majalis shoots, based on 70 microsatellite alleles (Table 2). Eleven out of 20 populations consisted of only one MLG. In the nine populations that consisted of more than one genotype, two to four MLG were found (Table 1). All MLG were population-specific. No additional genotypes were found in population 12a among 20 additional shoots sampled every 20 cm along a 4-m axis. In the three largest populations, larger than the plot area studied, additional sampling rendered one or two additional genotypes (populations 10, 14 and 15a in Table 1). A histogram of genetic distances among MLG (Arnaud-Haond and Belkhir, 2007; Arnaud-Haond et al., 2007) shows that allelic differences between MLG are large (Fig. 2). Only one MLG pair, which belonged to population 15b, differed at a single allele.
Fig. 2.
Frequency distribution of allele differences among the 40 different C. majalis multilocus genotypes based on seven microsatellite loci (locus CK41 excluded for the calculations).
Within-population genetic variation
Population G : N ratios varied between 0·05 and 0·21 (mean = 0·09; N = 20; standard deviation = 0·05). Multilocus linkage disequilibrium (mean rD = 0·82; N = 9; s.d. = 0·20) and genotypic diversity (mean G = 0·25; N = 9; s.d. = 0·30) were significantly different from the expectation under random sexual recombination in the nine populations with more than one genotype (see Table 1). There was a significant heterozygote excess in the populations [FIS = −0·48 (where FIS measures the deviations from panmixia within each population), P < 0·001; HO = 0·53 > HS = 0·36 (where HO and HS are, respectively, the average observed and expected heterozygosity within each population)]. This heterozygote excess was foremost the result of the maximal negative FIS values in monoclonal populations and was limited but still significant in the populations consisting of more than one MLG (FIS = −0·12; P < 0·001; HO = 0·54 > HS = 0·47). Variance in FIS among loci in the latter analysis was large (mean s.d. = 0·58), as simulations predict when sex occurs (Balloux et al., 2003). As the contribution of a given locus to FIS varied among populations (not shown), variance is not caused by null alleles.
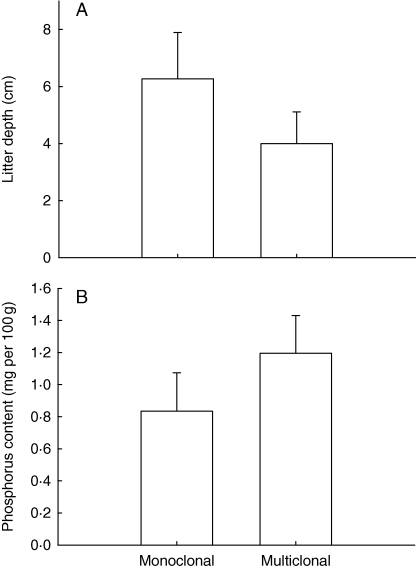
The occurrence of multiple genotypes, assumed to be derived from sexual recombination (genets), was significantly affected by local environmental variables. The second PCA axis, representing the negatively correlated variables litter depth and soil phosphorus content (rPearson = −0·60; N = 20; P < 0·01), was selected in the logistic regression model (Wald χ2 = 5·77; P = 0·01). The occurrence of multiple genets coincided with reduced litter depth (two-sample independent t-statistic = 3·56; P < 0·01) and increased phosphorus soil content (t = −3·40; P < 0·01; means and standard deviations are depicted in Fig. 3). None of the other environmental variables studied (Table 3) did differ significantly between mono- and multiclonal populations (α = 0·05).
Fig. 3.
Relationships between the environmental parameters litter depth (A) and soil-soluble phosphorus content (B), and the occurrence of multiple genets. Means and s.d. are depicted.
Among-population genetic variation
Genetic differentiation among populations was very high [FST = 0·52; HS = 0·36 < HT = 0·75 (where HS and HT are, respectively, the expected heterozygosity or gene diversity within each population and over all populations according to Nei (1978)]. FST according to Weir and Cockerham (1984) was almost identical (0·51, P < 0·001).
Reproductive success
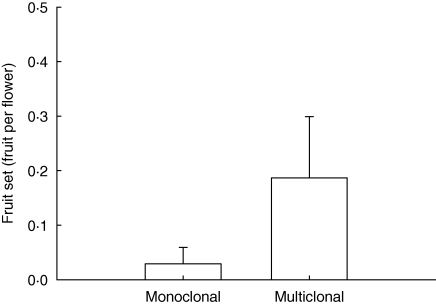
Fruit set was low overall (mean = 0·10, s.d. = 0·11; Table 1). Fruit set was absent or nearly absent in monoclonal populations and was significantly reduced compared with multiclonal populations (t = −3·90; P < 0·01; means and standard deviations are depicted in Fig. 4). There was no significant difference between mono- and multiclonal populations in flowering shoot density (range: 0–27 m−2; mean = 6·48; N = 20; s.d. = 7·71) or the density of all shoots (range: 18–212 m−2; mean = 88·96; N = 20; s.d. = 48·02).
Fig. 4.
Fruit set in mono- and multiclonal C. majalis populations in Belgium. Fruit set increases in multiclonal populations. Means and s.d. are depicted.
DISCUSSION
Although most clonal plant species maintain moderate to high levels of genetic variation through sexual reproduction (Ellstrand and Roose, 1987; Stehlik and Holderegger, 2000; Richards et al., 2004), the studied C. majalis populations essentially rely on clonal replication. Clonal diversities are very low (55 % of the populations studied consisted of a single clone) and the degree of linkage disequilibrium is high (clonal replication mimics complete physical linkage; Halkett et al., 2005). However, the small heterozygote excess in multiclonal populations, large variance in FIS across loci and the fact that only few genotypes differed at a single allele (Fig. 2), suggests that different genotypes result from rare sexual reproduction rather than somatic mutations (Balloux et al., 2003; Halkett et al., 2005).
The mean G : N ratio (0·09) is indeed exceptionally low compared with values reported for other clonal herbs of temperate forests [Anemone nemorosa: 0·91 and 0·95 (Stehlik and Holderegger, 2000; Rusterholz et al., 2009); Viola riviniana: 0·93 (Auge et al., 2001); Circea lutetiana: 0·82 (Verburg et al., 2000); Trillium cuneatum: 0·81 (Gonzales et al., 2008); Maianthemum bifolium: 0·55 and 0·70 (Arens et al., 2005; Honnay et al., 2006); Mercurialis perennis 0·52 (Vandepitte et al., 2009) and 0·43 in Paris quadrifolia (Jacquemyn et al., 2006)], but agrees with other studies on Convallaria species. Analysing three samples per population, Chwedorzewska et al. (2008), mostly found identical AFLP genotypes within C. majalis populations in Poland. Araki et al. (2007) reported a genotype spreading over 40 m2 in a large population of the closely related C. keiskei in Japan (Araki et al., 2009).
The relationship between the occurrence of multiple genotypes and environmental factors suggests that the rare occurrence of sexual recruitment is mediated by the environment. Populations growing at sites with a thin litter layer and higher phosphorus content were characterized by higher genotypic diversity (but note that phosphorus content was generally low). These results are in agreement with other studies that have shown that seedling recruitment increases as the litter depth decreases, or as the proportion of bare soil increases (Facelli and Pickett, 1991; Eriksson, 1995; Lennartsson and Oostermeijer, 2001; Dupuy and Chazdon, 2008; de Vere et al., 2009). As seed viability per se is high in these C. majalis populations (K. Vandepitte et al., unpubl. res.), a thick litter layer may inhibit seed recruitment and therefore decrease clonal diversity because clones that disappear over time are not replaced. A thinner litter layer, in contrast, may promote both recruitment from seeds and improve the survival chances of clonal lineages by providing space for new ramets to establish. The higher soil phosphorus level apparently associated with thinner litter depths may be due to the locally higher organic matter mineralization rate (Harrison, 1987; Kaspari and Yanoviak, 2008).
The restricted dispersal of clonal offspring, clonal replication and reproductive isolation probably all contributed to genetic differentiation (FST = 0·52, P < 0·001). Transfer of vegetative plant parts such as rhizomes by animal activity would relocate genotypes, contributing to a reduction of FST. All genotypes were population-specific, however. Furthermore, the replication of genotypes in a population results in low within-population gene diversity (HS; Nei, 1978), which is an artefact that adds to FST. When the influence of clonality is removed, FST drops but remains significant (FST = 0·17, P < 0·001; clonal replicates and monoclonal populations not included). This could stem from limited pollen exchange among isolated populations situated in forest fragments that have been separated by agricultural land for at least one century (Jacquemyn et al., 2003, Honnay et al., 2006).
Fruiting success was almost zero in monoclonal populations, suggesting that the availability of different genotypes is critical to maintain sexual reproduction. Some genetic studies of other obligate outcrossing clonal herbs support the idea of decreasing fruit set with decreasing genetic diversity (Honnay et al., 2006; Araki et al., 2007). Pollination experiments in Linnaea borealis have confirmed that low reproductive success in within-population crosses is due to the absence of compatible genotypes in populations with no flower colour variation, presumed monoclonal (Scobie and Wilcock, 2009). Fruiting success seems generally maintained in the study populations, as long as some genotypic variation is present, while monoclonality appears to result in sexual extinction in a number of populations (e.g. 1, 7, 9, 11b and 12b). That some fruit set is still present in some monoclonal populations (e.g. 2 or 8) may result from a very small amount of selfing within shoots or clones (incomplete self-incompatibility), pollen flow, or our inability to detect some genotypes. Furthermore, oversized clones may reduce mate availability and abundant within-clone pollinations can reduce reproductive success through stigma saturation and style clogging (Dejong et al., 1993; Charpentier, 2002).
It can be concluded that low reproductive success and sporadic sexual recruitment seem to maintain mainly clonal growth in the C. majalis populations studied. At face value, the results indicate that when leaf litter accumulates, the negative interaction between genotypic diversity and reproductive success in this obligate outcrossing plant might result in complete abandonment of sexual reproduction. Furthermore, a lack of gene flow between populations is likely to make it impossible for isolated monoclonal populations to restore genotypic diversity through sexual reproduction. As monoclonality has been shown to reduce disease resistance (Schmid, 1994) and the potential to adapt to changing conditions (Reusch et al., 2005) in other clonal plant species, the long-term persistence of remnant populations through merely clonal reproduction is uncertain. To confirm the role of the depth of the litter layer on the genotypic diversity through sexual recruitment, a seed sowing experiment should compare the long-term establishment of new clones and their influence on genotypic diversity between high- and low-litter plots.
ACKNOWLEDGEMENTS
We thank Khosro Mehdikhanlou and Rita Degraer for help in the field and the people responsible for Hondsbossen (Natuurpunt) to perform research there. This study is funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
LITERATURE CITED
- Agapow PM, Burt A. Indices of multilocus linkage disequilibrium. Molecular Ecology Notes. 2001;1:101–102. [Google Scholar]
- Araki K, Ohara M. Reproductive demography of ramets and genets in a rhizomatous clonal plant Convallaria keiskei. Journal of Plant Research. 2008;121:147–154. doi: 10.1007/s10265-007-0141-9. [DOI] [PubMed] [Google Scholar]
- Araki K, Yamada E, Ohara M. Breeding system and floral visitors of Convallaria keiskei. Plant Species Biology. 2005;20:149–153. [Google Scholar]
- Araki K, Lian CL, Shimatani K, Ohara M. Development of microsatellite markers in a clonal perennial herb, Convallaria keiskei. Molecular Ecology Notes. 2006;6:1144–1146. [Google Scholar]
- Araki K, Shimatani K, Ohara M. Floral distribution, clonal structure, and their effects on pollination success in a self-incompatible Convallaria keiskei population in northern Japan. Plant Ecology. 2007;189:175–186. [Google Scholar]
- Araki K, Shimatani K, Ohara M. Dynamics of distribution and performance of ramets constructing genets: a demographic–genetic study in a clonal plant, Convallaria keiskei. Annals of Botany. 2009;104:71–79. doi: 10.1093/aob/mcp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arens P, Grashof-Bokdam CJ, van der Sluis T, Smulders MJM. Clonal diversity and genetic differentiation of Maianthemum bifolium among forest fragments of different age. Plant Ecology. 2005;179:169–180. [Google Scholar]
- Arnaud-Haond S, Belkhir K. GENCLONE: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. Molecular Ecology Notes. 2007;7:15–17. [Google Scholar]
- Arnaud-Haond S, Duarte CM, Alberto F, Serrao EA. Standardizing methods to address clonality in population studies. Molecular Ecology. 2007;16:5115–5139. doi: 10.1111/j.1365-294X.2007.03535.x. [DOI] [PubMed] [Google Scholar]
- Auge H, Neuffer B, Erlinghagen F, Grupe R, Brandl R. Demographic and random amplified polymorphic DNA analyses reveal high levels of genetic diversity in a clonal violet. Molecular Ecology. 2001;10:1811–1819. doi: 10.1046/j.0962-1083.2001.01311.x. [DOI] [PubMed] [Google Scholar]
- Balloux F, Lehmann L, de Meeus T. The population genetics of clonal and partially clonal diploids. Genetics. 2003;164:1635–1644. doi: 10.1093/genetics/164.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt B, Hermy M, Deckers J. Migration of herbaceous plant species across ancient-recent forest ecotones in central Belgium. Journal of Ecology. 1999;87:628–638. [Google Scholar]
- Brown AHD, Feldman MW, Nevo E. Multilocus substructure of natural populations of Hordeum spontaneum. Genetics. 1980;96:523–536. doi: 10.1093/genetics/96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier A. Consequences of clonal growth for plant mating. In: Stuefer JF, Erschbamer B, Huber H, Suzuki J-I, editors. Ecology and Evolutionary Biology of Clonal Plants: Proceedings of Clone 2000, an International Workshop held in Obergurgl, Austria, 20–25 August 2000. Berlin: Springer; 2002. pp. 521–530. [Google Scholar]
- Chwedorzewska KJ, Galera H, Kosinski I. Plantations of Convallaria majalis L. as a threat to the natural stands of the species: genetic variability of the cultivated plants and natural populations. Biological Conservation. 2008;141:2619–2624. [Google Scholar]
- Dejong TJ, Waser NM, Klinkhamer PGL. Geitonogamy: the neglected side of selfing. Trends in Ecology & Evolution. 1993;8:321–325. doi: 10.1016/0169-5347(93)90239-L. [DOI] [PubMed] [Google Scholar]
- Dupuy JM, Chazdon RL. Interacting effects of canopy gap, understory vegetation and leaf litter on tree seedling recruitment and composition in tropical secondary forests. Forest Ecology and Management. 2008;255:3716–3725. [Google Scholar]
- Eckert CG. The loss of sex in clonal plants. Evolutionary Ecology. 2000;15:501–520. [Google Scholar]
- Ehrlen J, Munzbergova Z, Diekmann M, Eriksson O. Long-term assessment of seed limitation in plants: results from an 11-year experiment. Journal of Ecology. 2006;94:1224–1232. [Google Scholar]
- Ellstrand NC, Roose ML. Patterns of genotypic diversity in clonal plant species. American Journal of Botany. 1987;74:123–131. [Google Scholar]
- Eriksson O. Dynamics of genets in clonal plants. Trends in Ecology & Evolution. 1993;8:313–316. doi: 10.1016/0169-5347(93)90237-J. [DOI] [PubMed] [Google Scholar]
- Eriksson O. Seedling recruitment in deciduous forest herbs: the effect of litter, soil chemistry and seed bank. Flora. 1995;190:65–70. [Google Scholar]
- Eriksson O. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecologica: International Journal of Ecology. 1999;20:61–66. [Google Scholar]
- Facelli JM, Pickett STA. Plant litter: its dynamics and effects in plant community structure. Botanical Review. 1991;57:1–32. [Google Scholar]
- Gonzales E, Hamrick JL, Smouse PE. Comparison of clonal diversity in mountain and Piedmont populations of Trillium cuneatum (Melanthiaceae – Trilliacea), a forest understorey species. American Journal of Botany. 2008;95:1254–1261. doi: 10.3732/ajb.2007159. [DOI] [PubMed] [Google Scholar]
- Goudet J. FSTAT (Version 1.2): a computer program to calculate F-statistics. Journal of Heredity. 1995;86:485–486. [Google Scholar]
- Halkett F, Simon JC, Balloux F. Tackling the population genetics of clonal and partially clonal organisms. Trends in Ecology & Evolution. 2005;20:194–201. doi: 10.1016/j.tree.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Handel SN. The intrusion of clonal growth patterns on plants breeding systems. The American Naturalist. 1985;125:367–384. [Google Scholar]
- Harrison . Soil organic phosphorus: a review of world literature. Wallingford: CAB International; 1987. [Google Scholar]
- Honnay O, Bossuyt B. Prolonged clonal growth: escape route or route to extinction? Oikos. 2005;108:427–432. [Google Scholar]
- Honnay O, Jacquemyn H, Roldan-Ruiz I, Hermy M. Consequences of prolonged clonal growth on local and regional genetic structure and fruiting success of the forest perennial Maianthemum bifolium. Oikos. 2006;112:21–30. [Google Scholar]
- Jacquemyn H, Honnay O. Mating system evolution under strong clonality: towards self-compatibility or self-incompatibility? Evolutionary Ecology. 2008;22:483–486. [Google Scholar]
- Jacquemyn H, Butaye J, Hermy M. Influence of environmental and spatial variables on regional distribution of forest plant species in a fragmented and changing landscape. Ecography. 2003;26:768–776. [Google Scholar]
- Jacquemyn H, Brys R, Honnay O, Hermy M, Roldan-Ruiz I. Local forest environment largely affects below-ground growth, clonal diversity and fine-scale spatial genetic structure in the temperate deciduous forest herb Paris quadrifolia. Molecular Ecology. 2005;14:4479–4488. doi: 10.1111/j.1365-294X.2005.02741.x. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Honnay O, Hermy M, Roldan-Ruiz I. Sexual reproduction, clonal diversity and genetic differentiation in patchily distributed populations of the temperate forest herb Paris quadrifolia (Trilliaceae) Oecologia. 2006;147:434–444. doi: 10.1007/s00442-005-0287-x. [DOI] [PubMed] [Google Scholar]
- Kaspari M, Yanoviak SP. Biogeography of litter depth in tropical forests: evaluating the phosphorus growth rate hypothesis. Functional Ecology. 2008;22:919–923. [Google Scholar]
- Klimes L, Klimesova J, Hendriks R, Van Groenedael J. Clonal plant architecture: a comparative analysis of form and function. In: de Kroon H, Groenendael J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. pp. 1–29. [Google Scholar]
- Kosinski I. The influence of shoot harvesting on the size and fecundity of Convallaria majalis L. Acta Societatis Botanicorum Poloniae. 2001;70:303–312. [Google Scholar]
- Kudoh H, Shibaike H, Takasu H, Whigham DF, Kawano S. Genet structure and determinants of clonal structure in a temperate deciduous woodland herb, Uvularia perfoliata. Journal of Ecology. 1999;87:244–257. [Google Scholar]
- Lennartsson T, Oostermeijer JGB. Demographic variation and population viability in Gentianella campestris: effects of grassland management and environmental stochasticity. Journal of Ecology. 2001;89:451–463. [Google Scholar]
- McCune B, Mefford MJ. PC-ORD: multivariate analysis of ecological data. Version 5.10. Gleneden Beach, OR: MjM Software; 2006. [Google Scholar]
- van der Maarel E. Transformation of cover-abundance values for appropriate numerical treatment: alternatives to the proposals by Podani. Journal of Vegetation Science. 2007;18:767–770. [Google Scholar]
- de Meeûs T, Balloux F. Clonal reproduction and linkage disequilibrium in diploids: a simulation study. Infection, Genetics and Evolution. 2004;4:345–351. doi: 10.1016/j.meegid.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average hetrozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AL, Miller RH, Keeney DR. Methods of soil analysis. Madison, WI: Soil Science Society of America; 1982. Part 2. Chemical and microbiological properties. [Google Scholar]
- Rasmussen KK, Kollmann J. Low genetic diversity in small peripheral populations of a rare European tree (Sorbus torminalis) dominated by clonal reproduction. Conservation Genetics. 2008;9:1533–1539. [Google Scholar]
- Reusch TBH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences of the USA. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CL, Hamrick JL, Donovan LA, Mauricio R. Unexpectedly high clonal diversity of two salt marsh perennials across a severe environmental gradient. Ecology Letters. 2004;7:1155–1162. [Google Scholar]
- Rusterholz HP, Kissling M, Baur B. Disturbances by human trampling alter the performance, sexual reproduction and genetic diversity in a clonal woodland herb. Perspectives in Plant Ecology Evolution and Systematics. 2009;11:17–29. [Google Scholar]
- Schmid B. Effects of genetic diversity in experimental stands of Solidago altissima: evidence for the potential role of pathogens as selective agents in plant populations. Journal of Ecology. 1994;82:165–175. [Google Scholar]
- Scobie AR, Wilcock CC. Limited mate availability decreases reproductive success of fragmented populations of Linnaea borealis, a rare, clonal self-incompatible plant. Annals of Botany. 2009;103:835–846. doi: 10.1093/aob/mcp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown J. The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. International Journal of Plant Sciences. 2008;169:157–168. [Google Scholar]
- Stehlik I, Holderegger R. Spatial genetic structure and clonal diversity of Anemone nemorosa in late successional deciduous woodlands of Central Europe. Journal of Ecology. 2000;88:424–435. [Google Scholar]
- Tutin TG, Burges NA, Chater OA, et al. Flora Europaea. 2nd edn. Vol. 1. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Vandepitte K, Jacquemyn H, Roldan-Ruiz I, Honnay O. Landscape genetics of the self-compatible forest herb Geum urbanum: effects of habitat age, fragmentation and local environment. Molecular Ecology. 2007;Vol. 16:4171–4179. doi: 10.1111/j.1365-294X.2007.03473.x. [DOI] [PubMed] [Google Scholar]
- Vandepitte K, Roldan-Ruiz I, Leus L, Jacquemyn H, Honnay O. Canopy closure shapes clonal diversity and fine-scale genetic structure in the dioecious understorey perennial Mercurialis perennis. Journal of Ecology. 2009;97:404–414. [Google Scholar]
- Verburg R, Maas J, During HJ. Clonal diversity in differently-aged populations of the pseudo-annual clonal plant Circaea lutetiana L. Plant Biology. 2000;2:646–652. [Google Scholar]
- de Vere N, Jongejans E, Plowman A, Williams E. Population size and habitat quality affect genetic diversity and fitness in the clonal herb Cirsium dissectum. Oecologia. 2009;159:59–68. doi: 10.1007/s00442-008-1203-y. [DOI] [PubMed] [Google Scholar]
- Watkinson AR, Powell JC. Seedling recruitment and the maintenance of clonal diversity in plant populations: a computer simulation of Ranunculus repens. Journal of Ecology. 1993;81:707–717. [Google Scholar]
- Weir BS. Inferences about linkage disequilibrium. Biometrics. 1979;35:235–254. [PubMed] [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Westhoff V, van der Maarel E. The Braun–Blanquet approach. In: Whittaker RH, editor. Handbook of vegetation science. The Hague: Dr W. Junk B.V. Publishers; 1973. pp. 619–726. Part V. Ordination and classification of vegetation. [Google Scholar]
- Zhu YY, Chen HR, Fan JH, et al. Genetic diversity and disease control in rice. Nature. 2000;406:718–722. doi: 10.1038/35021046. [DOI] [PubMed] [Google Scholar]