Abstract
Aortic aneurysms are common among the elderly population. Large majority of aortic aneurysms are located at two distinct aneurysm-prone regions—the abdominal aorta and thoracic aorta involving the ascending aorta. In this study, we combined two factors that are associated with human aortic aneurysms—hypertension and degeneration of elastic lamina—to induce an aortic aneurysm in mice. Roles of hemodynamic conditions in the formation of aortic aneurysms were assessed using (1) two different methods for inducing hypertension, and (2) anti-hypertensive agents.
In nine-week-old C57BL/6J male mice, hypertension was induced by angiotensin-II or deoxycorticosterone acetate (DOCA)-salt hypertension; degeneration of elastic lamina was induced by infusion of beta-aminopropionitrile, a lysyl oxidase inhibitor. Irrespective of the methods for inducing hypertension, mice developed thoracic and abdominal aortic aneurysms (38-50% and 30-49 %, respectively). Aneurysms were found at the two aneurysm-prone regions with site-specific morphological and histological characteristics. Treatment with anti-hypertensive agent, amlodipine, normalized blood pressure and dramatically reduced aneurysm formation in the mice that received angiotensin-II and beta-aminopropionitrile. However, a treatment with captopril, angiotensin converting enzyme inhibitor, did not affect blood pressure or the incidence of aortic aneurysms in the mice that received deoxycorticosterone acetate-salt and beta-aminopropionitrile.
In summary, we have shown that a combination of hypertension and pharmacologically-induced degeneration of elastic laminas can induce both thoracic and abdominal aortic aneurysms with site-specific characteristics. The aneurysm formation in this model was dependent on hypertension, but not on direct effects of angiotensin-II to the vascular wall.
Keywords: aorta, aneurysm, hypertension, angiotensin-II, lysyl oxidase, hemodynamics, remodeling
Introduction
Aortic aneurysms are common among the elderly population, and their rupture results in severe mortality and morbidity. The primary purpose of surgical intervention for unruptured aortic aneurysms is to prevent future rupture. However, surgical intervention still carries significant risks of mortality and morbidity. Therefore, pharmacological stabilization of aneurysms that prevents growth and rupture of aortic aneurysms has been vigorously sought.1 In order to develop such strategy, underlying mechanisms of aortic aneurysm formation and growth need to be elucidated in an animal model that recapitulates key features of human aortic aneurysms.
Clinically, systemic hypertension is closely associated with aortic aneurysm formations.2, 3 However, a causal relationship between hypertension and aortic aneurysm has not been completely established. Degeneration and disorganization of elastic lamina are characteristic histological changes observed in both thoracic and abdominal aortic aneurysms in humans.4, 5 Incidence of aortic aneurysms increases with age,6, 7 and aging-related degeneration of elastic lamina is often considered as a precursory change that precedes aneurysm formation.8
Experimentally, degeneration of elastic lamina can be induced by administration of beta-aminopropionitrile (BAPN), an inhibitor of lysyl oxidase.9 Lysyl oxidase cross-links elastin fibers and collagen fibers, and plays a critical role in maintaining homeostasis of elastic lamina. With aging, lysyl oxidase activity decreases.10 BAPN is referred to as a lathyrogen because its effects closely mimic human aging.11 Degeneration of elastic laminas has been observed in both lysyl oxidase knockout mice and blotchy mice, which have decreased lysyl oxidase activity.12, 13 Some of the mice show aneurysmal changes in large arteries.12, 13 These findings suggest a possible mechanistic link between aneurysm formation and degeneration of elastic lamina caused by aging or reduction in lysyl oxidase activity.
In this study, we show that a combination of hypertension and degeneration of elastic lamina by lysyl oxidase inhibitor, BAPN, can cause both thoracic and abdominal aortic aneurysms in mice. We used two well-established methods of pharmacologically induced hypertension— angiotensin-II induced hypertension and deoxycorticosterone acetate (DOCA)-salt hypertension. Similar to human aortic aneurysms, aortic aneurysms in this model developed at the ascending thoracic aorta and abdominal aorta7 with site-specific morphological and histological characteristics. Furthermore, we assessed the roles of hypertension on aneurysm formation by utilizing amlodipine, an anti-hypertensive agent. Potential contributions to aneurysm formation from angiotensin-II locally produced in the vascular wall were assessed by using captopril (angiotensin-converting enzyme inhibitor) in the mice that received DOCA-salt treatment and BAPN.
Methods
Detailed methods are described in Online Supplements. Please see “http://hyper.ahajournals.org.”
Induction of aortic aneurysm by angiotensin-II and BAPN
In nine-week-old C57BL/6J male mice (Jackson Laboratory), hypertension was induced by angiotensin-II (1000 ng/kg/min)14 or DOCA-salt treatment.14, 15 BAPN (150 mg/kg/day), a lysyl oxidase inhibitor, was administered for the first two weeks through a subcutaneously implanted osmotic-pump (Alzet, Durect Corp) to induce degeneration of elastic laminas. Mice were sacrificed six weeks after the surgery. Aneurysms were defined as a localized dilation of aorta greater than 50% of its adjacent intact portion of aorta.16 One group of mice received an anti-hypertensive agent, amlodipine (5 mg/kg/day) in addition to angiotensin-II and BAPN. Additional mice received captopril (angiotensin-converting enzyme inhibitor, 6 mg/kg/day15) in addition to DOCA-salt treatment and BAPN.
Statistical analysis
Data were presented as mean ± SD. Differences between multiple groups were analyzed by one-way ANOVA, followed by the Tukey-Kramer post hoc test. Chi-square test was used to analyze categorical data. Statistical significance was taken at P < 0.05.
Results
Combination of angiotensin-II induced hypertension and lysyl oxidase inhibition by BAPN resulted in aortic aneurysm formations
Forty-five mice received angiotensin-II for six weeks and BAPN for two weeks. A total of 16 mice died before six weeks from ruptured aortic aneurysms (15/16) or dissecting aneurysm (1/16). 64% of the mice (29/45) survived for six weeks. Including ruptured and unruptured aneurysm, 71% (32/45) of the mice developed aortic aneurysms during the six-week period. 38% (17/45) of the mice developed thoracic aortic aneurysms (Figure 1A). Nine thoracic aortic aneurysms were found as ruptured aneurysms, indicating a 53% rupture rate (9/17). 49% of the mice (22/45) developed abdominal aortic aneurysms (Figure 1A). Six abdominal aortic aneurysms were found as ruptured aneurysms, representing a 27% rupture rate (6/22). Seven mice out of the 45 (16%) had both thoracic and abdominal aortic aneurysms.
Figure 1. Combination of angiotensin-II induced hypertension and lysyl oxidase inhibition by BAPN resulted in aortic aneurysm formation in mice.
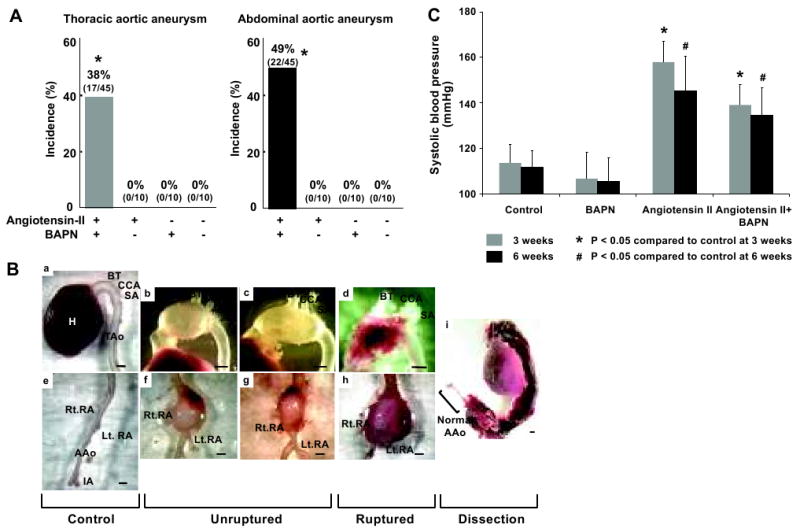
A: Incidence of aortic aneurysms. 38% and 49% of the mice that received angiotensin-II and BAPN developed thoracic and abdominal aortic aneurysms, respectively. No aneurysm formation was found in the mice that received angiotensin-II or BAPN alone. B: Representative aortic aneurysms. Macroscopically, thoracic and abdominal aortic aneurysms in this model resembled human aortic aneurysms with their site-specific morphology. Thoracic aortic aneurysms were saccular-shaped with localized dilation at the great curvature, while abdominal aortic aneurysms were fusiform-shaped aneurysms with a thick vascular wall. a: normal thoracic aorta, b-c: unruptured thoracic aortic aneurysm, d: ruptured thoracic aortic aneurysm, e: normal abdominal aorta, f-g: unruptured abdominal aortic aneurysm, h: ruptured abdominal aortic aneurysm, i: dissecting aortic aneurysm. Scale bar: 1mm. C: Systolic blood pressure in the mice that received both angiotensin-II and BAPN, and the mice that received BAPN or angiotensin-II alone. Systolic blood pressures of mice that received angiotensin-II alone or combination of angiotensin-II and BAPN are significantly higher than systolic blood pressures of mice in the control group at 3 and 6 weeks. Mean ± SD, *: P < 0.05 compared with control. TAo : thoracic aorta, AAo : abdominal aorta, BT : brachiocephalic trunk, CCA : left common carotid, artery, SA : left subclavian artery, RA : renal artery, IA : iliac arteries, Rt. : right, Lt. : left.
One mouse had a dissecting aneurysm, which extended over the entire thoracic and abdominal aortas (Figure 1Bi). Two animals developed small isolated aneurysms at the distal descending thoracic aorta. Except for these three aneurysms, all aneurysms were localized at the two distinct regions of the aorta that are known to be aneurysm-prone regions of the aorta in humans—the thoracic aorta involving the ascending aorta and the abdominal aorta. In the following sections, thoracic aortic aneurysms refer to aneurysms that involve the ascending aorta and arch. The two small aneurysms at the distal descending thoracic aorta are referred to as descending thoracic aortic aneurysms.
Mice treated with angiotensin-II (n=10) or BAPN (n=10) or phosphate-buffered saline (PBS) (n=10) alone did not develop aneurysm.
Macroscopically, thoracic and abdominal aortic aneurysms in this model resembled human aortic aneurysms with their site-specific morphology (Figure 1B).7, 17 Thoracic aortic aneurysms were saccular-shaped with localized dilation at the great curvature (Figure 1Bb-d). In contrast, abdominal aortic aneurysms were fusiform-shaped aneurysms with a thick vascular wall and an intramural thrombus (Figure 1Bf-h). Figure 1Bi shows a dissecting aneurysm that extended over the ascending aorta and the abdominal aorta (1/45).
Systolic blood pressures of mice that received angiotensin-II alone or combination of angiotensin-II and BAPN were significantly higher than those of the control group at 3 and 6 weeks (Figure 1C). The time course of aneurysm formation and growth in this model is presented in Online Supplements Figure S1.
Histological characterization of aortic aneurysms
Control thoracic and abdominal aorta and representative thoracic and abdominal aneurysms are shown in Figure 2A-D. Thoracic aortic aneurysms showed two distinct parts of the vascular wall—thin wall and thick wall parts (Figure 2B). The thin wall part of the aneurysm formed a saccular-shaped thoracic aortic aneurysm. The thin aneurysm wall had only two or three layers of elastic lamina (B2-5). The thick wall part of the thoracic aortic aneurysm showed severe medial degeneration and adventitial thickening (B9-12). Thickening and disorganization of the media were accompanied by fragmentation and disruption of elastic laminas with widening space between the elastic laminas (B11). Thickened adventitia was collagen-rich and contained large numbers of inflammatory-like cells (B12). Neither atherosclerotic changes nor intramural thrombus was found. Smooth muscle cells were scarce in the thin wall (B7) while smooth muscle cells were abundant in the thickened part of the media (B14). Endothelial cell layer was intact in both thin and thick parts of thoracic aortic aneurysms (B8, B15). Fibroblasts were mainly present in the inner half of the thickened adventitia (B13).
Figure 2. Histological assessment of thoracic and abdominal aortic aneurysms.
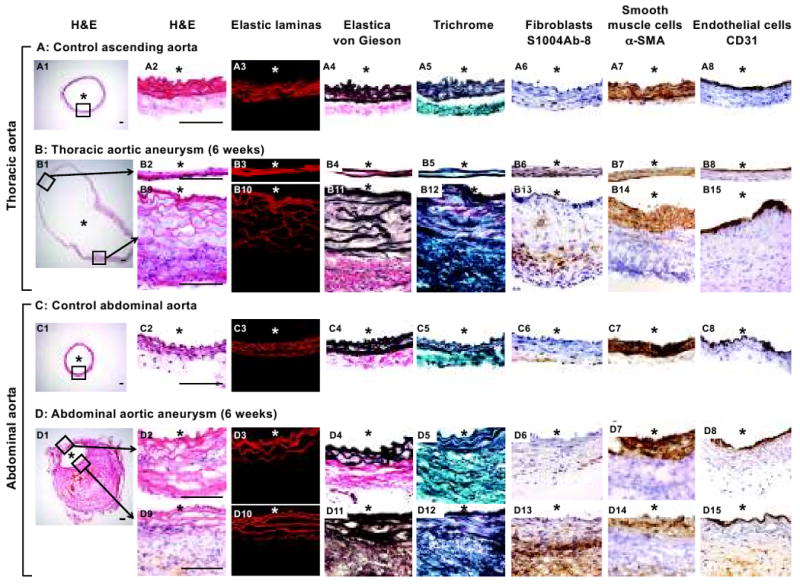
Thoracic and abdominal aortic aneurysms in this mouse model closely resembled human thoracic and abdominal aortic aneurysms with their site-specific histological characteristics. Control aortas, thoracic and abdominal aneurysms were stained for H&E, elastic laminas, Elastica von Gieson, Trichrome, fibroblasts, smooth muscle cells, and endothelial cells. *: lumen. Scale bar: 0.1mm.
Abdominal aortic aneurysms showed thickening of the vascular wall throughout the entire circumference and presence of an intramural thrombus (Figure 2D), resembling human abdominal aortic aneurysms.18 Thinning of the vascular wall was not observed. Degeneration of elastic lamina in the abdominal aortic aneurysm was much less than that of the thoracic aneurysms (D2-5, D9-12). Inflammatory cells were observed in the adventitia, especially around the intramural thrombus. Oil red O staining showed presence of lipids around the intramural thrombus, which is possibly an early sign of atherosclerosis (Online Supplements, Figure S2). Majority of fibroblasts were present around the intramural thrombus, and some of them infiltrated into the media (D13). Smooth muscle cell layers were mildly disorganized, losing tight alignment of the elastic lamina (D7, D14). Endothelial cell layer was generally intact (D8, D15).
Similarly to human aortic aneurysms, aortic aneurysms in this model showed inflammatory cell infiltration.18, 19 At one-week, numerous leukocytes were already detected in the adventitia, especially in the outer layer of the adventitia, in both the thoracic and abdominal aortas (Online Supplements, Figure S3).
Differences in distribution of inflammatory cells between thoracic and abdominal aortic aneurysms became apparent at six weeks (Figure 3). In thoracic aneurysms, numerous leukocytes were observed in the adventitia and media within both the thin and thick walls (B2, B6). In contrast, leukocytes were highly concentrated in the thick wall near the intramural thrombus in abdominal aortic aneurysms (D6), and the wall without intramural thrombus contained only a small number of leukocytes (D2). Majority of leukocytes (CD45+) appeared to be macrophages (CD68+) (B2-5, B6-9, D2-5, D6-9, Online Supplements, Figure S5A). Helper T-cells and B-cells were present, but scarce in both thoracic and abdominal aneurysms (B4-5, B8-9, D4-5, D8-9).
Figure 3. Inflammatory cells in thoracic and abdominal aortic aneurysms.
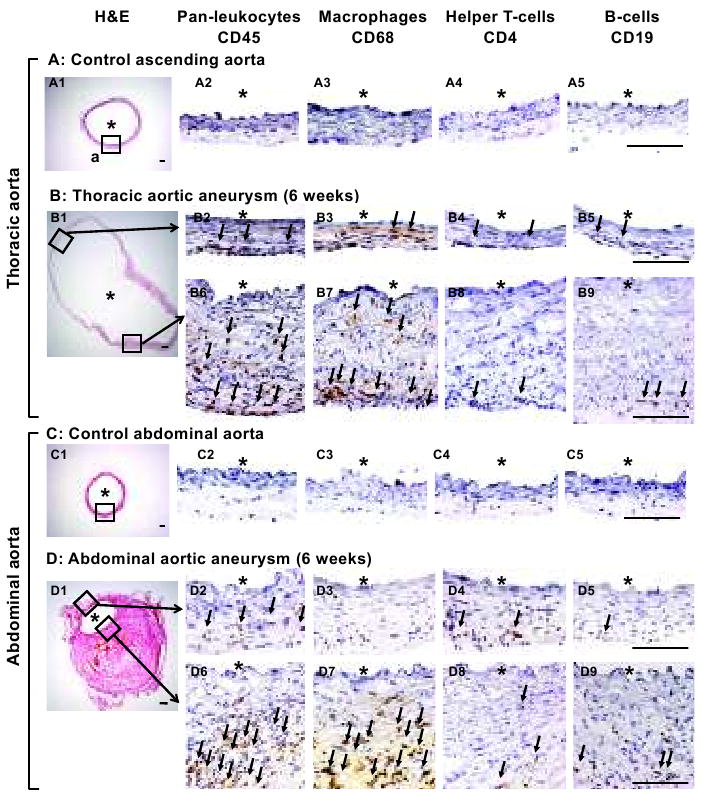
Stainings for pan-leukocytes (CD45), macrophages (CD68), helper T-lymphocytes (CD4) and B-lymphocytes (CD19). In thoracic aneurysms, leukocytes were observed in the adventitia and media (B2, B6). In contrast, leukocytes were highly concentrated in the thick wall near the intramural thrombus in abdominal aortic aneurysms (D6). In both thoracic and abdominal aneurysms, majority of leukocytes appeared to be macrophages (B2-5, B6-9, D2-5, D6-9). *: lumen. Scale bar: 0.1mm. Arrows point to positive cells.
Thoracic and abdominal aortas with pre-aneurysmal changes— localized dilation of the aorta that did not reach the 50% cutoff—had similar structural and histological changes, including inflammatory cell infiltration, to those with mature aneurysms (Online Supplements, Figure S3). Morphometric analysis and the grading of changes of elastic lamina, semi-quantification of leukocytes are shown in Online Supplements, Figure S4 and S5B.
Roles of hypertension in aneurysm formation in this model
Non-hemodynamic effects of angiotensin-II could potentially have contributed to the formation of aneurysms independently from its hypertensive effects.20-22 Therefore, to elucidate roles of hypertension and to assess potential contributions from non-hemodynamic effects of angiotensin-II in aneurysm formation in this model, we performed two lines of experiments. First, we treated the mice that were receiving angiotensin-II and BAPN with an anti-hypertensive agent, amlodipine (calcium channel blocker) to separate the hypertensive effect of angiotensin-II from its other effects (n=20). Second, we used deoxycorticosterone acetate (DOCA)-salt hypertension instead of angiotensin-II induced hypertension (n=10).
Reduction of blood pressure by the anti-hypertensive agent, amlodipine (Figure 4D), dramatically decreased incidence of thoracic and abdominal aortic aneurysms from 38% to 5% (P < 0.05) and from 49% to 0% (P < 0.05), respectively (Figure 4A-B). Thoracic and abdominal aortas treated with amlodipine showed a complete lack of the adventitial inflammation and medial degeneration (Figure 4C). Amlodipine did not affect blood flow in the thoracic or abdominal aorta (Figure 4E).
Figure 4. Roles of hypertension in aneurysm formation.
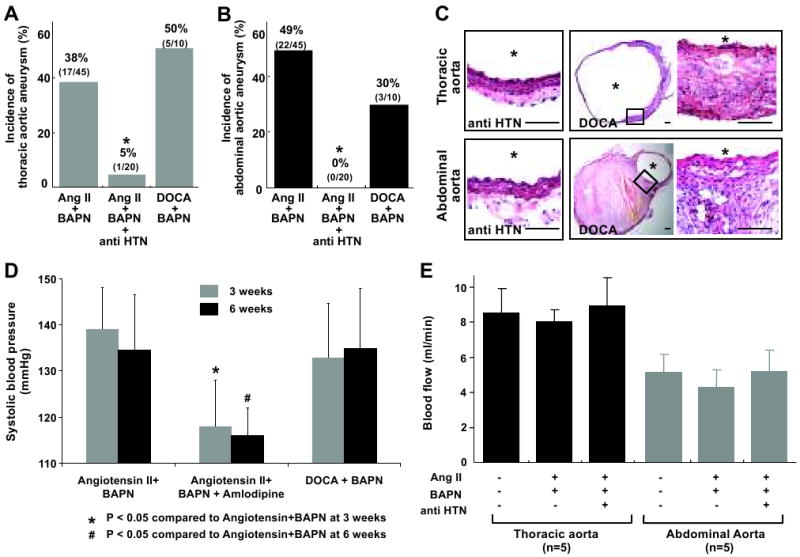
A & B: Incidence of thoracic and abdominal aortic aneurysms. Both angiotensin-II and DOCA-salt induced hypertension were able to induce aortic aneurysms when combined with the treatment of BAPN. Anti-hypertensive agent (anti-HTN), amlodipine, dramatically reduced the incidence of aortic aneurysms. *: P < 0.05 compared to angiotensin-II and BAPN group. C: Aortic aneurysms in the mice treated with DOCA-salt and BAPN were indistinguishable from those observed in mice that were treated with angiotensin-II and BAPN. Amlodipine treatment in the mice that were receiving angiotensin-II and BAPN resulted in a complete lack of adventitial inflammation and thickening of the media and adventitia. Scale bar: 0.1mm. *: lumen. D: Systolic blood pressure. Amlodipine significantly reduced blood pressure in the mice that were treated with angiotensin-II and BAPN. There was no difference in blood pressure between mice receiving DOCA-salt and BAPN and mice receiving angiotensin-II and BAPN. Mean ± SD. *: P < 0.05 compared to control. E: There was no significant effect of amlodipine on blood flow rates.
The combination of DOCA-salt hypertension and BAPN (n =10) successfully induced aortic aneurysms in both thoracic (5/10, 50%) and abdominal aortas (3/10, 30%) (Figure 4A-B, 4D). One mouse (1/10) developed both thoracic and abdominal aneurysms. In total, 70% of the mice developed aortic aneurysms. Histologically, thoracic and abdominal aortic aneurysms in the mice treated with DOCA-salt and BAPN (Figure 4C) were indistinguishable from the aortic aneurysms observed in mice that were treated with angiotensin-II and BAPN.
Since angiotensin-II levels in the vessel wall can be elevated and potentially contribute to aneurysm formation in DOCA-salt-treated mice,15 we treated mice that were receiving DOCA-salt treatment and BAPN with captopril (angiotensin-converting enzyme inhibitor) (n = 13). Captopril did not cause a significant reduction of blood pressure compared to the group that only received DOCA-salt treatment with BAPN (143 ±12 vs 140 ±12 mmHg). 69% of the mice that received DOCA-salt, BAPN, and captopril (9/13) developed aortic aneurysms. Five mice had thoracic aneurysms, and seven mice had abdominal aneurysms. Three mice had both thoracic and abdominal aneurysms.
Discussion
In this study, we showed that the combination of hypertension and degeneration of elastic lamina by lysyl oxidase inhibition in mice resulted in formation of aortic aneurysms that recapitulate key features of human aortic aneurysms with site-specific phenotypes. Using this model, we showed critical roles of high blood pressure in the formation of aortic aneurysms, establishing a causal link between hemodynamic conditions and aortic aneurysm formation in animals.
Daugherty et al. pioneered an abdominal aortic aneurysm model in genetically atherosclerosis-prone mice by continuously infusing angiotensin-II.23, 24 They used apolipoprotein E (ApoE)-knockout mice and fat-fed low-density lipoprotein (LDL)-receptor knockout mice.23, 24 Morphological and histological characteristics of angiotensin-II-induced abdominal aortic aneurysms in these knockout mice were similar to the abdominal aortic aneurysms in our model, indicating that common molecular mechanisms potentially exist between these two models in respect to abdominal aortic aneurysms. It should be noted that angiotensin-II infusion in ApoE-knockout or LDL-receptor knockout mice did not cause thoracic aortic aneurysm.23, 24 In contrast, aneurysm formation in our model occurred not only in abdominal aorta, but also in the thoracic aorta involving the ascending aorta, the segment of the thoracic aorta in which most human thoracic aneurysms are located.
Previously, Ikonomidis et al. showed that direct application of calcium chloride to the descending thoracic aorta through thoracotomy caused aneurysmal formation in the aortic segment that was exposed to calcium chloride.25 The advantage of their model is that aneurysmal dilatation occurred in almost all animals.25 However, the aneurysmal dilatation in their model was mild, i.e., 25% dilatation comparing to 50% in our model, and the location of aneurysms in thoracic aorta in their model differs from the common location of thoracic aneurysms in humans. More importantly, in our model, both abdominal and thoracic aneurysms were induced by the same pharmacological treatments. Our model may be more suitable for studying potential similarities and differences in the pathophysiology between thoracic and abdominal aortic aneurysms.
Although atherosclerosis is strongly linked to systemic hypertension, majority of the patients with thoracic aortic aneurysms are often free from systemic or local atherosclerosis 19, 26. While many of the abdominal aortic aneurysms in our model showed signs of early atherosclerosis, such changes were absent in thoracic aortic aneurysms in this model. Interestingly, abdominal aortas from the earlier time point revealed pre-aneurysmal changes without any sign of atherosclerosis. Atherosclerosis observed in abdominal aortic aneurysms in this model may not be part of a causative factor but rather a secondary change that follows aneurysm, as previously suggested.27, 28
Another advantage of this new model is the use of wild-type mice, which makes it easier to examine roles of different signaling pathways compared to using knockout and transgenic mice. While the successful induction of abdominal aortic aneurysms by angiotensin-II in ApoE-knockout or LDL-receptor knockout mice has been validated by several groups, the incidence of abdominal aneurysms in the wild-type mice treated with only angiotensin-II widely varied among published papers (0-39%),16, 23, 24, 29 making it difficult to compare the incidence of abdominal aneurysms between the wild-type and genetically manipulated mice when angiotensin-II alone is used.
In our model, the aneurysms at the two aneurysm-prone regions were induced by the same systemic pharmacological treatment, but they exhibited different morphological and histological features that closely resembled human aortic aneurysms at the respective locations. Morphological and histological differences observed between thoracic and abdominal aortic aneurysms in this model may suggest that differential responses to the combination of hypertension and lysyl oxidase inhibition at these two regions of the aorta lead to different phenotypes of aneurysms. Morphological and histological differences between thoracic and abdominal aortas in this model and in humans may be due to the differences in developmental origins of smooth muscle cells at these two segments of aorta.26, 27 Embryologically-programmed differences of vascular smooth muscle cells may determine different vascular responses to hemodynamic stimuli and degeneration of elastic lamina between thoracic and abdominal aortas,26, 27, 30 leading to site-specific phenotypes of aneurysms at the two regions. More importantly, phenotypic differences between thoracic and abdominal aortic aneurysms indicate that different pharmacological strategies may be needed to prevent growth and rupture of aneurysms at these two different locations.
Angiotensin-II can exert various effects on the vasculature in addition to its hypertensive effect.20-22 For the formation of abdominal aortic aneurysms in ApoE-knockout or fat-fed LDL-receptor knockout mice in response to angiotensin-II infusion, 31 non-hemodynamic effects of angiotensin-II, but not hypertensive effects, are required.31, 32 In contrast, the aneurysm formation observed in our model was dependent on systemic hypertension. In our model, normalization of blood pressure by an anti-hypertensive agent dramatically reduced the incidence of aneurysms and almost completely abolished histological changes associated with angiotensin-II and BAPN treatment. We were able to reproduce thoracic and abdominal aortic aneurysms when DOCA-salt hypertension was used instead of angiotensin-II. However, in DOCA-salt-treated mice, endogenous angiotensin-II that was produced in the vascular wall in response to systemic hypertension may have played a role in our model.15 Therefore, we treated the mice receiving DOCA-salt and BAPN with captopril, an angiotensin-converting enzyme inhibitor, to exclude potential confounding effects from the endogenous production angiotensin-II in DOCA-salt hypertensive mice. Captopril did not reduce the incidence of aortic aneurysm in DOCA-salt hypertensive mice, further suggesting critical roles of hypertension in this model.
Our data represent the first demonstration of the causal relationship between systemic hypertension and aortic aneurysm formation. One critical caveat to this study is that systolic blood pressure was measured under anesthesia as previously performed by others.23 Blood pressure measurement under anesthesia may underestimate effects of hypertensive and anti-hypertensive agents.
It should be noted that although our mouse model replicated key features of thoracic and abdominal aortic aneurysms in humans, aneurysms in this model did not form spontaneously but were induced by two pharmacological interventions, which potentially bypassed some of the early critical events that lead to aortic aneurysm in humans.
Perspectives.
We showed that the combination of pharmacologically induced hypertension and degeneration of elastic lamina by lysyl oxidase inhibition caused aneurysm formations at two aneurysm-prone regions of aorta that are common locations of aortic aneurysms in humans. Using this model, we established critical roles of hypertension in the formation of aortic aneurysms. Phenotypic differences between thoracic and abdominal aortic aneurysms in this model and in humans may indicate that different pharmacological strategies may be needed to prevent growth and rupture of aneurysms at these two different locations. Our model may be suitable to study potential similarities and differences in the pathophysiology between thoracic and abdominal aortic aneurysms.
Supplementary Material
Acknowledgments
We wish to thank Dr. William L. Young for providing mentoring and insightful suggestion and Mr. Mark Weinstein for his skillful technical assistance.
Sources of Funding: This study was funded by NIH R01NS055876 (TH) and American Heart Association Grant-in-Aid 0755102Y (TH).
Footnotes
Disclosures: No conflicts.
References
- 1.Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, Thompson MM. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33:2858–2864. doi: 10.1161/01.str.0000038098.04291.f6. [DOI] [PubMed] [Google Scholar]
- 2.Dapunt OE, Galla JD, Sadeghi AM, Lansman SL, Mezrow CK, de Asla RA, Quintana C, Wallenstein S, Ergin AM, Griepp RB. The natural history of thoracic aortic aneurysms. The Journal of thoracic and cardiovascular surgery. 1994;107:1323–1332. [PubMed] [Google Scholar]
- 3.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Tang PC, Coady MA, Lovoulos C, Dardik A, Aslan M, Elefteriades JA, Tellides G. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation. 2005;112:1098–1105. doi: 10.1161/CIRCULATIONAHA.104.511717. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 6.Albornoz G, Coady MA, Roberts M, Davies RR, Tranquilli M, Rizzo JA, Elefteriades JA. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–1405. doi: 10.1016/j.athoracsur.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 7.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 8.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 9.McCallum HM. Experimental Lathyrism In Mice. J Pathol Bacteriol. 1965;89:625–636. doi: 10.1002/path.1700890223. [DOI] [PubMed] [Google Scholar]
- 10.Behmoaras J, Slove S, Seve S, Vranckx R, Sommer P, Jacob MP. Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation research. 2008;11:883–889. doi: 10.1089/rej.2008.0760. [DOI] [PubMed] [Google Scholar]
- 11.Davies I, Schofield JD. Connective tissue ageing: the influence of a lathyrogen (beta-aminopropionitrile) on the life span of female C57BL/Icrfat mice. Exp Gerontol. 1980;15:487–494. doi: 10.1016/0531-5565(80)90057-1. [DOI] [PubMed] [Google Scholar]
- 12.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 13.Coutard M. Experimental cerebral aneurysms in the female heterozygous Blotchy mouse. Int J Exp Pathol. 1999;80:357–367. doi: 10.1046/j.1365-2613.1999.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gletsu N, Doan TN, Cole J, Sutliff RL, Bernstein KE. Angiotensin II-induced hypertension in mice caused an increase in insulin secretion. Vascul Pharmacol. 2005;42:83–92. doi: 10.1016/j.vph.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Weiss D, Taylor WR. Deoxycorticosterone acetate salt hypertension in apolipoprotein E-/- mice results in accelerated atherosclerosis: the role of angiotensin II. Hypertension. 2008;51:218–224. doi: 10.1161/HYPERTENSIONAHA.107.095885. [DOI] [PubMed] [Google Scholar]
- 16.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Akutsu K, Tamori Y, Sakamoto S, Yoshimuta T, Hashimoto H, Takeshita S. Differences in atherosclerotic profiles between patients with thoracic and abdominal aortic aneurysms. Am J Cardiol. 2008;101:696–699. doi: 10.1016/j.amjcard.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 19.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JA, Berliner JA, Nadler JL. Angiotensin II increases monocyte binding to endothelial cells. Biochem Biophys Res Commun. 1996;226:862–868. doi: 10.1006/bbrc.1996.1441. [DOI] [PubMed] [Google Scholar]
- 21.Yanagitani Y, Rakugi H, Okamura A, Moriguchi K, Takiuchi S, Ohishi M, Suzuki K, Higaki J, Ogihara T. Angiotensin II type 1 receptor-mediated peroxide production in human macrophages. Hypertension. 1999;33:335–339. doi: 10.1161/01.hyp.33.1.335. [DOI] [PubMed] [Google Scholar]
- 22.Keidar S. Angiotensin, LDL peroxidation and atherosclerosis. Life Sci. 1998;63:1–11. doi: 10.1016/s0024-3205(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor -/- mice. Ann N Y Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 25.Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, Spinale FG. A murine model of thoracic aortic aneurysms. J Surg Res. 2003;115:157–163. doi: 10.1016/s0022-4804(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 26.Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:339–352. doi: 10.1196/annals.1383.013. [DOI] [PubMed] [Google Scholar]
- 27.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 30.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 31.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 32.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Angiotensin II Infusion Promotes Abdominal Aortic Aneurysms Independent of Increased Blood Pressure in Hypercholesterolemic Mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


