Abstract
Objective
A decline in the inhibitory neurotransmitter γ-aminobutyric acid (GABA) may enhance cytokine release in Alzheimer’s disease (AD) resulting in neuroinflammation. We investigated the GABA-mediated suppression of the synergistic release of interleukin-6 (IL-6) due to interleukin 1-β (IL-1β) and tumor necrosis factor α (TNF-α).
Methods
Rat C6 astrocytoma cells were treated with IL-1β and TNF-α in the absence and presence of GABA. Activation of p38, degradation of IκB-α, and total cellular IL-6 were determined by Western analysis. IL-6 release and gene expression were measured by ELISA and RT-PCR, respectively.
Results
Although p38 and nuclear factor (NF)-κB are essential for the synergistic release of IL-6, GABA did not affect either p38 phosphorylation or IκB-α degradation. Additionally, GABA suppressed IL-6 release but did not alter cytokine-driven synergistic increases in IL-6 gene expression. Western blot analysis revealed that co-treatments with IL-1β and TNF-α resulted in an increase in intracellular IL-6 that was prevented by GABA.
Conclusion
GABA inhibition of IL-6 release appears to coincide with a reduction in cellular IL-6. The GABA suppression of IL-6 release may include inhibition of IL-6 gene translation.
Keywords: GABA, IL-1β, TNF-α, IL-6, Alzheimer disease
Introduction
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter in the CNS capable of mediating membrane hyperpolarization or depolarization. GABA may be responsible for developmental neuronal migration [1] and rapid inhibition of synaptic activity [2,3]. GABA also inhibits proinflammatory responses in T-cells [4] and interleukin-1β (IL-1β) induced interleukin-6 (IL-6) release [5]. Interestingly, individuals afflicted with Alzheimer disease (AD) have lower levels of GABA [6].
Several lines of evidence suggest a critical role for glia-derived cytokines in various neurodegenerative disorders. Cytokines are low molecular weight proteins secreted into the proximal extracellular fluid, where they exert their effects locally via autocrine or paracrine mechanisms. Numerous cytokines expressed in the CNS appear to contribute to inflammatory events implicated in a variety of neurodegenerative states. Accordingly, increased levels of IL-1 and IL-6 in the CNS have been noted in the development and progression of neurodegenerative diseases such as AD. Increased cytokine production therefore may plausibly initiate a recursive, or “self-propagating”, inflammatory process that contributes to senile plaque formation and consequent neurodegeneration. CNS levels of IL-1 and IL-6 increase in the early stages of AD and correlate with the severity of the manifestation of dementia. Enhanced IL-1 and IL-6 production in the senile plaques of AD thus represents an important factor in the progression of this disease [reviewed in 7,8].
We previously reported that IL-1β stimulates IL-6 release from rat C6 astrocytoma cells [5,9]. Significantly, agents which elevated intracellular cAMP and the catecholamines epinephrine and norepinephrine synergistically stimulated IL-1β induced IL-6 release [9]. Subsequently we noted that somatostatin and GABA each inhibits IL-1β induced IL-6 release from C6 cells [5]. Mechanistic features of inhibitory neurotransmitter interruption of IL-1β glial cell signaling remain currently undefined, however.
Inflammatory cytokines such as IL-1β have been linked to the progression of AD [10]. This observation suggests that inhibitory neurotransmitters such as GABA may alleviate neuroinflammation and thus reduce neurodegenerative processes. We now report that combined exposures of IL-1β and tumor necrosis factor-α (TNFα) result in a synergistic release of IL-6 from C6 cells. Although GABA inhibited the synergistic release of IL-6, it did not prevent cytokine-driven signaling events or transcription of the IL-6 gene. Interestingly GABA did reverse cytokine-related increases in intracellular IL-6, suggesting a novel mechanism involving suppression of translation.
Materials and methods
Chemicals and Reagents
γ-Aminobutyric acid (GABA) was obtained from Sigma-Aldrich (St. Louis, MO). Poly-D-lysine (PDL) hydrobromide was from BD Biosciences (Bedford, MA). RPMI-1640 and heat-inactivated fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). Recombinant rat IL-1β and TNF-α were from PeproTech Inc. (Rocky Hill, NJ). Primary antibodies to IκB-α, IκB-β, phosphorylated p38 (T180/Y182), and total p38 were obtained from Cell Signaling (Danvers, MA). Rabbit anti-rat IL-6 antibody was purchased from Cell Sciences (Canton, MA). Goat anti-rabbit horseradish peroxidase conjugated secondary antibody was from KPL (Gaithersburg, MD). SB203580, SB202190, and Bay 11-7082 were obtained from EMD Biosciences (La Jolla, CA). The rat C6 astrocytoma cell line was from the American Tissue Type Culture Collection (Rockville, MD). Rat IL-6 ELISA kits, M-PER, precasted 12% SDS-polyacrylamide gel electrophoresis (PAGE) gels, protease and phosphatase inhibitor cocktails were obtained from Pierce Biotechnology (Rockford, IL). Enhanced chemiluminescence (ECL)-Plus detection reagent was purchased from Amersham (Piscataway, NJ). PureLink Micro-to-Midi total RNA purification system and Superscript II reverse transcriptase, were obtained from Invitrogen.
C6 Glioma Cell Culture
Rat C6 glioma cells were maintained in continuous culture in humidified 95% air/5% CO2 in complete medium (RPMI-1640 with 10% heat-inactivated FBS, 25 mM HEPES pH 7.4, antibiotics). Cells were grown either in 25 cm2 or 75 cm2 flasks. After 3–4 days in culture, cells were removed from flasks with 0.25% trypsin/0.05% EDTA in Hanks-buffered salt solution. Trypsin was inactivated with addition of complete medium; cells were centrifuged and resuspended in complete medium. Cell densities and cellular viability were determined via trypan blue exclusion. Cells were seeded into tissue culture plates at densities noted. In all experiments, C6 glioma cells were used between passages 5–35.
ELISA Cytokine Quantification
Rat C6 glioma cells were seeded (1.0 × 106/well) on 24 well plates using poly-D-lysine (PDL) in 2 mL complete medium and allowed to incubate for 24 h. For each experiment, cells were rinsed twice with 2 mL serum-free RPMI 1640 and incubated in the absence or presence of agents for times and concentrations indicated. IL-6 release was determined by rat IL-6 ELISA (Pierce Biotechnology).
Collection of Cellular Protein Lysates
Rat C6 glioma cells were seeded (3.0 × 106/dish) on PDL-coated 35 × 10 mm dishes in 3 mL complete medium and allowed 24 h to attach. Cells were washed twice with 2 mL serum-free RPMI-1640 and incubated in serum-free RPMI-1640 in the absence or presence of agents. After treatment, cells were washed twice in 2 mL ice cold PBS, and scraped in 200 μL of M-PER® containing both phosphatase and protease inhibitors (Pierce). Protein-containing solutions were kept on ice and vortexed at 10 min intervals for a total time of 30 min. Lysates were then centrifuged at 14,000 × g for 10 min at 4 °C. Protein concentrations were determined by Micro-BCA protein assay (Pierce) using BSA as a standard. Protein lysates were diluted by 20% using a 2X SDS-PAGE loading buffer (10% w/v SDS, 2% v/v glycerol, 0.1% w/v bromophenol blue, 0.5 M Tris pH 6.8, 5% v/v β-ME), boiled for 3 min and stored at −70 C°.
RNA Extraction and semi-quantitative RT-PCR
Rat C6 glioma cells were seeded (3.0 × 106/dish) on PDL-coated 35 × 10 mm dishes in complete medium and allowed 24 h to attach. Cells were washed twice with 2 mL serum-free RPMI-1640 and incubated in the absence or presence of cytokines and GABA. After treatment, cells were washed twice in 2 mL ice cold PBS. Total RNA was then extracted using the Invitrogen PureLink total RNA purification system.
To detect the presence of mRNA encoding IL-6 and glyceraldehyde-3-phosphate dehydrogenase (G3PDH), semi-quantitative RT-PCR analyses were performed as described by Elsawa et al. [11] and Bost et al. [12]. One μg of total RNA was reverse transcribed using SuperScript II reverse transcriptase (Invitrogen), and a portion of the total cDNA was amplified by PCR using 94°C denaturation, 55°C annealing, and 72°C extension temperatures for 27 cycles. Positive and negative strand primers used for the amplification of each mRNA species are: G3PDH, GGAGCCAAACGGGTCATCATCTC and ATGCCTCTTCACCACCTTCTTG; IL-6, AGAGTTGTGCAATGGCAATTC and CCTTCTGTGACTCTAACTTCTCCA. Amplified products were electrophoresed on ethidium bromide stained gels and visualized under UV. Analyses of cDNA samples were corrected for expression of G3PDH.
SDS-PAGE Western Blot Analysis
Equal amounts of protein (5 to 25 μg) were loaded into each lane of 12% precast poly-acrylamide gels (Pierce) and subsequently transferred to a Hoffer SE 250 Minivertical Gel Electrophoresis Unit (Amersham) filled with Tris-HEPES (0.1 M HEPES, 0.1 M Tris, 0.1% w/v SDS) running buffer. Biotinylated and prestained protein ladders were used to determine molecular weight mobility and transfer efficiency, respectively. The proteins were electrophoretically separated at 4 °C using a constant voltage of 100 V. Following electrophoresis, the proteins were transferred (Hoefer TE22 Mighty Small TransPhor Tank, Amersham) to 0.45 μm nitrocellulose membranes at 4 °C using constant amperage of 360 mA for 90 min in Western transfer buffer (25 mM Tris, 192 mM glycine, 20% v/v methanol).
Following the transfer period, protein-containing membranes were washed once for 5 min in tris-buffered saline containing tween (TBST: 50 mM Tris pH 7.4, 150 mM NaCl, 0.1% v/v Tween-20) and subsequently blocked with TBST containing 5% nonfat milk (Nestlé Carnation) for 1 h. Membranes were then washed three times for 5 min in TBST and incubated 16 h with primary antibodies at 4 °C. Antibody solutions were removed and membranes washed in TBST three times for 5 min each. Membranes were then incubated with a secondary antibody conjugated to horseradish peroxidase (HRP) and anti-biotin. All antibodies were diluted in TBST containing 0.1% BSA. Proteins were visualized on the Typhoon multipurpose imager using the ECL-plus® detection reagent (Amersham).
Statistical Analysis
Statistical analyses consisted of analysis of variance (ANOVA), with significance confirmed using the Bonferroni test for multiple comparisons (GraphPad Instat, version 3.0). A P-value of ≤ 0.05 was considered significant. Where appropriate, data are expressed as the mean ± SEM of groups consisting of three to four observations and each experiment was performed at least three times.
Results
Synergistic release of IL-6 by TNF-α and IL-1β: p38 and NF-κB contribution
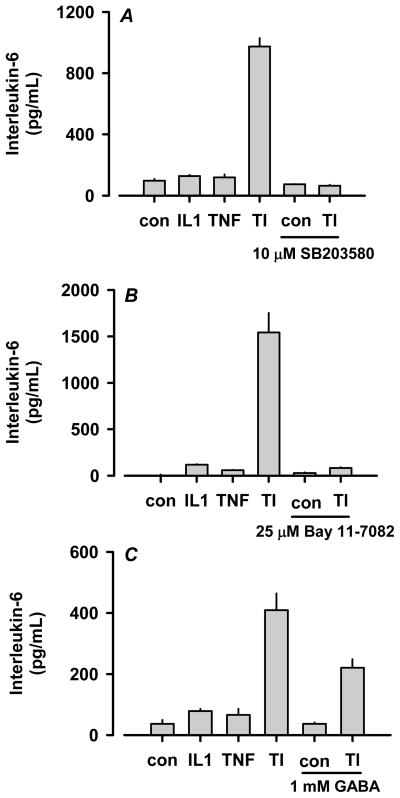
Using the 7TD1 bioassay, we reported that IL-1β stimulates IL-6 release from rat C6 astrocytoma cells [9]. We now report the synergistic effect of TNF-α and IL-1β (i.e., TI) on IL-6 release using ELISA quantification (Fig. 1). In addition, we previously reported that p38 is essential for the IL-1β-stimulated release of IL-6 [5]. To ascertain the roles of p38, NF-κB and GABA in the synergistic release of IL-6, C6 astrocytoma cells were pretreated with SB203580, Bay 11-7082 or GABA followed by co-treatment with each inhibitor and 100 ng/mL TNF-α and 50 ng/mL IL-1β. As shown in Fig. 1A, inhibition of p38 using the inhibitor SB203580 completely blocked the synergistic release of IL-6 by TI. Identical results were obtained with the related p38 inhibitor SB202190 (data not shown). Interruption of NF-κB signaling using Bay 11-7082 also completely inhibited TI-mediated IL-6 release (Fig. 1B). Finally, GABA was able to partially suppress the synergistic release of IL-6 (Fig. 1C). These data substantiate prominent roles for p38 and NF-κB in the TI-mediated synergistic release of IL-6 and confirm the GABA suppression of IL-6 release.
Figure 1.
Cytokine-mediated synergistic stimulation of IL-6 release from C6 astrocytoma cells is inhibited by GABA as well as p38 and NF-κB inhibitors in vitro. Rat C6 cells (1.0 × 106 cells/well in PDL-coated 24 well plates) were pretreated with 10 μM SB203580 (panel A), 25 μM Bay 11-7082 (panel B), or 1 mM GABA (panel C) for 1 h and subsequently co-treated with 100 ng/mL TNF-α/50 ng/mL IL-1β (i.e. TI) for 20 h. Conditioned media were removed and assayed for IL-6 using Pierce ELISA kits. TI significantly stimulated IL-6 release compared to control in each experiment (P ≤ 0.001). GABA, SB203580 and Bay 11-7082 each significantly reduced TI-stimulated IL-6 release (P ≤ 0.001). The data are presented as mean ± SEM of quadruplicate observations obtained from single representative experiments.
Phosphorylation of p38 and degradation of IκB-α
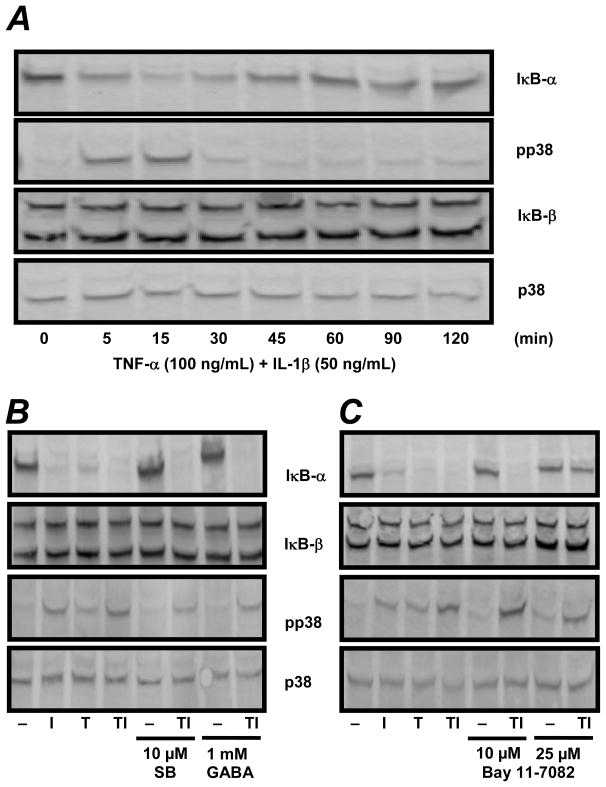
To clarify the mechanism underlying the synergistic release of IL-6 by TNF-α and IL-1β, p38 and NF-κB activation were investigated. The activity of NF-κB can be assessed through the degradation of its intrinsic inhibitor IκB [13]. C6 cells were treated with 100 ng/mL TNF-α and 50 ng/mL IL-1β for 5 to 120 minutes and cytosolic protein was assayed for IκB degradation by Western analysis. IL-1β and TNF-α each caused a rapid and transient decrease in IκB-α immunoreactivity at 15 and 30 min which coincided with maximal p38 phosphorylation (data not shown). Interestingly, neither cytokine affected IκB-β degradation at any time point (data not shown). The effect of combining TNF-α and IL-1β on p38, IκB-α, and IκB-β was then determined. For p38, the combination of both cytokines caused an increased activation of p38 at 5 min compared to either cytokine alone (Fig. 2A and data not shown). Further, we observed substantial IκB-α degradation at 5 min with a maximum disappearance at 15 min (Fig. 2A). We observed no degradation of the related NF-κB inhibitor IκB-β. Thus, TI induced a more rapid degradation of IκB-α and an earlier phosphorylation of p38 compared to either cytokine alone.
Figure 2.
Effects of SB203580, GABA and Bay 11-7082 on IκB-α degradation and p38 phosphorylation in C6 astrocytoma cells in vitro. Panel A: Rat C6 cells (3 × 106 cells/dish in PDL coated 35 × 10 mm dishes) were treated with 100 ng/mL TNF-α and 50 ng/mL IL-1β for 5–120 min. Panel B: Rat C6 cells (3 × 106 cells/dish in PDL coated 35 × 10 mm dishes) were pre-treated with 10μM SB203580 or 1 mM GABA for 1 h and subsequently co-treated with either SB203580 or GABA and TI (i.e. 100 ng/mL TNF-α and 50 ng/mL and IL-1β) for 15 min. Panel C: Cells were treated with 100 ng/mL TNF-α and 50 ng/mL IL-1β in the presence or absence of 10 μM or 25 μM Bay 11-7082 for 15 min. Cellular proteins were extracted and 25 μg of total protein separated via SDS-PAGE followed by Western analysis for IκB-α, IκB-β, phoshorylated p38 and total p38.
Effects of GABA on p38 and NF-κB activation
Although activation of NF-κB and MAPKs may be parallel events, Jang et al. [14] reported that the p38 inhibitor SB203580 reduced β-amyloid stimulated binding of NF-κB to its cognate DNA sequence in PC12 rat pheochromocytoma cells. This indicates that p38 is required for NF-κB transcriptional activity in neurons. To establish a role of p38 in IκB-α degradation, C6 cells were treated with 10 μM SB203580 for 1 h and then co-treated with SB203580, 100 ng/mL TNF-α and 50 ng/mL IL-1β. As shown in Fig. 2B (lanes 5 and 6), SB203580 did not suppress degradation of IκB-α. Furthermore, a 1 h pre-treatment with 1 mM GABA followed by a 15 min co-treatment with GABA, IL-β and TNF-α also failed to suppress the degradation of IκB-α or the phosphorylation of p38 (Fig. 2B, lanes 7 and 8). Taken together, these data suggest that glial p38 does not mediate NF-κB activation. Additionally, the GABA mediated suppression of synergistic IL-6 release may not involve either NF-κB or p38 activation.
To evaluate the inhibition of IκB-α degradation by Bay 11-7082 in the presence of TNF-α and IL-1β, C6 astrocytoma cells were co-treated with either 10 or 25 μM Bay 11-7082, 100 ng/mL TNF-α and 50 ng/mL IL-1β for 15 min. As shown in Fig. 2C, 25 μM (but not 10 μM) Bay 11-7082 prevented IκB-α degradation in the presence of TI (lanes 5–8). As expected, no changes in total p38 or IκB-β were observed. These data suggest that the ability of Bay 11-7082 to prevent the synergistic release of IL-6 is due to a blockade in IκB-α degradation.
Effects of TNF-α, IL-1β and GABA on IL-6 transcription
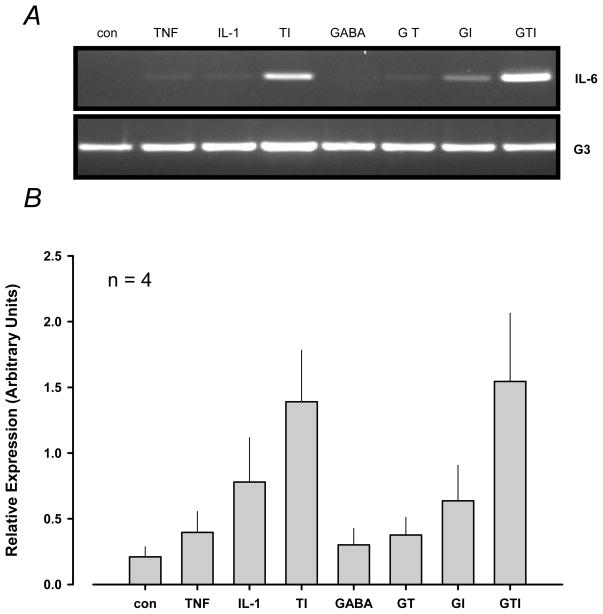
We next evaluated the effects of TNF-α, IL-1β and GABA on IL-6 transcription. C6 cells were pre-treated with 1 mM GABA for 1 h and then co-treated with GABA, 100 ng/mL TNF-α and 50 ng/mL IL-1β for 4 h. Although TNF-α and IL-1β alone caused modest increases in IL-6 transcription, the combination of both cytokines dramatically increased IL-6 expression (Fig. 3A). Interestingly, GABA did not inhibit IL-6 transcription due to any treatment. Relative expression levels were measured verifying visual observations in four experiments (Fig. 3B).
Figure 3.
Relative expression of IL-6 transcripts in C6 astrocytoma cells treated with GABA, TNF-α and IL-1β. Rat C6 cells (3 × 106 cells/dish in 35 × 10 mm dishes) were pre-treated with 1 mM GABA for 1 h and co-treated with either 1 mM GABA, 50 ng/mL IL-1β and 100 ng/mL TNF-α for 4 h. Panel A. Post stimulation, cellular RNA was extracted and 1 μg of total RNA was subsequently separated and analyzed via semi-quantitative RT-PCR. Panel B. IL-6 transcript relative expression levels were determined. TI significantly stimulated IL-6 transcription compared to control in each experiment (P ≤ 0.001). The data are presented as the observations obtained from four experiments.
Determination of total cellular content of IL-6
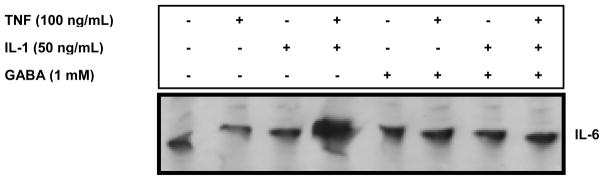
Because GABA reduced IL-6 release but not its gene expression, we evaluated IL-6 cellular content by Western analysis. C6 cells were pre-treated with 1 mM GABA for 1 h and then co-treated with GABA, 100 ng/mL TNF-α and 50 ng/mL IL-1β for 4 h. We observed greater IL-6 cellular content due to TNF-α and IL-1β combined exposures, consistent with the TI-mediated synergistic release of IL-6 (Fig. 4). GABA reversed the TI-driven accumulation of cellular IL-6 (lane 8), suggesting an effect on IL-6 mRNA translation.
Figure 4.
Cytokine-mediated stimulation of IL-6 content in C6 astrocytoma cells is inhibited by GABA in vitro. Rat C6 cells (3 × 106 cells/dish in 35 × 10 mm dishes) were pre-treated with 1 mM GABA for 1 h and subsequently co-treated with 50 ng/mL IL-1β, 100 ng/mL TNF-α and 1 mM GABA alone or in combination for 4 h. Post stimulation, cellular protein was extracted and 10 μg total protein subjected to SDS-PAGE followed by Western analysis for IL-6.
Discussion
Blockade of increased pro-inflammatory cytokine expression in AD may lead to novel treatments for this neurodegenerative disorder [15–17]. Elucidation of cytokine signal transduction pathways may identify potential pharmacological targets to prevent neuroinflammation associated with AD. Our previous results demonstrated that IL-1β induction of IL-6 release from C6 astrocytoma cells is synergistically enhanced by elevations in intracellular cyclic adenosine 3′–5′ monophosphate (cAMP). In addition, while catecholamines also synergistically augmented IL-1β-mediated IL-6 release, somatostatin and GABA inhibited this release [5,9]. The synergistic stimulation of IL-6 release by TNF-α and IL-1β (i.e. TI) demonstrated in the present study is relevant because increased levels of TNF-α and IL-1β have been documented in AD [10,18]. Additionally, dysfunction of inhibitory neurotransmitter systems (e.g., GABAergic) may facilitate the increased cytokine levels in AD [19].
We previously suggested that the p38 signaling module is essential for IL-1β stimulated release of bioactive IL-6 in C6 astrocytoma cells [5]. Recently Reyes-Garcia et al. [20] reported that GABA inhibits the production of IL-6 and IL-12 in macrophages. Similarly, we find that GABA inhibits the TI-mediated synergistic release of immunoreactive IL-6 from C6 cells. The IL-1β and TNF-α signaling pathways apparently converge with the activation of NF-κB [21]. Bourke et al. [22] reported that IL-1β activation of NF-κB is mediated through the long term degradation of IκB-β instead of a transient degradation of IκB-α. Interestingly, our data indicates that neither IL-1β nor TNF-α stimulates IκB-β degradation. Even though GABA caused an ~50% suppression of IL-6 release, it did not prevent IκB-α degradation nor p38 activation. In sharp contrast to GABA, 25 μM Bay 11-7082 produced an ~95% suppression of TI-mediated IL-6 release and complete prevention of IκB-α degradation. Consistent with our results, Loop et al. [23] reported that GABA does not affect TNF-α induced NF-κB DNA binding activity in Jurkat T cells.
Further, combined cytokine exposures shifted p38 phosphorylation and IκB-α degradation from 15 to 5 min. Our results suggest that the synergistic induction of IL-6 release due to TNF-α and IL-1β is a result of an accelerated effect on p38 and NF-κB activation. Apparent inhibition of either p38 or NFκB activation blocks TI-mediated increases in IL-6 release. Because the p38 inhibitor SB203580 blocks IL-6 release but did not prevent IκB-α degradation, NF-κB activation may be essential, but not sufficient, for the synergistic release of IL-6. In fact, the NF-κB inhibitor Bay 11-7082 completely inhibited IL-6 release but did not prevent p38 phosphorylation.
Synergistic increases in IL-6 release may result from accelerated effects on p38 and NF-κB activation resulting in increased IL-6 gene transcription. Thus, we hypothesized that GABA may suppress the synergistic release of IL-6 by inhibiting IL-6 gene expression. Consistent with the inability of GABA to alter either p38 or NFκB activation, we found that GABA did not inhibit the dramatic induction of TI-induced increases in IL-6 gene transcription.
Consequent to our RT-PCR results, we investigated possible GABA effects on intracellular IL-6 levels. We noted a large accumulation in intracellular IL-6 due to the combination of TNF-α and IL-1β. Furthermore, GABA inhibited the TI-mediated increase in intracellular IL-6. Because GABA did not prevent IL-6 gene transcription but did reduce IL-6 release and intracellular expression, we suggest that GABA may interrupt the translation of IL-6 mRNA.
GABA receptors are functionally classified as GABAA or GABAB. The ionotropic GABAA receptor conducts chloride ions and mediates fast inhibitory responses [24]. In addition, selective activation of the GABAA receptor is achieved by muscimol [25] or isoguvacine oxide [26]. In contrast to the ionotropic GABAA receptor, GABAB is a metabotropic G protein coupled receptor. While GABAA elicits fast inhibitory responses, GABAB is responsible for slower inhibitory responses [27,28]. Selective activation of the GABAB receptor is possible with the use of baclofen [29]. We are currently examining which receptor subtype is responsible for GABA suppression of IL-6 release.
Our major findings, therefore, are that [a] combined IL-1β/TNF-α treatment of C6 astrocytoma cells results in a substantial synergistic release of IL-6; [b] this synergistic release is particularly sensitive to the p38 inhibitor SB203580 and the NF-κB inhibitor Bay 11-7082; [c] GABA inhibits the synergistic release of IL-6 from C6 cells; [d] exposure of C6 cells to IL-1β/TNF-α results in more extensive phosphorylation of p38 and degradation of IκB-α compared to either cytokine alone; [e] GABA does not prevent p38 phosphorylation nor IκB-α degradation in the presence of IL-1β/TNF-α; [f] GABA does not prevent IL-6 transcription in the presence of IL-1β/TNF-α; and [g] GABA partial inhibition of TI-induced IL-6 release appears to coincide with a reduction in cellular IL-6, suggesting translational suppression.
The exogenous manipulation of inhibitory neurotransmitters could be of therapeutic benefit in AD. Indeed, administration of the inhibitory neuropeptide somatostatin analog octreotide enhances AD patient memory in story recall [30]. Validation of inhibitory neurotransmitter suppression of neuroinflammatory mediators of AD may lead to novel treatments of this catastrophic neurodegenerative disorder.
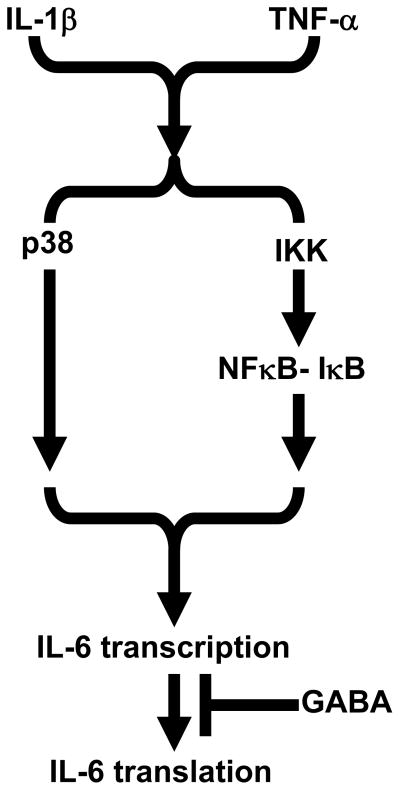
Figure 5.
Postulated mechanism of GABA suppression of IL-1β and TNF-α synergistic release of IL-6 in C6 astrocytoma cells in vitro. See Discussion for details.
Acknowledgments
Michelle Fong, Virag Patel, Christopher Mercado and Freidun Hadi are thanked for their excellent technical assistance. These experiments were supported by grant 1R15NS051198-01A from the National Institute of Neurological Disorders and Stroke (NINDS) at the National Institutes of Health (NIH). This manuscript is dedicated to the memory Dr. W. David Jarvis, our enthusiastic colleague and friend.
References
- 1.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- 2.Kolaj M, Bai D, Renaud LP. GABAB Receptor modulation of rapid inhibitory and excitatory neurotransmission from subfornical organ and other afferents to median preoptic nucleus neurons. J Neurophysiol. 2004;92:111–122. doi: 10.1152/jn.00014.2004. [DOI] [PubMed] [Google Scholar]
- 3.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. γ-Aminobutyric acid inhibits T cell autoimmunity and the developmental of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 5.Spangelo BL, Horrell S, Goodwin AL, Shroff S, Jarvis WD. Somatostatin and gamma-aminobutyric acid inhibit interleukin-1β-stimulated release of interleukin-6 from rat c6 glioma cells. Neuroimmunomodulation. 2004;11:332–340. doi: 10.1159/000079414. [DOI] [PubMed] [Google Scholar]
- 6.Bareggi SR, Franceschi M, Bonini L, Zecca L, Smirne S. Decreased CSF concentrations of homovanillic acid and γ-aminobutyric acid in Alzheimer’s disease. Arch Neurol. 1982;39:709–712. doi: 10.1001/archneur.1982.00510230035010. [DOI] [PubMed] [Google Scholar]
- 7.Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJM, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer’s Disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 8.Zhao B, Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res. 1998;52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Zumwalt J, Thunstrom B, Spangelo BL. Interleukin-1β and catecholamines synergistically stimulate interleukin-6 release from rat C6 glioma cells in vitro: A potential role for lysophosphatidylcholine. Endocrinology. 1999;140:888–896. doi: 10.1210/endo.140.2.6536. [DOI] [PubMed] [Google Scholar]
- 10.Griffin WST. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83(suppl):470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- 11.Elsawa SF, Bost KL. Murine gamma-herpesvirus-68-induced IL-12 contributes to the control of latent viral burden, but also contributes to viral-mediated leukocytosis. J Immunol. 2004;172:516–524. doi: 10.4049/jimmunol.172.1.516. [DOI] [PubMed] [Google Scholar]
- 12.Bost KL, Mason MJ. Thapsigargin and cyclopiazonic acid initiate rapid and dramatic increases of IL-6 mRNA expression and IL-6 secretion in murine peritoneal macrophages. J Immunol. 1995;155:285–296. [PubMed] [Google Scholar]
- 13.Uehara T, Matsuno J, Kaneko M, Nishiya T, Fujimuro M, Yokosawa H, Nomura Y. Transient nuclear factor kappaB (NF-kappaB) activation stimulated by interleukin-1 beta may be partly dependent on proteasome activity, but not phosphorylation and ubiquitination of the IkappaBalpha molecule in C6 glioma cells: Regulation of NF-kappaB linked to chemokine production. J Biol Chem. 1999;274:15875–15882. doi: 10.1074/jbc.274.22.15875. [DOI] [PubMed] [Google Scholar]
- 14.Jang JH, Surh YJ. Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 up-regulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic Biol Med. 2005;38:1604–1613. doi: 10.1016/j.freeradbiomed.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Ranaivo HR, Craft JM, Hu W, Guo W, Wing LK, Van Eldik LJ, Watterson DM. Glia as a therapeutic target: selective suppression of human amyloid-β-induced up-regulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci. 2006;26:662–670. doi: 10.1523/JNEUROSCI.4652-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allan SM, Pinteaux E. The interleukin-1 system: an attractive and viable therapeutic target in neurodegenerative disease. Curr Drug Targets CNS Neurol Disord. 2003;2:293–302. doi: 10.2174/1568007033482742. [DOI] [PubMed] [Google Scholar]
- 17.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- 18.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological diseases with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 19.Bell KFS, Ducatenzeiler A, Ribeira-da-Silva A, Duff K, Bennett DA, Cuello AC. The amyloid pathology progresses in a neurotransmitter specific matter. Neurobiol Aging. 2005;27:1644–1657. doi: 10.1016/j.neurobiolaging.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Akama KT, Van Eldik LJ. β-Amyloid stimulation of inducible nitric oxide synthase in astrocytes is interleukin-1β- and tumor necrosis factor-α (TNFα)-dependent, and involves a TNFα receptor-associated factor- and NFκB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- 22.Bourke E, Kennedy EJ, Moynagh PM. Loss of IκB-β Is associated with prolonged NF-κB activity in human glial cells. J Biol Chem. 2000;275:39996–40002. doi: 10.1074/jbc.M007693200. [DOI] [PubMed] [Google Scholar]
- 23.Loop T, Humar M, Pischke S, Hoetzel A, Schmidt R, Pahl HL, Geiger KK, Pannen BHJ. Thiopental inhibits tumor necrosis factor [alpha]-induced activation of nuclear factor [kappa]B through suppression of I[kappa]B kinase activity. Anesthesiology. 2003;99:360–367. doi: 10.1097/00000542-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Banks MI, Hardie JB, Pearce RA. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- 25.Murashita H, Tabuchi K, Sakai S, Uemaetomari I, Tsuji S, Hara A. The effect of the GABAA agonist muscimol on acoustic injury of the mouse cochlea. Neurosci. Lett. 2007;418:18–21. doi: 10.1016/j.neulet.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 26.Frolund B, Jeppesen L, Krogsgaard-Larsen P, Hansen JJ. GABAA agonists: resolution and pharmacology of (+)- and (−)- isoguvacine oxide. Chirality. 1995;6:434–438. doi: 10.1002/chir.530070608. [DOI] [PubMed] [Google Scholar]
- 27.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 28.Federici M, Geracitano R, Tozzi A, Longone P, Di Angelantonio S, Bengston CP, Bernardi G, Mercuru NB. Trace amines depress GABAB response in dopaminergic neurons by inhibiting G-βγ-gated inwardly rectifying potassium channels. Mol. Pharmacol. 2005;67:1283–1290. doi: 10.1124/mol.104.007427. [DOI] [PubMed] [Google Scholar]
- 29.Bartoletti M, Gubellini C, Ricci Fm Gaiardi M. The GABAB agonist baclofen blocks the expression of sensitization to the locomotor stimulant effect of amphetamine. Behav. Pharmacol. 2004;15:397–401. doi: 10.1097/00008877-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Craft S, Asthana S, Newcomer JH, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Perova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer’s disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]