Abstract
The neurochemical profile of the striatum of R6/2 Huntington's disease mice was examined at different stages of pathogenesis using in vivo 1H NMR spectroscopy at 9.4 T. Between 8 and 12 weeks, R6/2 mice exhibited distinct changes in a set of 17 quantifiable metabolites compared with littermate controls. Concentrations of creatine, glycerophosphorylcholine, glutamine and glutathione increased and N-acetylaspartate decreased at 8 weeks. By 12 weeks, concentrations of phosphocreatine, taurine, ascorbate, glutamate, and myo-inositol increased and phophorylethanolamine decreased. These metabolic changes probably reflected multiple processes, including compensatory processes to maintain homeostasis, active at different stages in the development of HD. The observed changes in concentrations suggested impairment of neurotransmission, neuronal integrity and energy demand, and increased membrane breakdown, gliosis, and osmotic and oxidative stress. Comparisons between metabolite concentrations from individual animals clearly distinguished HD transgenics from non-diseased littermates and identified possible markers of disease progression. Metabolic changes in R6/2 striata were distinctly different from those observed previously in the quinolinic acid and 3NP models of HD. Longitudinal monitoring of changes in these metabolites may provide quantifiable measures of disease progression and treatment effects in both mouse models of HD and patients.
Keywords: In vivo 1H NMR spectroscopy, quantification, neurochemical profile, Huntington's disease, transgenic mice
Huntington's disease (HD) is characterized initially by selective degeneration of medium spiny GABAergic projection neurons in the neostriatum (Vonsattel et al. 1985), and involves progressive motor, cognitive, and psychiatric dysfunction (Huntington 1872; Nance 1998). Huntington's disease is caused by an expansion of the CAG repeat region beyond 37 trinucleotides with age of onset inversely proportional to repeat length (Huntington's Disease Collaborative Research Group. 1993; Duyao et al. 1993). The precise mechanism of HD pathophysiology is unknown, although substantial evidence exists for multiple degenerative pathways, including mitochondrial impairment, oxidative stress, caspase activation and apoptosis, impaired gene transcription, and altered protein activity and interactions (Landles and Bates 2004; Li and Li 2004; Beal 2005). Critical insights into the pathophysiology of HD can be derived from direct longitudinal studies of the CNS in pre-symptomatic and affected HD patients (Rosenblatt et al. 2003; Aylward et al. 2004). However, because of the inherent difficulties in accessing human CNS tissue, biochemical studies of HD have been performed on postmortem specimens, non-CNS tissues, such as lymphoblasts or muscle, or extracellular sources, such as serum or cerebrospinal fluid (Dunlop et al. 1992; Nicoli et al. 1993; Sawa et al. 1999; Lodi et al. 2000; Garseth et al. 2000).
In vivo 1H nuclear magnetic resonance (NMR) spectroscopy is a unique method for non-invasive quantification of tissue metabolites. It has been used to examine neurochemical changes in multiple neurological disorders, including epilepsy, brain tumors, multiple sclerosis, stroke, and Parkinson's and Alzheimer's disease (Bonavita et al. 1999). Previous 1H NMR spectroscopic studies in HD patients have reported changes in signal intensity ratios of four brain metabolites: N-acetylaspartate (NAA), total creatine (tCr), choline-containing compounds (Cho), and lactate (Jenkins et al. 1993; Koroshetz et al. 1997; Harms et al. 1997; Jenkins et al. 1998). In majority of studies, the tCr signal was used as an internal reference. However, reported changes in tCr levels (Sanchez-Pernaute et al. 1999) make interpretation of such ratios difficult.
Development of a number of mouse models of HD have provided rich resources for studying disease progression (Levine et al. 2004). Long echo-time in vivo 1H NMR spectroscopy has been used to monitor the decrease of NAA/tCr signal ratio during disease progression in several different genetic mouse models of HD (Jenkins et al. 2000; van Dellen et al. 2000; Jenkins et al. 2005). In addition, changes in signal intensity of Cho, taurine (Tau), and the sum of glutamine and glutamate (Glx) relative to tCr were reported for 12-week-old R6/2 and N171-82Q HD mice (Jenkins et al. 2005). In vitro 1H NMR spectroscopy of brain tissue extracts (Jenkins et al. 2000) and magic angle spinning 1H NMR spectroscopy of ex vivo brain samples (Tsang et al. 2006) were used to study neurochemical changes in multiple brain metabolites.
Recent technical and methodological developments in high-field in vivo NMR spectroscopy have dramatically increased the neurochemical information content achievable from in vivo 1H NMR spectra. Concentrations of up to 18 brain metabolites (termed a neurochemical profile) can be reliably quantified from the ultra-short echo-time 1H NMR spectra acquired at 9.4 T (Pfeuffer et al. 1999; Tkac et al. 2003, 2004). This approach was used to reveal alterations in the neurochemical profile of quinolinic acid-lesioned rat striatum (Tkac et al. 2001). However, this acute excitotoxic model of HD cannot address specific mechanisms of polyglutamine pathophysiology. Therefore, we decided to study a well established genetic model of HD using high performance in vivo 1H NMR spectroscopy at 9.4 T. The aim of the present study was to characterize the neurochemical profile of the striatum of R6/2 mice (Mangiarini et al. 1996) and monitor its changes at two time points during disease progression.
Methods
R6/2 Mice
Animal breeding and experiments were performed according to procedures approved by the University of Minnesota Institutional Animal Care and Use Committee. R6/2 founders were purchased from the Jackson Laboratories (Bar Harbor, ME, USA) and bred by crossing transgenic males and wild type (WT) females at 5 weeks of age. Offspring were genotyped according to established procedures (Mangiarini et al. 1996)and the Jackson Laboratory). In brief, DNA was purified from blood derived from the tail vein of recently weaned mice using standard practices (Gentra). PCR of tail DNA was performed according to protocols provided by the Jackson Laboratory using oligonucleotide primers OIMR494 (CGGCTGAGGCAGCAGCGGCTGT) and OIMR495 (GCAGCAGCAGCAGCAACAGCCGCCACCGCC) (University of Minnesota Microchemical Facility) in AM buffer, 10% DMSO, 200 mmol/L dNTP's, 10 ng/mL primers with 0.5 U/mL Taq polymerase (Boehringer Mannheim, Gaithersburg, MD, USA). Cycling conditions were 90″ @ 94°C, 35 cycles @ (30″ @ 94°C, 30″ @ 65°C, 90″ @ 72°C), 10′ @ 72°C. Reaction products were analyzed on a 3% agarose gel (Bio-Rad, Hercules, CA, USA) run at 150 V for 45 min. Transgene detection was facilitated using 100 bp oligonucleotide ladder (Bio-Rad) and illumination by GelStar (Cambrex Bio Science, Baltimore, MD, USA).
Animal preparation
Anesthesia was induced and maintained by flowing a gas mixture (O2 : N2O = 1 : 1) containing 1.5–2.0% of isoflurane through the chamber. For R6/2 mice, isoflurane levels were reduced to 1.25% during the experiment. Spontaneously breathing mice were fixed in a cylindrical chamber and the air temperature surrounding the chamber was maintained at 30°C by warm water circulation. The typical duration of the study for a single animal was approximately 1 h, but never exceeded 90 min. In vivo 1H NMR spectra were collected from 8 (n = 7) and 12 (n = 6) week-old R6/2 mice and from 8 (n = 8) and 12 (n = 9) week-old WT littermate controls.
In vivo 1H NMR spectroscopy
All experiments were performed on a 9.4 T/31 cm magnet (Magnex Scientific, Abingdon, UK) equipped with an 11 cm gradient coil insert (300 mT/m, 500 μs) and strong custom-designed second-order shim coils (Magnex Scientific, Abingdon, UK) (Tkac et al. 2004). The magnet was interfaced to a Varian INOVA console (Varian, Inc., Palo Alto, CA, USA). A quadrature surface RF coil with two geometrically decoupled single-turn coils (of 14 mm diameter) was used for both RF transmission and reception. All firstand second-order shim terms were automatically adjusted using FASTMAP with EPI readout (Gruetter 1993; Gruetter and Tkac 2000). Ultra-short echo-time STEAM (echo time TE = 2 ms, middle period TM = 20 ms, repetition time TR = 5 s, number of transients NT = 160) combined with outer volume suppression and VAPOR water suppression was used for localization (Tkac et al. 1999, 2004). Data were saved as arrays of FIDs (four transients per FID), corrected for the frequency drift, summed, and finally corrected for residual eddy current effects using the reference water signal. The position of the volume of interest (VOI) was selected based on multi-slice RARE images. VOIs were centered in the left striatum at the level of the anterior commissure. The size of the VOI (7–12 μL) was adjusted to fit the anatomical structure of striatum, exclude the lateral ventricle, and to minimize partial volume effects.
Quantification and statistics
In vivo 1H NMR spectra were analyzed using LCModel (Provencher 1993). The unsuppressed water signal measured from the same VOI was used as an internal reference for the quantification, assuming 80% brain water content. The LCModel analysis calculates the best fit to the experimental spectrum as a linear combination of model, solution spectra of brain metabolites. The following 19 metabolites were included in the basis set: alanine (Ala), ascorbate (Asc), aspartate, creatine (Cr), γ-aminobutyric acid (GABA), glucose (Glc), glutamate (Glu), glutamine (Gln), glutathione (GSH), glycerophosphorylcholine (GPC), myo-inositol (myo-Ins), lactate (Lac), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocreatine (PCr), phosphorylcholine (PCho), phosphorylethanolamine (PE), scyllo-inositol, and taurine (Tau). In addition, the spectrum of fast relaxing macromolecules, experimentally measured in the mouse brain with an inversion recovery experiment using a short repetition time (TR = 2.0 s, inversion time = 0.675 s), was included in the basis set as previously described (Pfeuffer et al. 1999; Tkac et al. 2004). Most of metabolites were quantified with Cramer-Rao lower bounds (CRLB) of 3–35%, corresponding to estimated errors in metabolite concentrations of 0.3–0.5 μmol/g. Aspartate and scyllo-inositol were quantified with CRLB > 35% in more than 50% of cases and therefore were eliminated from further analysis. Also Ala data from 12-week-old R6/2 mice were not used for further evaluation because of high CRLB. Because of the consistent quantification of GPC and PCho with CRLB below 35% from all individual animals, their concentrations were reported separately despite negative correlations between them in the LCModel fitting algorithm (r = −0.82).
Two different statistical procedures were applied to identify changes in metabolite levels. Only comparisons identified as significantly different in both tests were reported. Initially, two-tailed t-tests were used to compare metabolite concentrations from striata of WT and R6/2 mice of the same age and to compare metabolite concentrations of the same strains measured at 8 and 12 weeks. To correct for multiple comparisons, p-value thresholds p < 0.01 (*), p < 0.002 (**), and p < 0.0002 (***) were used to determine the statistical significance. In addition, p-values for metabolite changes were ranked and evaluated using a false discovery rate procedure (Benjamini and Hochberg 1995). The false discovery rate was set so that only one of fifty identified metabolite changes might be erroneous (q = 0.02). Analysis of the effects of age and genotype on the summed concentrations (Gln + Glu, Cr + PCr, GPC + PCho) and concentration ratios (Glu/Gln, PCr/Cr, GPC/PCho) was performed by two-way analysis of variance followed by post hoc tests with Bonferroni correction (two-way anova, Graphpad Prism, 4.01). For these additional post-test comparisons, p < 0.05 (*), p < 0.001 (**), p < 0.001 (***) were considered significant.
Results
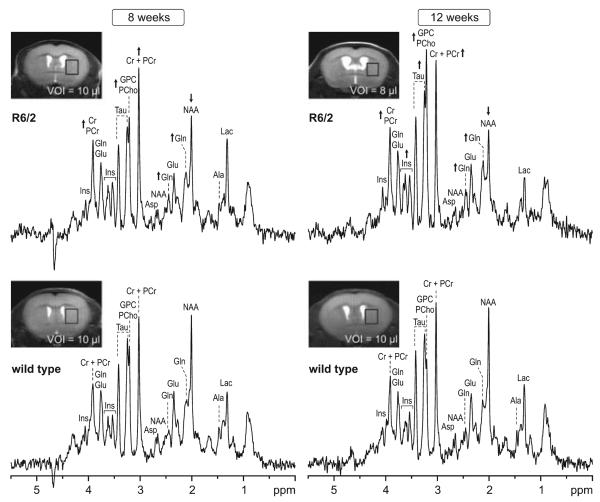
In vivo 1H NMR spectra were acquired from striata of R6/2 and WT mice at 8 and 12 weeks of age (Fig. 1). Corresponding MR images were used to position VOIs in left striatum (Fig. 1 insets). The VOI size was adjusted according to brain morphology, taking into account progressive striatal and cortical atrophy and enlarged ventricles of the R6/2 mice. Shimming resulted in water linewidths of 12–14 Hz with concomitant creatine linewidths of 10–12 Hz. Resulting high spectral resolution enabled unambiguous signal assignment. In addition to the commonly resolved signals of NAA, Cr + PCr, and choline-containing compounds (GPC + PCho), resonances of several other brain metabolites, such as Gln, Glu, myo-Ins, and Tau were clearly resolved. Changes between the neurochemical profiles of R6/2 and WT mice were evident from visual inspection of corresponding 1H NMR spectra (Fig. 1). At 8 weeks of age, reductions in NAA and increases in GPC + PCho, Gln, and Cr + PCr were apparent in spectra from R6/2 mice compared with WT controls. At 12 weeks of age, these changes were more pronounced and a considerable increase in the Tau signal was observed.
Fig. 1.
Representative in vivo 1H NMR spectra measured from striata of Huntington's disease transgenic R6/2 and wild type mice at 8 and 12 weeks of age. Insets: MR images with the selected volume of interest centered rostrocaudally at the level of the anterior commissure. Arrows indicate changes of a specific metabolite in R6/2 mice relative to age matched WT control. STEAM, TE = 2 ms, TR = 5 s, NT = 160. Processing: Gaussian multiplication (σ = 0.085 s), FT and zero-order phase correction. No water signal removal or baseline correction was applied.
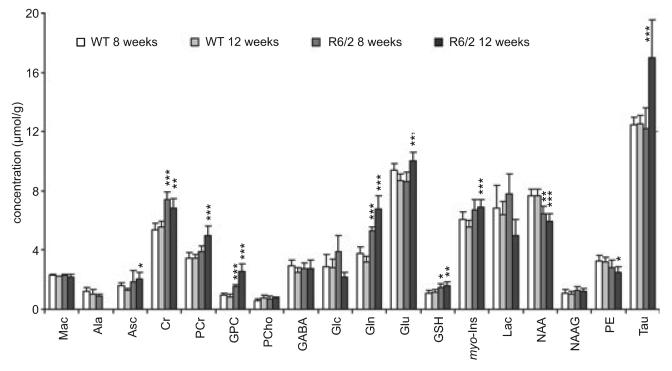
Neurochemical profiles composed of seventeen brain metabolites were reliably quantified using LCModel fitting analysis from each spectrum. To examine metabolite changes associated with HD, neurochemical profiles of R6/2 mice and WT controls measured at 8 and 12 weeks were compared (Fig. 2). At 8 weeks of age, the most striking changes associated with HD were increases in concentrations of Cr (+38%), Gln (+41%), and GPC (+60%) (Fig. 2, Table 1). Also notable was an increase in GSH (+36%) and a decrease in NAA levels (−16%). At 12 weeks of age, concentrations Gln and GPC continued to increase (+113% and +206%, respectively), Cr and GSH remained elevated and NAA decreased slightly (−22%). Additional concentration changes were observed in six more metabolites: increases in Asc, PCr, Glu, myo-Ins, Tau and a decrease in PE (Fig. 2, Table 1). An increase in Tau (36%) was the most noticeable change at 12 weeks (Figs 1 and 2). Ala, readily detected in WT controls, was below the detection threshold in the 12 week old R6/2 mice, suggesting a decrease in Ala concentration.
Fig. 2.
Metabolite concentrations quantified from striata of wild type and R6/2 mice at 8 and 12 weeks of age. Statistically significant differences are noted for comparisons between metabolite concentrations of R6/2 and wild type mice at each age. For comparisons between ages within each strain, see Table 1. Data represent mean ± SD, n = 6–9. Significance level: *p < 0.01, **p < 0.002, ***p < 0.0002.
Table 1.
Relative changes in striatal metabolite concentrations between WT and R6/2 mice at 8 and 12 weeks of age and between different ages of the same strain
| % Change in R6/2 relative to WT |
% Change at 12 weeks relative to 8 weeks |
|||
|---|---|---|---|---|
| Metabolite | At 8 weeks |
At 12 weeks |
WT | R6/2 |
| Mac | 1 | −2 | −3 | −6 |
| Ala | −25 | −15 | ||
| Asc | 16 | 62* | −21 | 10 |
| Cr | 38*** | 23** | 3 | −8 |
| PCr | 12 | 43*** | 0 | 28* |
| GPC | 60*** | 206*** | −13 | 66** |
| PCho | 19 | −3 | 33 | 8 |
| GABA | −7 | 10 | −15 | 0 |
| Glc | 33 | −23 | −2 | −44* |
| Gln | 41*** | 113*** | −15 | 28** |
| Glu | −9 | 16** | −8 | 17** |
| GSH | 36* | 39** | 8 | 10 |
| myo-Ins | 11 | 25*** | −8 | 3 |
| Lac | 14 | −23 | −6 | −36* |
| NAA | −16** | −22*** | 0 | −8 |
| NAAG | 16 | 13 | −6 | −8 |
| PE | −15 | −22* | −3 | −11 |
| Tau | −2 | 36*** | 1 | 39** |
Statistical comparisons were made using absolute metabolite concentrations (Fig. 2) and are repeated here for convenience. Significance level:
p < 0.01
p < 0.002
p < 0.0002.
Significant age-related changes in the neurochemical profile of WT mice were not found (Fig. 2, Table 1). A decrease in Glu concentration should be viewed only as a trend, because it did not pass both the statistical tests (Table 1). In the R6/2 mice, progressive increases in concentration of PCr, Gln, Glu, Tau and GPC were observed at 12 weeks relative to 8-week-old mice. Significant decreases in concentrations of Glc and Lac between 8 and 12 weeks were observed in R6/2 mice (Fig. 2, Table 1).
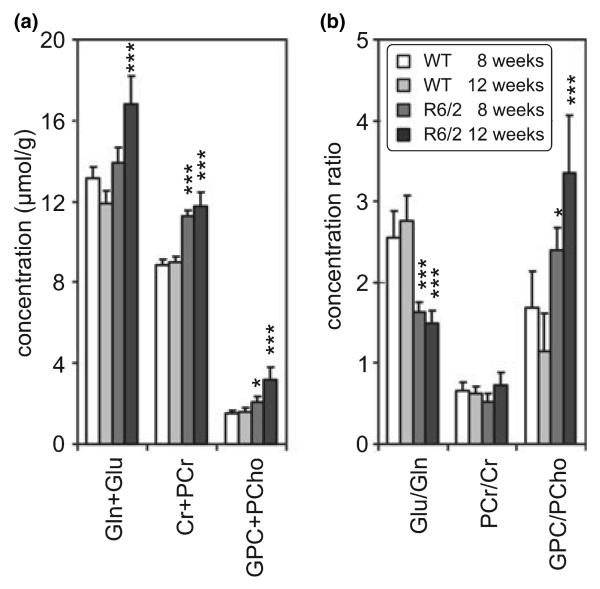
To facilitate comparison of these data with previously published spectral analyses reporting relative signal intensities of total Cr, choline-containing compounds and combined glutamate and glutamine (Glx), the sums Cr + PCr, GPC + PCho and Gln + Glu and the concentration ratios, PCr/Cr, GPC/PCho and Glu/Gln, were calculated (Fig. 3). Concentration of Cr + PCr was highly elevated in striatum of R6/2 mice at both ages compared with WT controls (Fig. 3a). However, the PCr/Cr ratio (Fig. 3b) changed between 8 and 12 weeks of age in the R6/2 mice, although neither of these differed significantly with respect to WT controls. Concentration of Gln + Glu did not change between R6/2 and WT mice at 8 weeks, but rose significantly at 12 weeks in R6/2 mice (Fig. 3a). At 8 and 12 weeks of age, the Glu/Gln ratio was significantly decreased (by −36% and −0.46%, respectively) in transgenics relative to controls (Fig. 3b). Both sum GPC + PCho and GPC/PCho ratio increased in striatum of R6/2 mice when compared with WT controls, reflecting the progressive increases in GPC (Fig. 2).
Fig. 3.
Summed concentrations (a) and concentration ratios (b) of selected pairs of metabolites (Gln and Glu, PCr and Cr, GPC and PCho) that are difficult to resolve at lower magnetic field strengths (mean ± SD). Metabolites quantified from striata of R6/2 and wild type mice at 8 and 12 weeks of age. Statistically significant differences are shown for comparisons between R6/2 and wild type mice at each age. Significance level: *p < 0.05, **p < 0.01, ***p < 0.001. Additionally, significant differences between 8 and 12-week-old R6/2 mice were found for Gln + Glu (p < 0.001), GPC + PCho (p < 0.001), PCr/Cr (p < 0.01) and GPC/PCho (p < 0.001).
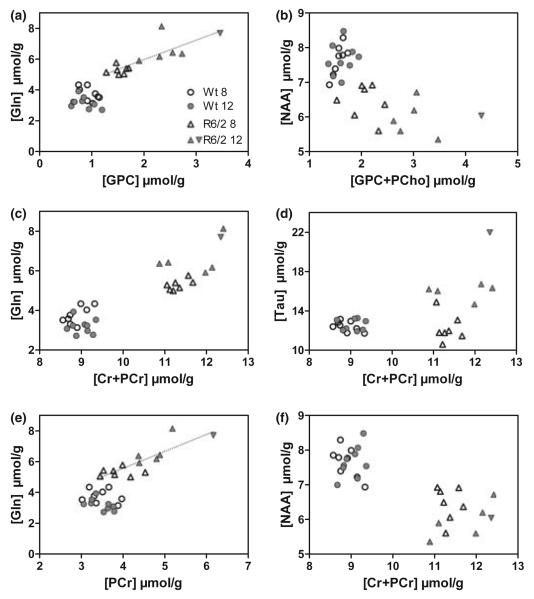
To visualize metabolite concentrations quantified from individual mice and to investigate the relationship between them, pairs of metabolites that changed the most were selected for scatter plots (Fig. 4). While all six scatter plots discriminate HD mice from WT controls at both ages, separation between those two groups was most evident for pairs of Gln versus Cr + PCr (Fig. 4c) and NAA versus Cr + PCr (Fig. 4f). In addition, clusters of data from 12 and 8 week-old R6/2 mice were distinguishable in all scatter plots (Figs. 4a–e) except the NAA versus Cr + PCr plot (Fig. 4f). In the R6/2, concentrations of GPC, Gln, PCr and Tau appear to increase progressively with age. Gln concentrations correlated with GPC (Fig. 4a, r = 0.81, p = 0.0008), PCr (Fig. 4e, r = 0.84, p = 0.0003) and Tau (not shown, r = 0.82, p = 0.0006). Variations in metabolite levels of Gln, GPC, PCr, and Tau were greater in the older, more severely effected R6/2 mice, suggesting differences in disease progression among individual animals. Indeed, the data points with the highest values of GPC, Tau, PCr, and second highest Gln value came from the same animal (Fig. 4, upside-down triangle).
Fig. 4.
Scatter plots of selected metabolite concentrations quantified from individual mice distinguished between R6/2 and wild type at 8 and 12 weeks of age. For the R6/2 data, dotted lines represent correlation between Gln and GPC (a; r = 0.81, p = 0.0008), Gln and PCr (e; r = 0.84, p = 0.0003), Gln and Tau (not shown, r = 0.82, p = 0.0006). To illustrate the ability to track individual animals, values from one particular R6/2 mouse are highlighted with an inverted, filled triangle.
Discussion
These data represent the first non-invasive, longitudinal measurements of absolute metabolite concentrations from a specific brain region in a transgenic mouse model of Huntington's disease. Striatal neurochemical profiles of 17 metabolites, were repeatedly measured from the same animals as disease progressed. This set of metabolites was similar to the previously reported in vitro studies of R6/2 mice using tissue extracts (Jenkins et al. 2000) or tissue samples (Tsang et al. 2006). In addition, in vivo measurements eliminated undesirable catabolic processes that occur during ex vivo sample handling and enabled detection of labile compounds, e.g., PCr. Precise metabolite quantification and high reproducibility resulted in identification of disease progression among individual animals and clearly distinguished HD transgenics from non-diseased littermates. Key methodological factors enabling precise metabolite quantification included: (i) increased sensitivity and chemical shift dispersion at 9.4 T; (ii) efficient automatic adjustment of magnetic field homogeneity (shimming); (iii) a pulse sequence providing high localization performance; (iv) minimization of T2 relaxation and J-evolution with ultra-short TE; and (v) LCmodel analysis that included the macromolecule spectrum in the basis set and the water signal as an internal reference. The resulting high-resolution spectra reliably quantified individual metabolites with similar spectral patterns, such as Gln and Glu, PCr and Cr or GPC and PCho.
By comparing striatal neurochemical profiles of HD transgenic and WT mice a pattern of disease-related changes in metabolic homeostasis at different ages became evident. At 8 weeks of age, metabolic changes reflected the mid-course disease phenotype. Increased concentrations of Gln, GPC, and Cr + PCr and decreased NAA and the Glu/Gln ratio clearly discriminated individual HD transgenic R6/2 mice from WT littermate controls. Surprisingly, the characteristic changes in Cr, Cr + PCr, GSH, NAA and Glu/Gln observed at 8 weeks were not further progressive with increasing age. By 12 weeks, as these animals approached the end of life (Mangiarini et al. 1996), additional metabolic changes characterized this later disease state. Concentrations of metabolites such as Tau, Lac and Glu only changed late in the animal's lifespan, potentially identifying a more advanced disease state. Concentrations of Gln, GPC and PCr, as well as the ratio GPC/PCho increased progressively with the age of R6/2 mice. Positive correlations between concentrations of Gln and PCr, GPC or Tau suggested a link between observed changes of these metabolites and the progress of the disease in individual HD transgenic mice. Changes observed at 12 weeks in metabolites, such as PCr, Asc, PE and myo-Ins, might have begun earlier, but failed to reach significance by our strict statistical criteria. Thus, we have identified metabolic markers that may characterize different stages of disease in this mouse model of HD.
Among individual R6/2 mice, concentrations of Gln correlated with those of GPC, PCr and Tau, suggesting parallel changes of the underlying mechanisms. As the R6/2 mice became older, the metabolite concentration ranges became broader and farther away from normative values. The scatter of points among mice of similar ages may indicate that disease progression was not identical among animals. While we have no independent measures of disease stage in these animals, we suggest that the concentration changes of these metabolites may be useful in monitoring disease progression. A multiparameter approach to follow metabolite changes may prove useful in monitoring disease progression, especially with respect to therapeutic trials. Indeed, characteristic changes in absolute brain metabolite concentrations have been reported for humans with adrenoleukodystrophy examined at 4 T (Oz et al. 2005).
A significant increase in Cr + PCr, typically reported as tCr, was observed at both ages. As tCr is typically used in reporting ratios of other metabolites, care must be taken in interpreting this type of data (Jenkins et al. 1993; Taylor-Robinson et al. 1996; Jenkins et al. 1998; Hoang et al. 1998; Jenkins et al. 2000, 2005). The increased Cr + PCr at 8 week was the result of Cr alone, and not PCr. Considering the homeostatic need to maintain normal PCr levels and the reported decrease of creatine kinase mRNA in R6/2 striatum (Luthi-Carter et al. 2002), an up-regulation of Cr may occur. An increase in kinetic parameters for the creatine kinase reaction has been reported in the 3NP model of HD (Kasparova et al. 2005). Decreased respiration and ATP production would also contribute to an increased demand for total creatine (Seong et al. 2005; Milakovic and Johnson 2005; Puranam et al. 2006). A similar compensatory homeostatic response was observed in the increase in the antioxidants, Asc and GSH probably in response to the reported oxidative stress in R6/2 (Tabrizi et al. 2000).
At 12 weeks, the pattern of metabolite changes was not simply an exaggeration of those seen at 8 weeks, as might be expected for a single mechanism mediating an increasingly progressive disease. Between 8 and 12 week, PCr levels and PCr/Cr ratio in the R6/2 striatum increased while Cr + PCr remained elevated. As disease phenotype continuously worsens with age in R6/2, as typified by the continuously increasing Gln, Tau and GPC concentrations, the increase in PCr/Cr ratio may represent a decrease in striatal energy demands rather than an improved energetic status or mitochondrial function. Indeed, striatal glucose utilization decreases between 8 and 12 weeks in R6/2 animals (Wang et al. 2005). Progressive tissue atrophy, as suggested by the elevated GPC, a marker of membrane breakdown and altered dendritic morphology, neuronal excitability and neurotrans-mission (Nitsch et al. 1992; Klapstein et al. 2001), may mediate the decreased energy demand. Similarly, PCr increased relative to Cr in chronically hypoxic rats or insulin hypoglycemia, conditions expected to decrease energy consumption (Thurston et al. 1977; Raman et al. 2005).
Different disease-related processes may be responsible for the changes in Glu and Gln observed at the two time points as well. At 8 weeks, the increased Gln may represent an imbalance in the Glu–Gln cycling through neurons and astrocytes reflecting compromised glutamatergic neurotrans-mission. Indeed, highly significant decreases in Glu/Gln ratio was observed at 8 weeks despite the unchanged sum of Gln and Glu. The increase in Gln steady-state concentration occurred despite the abnormal astrocytic glutamate uptake following down-regulation of glutamate transporters (Lievens et al. 2001; Behrens et al. 2002). The source of the increased Gln was not readily apparent, as both glutamine synthase and phosphate activated glutaminase activities are decreased in R6/2 (Butterworth et al. 1985; Lievens et al. 2001). Net Gln synthesis could not account for the continuous Gln increase, as the sum, Glu + Gln, was not increased at this time. While the excess polyglutamines incorporated in the Huntingtin protein or protein fragments would not be expected to contribute to the 1H NMR signal, an increase in soluble glutamine monomers as a consequence of Huntington degradation remains a remote possibility. At 12 weeks, Gln + Glu increased significantly. Astrocyte proliferation accompanies HD and could account for the increased Gln and myo-Ins, which are predominantly found in astrocytes (Norenberg and Martinez-Hernandez 1979; Brand et al. 1993). However, this is only locally active in R6/2 mice (Davies et al. 1999; Turmaine et al. 2000). The increased Glu could also reflect its accumulation as an osmolyte at this stage in the disease process. Indeed, other osmolytes, such as Tau and myo-Ins, are also prominently elevated at 12 weeks.
Tau is elevated in the putamen of HD patients (Gramsbergen et al. 1986) and in the cortex and striatum of aged R6/2 mice (Jenkins et al. 2000, 2005). We also found a 36% elevation in taurine in 12, but not 8-week-old R6/2 mice. Tau has been assigned numerous functions in the CNS, including neuromodulator, neurotransmitter, osmolyte and neuroprotectant (Foos and Wu 2002). Appreciable amounts of Tau are released from astrocytes in response to hyposmolarity, hypoxia, ischemia, oxidative stress, and metabolic toxins (Reymond et al. 1996; Saransaari and Oja 2000; Foos and Wu 2002). Strikingly, the increased Tau, Gln, Glu, PCr, total Cr, GPC and myo-Ins and decreased Lac observed in R6/2 mice at 12 weeks resemble the pattern of changes in these metabolites observed by both analytical methods and ex vivo 1H NMR spectroscopy following hypernatremia (Thurston et al. 1983; Heilig et al. 1989). Hypernatremia could result from insufficient energy to power the Na–K–ATPase to maintain intracellular ion concentrations or the reported decrease in Na–K–ATPase mRNA in R6/2 mice (Luthi-Carter et al. 2002).
The decrease in NAA concentration and subsequent increase in Tau concentration verify that conclusions reached in earlier HD studies, reporting signal ratios relative to tCr signal, indeed reflected decreases in NAA and increases in Tau (Jenkins et al. 2000, 2005). However, the reported 50–60% decrease of NAA/tCr (Jenkins et al. 2000, 2005) is attributable to a 16–22% drop in NAA combined with a 27–31% increase in Cr + PCr demonstrated here. The decreased levels of NAA in R6/2 mice may reflect mitochondrial impairment (Bates et al. 1996), decreased neuronal integrity or neuronal dysfunction as consistently observed in neurological diseases (Demougeot et al. 2004).
The largest percent change in a metabolite was the increase in GPC at 12 weeks. Increases in GPC levels in cortical gray matter measured analytically from severely demented Alzheimers disease brains have been attributed to membrane breakdown (Nitsch et al. 1992). However, this same study did not find increases in GPC in human Huntington's disease cortical gray matter. Alternatively, elevated GPC levels could result from inactivation of its catabolism. GPC cholinephosphodiesterase, an enzyme that catalyzes conversion of GPC into glycerol and PCho, undergoes oxidative inactivation (Sok 1998). GPC cholinephosphodiesterase activity, selectively enriched in oligodendrocytes, was decreased in three different rodent models of demyelination (Yuan et al. 1992). Oxidative damage is characteristic of R6/2 brains (Tabrizi et al. 2000) and white matter damage occurs in HD (Ciarmiello et al. 2006). Thus, the possibility exists that the increase in GPC but not PCho in R6/2 striatum may reflect metabolic changes in white matter tracts that traverse this region.
The pattern of metabolite changes observed in the R6/2 mouse contrast with those observed in two other models of HD. The transgenic R6/2 mouse overexpresses an N-terminal fragment of human Huntingtin with 150 or more CAG repeats and is considered to model juvenile onset HD, the severest form of the disease. quinolinic acid-induced excitotoxicity has been proposed as a model of the final steps in the cell death pathway in HD (Beal et al. 1986). Chronic exposure to the complex II inhibitor, 3NP, has been used to mimic the metabolic insufficiency proposed to accompany HD (Brouillet et al. 1993). In normal rats and mice, basal levels of metabolites detectable by 1H NMR spectrosocpy are comparable, making comparisons of these disease models plausible (Pfeuffer et al. 1999; Tkac et al. 2004). While NAA decreases in all three models, other metabolites do not change in similar manner. In both QA-treated rats and R6/2 mice at 8 weeks, Gln increased and Glu decreased. Other metabolite changes, however, are not similar. By 12 weeks in R6/2, Glu concentration increased. In R6/2, Tau, GSH and Cr + PCr increased, while in the QA-treated rats, these metabolites decreased (Tkac et al. 2001). Lac, GPC + PCho, myo-Ins, and PE remained unchanged in QA-treated rats despite changes in R6/2 (Tkac et al. 2001). Total Cr and choline-containing compounds also decreased in the striatum of the 3NP-treated baboon (Dautry et al. 1999). These comparisons suggest that acute, relatively synchronous excitotoxic cell death produces a metabolic pattern quite distinct from that observed in the transgenic animal where cell death, if present, will affect only a small proportion of neurons at any one time. The distinct metabolite profile of the R6/2 mouse indicated that if this is a faithful model of HD, the neurodegenerative mechanisms may be more complex than either TCA and electron transport impairment or excitotoxicty. Thus, the metabolic changes observed in R6/2 may reflect the ongoing balance between multiple processes: initial compensatory changes, secondary consequences, and in later stages, the denouement to cellular dysfunction and death.
Acknowledgements
We thank Dr Jean H. Thurston for insightful discussions and Dorothee Aeppli, Ph.D., Biostatistics Consulting Lab for statistical consultations. This work was supported by NIH F30-MH12157, NIH P41 RR08079, the Keck Foundation, the MIND Institute, the Biomedical Engineering Institute of the University of Minnesota and the Huntington's Disease Society of America.
Abbreviations used
- Ala
alanine
- Asc
ascorbate
- Cho
choline-containing compounds
- Cr
creatine
- CRLB
Cramer-Rao lower bounds
- FID
free induction decay
- GABA
γ-aminobutyric acid
- Glc
glucose
- Gln
glutamine
- Glu
glutamate
- Glx
the sum of glutamine and glutamate
- GPC
glycerophosphorylcholine
- GSH
glutathione
- HD
Huntington's disease
- myo-Ins
myo-inositol
- Lac
lactate
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- NT
number of transients
- PCho
phosphorylcholine
- PCr
phosphocreatine
- PE
phosphorylethanolamine
- Tau
taurine
- tCr
total creatine
- TE
echo time
- TR
repetition time
- VOI
volume of interest
- WT
wild type
References
- Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neuro-degeneration. Ann. Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125:1908–1922. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Roy. Stat. Soc. B. 1995;1:289–300. [Google Scholar]
- Bonavita S, Di Salle F, Tedeschi G. Proton MRS in neurological disorders. Eur. J. Radiol. 1999;30:125–131. doi: 10.1016/s0720-048x(99)00051-0. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, Roy DS, Rosen BR, Beal MF. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J. Neurochem. 1993;60:356–359. doi: 10.1111/j.1471-4159.1993.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Butterworth J, Yates CM, Reynolds GP. Distribution of phosphate-activated glutaminase, succinic dehydrogenase, pyruvate dehydrogenase and gamma-glutamyl transpeptidase in postmortem brain from Huntington's disease and agonal cases. J. Neurol. Sci. 1985;67:161–171. doi: 10.1016/0022-510x(85)90112-1. [DOI] [PubMed] [Google Scholar]
- Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington's disease. J. Nucl. Med. 2006;47:215–222. [PubMed] [Google Scholar]
- Dautry C, Conde F, Brouillet E, Mittoux V, Beal MF, Bloch G, Hantraye P. Serial 1H NMR spectroscopy study of metabolic impairment in primates chronically treated with the succinate dehydrogenase inhibitor 3-nitropropionic acid. Neurobiol. Dis. 1999;6:259–268. doi: 10.1006/nbdi.1999.0244. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, Raza AS, Mahal A, Mangiarini L, Bates GP. From neuronal inclusions to neurodegeneration: neuropathological investigation of a transgenic mouse model of Huntington's disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:981–989. doi: 10.1098/rstb.1999.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen A, Welch J, Dixon RM, Cordery P, York D, Styles P, Blakemore C, Hannan AJ. N-Acetylaspartate and DARPP-32 levels decrease in the corpus striatum of Huntington's disease mice. Neuroreport. 2000;11:3751–3757. doi: 10.1097/00001756-200011270-00032. [DOI] [PubMed] [Google Scholar]
- Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. J. Neurochem. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- Dunlop DS, Mc Hale DM, Lajtha A. Decreased brain N-acetylaspartate in Huntington's disease. Brain Res. 1992;580:44–48. doi: 10.1016/0006-8993(92)90925-y. [DOI] [PubMed] [Google Scholar]
- Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat. Genet. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Foos TM, Wu JY. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem. Res. 2002;27:21–26. doi: 10.1023/a:1014890219513. [DOI] [PubMed] [Google Scholar]
- Garseth M, Sonnewald U, White LR, Rod M, Zwart JA, Nygaard O, Aasly J. Proton magnetic resonance spectroscopy of cerebrospinal fluid in neurodegenerative disease: indication of glial energy impairment in Huntington chorea, but not Parkinson disease. J. Neurosci. Res. 2000;60:779–782. doi: 10.1002/1097-4547(20000615)60:6<779::AID-JNR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Veenma-Van der Duin L, Venema K, Korf J. Cerebral cation shifts and amino acids in Huntington's disease. Arch. Neurol. 1986;43:1276–1281. doi: 10.1001/archneur.1986.00520120056016. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Harms L, Meierkord H, Timm G, Pfeiffer L, Ludolph AC. Decreased N-acetyl-aspartate/choline ratio and increased lactate in the frontal lobe of patients with Huntington's disease: a proton magnetic resonance spectroscopy study. J. Neurol. Neurosurg. Psychiatry. 1997;62:27–30. doi: 10.1136/jnnp.62.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig CW, Stromski ME, Blumenfeld JD, Lee JP, Gullans SR. Characterization of the major brain osmolytes that accumulate in salt-loaded rats. Am. J. Physiol. 1989;257:F1108–F1116. doi: 10.1152/ajprenal.1989.257.6.F1108. [DOI] [PubMed] [Google Scholar]
- Hoang TQ, Bluml S, Dubowitz DJ, Moats R, Kopyov O, Jacques D, Ross BD. Quantitative proton-decoupled 31P MRS and 1H MRS in the evaluation of Huntington's and Parkinson's diseases. Neurology. 1998;50:1033–1040. doi: 10.1212/wnl.50.4.1033. [DOI] [PubMed] [Google Scholar]
- Huntington G. On chorea. Adv. Neurol. 1872;1:33–35. [Google Scholar]
- Huntington's Disease Collaborative ResearchGroup A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington's disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Rosas HD, Chen YC, Makabe T, Myers R, MacDonald M, Rosen BR, Beal MF, Koroshetz WJ. 1H NMR spectroscopy studies of Huntington's disease: correlations with CAG repeat numbers. Neurology. 1998;50:1357–1365. doi: 10.1212/wnl.50.5.1357. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Klivenyi P, Kustermann E, Andreassen OA, Ferrante RJ, Rosen BR, Beal MF. Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington's disease mice. J. Neurochem. 2000;74:2108–2119. doi: 10.1046/j.1471-4159.2000.0742108.x. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Andreassen OA, Dedeoglu A, Leavitt B, Hayden M, Borchelt D, Ross CA, Ferrante RJ, Beal MF. Effects of CAG repeat length, HTT protein length and protein context on cerebral metabolism measured using magnetic resonance spectroscopy in transgenic mouse models of Huntington's disease. J Neurochem. 2005;95:553–562. doi: 10.1111/j.1471-4159.2005.03411.x. [DOI] [PubMed] [Google Scholar]
- Kasparova S, Sumbalova Z, Horecky J, Bystricky P, Mlynarik V, Gvozdjakova A, Liptaj T. New magnetic resonance spectroscopy biomarker for monitoring neurodegenerative diseases: animal models. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2005;149:373–376. doi: 10.5507/bp.2005.061. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J Neurophysiol. 2001;86:2667–2677. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington's disease and effects of coenzyme Q10. Ann Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington's disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5:958–963. doi: 10.1038/sj.embor.7400250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Cepeda C, Hickey MA, Fleming SM, Chesselet MF. Genetic mouse models of Huntington's and Parkinson's diseases: illuminating but imperfect. Trends Neurosci. 2004;27:691–697. doi: 10.1016/j.tins.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Li SH, Li XJ. Huntingtin and its role in neuronal degeneration. Neuroscientist. 2004;10:467–475. doi: 10.1177/1073858404266777. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lodi R, Schapira AH, Manners D, Styles P, Wood NW, Taylor DJ, Warner TT. Abnormal in vivo skeletal muscle energy metabolism in Huntington's disease and dentatorubropallidoluysian atrophy. Ann. Neurol. 2000;48:72–76. [PubMed] [Google Scholar]
- Luthi-Carter R, Hanson SA, Strand AD, Bergstrom DA, Chun W, Peters NL, Woods AM, Chan EY, Kooperberg C, Krainc D, Young AB, Tapscott SJ, Olson JM. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum Mol Genet. 2002;11:1911–1926. doi: 10.1093/hmg/11.17.1911. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Milakovic T, Johnson GV. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem. 2005;280:30773–30782. doi: 10.1074/jbc.M504749200. [DOI] [PubMed] [Google Scholar]
- Nance MA. Huntington disease: clinical, genetic, and social aspects. J. Geriatr. Psychiatry Neurol. 1998;11:61–70. doi: 10.1177/089198879801100204. [DOI] [PubMed] [Google Scholar]
- Nicoli F, Vion-Dury J, Maloteaux JM, Delwaide C, Confort-Gouny S, Sciaky M, Cozzone PJ. CSF and serum metabolic profile of patients with Huntington's chorea: a study by high resolution proton NMR spectroscopy and HPLC. Neurosci. Lett. 1993;154:47–51. doi: 10.1016/0304-3940(93)90168-k. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl Acad. Sci. USA. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Oz G, Tkac I, Charnas LR, Choi IY, Bjoraker KJ, Shapiro EG, Gruetter R. Assessment of adrenoleukodystrophy lesions by high field MRS in non-sedated pediatric patients. Neurology. 2005;64:434–441. doi: 10.1212/01.WNL.0000150906.52208.E7. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J. Magn. Reson. 1999;141:104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Puranam KL, Wu G, Strittmatter WJ, Burke JR. Polyglutamine expansion inhibits respiration by increasing reactive oxygen species in isolated mitochondria. Biochem. Biophys. Res. Commun. 2006;341:607–613. doi: 10.1016/j.bbrc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res. Dev. Brain Res. 2005;156:202–209. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Reymond I, Almarghini K, Tappaz M. Immunocytochemical localization of cysteine sulfinate decarboxylase in astrocytes in the cerebellum and hippocampus: a quantitative double immunofluorescence study with glial fibrillary acidic protein and S-100 protein. Neuroscience. 1996;75:619–633. doi: 10.1016/0306-4522(96)00256-4. [DOI] [PubMed] [Google Scholar]
- Rosenblatt A, Abbott MH, Gourley LM, Troncoso JC, Margolis RL, Brandt J, Ross CA. Predictors of neuropatho-logical severity in 100 patients with Huntington's disease. Ann. Neurol. 2003;54:488–493. doi: 10.1002/ana.10691. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Garcia-Segura JM, del Barrio Alba A, Viano J, de Yebenes JG. Clinical correlation of striatal 1H MRS changes in Huntington's disease. Neurology. 1999;53:806–812. doi: 10.1212/wnl.53.4.806. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Taurine and neural cell damage. Amino Acids. 2000;19:509–526. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr., Greenamyre JT, Snyder SH, Ross CA. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nature Med. 1999;5:1194–1198. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- Seong IS, Ivanova E, Lee JM, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum. Mol. Genet. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- Sok DE. Ascorbate-induced oxidative inactivation of Zn2+-glycerophosphocholine cholinephosphodiesterase. J. Neurochem. 1998;70:1167–1174. doi: 10.1046/j.1471-4159.1998.70031167.x. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Workman J, Hart PE, Mangiarini L, Mahal A, Bates G, Cooper JM, Schapira AHV. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann. Neurol. 2000;47:80–86. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson SD, Weeks RA, Bryant DJ, Sargentoni J, Marcus CD, Harding AE, Brooks DJ. Proton magnetic resonance spectroscopy in Huntington's disease: evidence in favour of the glutamate excitotoxic theory. Mov. Disord. 1996;11:167–173. doi: 10.1002/mds.870110209. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Hauhart RE, Dirgo JA, McDougal DB., Jr. Insulin and brain metabolism. Absence of direct action of insulin on K+ and Na+ transport in normal rabbit brain. Diabetes. 1977;26:1117–1119. doi: 10.2337/diab.26.12.1117. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Hauhart RE, Schulz DW. Effect of chronic hypernatremic dehydration and rapid rehydration on brain carbohydrate, energy, and amino acid metabolism in weanling mice. J. Neurochem. 1983;40:240–245. doi: 10.1111/j.1471-4159.1983.tb12677.x. [DOI] [PubMed] [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tkac I, Keene CD, Pfeuffer J, Low WC, Gruetter R. Metabolic changes in quinolinic acid-lesioned rat striatum detected non-invasively by in vivo (1)H NMR spectroscopy. J. Neurosci. Res. 2001;66:891–898. doi: 10.1002/jnr.10112. [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn. Reson. Med. 2003;50:24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- Tkac I, Henry PG, Andersen P, Keene CD, Low WC, Gruetter R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9.4 T. Magn. Reson. Med. 2004;52:478–484. doi: 10.1002/mrm.20184. [DOI] [PubMed] [Google Scholar]
- Tsang TM, Woodman B, McLoughlin GA, Griffin JL, Tabrizi SJ, Bates GP, Holmes E. Metabolic characterization of the R6/2 transgenic mouse model of Huntington's disease by high-resolution MAS 1H NMR spectroscopy. J. Proteome. Res. 2006;5:483–492. doi: 10.1021/pr050244o. [DOI] [PubMed] [Google Scholar]
- Turmaine M, Raza A, Mahal A, Mangiarini L, Bates GP, Davies SW. Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington's disease. Proc. Natl Acad. Sci. USA. 2000;97:8093–8097. doi: 10.1073/pnas.110078997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, Saint-Pierre M, Brownell AL. Cerebral PET imaging and histological evidence of transglutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntington's disease. J Neurol Sci. 2005;231:57–66. doi: 10.1016/j.jns.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Yuan J, McCartney DG, Monge M, Espinosa de Los MA, Zalc B, de Vellis J, Kanfer JN. Glycerophosphorylcholine phosphocholine phosphodiesterase activity in cultured oligodendrocytes, astrocytes, and central nervous tissue of dysmyelinating rodent mutants. J. Neurosci. Res. 1992;31:68–74. doi: 10.1002/jnr.490310110. [DOI] [PubMed] [Google Scholar]