Abstract
Purpose
The EGF receptor (ERBB1) and related family member HER2/neu (ERBB2) are often overexpressed in aggressive breast cancers and their overexpression is correlated with poor prognosis. Clinical studies using ERBB inhibitors have focused on tumor growth effects, but ERBBs can contribute to malignancy independent of their effects on tumor growth. Our studies were designed to evaluate the effect of ERBB inhibition on tumor cell motility and intravasation in vivo using clinically relevant small molecule inhibitors.
Experimental Design
Using in vivo mouse models of breast cancer, we test the effects of ERBB1 and ERBB2 inhibitors AC480 and lapatinib, ERBB1 inhibitor gefitinib, and ERBB2 inhibitor AG825 on in vivo tumor cell invasive properties in mammary fat pad tumors.
Results
ERBB1 and ERBB2 inhibition rapidly (within 3 hours) inhibits both tumor cell motility and intravasation. Using gefitinib, ERBB1 inhibition rapidly inhibits tumor cell motility and invasion but not intravasation, while ERBB2 inhibition by AG825 rapidly blocks intravasation.
Conclusions
ERBB1 and ERBB2 inhibition can rapidly block tumor cell invasive properties. In addition, we differentiate for the first time the contributions of ERBB1 and ERBB2 to the key metastatic properties of in vivo tumor cell invasion and intravasation. These experiments temporally and molecularly separate two key stages in tumor cell entry into blood vessels: invasion and intravasation. These results indicate that ERBB inhibition should be considered for blocking other tumor cell malignant properties besides growth.
Keywords: ERBBs, metastasis, intravital imaging, motility, intravasation
Introduction
Metastatic spread is complex, requiring stromal invasion, intravasation (entry of cells into the vasculature), arrest at a metastatic site, and growth of a metastasis. The development of therapies that target specific steps in the cascade is growing, with the current therapeutic armamentarium focused on inhibiting growth (1). The EGF receptor (ERBB1) and related family member HER2/neu (ERBB2) are often overexpressed in aggressive breast cancers and their overexpression is correlated with poor prognosis (2–4). In addition to their well-characterized contributions to cell proliferation and survival, ERBB1 and ERBB2 also contribute to other characteristics of aggressive tumors such as local invasion and intravasation, potentially independent of their effects on growth (5–7). Important for the optimization of anti-ERBB treatments in cancer is a clear in vivo identification of the specific tumor properties that are dependent on ERBB1 and ERBB2.
The interpretation of studies that utilize stable, long term alteration of ERBB1 or ERBB2 expression is limited by the time (weeks to months) required to produce a tumor or metastasis. During that time, the altered ERBB expression can cause dramatic changes in gene expression within the tumor cells, which may in turn induce changes in the surrounding tumor stroma. The availability of drugs targeted to ERBBs that rapidly act to inhibit ERBB activity provides a novel opportunity to examine cellular processes that are more directly dependent upon ERBB activity. In this manuscript, we make use of ERBB-targeted drugs to rapidly inhibit ERBB function in order to dissect the contributions of ERBB1 and ERBB2 to invasion and intravasation at the primary tumor site. We find that ERBB1 is important for local stromal invasion while ERBB2 is more directly important for intravasation.
Materials and Methods
Cell culture
MTLn3 cells expressing GFP and human ERBB1 were generated (MTLn3E) and propagated as described previously(6). Leibowitz L-15 media supplemented with 0.3%BSA was used as serum-free starvation medium. MDA-MB-231-4173 cells (in vivo selected lung metastatic MDA-MB-231 cells) generously provided by Joan Massague (8) were transduced with a GFP-expressing lentivirus and GFP expressing transductants selected by FACS. MDA-MB-231 cells were cultured in Dulbecco's modified Eagle's medium, high glucose supplemented with 10% FBS. 1R, 5R and Control (pBabe) vectors for downregulation of surface ERBB1 and ERBB2 expression, respectively, were used as described previously (9).
Inhibitors
Gefitinib (Iressa), lapatinib (GW572016), and AC480 (also described as BMS-599626 [Ambit Biosciences]) were kindly provided by AstraZeneca, GlaxoSmithKline, and Bristol Myers Squibb, respectively. AG825 was purchased from Tocris, Inc.
Tumor formation and drug treatment
One million MTLn3E or MDA-MB-231 cells were injected under the second nipple from the rear of 4 to 6-week-old SCID mice. For PyMT tumors, mice carrying the polyoma middle T oncogene under the control of the MMTV promoter and expressing GFP in the mammary gland (10) were used. For all tumors, analysis was performed when tumor diameters were between 1.5 and 2 cm (roughly 35–40 days for MTLn3E or 50–57 days for MDA-MB-231). Mice were treated with carrier alone (0.5% hydroxypropylmethylcellulose, 0.1% Tween 80 for gefitinib or 50% propylene-glycol for AC480 and lapatinib) or carrier containing the inhibitor (100mg/kg). AG825 treatment was administered via IP injection in 10% DMSO at 20mg/kg. To test the effects of drug treatment on cell viability, cells were seeded at low density on 10 cm plates and allowed to attach. To mimic 3 hour treatment by oral gavage, the medium was changed to one containing 10 uM drug or DMSO control for 3 hours and then replaced with fresh medium. Cells were allowed to grow and form colonies for several days and the number of colonies counted.
In vivo imaging
For a detailed protocol see Unit 19.7 of Current Protocols in Cell Biology (11). Mice were treated with carrier or drug 3 hours prior to the start of the imaging session. Multiple fields were imaged for each animal and the number of moving cells per field were counted and compared. For each field, a 30 minute z-stack time-lapse series was collected and analyzed.
In vivo invasion and intravasation
MTLn3E tumor bearing mice were treated via oral gavage with the appropriate carrier compound or drug for 3 hours prior to beginning of the needle collection assay. The in vivo invasion and intravasation assays were performed as described previously (6, 10).
Tumor histology and immunohistochemistry
Sections from formalin fixed paraffin embedded samples were cut and processed for H&E or immunohistochemistry. Serial sections were incubated with either anti-phospho-ERBB1 (Tyr845, Cell Signaling Technology, Beverly, MA), or -phospho-ERBB2 (pNeu -1248, Santa Cruz Biotechnology, CA), and stained using the NBT-BCIP detection method (Vector Laboratories, Ltd., Burlingame, Calif.). Samples were then dehydrated, mounted and for each tumor the same area on all sections was imaged on a Zeiss Axioscop2 light microscope, under identical imaging conditions.
FACS Analysis
Cells were detached with PBS+2mM EDTA and then incubated in the cold with primary antibodies against ERBB1 (Neomarkers MS-316-P0) or ERBB2 (Neomarkers MS-229-P0) in PBS+BSA. Primary antibody binding was detected using PE-labeled goat anti-mouse secondary antibody (Jackson Immunoresearch 115-116-146).
Results
We first evaluated the effect of the ERBB1 and ERBB2 inhibitor AC480 (previously published as BMS-599626) (12) on the highly metastatic mammary adenocarcinoma MTLn3E cells(6, 13). Consistent with in vitro studies with other cell lines(12), concentrations in the 1 uM micromolar range were sufficient in vitro to block EGF-induced phosphorylation of ERBB1 and ERBB2 (Supplementary Fig. 1A), lamellipod extension, chemotaxis and invasion (Supplementary Fig. 1B–E), while inhibition of proliferation required higher concentrations (Supplementary Fig. 1F). To determine the effects of ERBB1 and ERBB2 inhibition on cell behavior in vivo, mice bearing MTLn3E xenograft tumors(6) were given 100mg/kg of AC480 via oral gavage for 3 hours (12). Using immunohistochemistry with phospho-ERBB antibodies, we confirmed that both ERBB1 and ERBB2 are phosphorylated in vehicle-treated primary tumors, and that inhibition of endogenous ERBB1/2 phosphorylation in the tumor was complete by 3 hours after oral gavage with AC480 (Fig. 1A), consistent with pharmacodynamic data indicating that plasma concentrations reach > 1uM after 3 hours(12). We therefore performed further in vivo analyses at this time point.
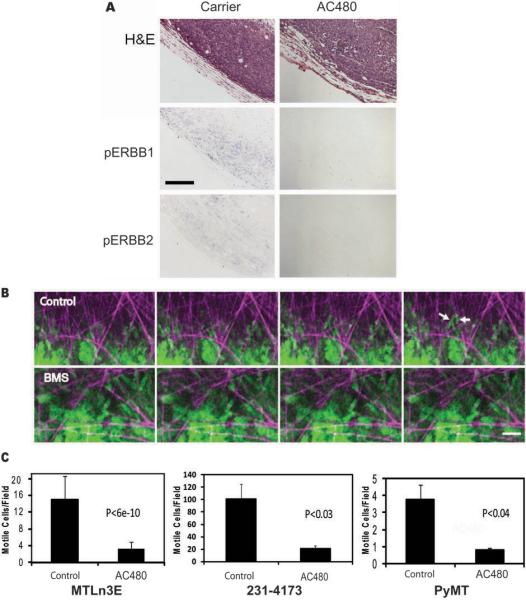
Figure 1. Inhibition of ERBB signaling blocks in vivo motility of tumor cells.
A, AC480 inhibits phosphorylation of ERBB1 and ERBB2. Serial sections from MTLn3E tumors from animals treated with 100 mg/kg AC480 or carrier by oral gavage were stained with H&E, for phospho-ERBB1, or for phospho-ERBB2, as described in Materials and Methods. Results are representative of samples stained from 10 AC480 and 10 carrier treated animals. Bar, 100 um. B, Representative motility images from Supplementary Movie 1 show MTLn3E tumor cell movement in a carrier treated animal (Control), with several cells (green) moving on matrix fibers (purple). Moving cells are marked with white arrows. Movement in the tumor of an AC480 treated animal (BMS) is rarely seen and nonmotile clusters of cells arrested on matrix fibers are often observed. Images at 9 min intervals are shown. Bar, 42 um. C. Quantitation of in vivo motility of cells in MTLn3E xenografts, MDA-MB-231 xenografts and PyMT transgenic tumors. The tumors of AC480 treated (AC480) or carrier treated (Control) animals were imaged using intravital microscopy as in B. Data were acquired in z-series time-lapse format at 1 min intervals and analyzed as described in Materials and Methods. Data are mean and SEM of 13 measurements from 4 animals (MTLn3E), 16 measurements from 5 animals (PyMT), and 12 measurements from 4 animals (MDA-MB-231).
To evaluate whether the endogenous motility and invasiveness of cancer cells in the primary tumor was dependent on ERBB activity, we utilized intravital multiphoton microscopy(14) to directly image cells in tumors generated by GFP-expressing tumor cells. Individual cells were followed in time lapse z-series by GFP fluorescence. In the tumors of animals treated with carrier alone, > 10 moving cells per field were observed on average, often invading along extracellular matrix fibers (Fig. 1B, C and Supplementary Movie 1). AC480 treatment resulted in an 80% reduction in the number of cells moving per field in the tumors (Fig. 1B, C and Supplementary Movie 1). Thus, in parallel with reduced ERBB1 and ERBB2 phosphorylation, AC480 inhibited endogenous breast tumor cell motility in the primary tumor. Studies with a second aggressive breast cancer model, the transgenic PyMT model(10), confirmed the importance of ERBB signaling for endogenous tumor cell motility and invasiveness (Fig. 1C and Wyckoff et al (10)). To extend these findings to human cells, we utilized MDA-MB-231 cells. The measurement of in vivo motility in the primary tumors using intravital imaging revealed that treatment of animals with AC480 dramatically reduces the numbers of moving cells in this model as well (Fig 1C). While the motility of MDA-MB-231 cells was several-fold higher than that of MTLn3E cells, the relative decrease in motility was similar. In summary, blockade of ErbBs resulted in inhibition of in vivo motility in both rat and human xenograft tumor models as well as a transgenic mouse model.
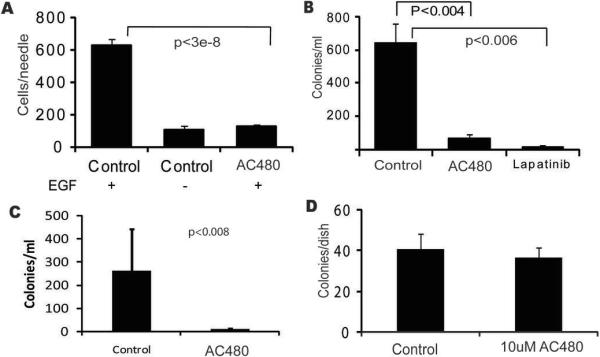
Inhibition of in vivo responses to direct EGF stimulation was confirmed by measuring in vivo tumor cell invasion into microneedles filled with Matrigel and EGF(10). Treatment with AC480 reduced EGF-induced in vivo invasion to background levels (Fig. 2A). An important consequence of tumor cell invasion and motility is the ability to enter tumor blood vessels, or intravasate (15). Intravasated tumor cells can then be transported to distant organs, resulting in the formation of metastases that can lead to patient mortality. To test the ability of AC480 to block intravasation, blood from the right atria of animals carrying MTLn3E or MDA-MB-231 xenograft tumors was collected and numbers of tumor cells per milliliter were scored(6). We found that AC480 treatment resulted in a greater than 80% decrease in the number of intravasated MTLn3E (Fig. 2B) or MDA-MB-231 (Fig. 2C) cells. Cells exposed to AC480 for 3 hours showed similar survival post-treatment to DMSO controls (Fig. 2D), demonstrating that the effect of AC480 on intravasation was not due to altered cell survival. In order to confirm that the observed effects of AC480 treatment are caused by ERBB inhibition and not by off-target effects, we treated tumor bearing animals with a different ERBB1 and ERBB2 inhibitor, lapatinib (GW572016)(16). Lapatinib treatment also significantly reduced intravasation of tumor cells (Fig. 2B), indicating that the inhibition of intravasation reflects inhibition of ERBB signaling. In order to determine if there were individual contributions of ERBB1 and ERBB2 to these in vivo tumor cell properties, we next evaluated the effects of selective ERBB1 or ERBB2 inhibition.
Figure 2. Inhibition of ERBB signaling blocks in vivo invasion and intravasation.
A, AC480 blocks EGF-induced in vivo invasion. Tumor cell in vivo invasion in MTLn3E tumors in response to EGF or buffer in carrier treated (Control) or AC480 treated (AC480) animals was measured as described in Materials and Methods. Mean and SEM of 11 measurements from 4 animals for controls and 8 measurements from 3 animals for AC480 is shown. B, Intravasation of MTLn3E tumor cells is reduced by 2 different ERBB1 and ERBB2 inhibitors. Mean and SEM of measurements from 18 carrier-treated animals (Control), 17 AC480 treated animals (AC480) and 9 lapatinib treated animals. C, Intravasation of MDA-MB-231 cells is reduced by treatment of tumor-bearing animals with AC480 (AC480) as compared to carrier-treated animals (Control). Bars represent averages and SEM for 12 control and 7 AC480-treated animals. D, AC480 treatment does not affect cell viability during the intravasation measurement. Cells were treated as described in Materials and Methods, and colonies of AC480-treated cells were counted and plotted in comparison to DMSO-treated controls (Control). Mean and SEM of 5 separate experiments.
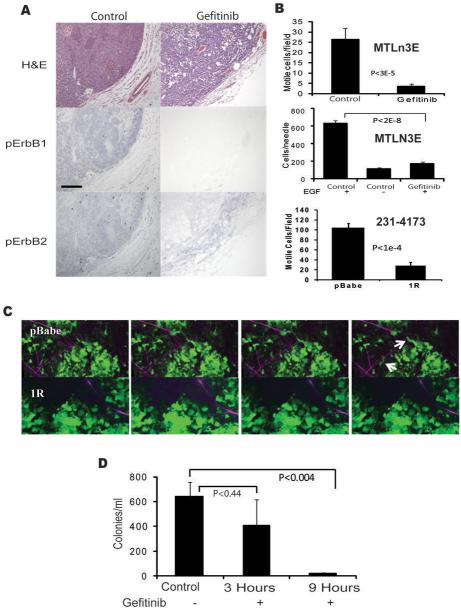
Gefitinib (Iressa), a highly selective inhibitor for the ERBB1 kinase activity(17), blocks EGF-stimulated ERBB1 and ERBB2 phosphorylation, lamellipod extension, chemotaxis, and invasion of MTLn3E cells in vitro at lower concentrations than proliferation (Supplementary Fig. 2). The effect on EGF-stimulated ERBB2 phosphorylation is a result of inhibition of ERBB1 kinase activity but not a direct effect on ERBB2(18). In vivo, immunostaining for phosphorylated forms of ERBB1 and ERBB2 demonstrated that gefitinib treatment strongly inhibited ERBB1 phosphorylation, with partial inhibition of ERBB2 phosphorylation (Fig. 3A). The in vivo motility of tumor cells in the primary tumors was significantly reduced by gefitinib treatment, demonstrating that the endogenous in vivo motility is ERBB1 dependent (Fig. 3B, top). In addition, the number of cells invading in vivo in response to EGF was reduced to levels similar to the buffer control group in gefitinib treated animals (Fig. 3B, middle), confirming that the gefitinib treatment was fully blocking in vivo responses to EGF. As an alternative method for evaluating the role of ERBB1 in cell motility, we suppressed the surface expression of ERBB1 using a single chain antibody that retains ERBB1 in the endoplasmic reticulum (9). MDA-MB-231 cells transduced with the 1R anti-ERBB1 scFv showed a 90% reduction in cell surface ERBB1 compared to cells transduced with the pBabe empty vector control. Suppression of surface expression of ERBB1 reduced motility by 70 % (Fig. 3B bottom, 3C and Supplementary Movie 2), confirming that cell surface ERBB1 is important for spontaneous cell motility in the primary tumor site. Thus ERBB1 signaling is critical for endogenous motility and invasion in the primary tumor. However, although ERBB1 phosphorylation, endogenous motility and EGF-induced in vivo invasion were blocked, there was not a statistically significant inhibition of intravasation 3 hours after gefitinib treatment (Fig. 3D). In order to intravasate, tumor cells must invade the neighboring stroma and approach blood vessels. Given that in vivo motility and invasion were inhibited by gefitinib, we hypothesized that gefitinib might be able reduce the efficiency of approach to blood vessels while not affecting intravasation directly. To test this hypothesis, we extended the treatment time to 9 hours, which resulted in significantly reduced intravasation efficiency (Fig. 3D), consistent with ERBB1 being important for invasion from the primary tumors towards blood vessels prior to intravasation but not for the intravasation event itself.
Figure 3. ERBB1 inhibition distinguishes in vivo motility and invasion from intravasation.
A, Gefitinib (100 mg/kg) completely inhibits ERBB1 phosphorylation but not ERBB2 phosphorylation. MTLn3E tumors from animals treated with 100 mg/kg gefitinib or carrier by oral gavage were stained with H&E, for phospho-ERBB1, or for phospho-ERBB2, as described in Materials and Methods. Results are representative of samples from 9 gefitinib and 10 carrier treated animals. Bar, 100 um. B, In vivo motility (top) and invasion (middle) of cells in MTLn3E xenografts treated with Gefitinib and in vivo motility of cells in MDA-MB-231 xenografts (bottom) with intracellular retention of ERBB1. Means and SEM of at least 15 fields from 3 animals (top), 10 measurements from 6 animals, and 7 fields from 4 animals are plotted. C, Representative motility images from Supplementary Movie 2 show MDA-MB-231 cell movement in empty vector MDA-MB-231 cells (pBabe), and 1R-expressing animals (1R) with cells (seen in green) moving towards vessels (dark areas on top for both the pBABE and the 1R images). Moving cells are marked with arrows. Individual frames are 5 minutes apart. Bar=42μm. D, Gefitinib requires longer treatment to block intravasation. Intravasation was measured in gefitinib-treated animals after 3 hours or 9 hours (with treatments 9 and 5 hours before measurement). Mean and SEM of measurements from 34 control animals (Control), 9 (3 Hours) and 13 (9 Hours) gefitinib-treated animals.
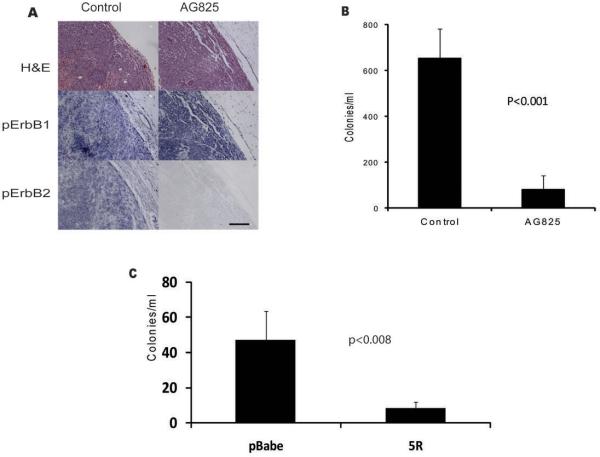
Since the ERBB1 and ERBB2 inhibitors were able to inhibit intravasation rapidly while gefitinib did not, this suggested that ERBB2 could be more directly involved in intravasation than ERBB1. We therefore evaluated the effect of selectively inhibiting ERBB2 function, using the ERBB2 inhibitor AG825(19). AG825 had no effect on in vitro invasion or proliferation of MTLn3B1 cells at concentrations up to 10 uM (data not shown). Treatment of animals with AG825 resulted in strong inhibition of ERBB2 phosphorylation with limited effects on ERBB1 phosphorylation (Fig. 4A), consistent with a selective in vivo inhibition of ERBB2 signaling. Correlated with the inhibition of ERBB2 was a strong inhibition of intravasation (Fig. 4B), demonstrating that ERBB2 contributes directly to intravasation. As an alternative approach we reduced the levels of ErbB2 on the cell surface of MDA-MB-231 cells by expressing a gene encoding a single-chain antibody that binds specifically to the extracellular portion of ErbB2 and prevents its transit through the endoplasmic reticulum(20). FACS analysis demonstrated a greater than 90% decrease in cell surface ERBB2 in cells expressing the 5R scFv compared to cells transduced with the pBabe empty vector control. The number of circulating tumor cells in mice with orthotopic xenografts of MDA-MB-231 cells expressing this 5R single chain antibody were significantly reduced compared to the empty vector (pBabe) controls (Fig. 4C).
Figure 4. Inhibition of ERBB2 blocks intravasation.
A, Treatment with AG825 reduces ERBB2 phosphorylation with limited effects on ERBB1 phosphorylation. One hour after intraperitoneal injection of 20 mg/kg AG825 (AG825) or carrier (Control), tumors were stained for H&E, phospho-ERBB1 or phospho-ERBB2, as described in Materials and Methods. Results are representative of samples from 6 AG825 and 10 carrier treated animals. Bar, 100uM. B, 20mg/kg intraperitoneal administration of AG825 (AG825) resulted in a significant reduction in the number of intravasating cells compared to carrier treatment (Control). Data are the mean and SEM for 6 AG825-treated and 34 control animals. C, Intravasation of MDA-MB-231 empty vector control cells (pBabe) and 5R-expressing ErbB2 downregulated cells (5R). Data are means and SEM for 8 animals in each group, p<0.008.
Discussion
In this paper we have examined the roles of ERBB1 and ERBB2 in invasion and intravasation at the primary tumor. Because these processes can be extremely sensitive to changes in tumor structure and microenvironment, we have used both drugs and stable retention in the endoplasmic reticulum to inhibit ERBB1 and/or ERBB2 in vivo in the primary tumor. Both approaches demonstrate that ERBB1 makes a major contribution to spontaneous tumor cell motility in the primary tumor microenvironment. Our work complements studies using alteration of ERBB expression to demonstrate a role for ERBB1 and ERBB2 in tumor cell invasion, intravasation, and metastasis (5–7, 13, 21, 22). The direct imaging of spontaneous motility and invasion demonstrates an important role for ERBB1 in in vivo invasion and motility. The rapid change in motility following inhibition of ERBB1 using both ERBB1 and ERBB2 inhibitors as well as the ERBB1-selective drug gefitinib supports a direct role for ERBB1 rather than indirect effects on tumor microenvironment due to altered gene expression. If ERBB1 plays a direct role in stromal invasion towards blood vessels, invasion could be stimulated by endogenous gradients of EGF, and consistent with this possibility, we find cellular sources of EGF in the stroma (data not shown).
Although ERBB1 inhibition does block both spontaneous tumor cell motility and in vivo invasion in response to an applied gradient of EGF, it does not directly block intravasation. Longer treatment with gefitinib (9 hours) was needed to produce a significant reduction in intravasation. This temporal difference between the effects of gefitinib on motility and intravasation suggests that intravasation occurs after, and depends upon, ERBB1-mediated invasion. Such a temporal sequence suggests that tumor cells must transit the loose connective tissue stroma prior to intravasation. This is consistent with the physical arrangement of the tumor microenvironment; the primary tumor mass is separated from the vasculature by loose connective tissue barriers of varying thickness.
In contrast to the indirect dependence of intravasation on ERBB1 function, we find that ERBB2 is more directly involved in the intravasation process. Two ERBB1 and ERBB2 inhibitors, AC480 and lapatinib, blocked intravasation within 3 hours of oral gavage. This conclusion was further reinforced by intraperitoneal injection of AG825, an ERBB2-specific inhibitor, which was found to inhibit intravasation with 1 hour of treatment. ERBB2 phosphorylation in the primary tumor was strongly inhibited while significant ERBB1 phosphorylation remained, consistent with a requirement for ERBB2 activation during intravasation. The importance of surface ERBB2 for intravasation was confirmed using retention of ERBB2 in the endoplasmic reticulum. Although the analyses using intracellular antibodies argue for the importance of ERBB1 and ERBB2 signaling in the tumor cells, it is also possible that the drugs are affecting other cells in the tumor microenvironment, such as endothelial cells, and through them affecting either invasion or intravasation.
The distinct contributions of ERBB1 and ERBB2 to invasion and intravasation may reflect different microenvironments stimulating intravasation and invasion. ERBB2 has been shown to be important for chemotaxis to a variety of chemoattractants including EGF and heregulin(6, 23). Consistent with the in vitro data, we find that AG825 inhibits in vivo invasion in response to EGF (data not shown). Thus ErbB2 activation contributes to both invasion and intravasation, and there is no direct evidence that different intracellular pathways are activated by ErbB2 under these two conditions. Rather, other ligands that do not act via ERBB1, such as heregulin (via ERBB3(5)) that can be present in serum and around blood vessels, could stimulate intravasation via ERBB2 in the absence of ERBB1 activation (or in the presence of gefitinib).
These studies have clinical implications since inhibition of invasion and intravasation could have significant effects on the ability of tumor cells to spread and metastasize without necessarily affecting proliferation. On the order of 30% of ERBB1 or ERBB2 expressing tumors have shown reduction in tumor size in response to ERBB inhibition(24). Our results suggest that clinical trials directly evaluating tumor invasion and spread might reveal an additional patient population whose tumor aggressiveness might be reduced independent of effects on tumor growth.
Statement of Translational Relevance.
The EGF receptor family (ERBBs) is overexpressed in a wide variety of tumor types and is correlated with poor prognosis. Consequently, there has been a significant investment of resources in the development of drugs to target these molecules. However, clinical trials of these drugs have shown limited efficacy using tumor size or growth as an endpoint. Previous studies of ERBB function using either altered expression levels of ERBBs or tumor growth are long term and do not differentiate the direct action of ERBBs on cell behavior from downstream effects of altered gene expression. We demonstrate for the first time in vivo, using small molecule inhibitors to rapidly inhibit ERBB1 and/or ERBB2 within 3 hours, that tumor cell motility, invasion and intravasation are directly dependent on ERBB function in the primary tumors of 3 different breast cancer models. We therefore propose that these drugs may have potential for inhibition of tumor cell invasion independent of their effects on tumor growth.
Supplementary Material
Acknowledgements
J.E.S. is the Betty and Sheldon Feinberg Senior Faculty Scholar in Cancer Research. We thank Dr. Joan Massague for supplying the MDA-MB-231 cells. We thank Tai Wong for comments and advice regarding the use of AC480, Mazen Sidani for blind analysis of cell motility, Radma Mahmood and Jonathan Peled for help in immunohistochemistry, and the Segall, Condeelis, and Cox labs for comments and suggestions.
Grant Support: DOD BC061403 (D. Kedrin), CA100324 (J. Wyckoff and J.E. Segall) and CA77522 (J.E.Segall), CA80195 (C.L. Arteaga), Novartis Research Foundation (N.E. Hynes).
Footnotes
Conflicts of interest: none
References
- 1.Arteaga CL, Baselga J. Tyrosine kinase inhibitors: why does the current process of clinical development not apply to them? Cancer Cell. 2004;5:525–31. doi: 10.1016/j.ccr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Hoadley KA, Weigman VJ, Fan C, et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics. 2007;8:258. doi: 10.1186/1471-2164-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieto Y, Nawaz F, Jones RB, Shpall EJ, Nawaz S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J Clin Oncol. 2007;25:4405–13. doi: 10.1200/JCO.2006.09.8822. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Fletcher JA. The HER-2/neu Oncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy. Oncologist. 1998;3:237–52. [PubMed] [Google Scholar]
- 5.Xue C, Liang F, Mahmood R, et al. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res. 2006;66:1418–26. doi: 10.1158/0008-5472.CAN-05-0550. [DOI] [PubMed] [Google Scholar]
- 6.Xue C, Wyckoff J, Liang F, et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66:192–7. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- 7.Zhan L, Xiang B, Muthuswamy SK. Controlled activation of ErbB1/ErbB2 heterodimers promote invasion of three-dimensional organized epithelia in an ErbB1-dependent manner: implications for progression of ErbB2-overexpressing tumors. Cancer Res. 2006;66:5201–8. doi: 10.1158/0008-5472.CAN-05-4081. [DOI] [PubMed] [Google Scholar]
- 8.Minn AJ, Gupta GP, Padua D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–5. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jannot CB, Beerli RR, Mason S, Gullick WJ, Hynes NE. Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cells. Oncogene. 1996;13:275–82. [PubMed] [Google Scholar]
- 10.Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 11.Kedrin D, Wyckoff J, Sahai E, Condeelis J, Segall JE. Unit 19.7 Imaging Tumor Cell Movement in Vivo. Current Protocols in Cell Biology. 2007;19:7. doi: 10.1002/0471143030.cb1907s35. [DOI] [PubMed] [Google Scholar]
- 12.Wong TW, Lee FY, Yu C, et al. Preclinical antitumor activity of BMS-599626, a pan-HER kinase inhibitor that inhibits HER1/HER2 homodimer and heterodimer signaling. Clin Cancer Res. 2006;12:6186–93. doi: 10.1158/1078-0432.CCR-06-0642. [DOI] [PubMed] [Google Scholar]
- 13.Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 2005;5:14. doi: 10.1186/1472-6750-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 15.Wyckoff JB, Wang Y, Lin EY, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer research. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 16.Spector NL, Xia W, Burris H, 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–12. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Averbuch SD. ZD1839 (`Iressa') as an anticancer agent. Drugs. 2000;60(Suppl 1):33–40. doi: 10.2165/00003495-200060001-00004. discussion 1–2. [DOI] [PubMed] [Google Scholar]
- 18.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–95. [PubMed] [Google Scholar]
- 19.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–8. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 20.Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Molecular and cellular biology. 1995;15:1182–91. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamoune A, Kassis J, Kharait S, et al. DU145 human prostate carcinoma invasiveness is modulated by urokinase receptor (uPAR) downstream of epidermal growth factor receptor (EGFR) signaling. Experimental cell research. 2004;299:91–100. doi: 10.1016/j.yexcr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. The Journal of biological chemistry. 1998;273:28238–46. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 23.Spencer KS, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. The Journal of cell biology. 2000;148:385–97. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.