Abstract
Background and Aims
Soil microbes have been demonstrated to play an important role in favouring plant iron (Fe) uptake under Fe-limiting conditions. However, the mechanisms involved are still unclear. This present study reported the effects of plant Fe status on the composition of siderophore-secreting microbes in the rhizosphere, and their potential function in improving plant Fe nutrition.
Methods
An Fe-efficient plant, red clover (Trifolium pratense ‘Kenland’) was cultured in a calcareous soil to obtain rhizosphere soils with (Fe-sufficient) or without (Fe-stressed) foliar FeEDTA spraying. The siderophore-producing ability of rhizospheric microbes was measured. The bioavailability of the siderophore-solubilized Fe from iron oxides/hydroxides was tested in hydroponic culture.
Key Results
In rhizosphere soil, the number of microbes that secreted siderophores quickly was more in the Fe-stressed treatment than in the Fe-sufficient one, while the number of microbes that did not secret siderophores was the opposite. A significantly higher concentration of phenolics was detected in the rhizosphere soil of Fe-stressed plants. Moreover, after the soil was incubated with phenolic root exudates, the composition of the siderophore-secreting microbial community was similar with that of the rhizosphere of Fe-stressed plant. Additionally, the siderophores produced by a rhizospheric microbe isolated from the Fe-stressed treatment can well solubilize iron oxides/hydroxides, and the utilization of the siderophore-solubilized Fe by plant was even more efficient than EDTA-Fe.
Conclusions
Iron-deficiency stress of red clover would alter the composition of siderophore-secreting microbes in the rhizosphere, which is probably due to the phenolics secretion of the root, and may in turn help to improve the solubility of Fe in soils and plant Fe nutrition via elevated microbial siderophore secretion.
Keywords: Iron, Fe stsatus, phenolic compound, rhizobia, rhizosphere, root exudates, siderophores, Trifolium pratense
INTRODUCTION
Although the total iron (Fe) content in soils usually far exceeds plant requirement for Fe, its bioavailability in the soil, especially in calcareous soils, is often severely limited. Fe-efficient plant species and genotypes have evolved different strategies to enhance Fe uptake. Römheld and Marschner (1986) classified these strategies as Strategy I in nongraminaceous monocots and dicots, and Strategy II in graminaceous monocots. Strategy I plants respond to Fe-deficiency stress typically by inducing ferric chelate reductase and Fe(II) transporter in roots system, by acidifying the rhizosphere medium and exuding organic compounds such as phenolics (Hell and Stephan, 2003; Morrissey and Guerinot, 2009). While Strategy II plants respond to Fe-deficiency stress by releasing phytosiderophores and inducing a specific plasmalemma Fe3+–phytosiderophore transporter in roots system (Hell and Stephan, 2003; Morrissey and Guerinot, 2009). In recent years, however, lines of evidence have shown that the above Fe-deficiency-induced responses are insufficient for plants to avoid Fe deficiency under Fe-limited conditions. Masalha et al. (2000) firstly demonstrated that the sunflower and maize grown in sterile soil showed poor growth and lower tissue, Fe concentration compared with non-sterile soil-grown plants. Similarly, Rroco et al. (2003) and Jin et al. (2006) reported that Fe acquisition and growth of rape (Brassica napus and red clover (Trifolium pratense) were significantly reduced when the plant was grown in sterile soil, but normal growth could be restored by adding Fe-EDDHA to the sterile soil or spraying EDTA-Fe to the leaves. It, therefore, appears that soil microbial activity plays an important role in favouring plant Fe uptake. However, the actual mechanism by which soil microbes contribute to plant Fe acquisition remains unknown.
Yang and Crowley (2000) found that the microbial community in barley (a Strategy II plant) rhizosphere varied with the plant's Fe nutritional status, and it was proposed to be due to the changes of root exudates. In addition, Robin et al. (2006, 2007) also demonstrated that a transgenic tobacco (a Strategy I plant) over-accumulating iron, lead to a reduced availability of iron in the rhizosphere and shifts in the microbial community. Previously, it has been found that, when the soil solution of a calcareous soil was incubated on an agar plate containing phenolic root exudates from Fe-deficient red clover, only a few microbial species thrived while growth of the rest is inhibited, and the majority of the microbes which thrived can secrete siderophores under Fe-deficient conditions (Jin et al., 2006, 2008b). Generally, siderophores produced by soil microbes are seen as one of the microbial functions most supportive of Fe acquisition by plants, because siderophores have a high affinity for chelating Fe(III), and the resulted chelates have been proven to be an efficient bioavailable Fe source for plants (Chen et al., 1998; Lemanceau et al., 2009). However, in our previous studies, phenolics secretion was measured and collected from the hydroponic-grown Fe-deficient red clover, and the soil microbes was characterized by incubating the soil solution on the agar plate containing phenolic root exudates (Jin et al., 2006, 2008b), so it still remains open whether red clover grown in calcareous soils really secrets phenolic compounds and the microbial community structure in the rhizosphere is actually altered to have more siderophore-secreting microbes to favour plant Fe nutrition.
In the present study, to investigate the siderophore-producing ability of the microbes, and their potential relationship with phenolic root exudates red clover, an Fe-efficient plant was cultured in a calcareous soil to obtain rhizosphere soils with or without foliar FeEDTA spraying. In addition, a microbe isolated from the rhizosphere of the plant without foliar Fe supply was investigated for its ability to secret siderophores and solubilize iron oxides/hydroxides, and how the plant utilized the dissolved Fe.
MATERIALS AND METHODS
Soil cultivation
A loess-derived loam soil with a pH of 7·2 and 0·9 mg Fe kg−1 soil of DPTA-extractable Fe (Lindsay and Norvell, 1978) used the pot experiments was prepared as follows. After the air-dried soil had been ground to pass through a 2-mm sieve, chemical fertilizers were mixed into the soil at a rate of 100 mg N kg−1 soil in the form of Ca(NO3)2, 100 mg K kg−1 soil in the form of K2SO4, and 150 mg P kg−1 soil in the form of KH2PO4.
Red clover (Trifolium pratense L. ‘Kenland’) seeds were sown at a depth of 1 cm, with five seeds per pot filled with 0·8 kg soil. During plant growth, soil moisture was maintained at 15 % (wt/wt) with deionized water added daily. After 16 d growth, the seedlings were thinned to three plants with similar size per pot. To avoid soil contamination by FeEDTA during foliar Fe-spraying treatment as described below, the soil surface of each pot was covered with tinfoil, leaving holes for seedlings to grow through. The plants were grown in a growth chamber at a humidity of 70 %, with a daily cycle of a 26 °C, 14 h day and a 23 °C, 10-h night. The daytime light intensity was 250–300 µmol photons m−2 s−1. After 30 d growth, the pots were divided into two plots. In one plot, the leaves were sprayed with 100 µm FeEDTA solution every other day (called Fe-sufficient), while in the other plot the leaves were sprayed with deionized water instead (called Fe-stressed).
Sampling
After 60 d growth, plants were pulled out together with the associated soil from the pots. Then 2 g of bulk soil was sampled from each pot and the excessive bulk soil was removed from the roots by shaking. The soil adhering firmly on the root surface was taken as the rhizosphere soil. The relatively young parts of the roots (i.e. <5 cm from the root tips) were excised and collected from each pot to form one mixed rhizosphere sample. The rhizosphere samples were used directly to measure microbial siderophore secretion and the concentration of phenolics.
Measurement of total water-soluble phenolics and numeration of the siderophore-secreting microbial colonies
Total water-soluble soil phenolics was determined according to DeForest et al. (2005). For siderophore secretion analysis, 1 g of the rhizosphere sample was moderately agitated in 20 mL of sterile 0·9 % NaCl solution for 10 min first. Then the microbial suspension in serial dilutions was spread on agar plates with 20 µm FeCl3 or 1 % (v/v) CAS (chrome azurol S) indicator. The agar medium consisted of (mg L−1): K2HPO4, 91; NH4Cl, 1000; MgSO4·7H2O, 63; CaCl2, 50; succinate, 1000; NaCl, 100; l-tyrosine, 100; l-methionine, 100. The pH of the medium was adjusted to 6·0 with NaOH. The CAS indicator was prepared according to Schwyn and Neilands (1987). The CAS assay is based on the removal of Fe3+ from an intensely pigmented complex by a competing ligand such as siderophore. When the siderophores forms a complex with Fe3+, the release of the free dye is indicated by the colour change from blue to orange (Schwyn and Neilands, 1987). The plates were incubated at 30 °C. The microbial colonies with an orange halo with a diameter >5 mm were counted at the 24th, 36th and 48th hour. The new colonies formed during the first 24 h, the 24–36th and the 36–48th were called quicker-siderophore-secretors (QSSs), middle-siderophore-secretors (MSSs) and slower-siderophore-secretors (SSSs), respectively. The colonies on the agar plate with FeCl3 were counted to calculate the total number of soil microbes which could be cultured and the non-siderophore-secretors (NSSs). The roots in NaCl solution were collected and weighed. Then the amount of rhizosphere soil was calculated by subtracting the root weight. Additionally, a single QSS colony from the agar plate of the Fe-stressed rhizosphere sample was randomly selected and stored at 4 °C for subsequent experiments.
Effect of the phenolic root exudates on the siderophore-producing microbes in the soil
Phenolic root exudates from hydroponically grown Fe-deficient red clover were collected as previously (Jin et al., 2007). Twenty-five grams of the calcareous soil were weighed into a tube. Five millilitres of distilled water containing 10 µmol (gallic acid equivalent) phenolics were mixed into the soil which was then incubated at 30 °C for 4 d. The siderophore-producing ability of the soil microbes was tested as described in a former section.
Growth of the isolated microbe in hydrous Fe(III)-oxides media
By analysing 16S rDNA nucleotide sequences, the isolated QSSs were identified as belonging to Pseudomonas (called Pseudomonas. sp. yy). The Fe-deficient medium used here was described by Hersman et al. (1996). The pH of the medium was adjusted to 6·85 with 10 m NaOH. To study the growth of this bacterium as a function of different Fe sources, the bacterial suspension was inoculated equally into the above media containing FeCl3 (10 µm) or hydrous Fe(III)-oxides (200 µg mL−1). Before and after incubation, the soluble Fe concentration in the culture media was determined via inductively coupled plasma atomic emission spectrometry (ICP-AES) (IRIS Advantage, Thermo Jarrell Ash Co., USA). The hydrous Fe(III)-oxides were prepared as previously (Jin et al., 2009). The growth was measured by turbidimetry at 600 nm.
Effect of siderophores on Fe solubilization
The bacterial suspension was inoculated into 1 L of the Fe-deficient Hersman medium. Two days later, cells were removed by centrifugation (10 000 g, 10 min), and the supernatant was filtered through a column filled with a cationic exchange resin, Amberlite IR-120B (H+) (Chang et al., 1999). The cation fraction was eluted with 2 m NH4OH and concentrated by rotary evaporation at 35 °C. The residue was dissolved in 3 mL distilled water and stored at 4 °C for later use.
The Fe-solubilizing capacity of siderophores secreted by the isolated microbes was conducted as described by Chang et al. (1999) with some modifications. In brief, 2 mL of 0·5 m sodium acetate buffer, pH 5·6, was added to 0, 50, 100, 150, 200, 250 µL of the samples containing siderophores. The pH of the buffered solution was then checked and adjusted with HCl to pH 5·6 ± 0·2. After addition of 0·5 mL of 5 mm Fe(OH)3 solution (prepared by neutralizing FeCl3 with NaOH to pH 6·5–7·0), making up a loss with distilled water to 3·0 mL in all, the tubes were incubated at 55 °C for 5 h, with occasional mixing by vortex. The reaction mixture was finally centrifuged at 10 000 g for 10 min at 25 °C and the dissolved Fe in the supernatant was determined.
Utilization of solubilized Fe by red clover
To investigate whether the Fe solubilized by microbial-borne siderophores can be utilized by a plant, a solution culture experiment was conducted in the growth chamber under the same growing conditions described earlier. The composition of the nutrient solution and plant culture method is described in detail in Jin et al. (2008a). Briefly, uniform 1-week-old red clover seedlings were grown with 20 µm EDTA-Fe (+Fe) or 20 µm siderophore − Fe complex (+Fe-S) as Fe sources, and −Fe as control in a 100-mL bottle. The nutrient solution was renewed every other day. After 4 weeks culture, the chlorophyll content of newly formed leaves was analysed with a chlorophyll meter (SPAD-502, Minolta), and the chlorophyll content was recorded as a SPAD reading. The plants were harvested and separated into roots and shoots. The dry weights were recorded after the samples were dried in an oven at 75 °C for 2 d. The ground shoot and root samples were digested in 4 mL of mixed acids of HClO4 and HNO3 at a ratio of 1 : 4 and then diluted to 50 mL with deionized water. The Fe concentration in the digestion was measured by ICP-AES.
Statistics
Statistical analyses were conducted with SAS software (SAS Institute, Cary, NC). Means were compared by t-test at P < 0·05 in all cases.
RESULTS
Effect of plant Fe status on the composition of siderophore-secreting microbes in rhizosphere
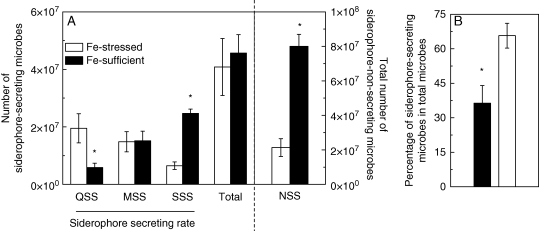
Although there was no significantly difference in the total number of siderophore-secreting microbes between the Fe-sufficient and Fe-stressed treatment, the population of QSSs in the Fe-stressed treatment was larger, while the population of SSSs was smaller (on left of Fig. 1A), and the population of NSSs in the Fe-sufficient treatment was much larger than that in the Fe-stressed treatment (on right of Fig. 1A). As a result, the percentage of siderophore-secreting microbes was much higher, being about 65 % of all the microbes in the Fe-stressed treatment (Fig. 1B).
Fig. 1.
(A) The siderophore-secreting ability of the microbes from the rhizosphere soil of Fe-stressed and Fe-sufficient red clover; (B) the percentage of soil microbes that secrete siderophores. The microbial suspension in serial soil dilutions was spread on agar plates with or without CAS indicator. The plates were incubated at 30 °C, and the siderophore-secreting ability of the soil microbes was conducted as described in Materials and methods. QSS, MSS and SSS are quicker-, middle- and slower-secreting siderophores, respectively. *, Significant differences (P < 0·05) between two treatments at each time point (n = 4).
Phenolics accumulation in the rhizosphere and its potential effect on the composition of siderophore-secreting microbes
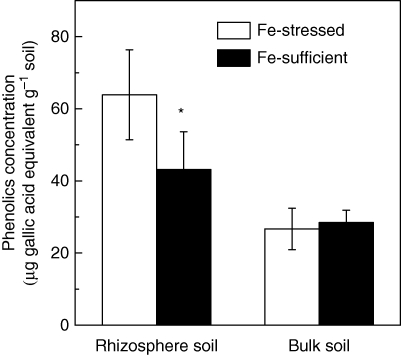
Compared with that of the Fe-sufficient plant, the concentration of phenolics in the rhizosphere soil of the Fe-stressed plant was significantly higher. However, no obvious difference was found between the bulk soils of the two treatments (Fig. 2).
Fig. 2.
Phenolics concentration in rhizosphere soil of Fe-stressed and Fe-sufficient red clover. The phenolics in the soil were extracted with deionized water on an orbital-action shaker for 18 h. The concentration of phenolics was mesured using Folin–Ciocalteu reagent. The concentration of all the phenolic compounds was expressed as an equivalent of gallic acid. *, Significant differences (P < 0·05) between two treatments at each time point (n = 4).
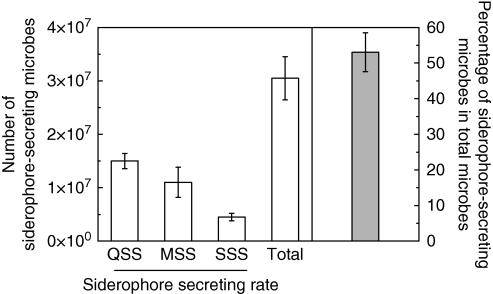
In an in vitro experiment, the calcareous soil was incubated with phenolic root exudates for 4 d and the siderophore-producing ability of microbes of the incubated soil was also quantified. Interestingly, the siderophore-secreting microbial composition of the soil treated with phenolics (Fig. 3) was similar with that of the rhizosphere soil of the Fe-stressed plant (Fig. 1).
Fig. 3.
The siderophore-secreting ability of the microbes from the soil incubated with phenolic root exudates. Twenty-five grams of air-dried calcareous soil were mixed with 5 mL of distilled water containing 10 µmol (gallic acid equivalent) phenolics. After 4 d incubation at 30 °C, the siderophore-secreting ability of the soil microbes was conducted as described in Materials and methods (n = 4). QSS, MSS and SSS are quicker-, middle- and slower-secreting siderophores, respectively.
Growth of isolated QSS in FeCl3 or hydrous Fe(III)-oxides media
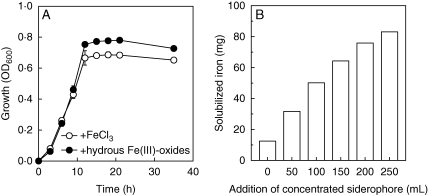
The Fe concentrations in autoclaved hydrous Fe(III)-oxides media, before inoculation, were 0·30 µm, which could be taken as Fe deficient. The growth of P. yy was similar in FeCl3 and hydrous Fe(III)-oxides media in the initial 9 h and even better in hydrous Fe(III)-oxides medium thereafter (Fig. 4A). The Fe concentration after cultivation in the hydrous Fe(III)-oxides medium was 13·1 µm, indicating that a significant amount of Fe had dissolved from the iron oxides.
Fig. 4.
(A) Growth kinetics of Pseudomonas sp. yy in FeCl3 (10 µm) or hydrous Fe(III)-oxides (200 mg L−1) media, as measured by absorbance at 600 nm. (B) Solubilization of Fe(OH)3 by concentrated siderophore compounds secreted by Pseudomonas sp. yy.
To verify further the solubility of the siderophores on the insoluble Fe, the siderophores were collected from the microbe-cultured Fe-deficient medium. As shown in Fig. 4B, the amount of the Fe dissolved from Fe(OH)3 by the siderophores increased with the amount of concentrated siderophores added.
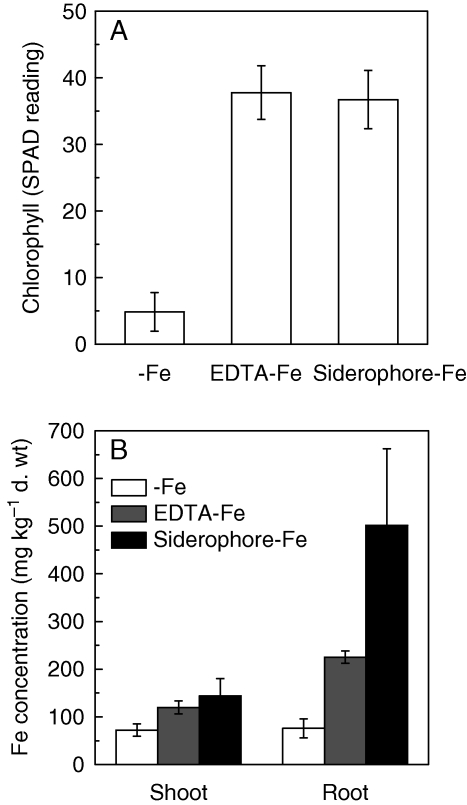
Utilization of siderophore-Fe by red clover
Four weeks of the −Fe treatment severely led to chlorosis of the new leaves in red clover, and yielded a SPAD reading of only 5, while for leaves of plants with EDTA-Fe or siderophore-Fe as Fe source, SPAD readings were approx. 40 (Fig. 5A). Interestingly, the Fe concentrations in roots and shoots with siderophore-Fe treatment were higher than when EDTA-Fe was the Fe source, particularly in roots where it increased >1-fold, although both were much higher than in the −Fe treatment (Fig. 5B). These results suggested that siderophore-Fe should be an efficient Fe source for plant growth.
Fig. 5.
(A) Chlorophyll synthesis and (B) Fe concentration in red clover plants after 4 weeks culture in −Fe, +EDTA-Fe (20 µm) or +siderophore ‐ Fe complexes (20 µm). Data are means ± s.d. (n = 3).
DISCUSSION
The root exudates have been proposed to selectively influence the growth of the microbes that colonize the rhizosphere by altering the chemical properties of the soil adjacent to the plant roots and serving as selective growth substrates for soil microbes. For example, the Strategy II plant roots release phytosiderophores to the rhizosphere under Fe-deficient conditions, and the phytosiderophores can influence the availability of iron to microbes too (Marschner and Crowley, 1998). Therefore, it was proposed that variation in the microbial community in the barley rhizosphere when the Fe-nutritional status of the plant is altered can be attributed to phytosiderophore exudation (Yang and Crowley, 2000).
By using 16S ribosomal DNA (rDNA) fingerprints generated by PCR-denaturing gradient gel electrophoresis, it was also found that the microbial community structure in the red clover rhizosphere was different under a different plant Fe nutritional status (data not shown). For strategy I plants, roots secrete phenolics under Fe-deficient conditions (Römheld and Maschner, 1986). As the chemical and physiological properties of phenolics are completely different from that of the phytosiderophore, their effect on the rhizosphere microbial community structure should, theoretically, be different too. Previously, one of the main components of the phenolic root exudates of Fe-deficient red clover had been identified to be a kind of isoflavone (Zheng et al., 2000). It has been widely demonstrated that isoflavones are toxic to many strains of microbes while some special strains can tolerate the isoflavones and use them as carbon sources for growth (Ozan et al., 1997; Flythe and Isabelle, 2010). In addition, many other phenolic compounds also have similar effects on the microbes (Blum et al., 2000; Rauha et al., 2000). Therefore, the difference observed in the composition of siderophore-secreting microbes in the rhizosphere when the plant Fe nutritional status differs (Fig. 1) may be related to the phenolic root exudates via its antimicrobial and/or growth beneficial effects. However, there is no report on the actual phenolic content in the rhizosphere of plant grown under Fe-deficient soil, although phenolics exudates have been frequently identified in solution culture (e.g. Olsen and Brown, 1980; Olsen et al., 1981; Jin et al., 2007, 2008b). As expected, the phenolics concentration in the rhizosphere of Fe-stressed red clover was significantly higher than that of an Fe-sufficient one (Fig. 2), therefore growth repression of NSSs in the rhizosphere of Fe-stressed plants may be due to the accumulation of phenolics and their antimicrobial effect. This possible link between Fe-deficiency-induced secretion of phenolics and the alteration in the structure of the microbial community was further verified by in vitro incubating the soil with phenolic root exudates, in which the percentage of NSSs was significantly reduced while the percentage of siderophore-secreting microbes significantly increased (Fig. 3). Actually, it is much better to provide evidence to confirm the above link by revealing the in vitro effect of Fe-sufficient root exudates on the composition of siderophore-secreting microbes in soil. However, the amount of root exudates from Fe-sufficient plants is much lower than that from Fe-deficient plants (Jin et al., 2007), so it is very difficult to collect enough root exudates from Fe-sufficient plants to conduct a control treatment. Anyway, in the soil incubated without addition of root exudates, it was found that the total number of the siderophore-secreting microbes was much lower, being <5 % of the microbes overall (data not shown). Therefore, it was deduced that the stimulation of QSS growth and the inhibition of the NSS growth in the rhizosphere of Fe-stressed red clover should be attributed to the phenolic root exudates.
Siderophore production by soil microbes is taken as the microbial activity most supportive of Fe acquisition by plants (Raymond et al., 1984). Interestingly, by comparing the amount and kind of siderophore-secreting microbes in the rhizosphere of red clover with and without foliar FeEDTA supply, it was demonstrated for the first time that, under Fe-deficient conditions, there are more microbes in the rhizosphere that can secret siderophores quickly (QSSs in Fig. 1). Moreover, during the process of the siderophore-secreting test on the CAS agar plates, it was also observed that the orange halos of the QSSs colonies were generally larger than those of other microbial colonies, indicating that more siderophores were produced by QSSs. Therefore, it is reasonable to deduce that more QSSs in the rhizosphere of Fe-stressed plant (Fig. 1) may facilitate the production of more soluble Fe through microbial siderophore secretion. On the other hand, however, soil microbes in the rhizosphere also need soluble Fe for normal growth; therefore, the microbes which colonized the rhizosphere soil were thought to compete with the plants for a source of soluble Fe (Marschner et al., 1997). The interesting thing is that the population of NSSs in the rhizosphere of the Fe-stressed plants was much smaller than in that of the Fe-sufficient plants (Fig. 1), meaning that fewer siderophores produced by microbes will be consumed by NSSs in the Fe-stressed rhizosphere soil. Thus, it is thought that fewer NSSs in the rhizosphere of Fe-stressed plant should be taken as a beneficial factor favouring plant Fe uptake by reducing consumption of soluble Fe by NSSs.
To verify further the potential role of the siderophore-secreting microbes in plant iron uptake, a QSS from the rhizosphere of the Fe-stressed plant was isolated, and it was identified as belonging to Pseudomonas. Although the soluble Fe in hydrous Fe(III)-oxides media is really lower, the growth of P. yy was similar to FeCl3 media (Fig. 4A), indicating that the growth of these this bacteria should also be good in soluble-Fe-limited calcareous soil. This behaviour is advantageous for the bacteria as it aids the Fe uptake of Fe-deficiency-stressed plants from the rhizosphere soil. Under Fe-deficient conditions, it has been reported that the siderophores secreted by Pseudomonas, called pyoverdines, show a high affinity for Fe(III) with a stability constant of 30·8 under room temperature (Meyer and Abdallah, 1978; Boukhalfa and Crumbliss, 2002). Therefore, although the real in situ concentrations of siderophores in the investigation of Fe solubilization (Fig. 4B) were not measured, all of the siderophores in the solution should be expected to dissolve Fe from the insoluble hydrous Fe(III)-oxides. Here, it was found that dissolved Fe can be easily utilized by plants (Fig. 5), indicating the importance of this microbe activity in plant Fe nutrition. However, so far, direct evidence demonstrating the role of siderophore-secreting microbes in plant iron uptake in the soil environment is still limited. In future, comparison between plant Fe uptake from sterile soils inoculated with a siderophore-secreting microbe and its mutant which does not secrete siderophores may be helpful to verify the above process.
Interestingly, in the present research it was found that the higher Fe accumulation by plants supplemented with siderophore-Fe than by those supplemented with EDTA-Fe was recorded in both shoots and roots (Fig. 5B, suggesting that the siderophore-Fe was incorporated in the root in a more efficient way. This finding is in accordance with Vansuyt et al. (2007), who also found that the Fe concentration in arabidopsis plants fed with Fe-pyoverdine was higher than in those fed with EDTA-Fe. In most instance, the redox potential E (mV) of siderophore − Fe3+ chelates is generally lower and is within the range of −330 mV to −750 mV (Boukhalfa and Crumbliss, 2002). However, the redox potential of most biological reductants is higher than this range, e.g. NADPH (E = −320 mV) which has been suggested to be the electron donor of Fe(III) through ferric chelate reductase (Robinson et al., 1999). Bienfait et al. (1983) and Römheld and Marschner (1983) have demonstrated little reduction of FOB-Fe (FOB, ferrioxamine B, is a kind of microbial siderophore) by bean and peanut. By using an IRT1 arabidopsis mutant, Vansuyt et al. (2007) demonstrated that iron acquisition from Fe-pyoverdine by arabidopsis was not through IRT1 transport. Therefore, the siderophore − Fe3+ chelates are probably directly acquired by a kind of unidentified plasmalemma transporter.
In conclusion, the present results imply that the phenolic compounds exuded from red clover roots under Fe-deficient conditions may selectively modify the microbial community structure in favouring more siderophore-secreting microbes, which helps to improve the solubility of insoluble iron and plant iron nutrition via microbial siderophores. We believe that such an interrelated scheme plays a very important ecological role in plant Fe acquisition in calcareous soils.
ACKNOWLEDGEMENTS
This work was financially supported by National Natural Science Foundation of China (No. 30625026).
LITERATURE CITED
- Bienfait HF, Bino AM, van der Bliek AM, Duivenvoorden JF, Fontaine JM. Characterization of ferric reducing activity in roots of Fe-deficient Phaseolus vulgaris. Physiologia Plantarum. 1983;59:196–202. [Google Scholar]
- Blum U, Staman KL, Flint LJ, Shafer SR. Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. Journal of Chemical Ecology. 2000;26:2059–2078. [Google Scholar]
- Boukhalfa H, Crumbliss AL. Chemical aspects of siderophore mediated iron transport. Biometals. 2002;15:325–339. doi: 10.1023/a:1020218608266. [DOI] [PubMed] [Google Scholar]
- Chang YC, Ma JF, Iwashita T, Matsumoto H. Effect of Al on the phytosiderophore-mediated solubilization of Fe and uptake of Fe-phytosiderophore complex in wheat (Triticum aestivum) Physiologia Plantarum. 1999;106:62–68. [Google Scholar]
- Chen LM, Dick WA, Streeter JG, Hoitink HAJ. Fe chelates from compost microorganisms improve Fe nutrition of soybean and oat. Plant and Soil. 1998;200:139–147. [Google Scholar]
- DeForest L, Zak DR, Pregitzer KS, Burton AJ. Atmospheric nitrate deposition and enhanced dissolved organic carbon leaching: test of a potential mechanism. Soil Science Society of America Journal. 2005;69:1233–1237. [Google Scholar]
- Flythe M, Isabelle I. Antimicrobial effect of red clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium. Clostridium sticklandii. Current Microbiology. 2010 doi: 10.1007/s00284-010-9586-5. doi 10·1007/s00284-010-9586-5. [DOI] [PubMed] [Google Scholar]
- Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta. 2003;216:541–551. doi: 10.1007/s00425-002-0920-4. [DOI] [PubMed] [Google Scholar]
- Hersman L, Maurice P, Sposito G. Iron acquisition from hydrous Fe(III)-oxides by an aerobic Pseudomonas sp. Chemical Geology. 1996;132:25–31. [Google Scholar]
- Jin CW, He YF, Tang CX, Wu P, Zheng SJ. Mechanisms of microbial enhanced iron uptake in red clover. Plant, Cell & Environment. 2006;29:888–897. doi: 10.1111/j.1365-3040.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Jin CW, You GY, Tang CX, Wu P, Zheng SJ. Iron-deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover (Trifolium pratense L.) Plant Physiology. 2007;144:278–285. doi: 10.1104/pp.107.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Chen WW, Meng ZB, Zheng SJ. The iron-deficiency-induced increase of root branching is important for enhancing ferric chelate reductase activity. Journal of Integrative Plant Biology. 2008a;50:1557–1562. doi: 10.1111/j.1744-7909.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- Jin CW, You GY, Zheng SJ. The iron deficiency-induced phenolics secretion plays multiple important roles in plant iron acquisition underground. Plant Signaling and Behavior. 2008b;3:60–61. doi: 10.4161/psb.3.1.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. Elevated carbon dioxide improves plant Fe nutrition through enhancing the Fe-deficiency-induced responses under Fe-limited conditions in tomato. Plant Physiology. 2009;150:272–280. doi: 10.1104/pp.109.136721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanceau P, Expert D, Gaymard F, Bakker PAHM, Briat JF. Role of iron in plant-microbe interactions. Advances in Botanical Research. 2009;51:491–549. [Google Scholar]
- Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Science Society of America Journal. 1978;42:421–428. [Google Scholar]
- Marschner P, Crowley DE. Phytosiderophores decrease iron stress and pyoverdine production of Pseudomonas fluorescens Pf-5 (pvdinaZ) Soil Biology and Biochemistry. 1998;30:1275–1280. [Google Scholar]
- Marschner P, Crowley DE, Sattelmacher B. Root colonization and iron nutritional status of a Pseudomonas fluorescens in different plant species. Plant and Soil. 1997;196:311–316. [Google Scholar]
- Masalha J, Kosegarten H, Elmaci Ö, Mengel K. The central role of microbial activity for iron acquisition in maize and sunflower. Biology and Fertility of Soils. 2000;30:433–439. [Google Scholar]
- Meyer JM, Abdallah MA. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. Journal of General Microbiology. 1978;107:319–328. [Google Scholar]
- Morrissey J, Guerinot ML. Iron uptake and transport in plants: the good, the bad, and the lonome. Chemical Reviews. 2009;109:4553–4567. doi: 10.1021/cr900112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RA, Brown JC. Factors related to iron uptake by dicotyledonous and monocotyledonous plants. Journal of Plant Nutrition. 1980;2:629–660. [Google Scholar]
- Olsen RA, Bennett JH, Blume D, Brown JC. Chemical aspects of the Fe stress response mechanism in tomatoes. Journal Plant Nutrition. 1981;3:905–921. [Google Scholar]
- Ozan A, Safir GR, Nair MG. Persistence of isoflavones formononetin and biochanin A in soil and their effects on soil microbe populations. Journal of Chemical Ecology. 1997;23:247–258. [Google Scholar]
- Rauha JP, Remes S, Heinonen M, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology. 2000;56:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- Raymond KN, Mueller G, Matzanke BF. Complexation of iron by siderophores: a review of their solution and structural chemistry and biological function. Topics in Current Chemistry. 1984;123:49–102. [Google Scholar]
- Robin A, Mougel C, Siblot S, Vansuyt G, Mazurier S, Lemanceau P. Effect of ferritin overexpression in tobacco on the structure of bacterial and pseudomonad communities associated with the roots. FEMS Microbiology Ecology. 2006;58:492–502. doi: 10.1111/j.1574-6941.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Robin A, Mazurier S, Mougel C, et al. Diversity of root-associated fluorescent pseudomonads as affected by ferritin overexpression in tobacco. Environmental Microbiology. 2007;9:1724–1737. doi: 10.1111/j.1462-2920.2007.01290.x. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Römheld V, Marschner H. Mechanism of iron uptake by peanut plants. I. FeIII reduction, chelate splitting and release of phenolics. Plant Physiology. 1983;71:949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Marschner H. Mobilization of iron in the rhizosphere of different plant species. Advance in Plant Nutrition. 1986;2:155–204. [Google Scholar]
- Rroco E, Kosegarten H, Harizaj F, Imani J, Mengel K. The importance of soil microbial activity for the supply of iron to sorghum and rape. European Journal of Agronomy. 2003;19:487–493. [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry. 1987;160:7–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P. Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Molecular Plant–Microbe Interactions. 2007;20:441–447. doi: 10.1094/MPMI-20-4-0441. [DOI] [PubMed] [Google Scholar]
- Yang CH, Crowley DE. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Applied and Environmental Microbiology. 2000;66:345–351. doi: 10.1128/aem.66.1.345-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Arakawa Y, Yahara S, Yamamoto Y, Matsumoto H, Masaoka Y. Resistant mechanism to iron deficiency in red clover. II. The characteristics and functions of root exudates. Abstracts of the Annual Meeting of Japanese Society of Soil Science and Plant Nutrition. 2000;46:72. [Google Scholar]