SUMMARY
Gonadotropin-dependent, or central, precocious puberty is caused by early maturation of the hypothalamic–pituitary–gonadal axis. In girls, this condition is most often idiopathic. Recently, a G protein–coupled receptor, GPR54, and its ligand, kisspeptin, were described as an excitatory neuroregulator system for the secretion of gonadotropin-releasing hormone (GnRH). In this study, we have identified an autosomal dominant GPR54 mutation — the substitution of proline for arginine at codon 386 (Arg386Pro) — in an adopted girl with idiopathic central precocious puberty (whose biologic family was not available for genetic studies). In vitro studies have shown that this mutation leads to prolonged activation of intracellular signaling pathways in response to kisspeptin. The Arg386Pro mutant appears to be associated with central precocious puberty.
Puberty represents a complex biologic process of sexual development that can be influenced by genetic, nutritional, environmental, and socioeconomic factors.1 The activation of pulsatile hypothalamic GnRH secretion is a key event in the onset of puberty.2 A network of hypothalamic neurons is critical for GnRH release and, consequently, pituitary gonadotropin secretion and gonadal steroid production during pubertal maturation.3
Precocious puberty is defined as the development of secondary sexual characteristics before the age of 8 years in girls and 9 years in boys.4 There are several causes of precocious puberty, and it is of utmost importance to distinguish between central (gonadotropin-dependent) precocious puberty, which results from premature activation of the hypothalamic–pituitary–gonadal axis, and gonadotropin-independent precocious puberty. Central precocious puberty has a striking predominance among girls, and most of these cases are considered idiopathic.1,4 However, it is known that genetic factors play a fundamental role in the timing of pubertal onset, as illustrated by the similar age at menarche among members of an ethnic group and in mother–daughter, monozygotic-twin, and sibling pairs.5 More recently, de Vries et al.6 reported a 27.5% prevalence of familial central precocious puberty; segregation analysis in these families suggested autosomal dominant transmission with incomplete sex-dependent penetrance.
The kisspeptin–GPR54 signaling complex has been proposed as a gatekeeper of pubertal activation of GnRH neurons and the reproductive axis.7-9 Loss-of-function point mutations and deletions in GPR54 have been identified in patients with familial or sporadic isolated hypogonadotropic hypogonadism.7-9 In addition, Gpr54-knockout mice had a similar failure of sexual maturation.8,10 In this study, we hypothesized that gain-of-function mutations of the human GPR54 receptor might be associated with premature activation of GnRH release, leading to central precocious puberty.
CASE REPORT
An 8-year-old adopted girl was referred to the Developmental Endocrinology Unit of Clinicas Hospital, São Paulo, for evaluation of precocious puberty. Premature breast development with slow progression had been observed since birth. At 7 years of age, the development of breasts accelerated and pubic hair was noted. The patient’s medical history was unremarkable. She had no exposure to sex steroids, according to her family. She had no neurologic symptoms. Additional pubertal signs such as acne, oily skin, axillary hair, and menstrual bleeding were absent. There were no café au lait spots. At 8 years of age, her height was 131.5 cm and her weight 26.7 kg. The mid-parental height was not available. Breast development was Tanner stage 4 and pubic hair Tanner stage 2. Bone age was 11 years (according to the method of Greulich and Pyle11).
The basal luteinizing hormone level was less than 0.6 IU per liter (normal prepubertal levels, <0.6 to 0.7 IU per liter), and the basal follicle-stimulating hormone level was 2.6 IU per liter (normal prepubertal levels, <1.0 to 7.2 IU per liter). In addition, luteinizing hormone and follicle-stimulating hormone levels after stimulation with GnRH were 6.4 IU per liter and 5.9 IU per liter, respectively (typical luteinizing hormone level after puberty, >6.9 IU per liter).12 The peak ratio of luteinizing hormone to follicle-stimulating hormone after GnRH stimulation was 1.08.13 The luteinizing hormone level 2 hours after a depot injection of leuprolide acetate was 8.5 IU per liter (typical level after puberty, >10 IU per liter).14 The serum estradiol level was 22 pg per milliliter (80 pmol per liter) (normal prepubertal level, <13 pg per milliliter [47 pmol per liter]). Detailed methods for the hormonal assays are given in the Supplementary Appendix (available with the full text of this article at www.nejm.org).
Pelvic ultrasonography showed no masses. Ovarian and uterine volumes were enlarged in relation to the chronologic age (right ovary, 4.4 cm3; left ovary, 3.6 cm3; uterus, 5.4 cm3). The results of magnetic resonance imaging of the central nervous system were normal.
Despite the fact that the basal luteinizing hormone level was within the normal prepubertal range and the GnRH-stimulated level was not diagnostic of gonadotropin-dependent precocious puberty, the absence of either a primary ovarian disorder or a neurogenic cause of the patient’s symptoms favored the diagnosis of idiopathic central precocious puberty. Therefore, treatment with a GnRH analogue (3.75 mg per month of a depot suspension of leuprolide acetate) was initiated and maintained for 4 years. The diagnosis of central precocious puberty was confirmed by a satisfactory response to the treatment, including partial regression of breast development (to Tanner stage 3), decrease in growth velocity, arrest of bone maturation, reduction of ovarian size, and gonadotropin suppression along with a return to prepubertal estradiol levels. Spontaneous menarche occurred at age 12 and was followed by regular menses. The patient’s adult height is 152.2 cm.
Methods
DNA ANALYSIS
Approval of the DNA-analysis methods was provided by the ethics committee of Clinicas Hospital. Written informed consent was provided by the parents of our patient and those of 53 unrelated children with idiopathic central precocious puberty, as well as by all 150 ethnically matched adult control subjects. All of the children provided oral assent. Genomic DNA was extracted from peripheral-blood leukocytes, and the entire coding region as well as the exon–intron boundaries of GPR54 (GenBank accession number, NM_032551) were amplified and sequenced on an automated sequencer.8
GENERATION OF ARG386PRO GPR54 THROUGH SITE-DIRECTED MUTAGENESIS
The Arg386Pro GPR54 mutant was generated in vitro by means of site-directed mutagenesis with the QuikChange II Site-Directed Mutagenesis Kit (Stratagene) and the mammalian expression vector pCMVsport6, containing the full-length wild-type human GPR54 as a template.8 We confirmed the presence of the mutation by using direct sequencing.
STUDIES OF GPR54 SIGNALING
Kidney-fibroblast cells from the African green monkey (COS-7 cells) were transiently transfected with 50 ng of wild-type GPR54, Arg386Pro GPR54, or an empty vector (pCMVsport6) using Gene-PORTER Transfection Reagent (Gene Therapy Systems). Total inositol phosphate and phosphorylation of extracellular signal–regulated kinase were measured at various points after stimulation with increasing levels of kisspeptin.15 (For details, see the Methods section of the Supplementary Appendix.)
RESULTS
DNA ANALYSIS
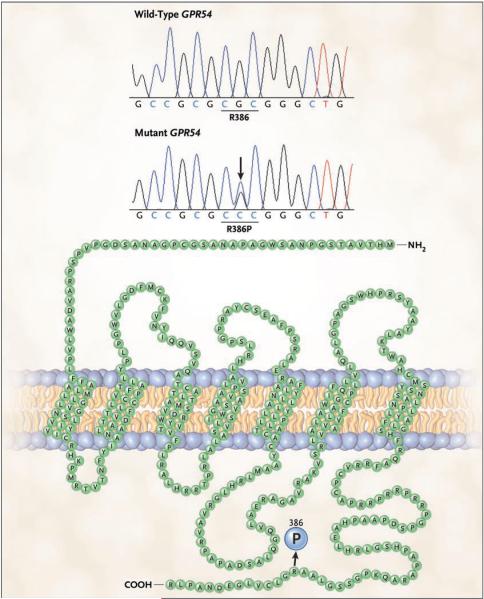
Automated sequencing of the patient’s genomic DNA for GPR54 revealed a heterozygous substitution of cytosine for guanine at nucleotide position 1157 in exon 5, resulting in the substitution of proline for arginine at codon 386 (Arg386Pro) in the carboxy-terminal tail of the receptor (Fig. 1). The Arg386Pro mutation generates a restriction site recognized by SmaI; the mutation was screened in the 300 chromosomes from the 150 ethnically matched controls who had a history of normal pubertal development and in the 106 chromosomes from the 53 unrelated children (50 girls and 3 boys) who had idiopathic central precocious puberty. The Arg386Pro mutation was absent in all controls as well as in the unrelated patients with precocious puberty.
Figure 1. Sequences of Wild-Type and Mutant GPR54.
The wild-type cytosine–guanine–cytosine (CGC) sequence in GPR54 exon 5 encodes arginine (R) at codon 386 (R386). The heterozygous substitution of C for G (shown for our patient with an arrow) results in the substitution of proline (P) for R (R386P) in the carboxy-terminal tail of the protein (shown embedded in a cellular membrane). In the nucleotide sequences (shown immediately under the two plots), T denotes the nucleotide thymidine.
FUNCTIONAL ANALYSIS OF ARG386PRO GPR54
Binding studies performed in COS-7 cells transfected with wild-type or Arg386Pro GPR54 and incubated with 125I-labeled kisspeptin revealed no significant effect of the amino acid substitution on the binding affinity of kisspeptin or the levels of expression of GPR54 (dissociation constant for wild-type GPR54, 4.4 nM; for Arg386Pro GPR54, 7.0 nM; maximal binding capacity for both types of GPR54, 20 nmol per milligram of protein) (Fig. S1 in the Supplementary Appendix).
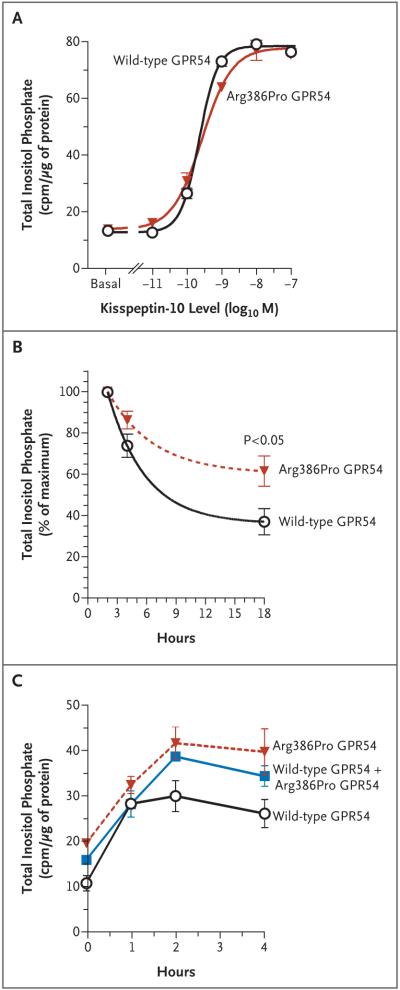
Basal inositol phosphate levels did not differ significantly in COS-7 cells transfected with wild-type GPR54 and those transfected with Arg386Pro GPR54. In addition, there was no significant difference in the dose–response curves or in the maximal responses of wild-type GPR54 and Arg-386Pro GPR54 to increasing concentrations of kisspeptin (10−11 to 10−7 M of kisspeptin-10) (Fig. 2A). The concentration of kisspeptin that provoked a response halfway between the baseline and maximum responses was 0.23 nM for wild-type GPR54 and 0.28 for Arg386Pro GPR54, and the maximal activity of inositol phosphate was 78.4 and 77.8 counts per minute per microgram of protein, respectively. However, a time-course study showed that inositol phosphate levels peaked at 2 hours for both wild-type and Arg386Pro GPR54, but the rate of the decline in inositol phosphate levels thereafter was slower in cells transfected with Arg386Pro GPR54, resulting in significantly higher inositol phosphate levels after 18 hours of stimulation with kisspeptin (Fig. 2B). This effect was also evident when both wild-type and Arg386Pro GPR54 were transfected into COS-7 cells (Fig. 2C).
Figure 2. Kisspeptin-Stimulated Production of Inositol Phosphate in COS-7 Cells Transfected with Wild-Type GPR54 or Arg386Pro GPR54.
Total inositol phosphate accumulation was measured in counts per minute (cpm) 45 minutes after stimulation with 10−11 to 10−7 M of kisspeptin-10 (Panel A). In a separate experiment, cells were stimulated with 10−9 M of kisspeptin-10 for 0, 2, 4, or 18 hours; the resulting inositol phosphate levels are expressed as the percentage of the maximal level (at 2 hours) (Panel B). Each point is the mean of five independent experiments, each performed in duplicate or triplicate. Finally, cells were transfected with 50 ng of wild-type GPR54 only, 50 ng of Arg386Pro GPR54 only, or 25 ng of wild-type GPR54 and 25 ng of Arg386Pro GPR54. The total inositol phosphate accumulation was measured after stimulation with 10−8 M of kisspeptin-10 for 0, 1, 2, or 4 hours (Panel C). I bars represent the standard errors.
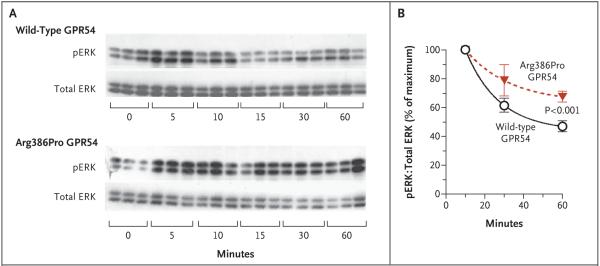
To confirm this prolonged course of action associated with the mutant receptor, we measured the time course of the phosphorylation of extracellular signal–regulated kinase, another downstream effector of the GPR54 signaling cascade, in response to kisspeptin. The levels of phosphorylated extracellular signal–regulated kinase in all cells peaked at 10 minutes, after which the levels declined. The rate of decrease of phosphorylated extracellular signal–regulated kinase levels was slower in cells transfected with Arg386Pro GPR54 than in those transfected with wild-type GPR54, such that the levels in the Arg386Pro GPR54–transfected cells remained significantly higher after 60 minutes of exposure to kisspeptin (Fig. 3). Kinetic studies of GPR54 binding similarly indicated that Arg386Pro GPR54 remains in the cell-surface plasma membranes for longer periods after kisspeptin stimulation than does the wild-type receptor (Fig. S2 in the Supplementary Appendix).
Figure 3. Time Course of Kisspeptin-Stimulated Phosphorylation of Extracellular Signal–Regulated Kinase (ERK) in COS-7 Cells Transfected with Wild-Type GPR54 or Arg386Pro GPR54.
Phosphorylated ERK (pERK) levels were measured by means of Western-blot analysis after stimulation with 3×10−9 M kisspeptin-10 for 0, 5, 10, 15, 30, or 60 minutes. Panel A shows representative Western blots. The intensity of the pERK bands was normalized to that of total ERK bands. Panel B shows the ratio of pERK to total ERK, expressed as percentage of the maximal level (at 10 minutes). Each point is the mean of five independent experiments; I bars represent the standard errors.
DISCUSSION
In humans, the onset of puberty requires an increase in the pulsatile release of GnRH from the hypothalamus.16 An excitatory neuronal system predominantly involved in the activation of GnRH secretion appears to be the kisspeptin–GPR54 signaling complex.7,8,10 Indeed, mammalian models suggest an important role of kisspeptin, since intermittent infusion of this protein results in early sexual maturation in rats and early GnRH release in monkeys.17,18
In our study, we identified a heterozygous GPR54 mutation, Arg386Pro, in a girl with idiopathic central precocious puberty. She had thelarche from birth, but with slow progression, suggesting an early, persistent, and mild increase in estrogen secretion. Isolated thelarche was ruled out on the basis of progressive secondary sexual development and accelerated growth and skeletal maturation.13 Although during her initial evaluation the patient showed borderline-pubertal luteinizing hormone levels after GnRH stimulation, approximately 8% of girls with gonadotropin-dependent precocious puberty have prepubertal luteinizing hormone levels after GnRH stimulation.12 The suppressive effects of using a depot suspension of GnRH agonist on the pituitary–gonadal axis in our patient confirmed the presence of central activation of the GnRH axis. Segregation analysis was not feasible in this adopted girl, because her biologic family was not available for genetic studies. Nonetheless, the absence of the Arg386Pro mutation in an ethnically matched population, as well as in American and European populations, suggests that the Arg386Pro mutation is not a GPR54 polymorphism.7,9,19
In vitro studies revealed no significant differences in the activity of Arg386Pro GPR54 and wild-type GPR54 in transfected cells under basal conditions, indicating that the Arg386Pro mutation does not generate a constitutively active receptor. Nor did cells transfected with mutant GPR54 and those transfected with wild-type GPR54 and incubated with kisspeptin differ significantly in the dissociation constant for kisspeptin binding, the response halfway between the baseline and maximum responses on the dose–response curve, or in the maximal binding capacity or responsiveness to kisspeptin, indicating that the affinity of mutant GPR54 for its ligand and the expression levels of the receptor on the surface of the transfected cells were not altered.
Nevertheless, in repeated time-course studies, the rate of decline in inositol-phosphate accumulation after kisspeptin stimulation was slower in cells transfected with Arg386Pro GPR54 than in cells transfected with wild-type GPR54, resulting in significantly higher inositol phosphate levels for as long as 18 hours. Similarly, the phosphorylation of extracellular signal–regulated kinase was prolonged, confirming the extended activation of intracellular signaling pathways by the mutant GPR54 in response to kisspeptin. These findings indicate a significant reduction in the rate of desensitization of the mutant GPR54. Kinetic studies of ligand binding revealed that the Arg-386Pro GPR54 remained on the plasma membrane of the cell surface after kisspeptin stimulation for longer periods than did the wild-type receptor, suggesting a reduced rate of internalization or degradation of the mutant receptor.
Desensitization of G protein–coupled receptors can occur through receptor phosphorylation, frequently on the carboxy-terminal tail, by intracellular kinases, leading to the uncoupling of the receptor from G proteins and other intracellular signaling pathways. Such phosphorylation may enhance the binding of arrestin, triggering receptor internalization.20 A similar mechanism of receptor activation involving impaired desensitization has been reported in a patient with adrenocorticotropic hormone–independent Cushing’s syndrome; the activation is due to a mutation in the carboxy-terminal tail of the melanocortin 2 receptor, a G-protein–coupled receptor affecting the signaling pathways of the G-protein alpha subunit Gs rather than Gq.21
Gain-of-function mutations in G protein–coupled receptors have been identified only in a few inherited disorders.22 Most of these mutations result in constitutive activation of the receptor and downstream cellular responses. However, constitutive activation of GPR54 might be expected to disrupt pulsatile GnRH release, thereby resulting in delayed — rather than precocious — puberty, since coordinated pulsatile GnRH release is critical for the onset of puberty. Indeed, continuous infusion of kisspeptin has been shown to decrease luteinizing hormone levels in agonadal juvenile male monkeys.23 In contrast, we describe a model of nonconstitutive receptor activation characterized by a reduction of the rate of GPR54 desensitization. This mechanism would result in an increased, prolonged cellular response and hence the release of an increased-amplitude pulse of GnRH in response to kisspeptin stimulation.
The increase in hypothalamic kisspeptin expression at puberty is believed to contribute to the maturation of the reproductive axis.24,25 We speculate that the decreased GPR54 desensitization seen for the Arg386Pro mutant might increase the stimulatory effects of kisspeptin on GnRH secretion, thus accelerating the maturation of the reproductive axis. Furthermore, the presence of breast development during the neonatal period in our patient might be consistent with neonatal activity of the kisspeptin–GPR54 system, as inferred from the presence of cryptorchidism and micropenis in a male infant with idiopathic hypogonadotropic hypogonadism due to a loss-of-function mutation in GPR54.26
In conclusion, we have identified an autosomal dominant Arg386Pro GPR54 mutation that prolongs intracellular GPR54 signaling in response to kisspeptin, which appears to be associated with a central precocious puberty phenotype.
Supplementary Material
Acknowledgments
Supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (05/50146-5, to Dr. Teles; and 05/04726-0, to Dr. Latronico), from Conselho Nacional de Desenvolvimento Científico e Tecnológico (300469/2005-5, to Dr. Latronico; and 300828/2005-5, to Dr. Mendonca), and from the National Institute of Child Health and Human Development and the National Institutes of Health (through cooperative agreement U54 HD28138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, to Dr. Kaiser).
We thank Dr. Ivo Jorge Prado Arnhold for his helpful discussions and comments.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Delemarre-van de Waal HA. Secular trend of timing of puberty. Endocr Dev. 2005;8:1–14. doi: 10.1159/000084082. [DOI] [PubMed] [Google Scholar]
- 2.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–51. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 3.Ojeda SR, Lomniczi A, Mastronardi C, et al. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–74. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 4.Papathanasiou A, Hadjiathanasiou C. Precocious puberty. Pediatr Endocrinol Rev. 2006;3(Suppl 1):182–7. [PubMed] [Google Scholar]
- 5.Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 2001;86:2364–8. doi: 10.1210/jcem.86.6.7603. [DOI] [PubMed] [Google Scholar]
- 6.de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89:1794–800. doi: 10.1210/jc.2003-030361. [DOI] [PubMed] [Google Scholar]
- 7.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 9.Semple RK, Achermann JC, Ellery J, et al. Two novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–55. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- 10.Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–63. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 11.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Vol. 51. Stanford University Press; Stanford, CA: 1959. pp. 103–5. [Google Scholar]
- 12.Brito VN, Batista MC, Borges MF, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab. 1999;84:3539–44. doi: 10.1210/jcem.84.10.6024. [DOI] [PubMed] [Google Scholar]
- 13.Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler GB., Jr. Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1988;67:474–9. doi: 10.1210/jcem-67-3-474. [DOI] [PubMed] [Google Scholar]
- 14.Brito VN, Latronico AC, Arnhold IJ, Mendonca BB. A single luteinizing hormone determination 2 hours after depot leuprolide is useful for therapy monitoring of gonadotropin-dependent precocious puberty in girls. J Clin Endocrinol Metab. 2004;89:4338–42. doi: 10.1210/jc.2003-031537. [DOI] [PubMed] [Google Scholar]
- 15.Bedecarrats GY, Linher KD, Kaiser UB. Two common naturally occurring mutations in the human gonadotropin-releasing hormone (GnRH) receptor have differential effects on gonadotropin gene expression and on GnRH-mediated signal transduction. J Clin Endocrinol Metab. 2003;88:834–43. doi: 10.1210/jc.2002-020806. [DOI] [PubMed] [Google Scholar]
- 16.Ebling FJ, Cronin AS. The neurobiology of reproductive development. Neuro-report. 2000;11:R23–R33. doi: 10.1097/00001756-200011090-00002. [DOI] [PubMed] [Google Scholar]
- 17.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–13. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 18.Navarro VM, Fernández-Fernández R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–86. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerrato F, Shagoury J, Kralickova M, et al. Coding sequence analysis of GNRHR and GPR54 in patients with congenital and adult-onset forms of hypogonadotropic hypogonadism. Eur J Endocrinol. 2006;155(Suppl 1):S3–S10. doi: 10.1530/eje.1.02235. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 21.Swords FM, Baig A, Malchoff DM, et al. Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Mol Endocrinol. 2002;16:2746–53. doi: 10.1210/me.2002-0099. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel AM, Weinstein LS. Inherited diseases involving G proteins and G protein-coupled receptors. Annu Rev Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- 23.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–6. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 24.Navarro VM, Castellano JM, Fernández-Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 25.Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–44. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.