Abstract
Background and Aims
Biological mutualisms rely on communication between partners, but also require protective measures against exploitation. Animal-pollinated flowers need to attract pollinators but also to avoid conflicts with antagonistic consumers. The view of flower visitors as mutualistic and antagonistic agents considers primarily the plants' interest. A classification emphasizing the consumer's point of view, however, may be more useful when considering animal's adaptations to flower visits which may include a tolerance against defensive floral scent compounds.
Methods
In a meta-analysis covering 18 studies on the responses of animals to floral scents, the animals were assigned to the categories of obligate and facultative flower visitors which considers their dependency on floral resources. Their responses on floral scents were compared.
Key Results
On average, obligate flower visitors, often corresponding to pollinators, were attracted to floral scent compounds. In contrast, facultative and mainly antagonistic visitors were strongly repelled by floral scents. The findings confirm that floral scents have a dual function both as attractive and defensive cues.
Conclusions
Whether an animal depends on floral resources determines its response to these signals, suggesting that obligate flower visitors evolved a tolerance against primarily defensive compounds. Therefore, floral scent bouquets in conjunction with nutritious rewards may solve the conflicting tasks of attracting mutualists while repelling antagonists.
Keywords: Attraction, benzenoids, floral scents, obligate and facultative flower visitors, plant defence, terpenes
INTRODUCTION
Plant volatiles serve as cues that mediate various interactions with animals. Scents emitted by vegetative plant parts effectively function as allelopathic agents, herbivore deterrents or as attractants for the herbivores' natural enemies, and flower volatiles are traditionally regarded as pollinator attracting signals (Pichersky and Gershenzon, 2002; Raguso, 2008b; Unsicker et al., 2009). The notion that floral traits such as colour, shape, nutritious rewards and scent are adaptations for efficient pollination by animals dates back to the classical work of Sprengel (1793) and Darwin (1862) and has stimulated numerous detailed investigations since then (Pellmyr, 2002; Dudareva and Pichersky, 2006). Non-pollinating visitors from several taxa potentially exploit rewards like nectar and pollen (Inouye, 1980), disturb true pollinators (Tsuji et al., 2004; Junker et al., 2007) or consume floral tissues (McCall and Irwin, 2006). The negative impact of such antagonists on plant reproduction may even exceed the benefits from mutualists (Herrera, 2000; Morris et al., 2007). Accordingly, defensive properties of flowers have recently received attention. Defensive traits include floral scents (Kessler and Baldwin, 2006; Junker and Blüthgen, 2008; Kessler et al., 2008; Raguso, 2008b; Willmer et al., 2009), unpalatable or even toxic nectar (Stephenson, 1981; Stephenson, 1982; Adler, 2000; Johnson et al., 2006; Liu et al., 2007) and non-volatile secondary metabolites (Johnson et al., 2008; Hanley et al., 2009). This study focuses on floral scents and explores their role as attractants and as repellents. Floral scent bouquets are complex blends composed of volatile substances from various biosynthetical pathways (most commonly mono- and sesquiterpenoids, benzenoids and aliphatics) and often with diverse functional groups (e.g. alcohols, aldehydes, esters, ethers and ketones) (Dudareva and Pichersky, 2006; Knudsen et al., 2006).
The dual role of floral traits (Irwin et al., 2004) may enable flowers to select their visitor spectrum in order to optimize pollination success. For instance, some floral scent compounds (e.g. linalool) commonly occurring in floral scent bouquets were shown to attract pollinators (Laloi et al., 2000; Andersson et al., 2002) but also repelled nectar thieves (Junker and Blüthgen, 2008). Similarly, iridoid glycosides in nectar of Catalpa speciosa (Stephenson, 1982) and phenolics in nectar of Aloe vryheidensis (Johnson et al., 2006) deterred antagonistic flower visitors while pollinators remained unaffected. Furthermore, Hanley et al. (2009) reported a trade-off between chemical (cyanogens) and morphological defences that exclude potential florivores.
Classifying flower visitors as mutualistic and antagonistic agents emphasizes the plants' need to maximize reproductive success. However, this classification may not be adequate to explain the diverse responses to floral traits by different flower visitors. Flower visitation by pollinators is driven by their interest as consumers, not necessarily as mutualists – their mutualistic service is merely a by-product of searching for resources or mating opportunities (Frame, 2003). To understand the behaviour of flower visitors towards floral traits, a different classification is proposed that emphasizes the consumers' perspective. ‘Obligate’ is distinguished from ‘facultative’ consumers of floral resources in contradiction to ‘mutualistic’ and ‘antagonistic’ agents. Obligate flower visitors are defined as those that require floral resources for at least part of their life-cycle, while facultative ones occasionally consume floral resources but are not obviously dependent on them. Given a reasonable knowledge of the animal species' natural history, the dependency on floral resources can usually be unequivocally assessed. In contrast, whether the relationship between an animal and a plant is mutualistic, commensalistic or antagonistic depends not only on the species involved, but also on the focal interaction. For example, ants are most often floral antagonists (e.g. Galen and Butchart, 2003) but in some comparatively rare cases they act as pollinating mutualists (e.g. Gomez and Zamora, 1992; Gomez et al., 1996), while their facultative use of floral resources is undisputed: they are omnivorous and feed on various resources, not just flower nectar (Blüthgen and Feldhaar, 2010). Furthermore, the positive or negative net effect of interactions between species may be variable over space and time (Bronstein, 1994), which makes clear classifications of flower visitors even more difficult.
Our meta-analysis examines whether the dependency of animals on floral resources determines their response to floral scents and it is hypothesized that obligate flower visitors are better adapted to potential floral defences. Possible consequences to flowers with respect to the dilemma to attract mutualists but repel antagonists are discussed.
MATERIALS AND METHODS
In addition to studies known to the authors, appropriate articles were found online using relevant search terms (combinations of the terms attraction, deterrence, floral scent/odour, herbivory, pollinator, repellence, terpenoids, volatiles) in Zoological Record, BIOSIS Previews and Google Scholar. All studies that contrasted the effect of floral volatiles to a scentless control and which gave information on the variance of experimental and control were included.
The classification of animal species as obligate and facultative flower visitors was either provided in the original studies, or was assigned based on the literature and/or information from entomologists by considering the resource spectrum of the animals (Supplementary Data 1, available online). Animals with an unclear status were excluded from the analysis. In studies that did not specifically deal with flower ecology, substances used in the tests were assigned as flower volatiles if they were listed in the extensive compilation by Knudsen et al. (2006). Scent was applied in natural concentrations in most studies, although this was not evident for all data. Information on the volume, mass or emission rate of scent compounds used in the bioassays was mostly provided in the literature (Supplementary Data 1). Koschier et al. (2000) applied several concentrations of their test substances; in this case, the results from the lowest concentration that are the most natural ones (e.g. 8700 ng linalool) were used.
The effect size of an animal's response to a scent was extracted from the original study as a log response ratio L = ln( ), with
), with  and
and  as the mean response of the focal organism to the experimental scent treatment and scentless control, respectively (Hedges et al., 1999). Log response ratio L was chosen to measure effect size because, unlike the commonly used Hedges' d, it assumes that a scent or bouquet has a proportional effect on the visitors' response, which is more appropriate when the response cannot be negative (Hedges et al., 1999; Morris et al., 2007). In the rare event that either
as the mean response of the focal organism to the experimental scent treatment and scentless control, respectively (Hedges et al., 1999). Log response ratio L was chosen to measure effect size because, unlike the commonly used Hedges' d, it assumes that a scent or bouquet has a proportional effect on the visitors' response, which is more appropriate when the response cannot be negative (Hedges et al., 1999; Morris et al., 2007). In the rare event that either  = 0 or
= 0 or 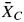 = 0, zero was replaced by 0·01 in order to calculate L. Using the standard deviation (s.d.) and the sample size n of treatment and control, the variances v of all L were computed using MetaWin 2·0 and v−1 was used as weight for the subsequent analysis of variance (ANOVA). First, a one-factorial weighted ANOVA was conducted with the complete dataset with L as response variable and the dependency of the animals on flower visits as explanatory variable. Secondly, in order to compare the magnitude of positive and negative responses by obligate and facultative flower visitors, the absolute values |L| of L (excluding cases where L = 0) were used in a weighted two-factorial ANOVA as response variable with the dependency of the animals and the sign of the response as first and second explanatory variable, respectively. Thirdly, reduced datasets comprising data on either the effects of aliphatics, benzenoids, mono- and sesquiterpenes or the effects of alcohols, aldehydes, esters, ethers, hydrocarbons and ketones were used to perform two-factorial weighted ANOVAs with the dependency and the biosynthetical origin or functional group as explanatory variables. Here, groups of compounds from different biosynthetical origins or with different functional groups where only included if sufficient data from both obligate and facultative flower visitors were available. The meta-analysis may have its limitations in the independency of data, since several observations were extracted from individual studies. In order to address this concern, the dataset was reduced to a single value (mean L) for each study prior to another ANOVA with mean L as response and the dependency as explanatory variables. In cases where a given study reported results from both obligate and facultative flower visitors, the mean for both groups was calculated separately.
= 0, zero was replaced by 0·01 in order to calculate L. Using the standard deviation (s.d.) and the sample size n of treatment and control, the variances v of all L were computed using MetaWin 2·0 and v−1 was used as weight for the subsequent analysis of variance (ANOVA). First, a one-factorial weighted ANOVA was conducted with the complete dataset with L as response variable and the dependency of the animals on flower visits as explanatory variable. Secondly, in order to compare the magnitude of positive and negative responses by obligate and facultative flower visitors, the absolute values |L| of L (excluding cases where L = 0) were used in a weighted two-factorial ANOVA as response variable with the dependency of the animals and the sign of the response as first and second explanatory variable, respectively. Thirdly, reduced datasets comprising data on either the effects of aliphatics, benzenoids, mono- and sesquiterpenes or the effects of alcohols, aldehydes, esters, ethers, hydrocarbons and ketones were used to perform two-factorial weighted ANOVAs with the dependency and the biosynthetical origin or functional group as explanatory variables. Here, groups of compounds from different biosynthetical origins or with different functional groups where only included if sufficient data from both obligate and facultative flower visitors were available. The meta-analysis may have its limitations in the independency of data, since several observations were extracted from individual studies. In order to address this concern, the dataset was reduced to a single value (mean L) for each study prior to another ANOVA with mean L as response and the dependency as explanatory variables. In cases where a given study reported results from both obligate and facultative flower visitors, the mean for both groups was calculated separately.
Additionally, 17 studies from which not all the data required could be extracted to estimate v (mean, s.d. and/or n of control and experimental) were subjected to a second ANOVA based on unweighted data (Supplementary Data 2a and 2b, available online). All statistical analyses were performed using R (Version 2.7.0, A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The meta-analysis included 18 publications that tested the effect of floral scent bouquets or individual synthetic substances that are common in flower odour blends on a broad spectrum of animals. In total, 425 observations (83 substances from seven chemical classes and bouquets from 31 plant species) were included in the analysis (Supplementary Data 1, available online). The designation of 24 obligate and 16 facultative visitor species was often consistent with their putative role as mutualists and antagonists, respectively. Most obligate visitors in this study were represented by putative pollinator taxa (butterflies, moths, bees, hoverflies and hummingbirds), but also included thrips for which it is unknown whether they are mutualistic, commensalistic or even antagonistic. Facultative flower visitors were represented by omnivorous ants that often act as non-pollinating nectar-thieves (Galen, 1983), herbivores such as Tettigoniidae, certain beetles belonging to the families Cerambycidae, Chrysomelidae, Mordellidae, Scarabaeidae, and bugs occasionally feeding on flowers, and generalist dipterans that use a variety of non-floral resources. For example, the study of Andrews et al. (2007) involved the facultative flower visitor Acalymma vittatum, a chrysomelid beetle that feeds on cucurbit leaves, fruits and flowers, and the obligate flower visitor Peponapis pruinosa, a squash bee that feeds exclusively on pollen and nectar of cucurbit flowers. A full list of the species involved, their dependency on flower visits (obligate and facultative), and assumed typical effect on flowers (mutualistic and antagonistic) is provided in Supplementary Data 1 (available online).
The meta-analysis revealed significantly different responses of obligate and facultative flower visitors to floral scents (Table 1A). Obligate flower visitors were attracted to the majority of floral scents, whereas facultative flower visitors were negatively affected (Fig. 1A). The magnitude of negative responses by facultative flower visitors was about four times stronger than the remaining responses (Table 1B and Fig. 1B). Flower visitors' responses differed between floral scent compounds from four pathways of plant secondary metabolism and between compounds with different functional groups (Table 1C, D). The strongly negative responses of facultative flower visitors towards monoterpenes and alcohols, ethers and ketones (Figs 2 and 3) are particularly remarkable. Their negative response was even found for linalool alone, the most common substance in the present analysis: facultative flower visitors negatively responded to this substance [95 % confidence interval (CI) based on the weighted data = −5·14 to −0·17], while obligate ones responded mainly positively (CI = −0·55 to 0·96; weighted ANOVA: F1,20 = 3·3, P = 0·085). However, results of these subsets examining the effects of linalool and different compound classes should be viewed with caution because of small sample sizes of some study groups.
Table 1.
Effect of the animals' dependency on floral resources on their responses to floral scents
| d.f. | F | P | |
|---|---|---|---|
| (A) Meta-analysis: parameter | |||
| Dependency | 1 | 52·6 | <0·001 |
| Residuals | 423 | ||
| (B) Dependency × sign of L | 1 | 7·5 | <0·01 |
| Dependency | 1 | 20·2 | <0·001 |
| Sign of L | 1 | 8·1 | <0·01 |
| Residuals | 364 | ||
| (C) Dependency × pathway | 3 | 16·6 | <0·001 |
| Pathway | 3 | 31·2 | <0·001 |
| Dependency | 1 | 130·2 | <0·001 |
| Residuals | 343 | ||
| (D) Dependency × functional group | 5 | 4·4 | <0·001 |
| Functional group | 6 | 33 | <0·001 |
| Dependency | 1 | 134·6 | <0·001 |
| Residuals | 298 |
Results of the weighted ANOVA for (A) effects of floral scents on animals with different dependencies in floral resources (complete dataset); (B) differences in the magnitude of positive and negative responses (|L|) on obligate and facultative flower visitors, complete dataset excluding cases where L = 0; (C) the effects of floral scents from different biosynthetical pathways (aliphatics, benzenoids, mono- and sesquiterpenes) on obligate and facultative flower visitors (using a subset of the data with these chemical classes only); (D) the effects of floral scents with different functional groups (alcohols, aldehydes, esters, ethers, hydrocarbons and ketones) on obligate and facultative flower visitors (using a subset of the data with these functional groups only).
Fig. 1.
Effects of floral scents on obligate and facultative flower visitors. Weighted mean and 95 % confidence intervals of the log response ratio L are shown. L describes the proportional difference between the mean effect of the scent treatment and the control. Sample sizes are given at the top of the figure. (A) Results of all data obtained from the literature. L > 0 indicates an attractive effect; L < 0 a repellent effect. A significant effect in either direction is indicated in cases where the confidence intervals do not include zero. (B) Magnitude of positive and negative responses by obligate and facultative flower visitors. Absolute values of log response ratios |L| are shown (excluding L = 0).
Fig. 2.
Effects of individual floral scent compounds derived from different biosynthetical pathways. Weighted mean and 95 % confidence intervals of the log response ratio L are shown. Effects of chemical classes were included if sufficient data for both obligate and facultative flower visitors were available. A significant effect in either direction is indicated in cases where the confidence intervals do not include zero.
Fig. 3.
Effects of individual floral scent compounds with different functional groups. Weighted mean and 95 % confidence intervals of the log response ratio L are shown. Effects of substances with certain functional groups were included if sufficient data for both obligate and facultative flower visitors were available. A significant effect in either direction is indicated in cases where the confidence intervals do not include zero.
Overall, the results were confirmed when the analysis was repeated with an extended dataset including 17 additional studies from which no variance of the response ratios could be additionally extracted (Supplementary Data 2a, b).
Observations on obligate flower visitors were dominated by thrips (11 species), facultative visitors by ants (four species). However, the log response ratios L of thrips (CI = 0·32 – 0·57) were slightly higher than those of the other obligate flower visitors (CI = 0·04 – 0·43) but they were not significantly different from each other (weighted ANOVA: F1,243 = 45·3, P = 0·095). Negative effects of floral scents on ants (CI = −0·33 to −0·17) were less pronounced than on other facultative flower visitors (CI = −5·1 to −3·18) (weighted ANOVA: F1,178 = 82·3, P < 0·001). Therefore, the over-representation of thrips and ants did not bias the general conclusions.
Different, often complex, methods were applied in the original studies in an effort to reveal the influence of floral odours on the study animals: 62 % of all observations derive from studies using scented traps, 22 % were based on olfactometer assays and 10 % on bait assays. The remaining observations were taken from toxicity tests (4 %) and food-choice tests (1 %) besides miscellaneous other experimental set-ups (1 %). Study design had a strong influence on the animals' responses (weighted ANOVA: F6,417 = 777·3, P < 0·001). However, the only significant differences in a post hoc comparison were found between toxicity tests versus all other designs and traps versus olfactometer tests (weighted pair-wise t-tests t ≥ 4·6, Bonferroni-corrected P ≤ 0·02). When data derived from toxicity tests were removed from the analysis (they include only tests with facultative flower visitors), the effect of the dependency was consistent to the complete dataset (weighted ANOVA: F1,406 = 62·7, P < 0·001). Hence, the toxicity tests did not bias our general finding. The contrast between obligate and facultative visitors was also consistent when the dataset was restricted to trap experiments (weighted ANOVA: F1,260 = 10·9, P = 0·001).
The difference between responses by facultative and obligate visitors remained significant (weighted ANOVA: F1,18 = 4·9, P = 0·04) in the analysis where the dataset was reduced to a single value (mean L) for each study (n = 13 and 7 for obligate and facultative flower visitors, respectively).
DISCUSSION
It was found that floral scents may act as filters (see Raguso, 2008a, b) that attract obligate flower visitors but repel facultative ones. The results suggest that the proposed dichotomy of dependencies on floral resources (obligate versus facultative) can explain responses to floral signals. In turn, such a framework allows hypotheses about the ecology and evolution of flower–animal interactions to be suggested: if the animals' and plants' interests meet, this may lead to coevolutionary trajectories wherein the attractive function serves as a mutualistic signal and the repellent function as an effective defence. This is true if obligate flower visitors often serve as mutualists, while facultative flower visitors typically represent antagonists. This dichotomy is tentatively supported in the present dataset (Supplementary Data 1, available online), but may require further investigations across species. The correspondence between dependence, net effect and response may provide a solution for the plants' dilemma of attracting pollinators while defending antagonists.
It has been hypothesized that attractive cues may have evolved from floral herbivore deterrents that occurred in early angiosperms (Pellmyr and Thien, 1986) and perhaps in other non-angiosperm taxa that may have been the first zoophilous plants during the Mesozoic (Ren et al., 2009). Some floral volatiles are emitted by archaic taxa as well as in modern angiosperms (Pellmyr et al., 1991; Goodrich and Raguso, 2009); hence it is probable that floral scents maintained this primary function. In a different context, terpenoids were shown to have harmful effects on various organisms (Gershenzon and Dudareva, 2007). The defensive function of individual floral scent compounds dissolved in nectar and of entire floral scent bouquets was recently shown for Nicotiana attenuata flowers (Kessler and Baldwin, 2006) and for a larger set of flowers repelling facultative nectar-thieving ants (Junker and Blüthgen, 2008; Willmer et al., 2009). As a consequence, obligate consumers of floral resources may have secondarily evolved a tolerance against repellent, deterrent or even toxic substances. This notion might be reflected in the finding that the magnitude of negative responses by facultative flower visitors is by far the largest compared with negative responses by obligate flower visitors and positive responses of both groups. Along with the view that pollination may represent a special case of phytophagy (Frame, 2003), one may thus hypothesize that plant–pollinator mutualism is the consequence of, rather than the prerequisite for, obligate flower visits.
Obligate flower visitors are believed to optimize their utilization of floral resources. This can be achieved by evolving accordant abilities like specialized mouth parts (Labandeira and Sepkoski, 1993), the capability to digest pollen (Roulston and Cane, 2000) and visual abilities like trichromacy (Chittka, 1996), but may also involve mechanisms to overcome flower defences or even the use of primarily defensive volatiles as host finding cues reinforced by learned associated rewards (Carlsson and Hansson, 2006; Riffell et al., 2008). In contrast, the selective pressure on facultative flower visitors to adapt to flowers is expected to be low or absent. Similarly, variable responses to defensive secondary metabolites are a common scenario in plant vegetative defences, where generalized herbivores are deterred, but specialized leaf-feeding herbivores tolerate and even utilize such defensive compounds as host-finding signals (Smallegange et al., 2007). While generalists may use alternative plants, specialists represent obligate consumers of their specific host, in analogy to obligate flower visitors. However, in the herbivory context, adaptations that enable specialist folivores to cope with defences of their host plants are usually species-specific results of co-evolution (Cornell and Hawkins, 2003). In the present definition of obligate flower visitors, no distinction is made between specialists that visit only flowers of a narrow taxonomic range and generalists with a wide host spectrum. Obligate flower visitors are animals that depend on the resources offered by flowers in general. In the present dataset, only a few specialist flower visitors are included where an innate attraction to scents would be expected, which may be the reason for the low magnitude of positive responses of obligate flower visitors. Andrews et al. (2007) tested the responses of Peponapis pruinosa, the specialist pollinator of Cucurbita moschata (Cucurbitaceae) to three floral scent compounds of this plant species and found a strong and significant attraction to two of these scents. It is assumed that specialized obligate flower visitors would be more attracted by the floral scents of its host plants (probably due to innate preferences) than generalists that may use these scents as learnt cues associated with rewards (Cunningham et al., 2004, 2006). Colour and shape may also facilitate the association of rewards with certain plant species but, in contrast to scent, these visual traits may not explain any aversions to a rewarded flower.
The finding that benzenoids are attractive while monoterpenes, alcohols, ethers and ketones are particularly repellent to facultative flower visitors may suggest that these compounds have been evolved for these respective functions. This idea is supported by the fact that defensive terpenoids are commonly produced by vegetative tissues, whereas the benzenoids and phenylpropanoids have direct biosynthetic links to floral colours (Pichersky and Gershenzon, 2002; Schie et al., 2006). The adaptive functions of benzenoids (attraction) and monoterpenes (defence) within floral scent bouquets are also suggested by Schiestl (2010). However, there are also examples of defensive benzenoids and attractive terpenoids, alcohols, ethers and ketones within the present study and in the literature (e.g. Kessler and Baldwin, 2006). Thus, phytochemicals that share certain chemical properties cannot be regarded as ecologically uniform, and the average trends of substances from a given biosynthetical pathway or with a certain functional group may not apply to all substances of a given group.
For an improved understanding of trait-mediated flower–visitor interactions, interests of both parties – plants and animals – need to be considered. Obligate outcrossing, animal pollinated flowers depend on pollen transferring mutualists, but may also attract commensalists and antagonists. Consumers visit flowers in search for resources, for which some of them evolved an obligate dependence and others are facultative consumers of flowers and floral rewards. Therefore, from the animals' perspective, flower visitors can be divided into obligate and facultative ones. The present meta-analysis revealed that the dependency on floral resources is a good predictor of the animals' response to floral signals.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We thank John Hilty and Konrad Fiedler for their help in assigning animals to obligate and facultative flower visitors and Bill Morris, Diego Vazquez, Jette Knudsen, Stefan Dötterl, Thomas Schmitt, Manja Wendt and Sara Leonhardt for discussions of earlier versions of the manuscript. Toshihiro Imai, Danny Kessler, William Kirk, Elisabeth H. Koschier and Nina Theis kindly provided additional data. This work was supported by the Deutsche Forschungsgemeinschaft (DFG BL 960/1-1) and a scholarship from Evangelisches Studienwerk e.V. Villigst to R.R.J.
LITERATURE CITED
- Adler LS. The ecological significance of toxic nectar. Oikos. 2000;91:409–420. [Google Scholar]
- Andersson S, Nilsson LA, Groth I, Bergström G. Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Botanical Journal of the Linnean Society. 2002;140:129–153. [Google Scholar]
- Andrews ES, Theis N, Adler LS. Pollinator and herbivore attraction to Cucurbita floral volatiles. Journal of Chemical Ecology. 2007;33:1682–1691. doi: 10.1007/s10886-007-9337-7. [DOI] [PubMed] [Google Scholar]
- Blüthgen N, Feldhaar H. Food and shelter: how resources influence ant ecology. In: Lach L, Parr C, Abbott K, editors. Ant ecology. Oxford: Oxford University Press; 2010. pp. 115–136. [Google Scholar]
- Bronstein JL. Conditional outcomes in mutualistic interactions. Trends in Ecology and Evolution. 1994;9:214–217. doi: 10.1016/0169-5347(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Hansson B. Detection and coding of flower volatiles in nectar-foraging insects. In: Dudareva N, Pichersky E, editors. Biology of floral scent. Boca Raton, FL: CRC Press; 2006. pp. 147–198. [Google Scholar]
- Chittka L. Does bee color vision predate the evolution of flower color? Naturwissenschaften. 1996;83:136–138. [Google Scholar]
- Cornell HV, Hawkins BA. Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. American Naturalist. 2003;161:507–522. doi: 10.1086/368346. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Moore CJ, Zalucki MP, West SA. Learning, odour preference and flower foraging in moths. Journal of Experimental Biology. 2004;207:87–94. doi: 10.1242/jeb.00733. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Moore CJ, Zalucki MP, Cribb BW. Insect odour perception: recognition of odour components by flower foraging moths. Proceedings of the Royal Society London B. 2006;273:2035–2040. doi: 10.1098/rspb.2006.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilised by insects. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biology of floral scent. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- Frame D. Generalist flowers, biodiversity and florivory: implications for angiosperm origins. Taxon. 2003;52:681–685. [Google Scholar]
- Galen C. The effects of nectar thieving ants on seedset in floral scent morphs of Polemonium viscosum. Oikos. 1983;41:245–249. [Google Scholar]
- Galen C, Butchart B. Ants in your plants: effects of nectar-thieves and pollen fertility and seed-siring capacity in the alpine wildflower, Polemonium viscosum. Oikos. 2003;101:521–528. [Google Scholar]
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nature Chemical Biology. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- Gomez JM, Zamora R. Pollination by ants: consequences of quantitative effects on a mutualistic system. Oecologia. 1992;91:410–418. doi: 10.1007/BF00317631. [DOI] [PubMed] [Google Scholar]
- Gomez JM, Zamora R, Hodar JA, Garcia D. Experimental study of pollination by ants in mediterranean high mountain and arid habitats. Oecologia. 1996;105:236–242. doi: 10.1007/BF00328552. [DOI] [PubMed] [Google Scholar]
- Goodrich KR, Raguso RA. The olfactory component of floral display in Asimina and Deeringothamnus (Annonaceae) New Phytologist. 2009;183:457–469. doi: 10.1111/j.1469-8137.2009.02868.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Armbruster WS. Pollination and plant defence traits co-vary in Western Australian Hakeas. New Phytologist. 2009;182:251–260. doi: 10.1111/j.1469-8137.2008.02709.x. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- Herrera CM. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology. 2000;81:2170–2176. [Google Scholar]
- Inouye DW. The terminology of floral larceny. Ecology. 1980;61:1251–1253. [Google Scholar]
- Irwin RE, Adler LS, Brody AK. The dual role of floral traits: pollinator attraction and plant defense. Ecology. 2004;85:1503–1511. [Google Scholar]
- Johnson ET, Berhow MA, Dowd PF. Colored and white sectors from star-patterned Petunia flowers display differential resistance to corn earworm and cabbage looper larvae. Journal of Chemical Ecology. 2008;34:757–765. doi: 10.1007/s10886-008-9444-0. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Junker RR, Blüthgen N. Floral scents repel potentially nectar-thieving ants. Evolutionary Ecology Research. 2008;10:295–308. [Google Scholar]
- Junker R, Chung AYC, Blüthgen N. Interaction between flowers, ants and pollinators: additional evidence for floral repellence against ants. Ecological Research. 2007;22:665–670. [Google Scholar]
- Kessler D, Baldwin IT. Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. The Plant Journal. 2006;49:840–854. doi: 10.1111/j.1365-313X.2006.02995.x. [DOI] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. Field experiments with transformed plants reveal the sense of floral scents. Science. 2008;321:1200–1202. doi: 10.1126/science.1160072. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Koschier EH, De Kogel WJ, Visser JH. Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. Journal of Chemical Ecology. 2000;26:2643–2655. [Google Scholar]
- Labandeira CC, Sepkoski JJ. Insect diversity in the fossil record. Science. 1993;261:310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- Laloi D, Bailez O, Blight MM, Roger B, Pham-Delegue M-H, Wadhams LJ. Recognition of complex odors by restrained and free-flying honeybees, Apis mellifera. Journal of Chemical Ecology. 2000;26:2307–2319. [Google Scholar]
- Liu F, Chen J, Chai J, et al. Adaptive functions of defensive plant phenolics and a non-linear bee response to nectar components. Functional Ecology. 2007;21:96–100. [Google Scholar]
- McCall AC, Irwin RE. Florivory: the intersection of pollination and herbivory. Ecology Letters. 2006;9:1351–1365. doi: 10.1111/j.1461-0248.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- Morris WF, Hufbauer RA, Agrawal AA, et al. Direct and interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology. 2007;88:1021–1029. doi: 10.1890/06-0442. [DOI] [PubMed] [Google Scholar]
- Pellmyr O. Plant–animal interactions: an evolutionary approach. In: Herrera CM, Pellmyr O, editors. Pollination by animals. Oxford: Blackwell; 2002. pp. 157–184. [Google Scholar]
- Pellmyr O, Thien LB. Insect reproduction and floral fragrances: keys to the evolution of the angiosperms? Taxon. 1986;35:76–85. [Google Scholar]
- Pellmyr O, Tang W, Groth I, Bergström G, Thien LB. Cycad cone and angiosperm floral volatiles: inferences for the evolution of insect pollination. Biochemical Systematics and Ecology. 1991;19:623–627. [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Raguso RA. Start making scents: the challenge of integrating chemistry into pollination ecology. Entomologia Experimentalis et Applicata. 2008a;128:196–207. [Google Scholar]
- Raguso RA. Wake up and smell the roses: the ecology and evolution of floral scent. Annual Review of Ecology, Evolution and Systematics. 2008b;39:549–69. [Google Scholar]
- Ren D, Labandeira CC, Santiago-Blay JA, et al. A probable pollination mode before angiosperms: Eurasian, long-proboscid scorpionflies. Science. 2009;326:840–847. doi: 10.1126/science.1178338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell JA, Alarcon R, Abrell L, Davidowitz G, Bronstein JL, Hildebrand JG. Behavioral consequences of innate preferences and olfactory learning in hawkmoth–flower interactions. Proceeding of the National Academy of Sciences of the USA. 2008;105:3404–3409. doi: 10.1073/pnas.0709811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston TH, Cane JH. Pollen nutritional content and digestibility for animals. Plant Systematics and Evolution. 2000;222:187–209. [Google Scholar]
- van Schie CCN, Haring MA, Schuurink RC. Regulation of terpenoid and benzenoid production in flowers. Current Opinion in Plant Biology. 2006;9:203–208. doi: 10.1016/j.pbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Schiestl FP. The evolution of floral scent and insect chemical communication. Ecology Letters. 2010 doi: 10.1111/j.1461-0248.2010.01451.x. in press. doi: 10.1111/j.1461-0248.2010.01451.x. [DOI] [PubMed] [Google Scholar]
- Smallegange RC, van Loon JJA, Blatt SE, Harvey JA, Agerbirk N, Dicke M. Flower vs. leaf feeding by Pieris brassicae: glucosinolate-rich flower tissues are preferred and sustain higher growth rate. Journal of Chemical Ecology. 2007;33:1831–1844. doi: 10.1007/s10886-007-9350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel CK. Das entdeckte Geheimnis der Natur im Bau und in der Befruchtung der Blumen. Berlin: Facsimile-Druck. Mayer & Müller; 1793. [Google Scholar]
- Stephenson AG. Toxic nectar deters nectar thieves of Catalpa speciosa. American Midland Naturalist. 1981;105:381–383. [Google Scholar]
- Stephenson AG. Iridoid glycosides in the nectar of Catalpa speciosa are unpalatable to nectar thieves. Journal of Chemical Ecology. 1982;8:1025–1034. doi: 10.1007/BF00987883. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Hasyim A, Nakamura H, Nakamura K. Asian weaver ants, Oecophylla smaragdina, and their repelling of pollinators. Ecological Research. 2004;19:669–673. [Google Scholar]
- Unsicker SB, Kunert G, Gershenzon J. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Current Opinion in Plant Biology. 2009;12:1–7. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Willmer PG, Nuttman CV, Raine NE, et al. Floral volatiles controlling ant behaviour. Functional Ecology. 2009;23:888–900. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





