Abstract
T regulatory cells are critical for the prevention of autoimmunity. Specifically, Treg cells can control anti-chromatin antibody production in vivo, and this correlates with decreased ICOS expression on CD4+ T helper cells. Here we test the significance of high ICOS expression by T effector cells, firstly in terms of the anti-chromatin B cell response, and secondly on the ability of Treg cells to suppress T cell help. We bred CD4+ T cell receptor transgenic mice with mice that carry the Roquinsan/san mutation. The Roquin gene functions to limit ICOS mRNA such that CD4 T cells from mutant mice express elevated ICOS. Using an in vivo model, TS1.Roquinsan/san Th cells were compared with wild-type TS1 Th cells with regard to their ability to help anti-chromatin B cells in the presence or absence of Treg cells. Both TS1 and TS1.Roquinsan/san Th cells induced anti-chromatin IgMa antibodies, but the TS1.Roquinsan/san Th cells resulted in the recovery of more class-switched and germinal center B cells. Neither source of Th cells were capable of inducing long-lived autoantibodies. Treg cells completely suppressed anti-chromatin IgMa antibody production and reduced anti-chromatin B cell recovery induced by TS1 Th cells. Importantly, this suppression was less effective when TS1.Roquinsan/san Th cells were used. Thus, high ICOS levels on effector T cells results in autoimmunity by augmenting the autoreactive B cell response and by dampening the effect of Treg cell suppression.
Keywords: regulatory T cells, autoreactive B cells, ICOS expression
1. Introduction
Anti-chromatin antibodies are highly significant clinically; they are one of the diagnostic markers of the autoimmune disease systemic lupus erythematosus (SLE). We have used an immunoglobulin (Ig) transgenic model to study how anti-chromatin B cell responses are prevented in healthy individuals and how they are initiated and sustained in autoimmune disease [1]. While anti-chromatin B cells are present in the periphery of healthy mice, they are predominantly immature, excluded from the B cell follicles and have a reduced lifespan [2]. Importantly, we and others have shown that anti-chromatin B cells can differentiate into antibody secreting cells (ASCs) in vivo following provision of CD4+ T cell help [3–5].
Foxp3+ Treg cells are important for the control of autoimmunity in mice and humans [6], but the mechanism of suppression by Treg cells in vivo is still largely unknown [7]. We have shown that CD4+ CD25+ Treg cells can effectively block anti-chromatin antibody production in the face of T cell help [3, 4]. A third-party adoptive transfer model was used to track the fates of Treg, Th, and anti-chromatin B cells in vivo, and to study the effects of Treg cells upon the cognate interactions between anti-chromatin B cells and Th cells. Using this system, we showed that Treg cells allow both Th cells and anti-chromatin B cells to become initially activated and enter the B cell follicle, but this response is ultimately insufficient to induce autoantibody production [4]. Furthermore, Treg cells maintained this suppressive capability even in the context of preactivated, differentiated Th1 and Th2 cells [8]. Strikingly, however, Treg cell suppression consistently correlated with lower ICOS (inducible costimulator) expression levels on Th cells, even though other molecules that have been shown to be crucial to initiate and/or sustain a Th-B cell cognate interaction (CD154, CXCR5, and CD178) were unaffected by Treg cells [4].
The reduced levels of ICOS on effector cells in this setting was notable, firstly, because ICOS is typically upregulated with CD4+ T cell activation, and it is a critical molecule for Th-driven antibody production and germinal center development [9–12]. Indeed, ICOS is one of the signature molecules that is used to define the T follicular helper cell subset (TFH) [13].
Secondly, CD4+ T cells from lupus patients display high levels of ICOS [14], and two mouse models have correlated high levels of ICOS with autoimmunity [15, 16]. In one of these models, mice harbor a single nucleotide recessive mutation in the Roquin gene. This gene codes for a RING (really interesting new gene) finger-E3 ligase that negatively regulates ICOS [15, 17]. The Roquinsan/san CD4+ T cells have a T cell intrinsic defect that results in elevated levels of ICOS and CXCR5, thus resembling TFH cells [15]. The Roquinsan/san mice exhibit spontaneous germinal center formation, and lupus-like features including elevated anti-dsDNA antibodies and IgG-immune complex deposition in the kidneys [15].
Collectively, these observations suggested that the ability of Treg cells to modulate ICOS levels on effector CD4+ T cells might contribute to their ability to suppress anti-chromatin B cell responses. To understand the relationship between ICOS expression, Treg cell activity, and anti-chromatin antibody production in more detail, we have used CD4+ CD25− T cells from Roquinsan/san mice in an adoptive transfer model of anti-chromatin antibody production. Our study addresses whether CD4+ CD25− T cells from these mice have an altered ability to help anti-chromatin B cells, and whether their enhanced expression of ICOS affects their susceptibility to Treg cell suppression.
2. Materials and Methods
2.1. Mice
Male and female mice between 6–20 weeks of age were maintained and bred under specific-pathogen-free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited Wistar Institute under the supervision of the Institutional Animal Care and Use Committee (IACUC). TS1 BALB/c mice harbor a TCR transgene specific for the site 1 (S1) peptide of hemagglutinin (HA) from the PR8 influenza virus [18]. The Roquinsan/san mutation was originally on the C57BL/6 background [15] but was backcrossed six-eight generations onto the BALB/c background for this study. These mice were then mated to generate TS1.Roquinsan/san mice. TS1 TCR transgenic mice were mated to HA28 mice that express influenza HA as a neoself antigen. TS1×HA28 mice contain a distinct population of Treg cells [19]. Site-directed-(sd)-VH3H9.HACII.Igκ−/− BALB/c [8] mice were generated as a source of anti-chromatin B cells. Here, mice carrying a sd-VH3H9 tg [20] were crossed with HACII mice that express HA under the control of the class II promoter. These mice were then mated to be kappa deficient (κ−/−) such that the vast majority of the B cells express the anti-chromatin receptor VH3H9/lambda1 [3]. CB17 (Igb) mice were purchased from the Charles River Laboratory and were used as receipt mice in the cell transfer studies.
2.2. In vitro Treg cell inhibition assay
Responder T cells for in vitro proliferation assays were obtained from the peripheral lymph nodes by sorting for CD4+ CD25− cells from BALB/c or BALB/c Roquinsan/san mice and CFSE-labeled as previously described [4]. Treg cells were sorted for CD4+ CD25+ expression from the peripheral lymph nodes of BALB/c or BALB/c Roquinsan/san mice. 5 × 104 CD4+ CD25− cells were stimulated with 0.125 μg/ml of anti-CD3 (2C11), 5 × 105 CD3-depleted BALB/c splenocytes and with 5 × 104 Treg cells where indicated. After three days, CFSE-labeled cells were analyzed on a flow cytometer.
2.3. Purification of Th and Treg cells
Peripheral lymph node cells from TS1, TS1. Roquinsan/san, and TS1 × HA28 mice were removed and stained for CD4 and CD25 [4]. Th cells from TS1 and TS1.Roquinsan/san mice were sorted for CD4+ CD25− cells, and Treg cells from TS1 × HA28 mice were sorted for CD4+ CD25+ expression.
2.4. T and B cell transfers
1 × 106 Th cells (from TS1, TS1.Roquinsan/san mice) with or without Treg cells (from TS1 × HA28 mice) were injected with 1000 hemagluttinating units of purified PR8 (A/Puerto Rico/8/34) virus i.v. into recipient CB17 mice [3, 21]. All transferred T cells were detected with the anti-clonotypic antibody, 6.5 [18], unless noted otherwise. One day after giving T cells and virus, splenocytes from sd-VH3H9.HACII.Igκ−/− (Iga) mice were depleted of red blood cells and an aliquot was surface stained to determine the frequency of anti-chromatin B cells (B220+ Igλ1+) [8]. CB17 (Igb) recipient mice were injected with splenocytes containing 5 × 106 anti-chromatin B cells (Iga).
2.5. Chromatin ELISAs
ELISA plates (ThermoLabSystems) were coated with 2 μg/ml of chromatin (a generous gift of M. Monestier, Temple University, Philadelphia, PA) overnight at 4 °C. The remaining steps were performed at room temperature. All washes were conducted at least eight times in 1× PBS/0.05% Tween 20. Following the coating step, plates were washed, blocked with 1% BSA/PBS/azide for at least 1 h, and washed again. Sera were then added at increasing dilutions, as indicated in figure, and incubated for a minimum of 1 h. Plates were washed and incubated with developing Ab anti-IgMa biotin (DS-1) (BD Biosciences), for at least 1 h. Finally plates were washed, incubated with streptavidin–AP (Southern Biotechnology Associates) for at least 1 h, and washed. The plates were developed with ImmunoPure p-nitro-phenyl phosphate (Pierce) as the substrate for 14–18 hours. Absorbances were read at dual wavelength, 405/650 nm using a microplate reader.
2.6. Determination of serum antibody titers
The serum anti-chromatin antibody titers were graphed based on the raw optical density or the percent inhibition in optical density for the groups of mice that received Th and Treg cells. The percent inhibition calculation was determined as follows: % inhibition = (the optical density of TS1 + Treg cells or TS1.Roquinsan/san + Treg cells sample/optical density of the TS1 or TS1.Roquinsan/san Th group, respectively) × 100.
2.7. Flow Cytometry
Splenic cells were stained with the following Abs: anti-CD4-PerCP-Cy5.5 (RM4.5), anti-B220-PerCP-Cy5.5 (RA3-6B2) (BioLegend), anti-CXCR5-PE (2G8), anti-IgMa-biotin (DS-1), anti-IgDa-biotin (AMS-9.1), anti-IgG1a-biotin (10.9), anti-IgG2aa-biotin (8.3), anti-Fas-PE-Cy7 (Jo2) (BD Pharmingen), anti-ICOS-PE (7E.17G9), anti-CD40L-PE (MR1), anti-IL-2-PE (JES6-5A4), anti-IL-4-PE (11B11), anti-IFN-γ-FITC (XMG1.2), streptavidin-allophycocyanin rat-IgG2b-PE (eB149), rat-IgG2a (eBR2a), hamster IgG-PE (eBio299Arm), anti-Foxp3-FITC (FJK-16s) (eBioscience), PNA-FITC (Sigma), and anti-IL-21-PE (149204) (R & D systems). The 6.5 antibody (anti-clonotype for the TS1 T cells [18]) was purified from hybridoma supernatant and biotinylated. Intracellular staining for CD40L and Foxp3 was performed following the instructions in the Fix/Perm kit from eBioscience.
2.8. Immunostaining
Spleens were frozen, sectioned, and stained [22] with anti-B220-biotin (RA3-6B2) (BD Pharmingen) and PNA-FITC (Sigma). Secondary reagents were anti-FITC-horseradish peroxidase (HRP) and streptavidin-AP (Southern Biotechnologies).
2.9. Statistical Analyses
Statistical significance was determined via the Wilcoxon signed-rank test and significance was ascribed when p < 0.05.
3. Results
3.1. The BALB/c Roquinsan/san model
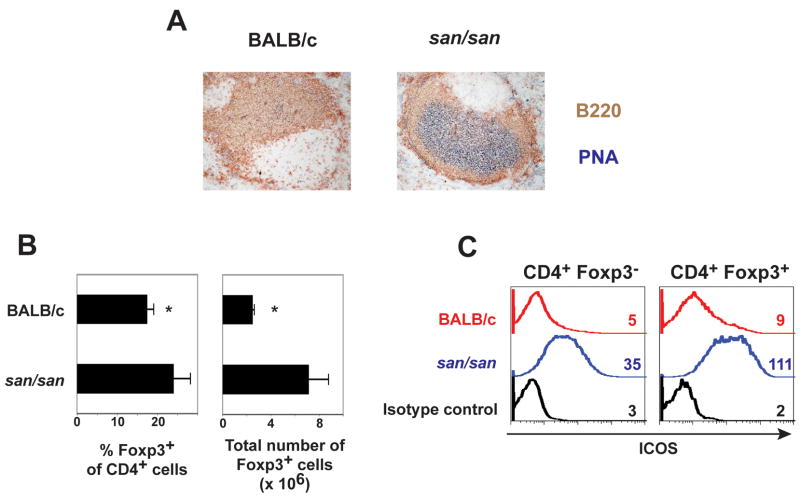
The original Roquinsan/san mice were derived from the C57BL/6 strain [15]. In order to evaluate the ability of T cells from Roquinsan/san mice to drive autoantibody production and to test their ability to be suppressed by Treg cells, we first needed to cross the mutation onto the BALB/c background, the strain in which the anti-chromatin Ig transgenic and TCR transgenic used in this study reside. BALB/c Roquinsan/san mice shared many features that were described in the autoimmune-prone C57BL/6 Roquinsan/san mice [15, 23, 24]. The spleens of BALB/c Roquinsan/san mice were enlarged (BALB/c Roquinsan/san 195.7 +/− 74.8 × 106 splenocytes vs. BALB/c 87.2 +/− 3.7 × 106 splenocytes (n=3; p <0.05)) and contained numerous germinal centers (Fig. 1A and data not shown). There was also a higher frequency and number of Foxp3+ cells as compared to control BALB/c mice (Fig. 1B). Moreover, many of the CD4+ T cells from BALB/c Roquinsan/san mice had elevated ICOS levels (Fig. 1C).
Figure 1.
Phenotype of BALB/c Roquinsan/san (san/san) mice A) This is representative immunohistochemical staining of the spleen showing B220 to mark B cells, and PNA to identify germinal centers (n=3). B) The graphs show the average percentage +/− standard deviation of Foxp3+ cells versus total CD4+ T cells (left) (n=5) and average total number of Foxp3 +/− the standard deviation in the spleen of the indicated mice (n=3). C) Graphs depict the ICOS expression levels on splenic CD4+ Foxp3− and CD4+ Foxp3+ T cells. The numbers designate the geometric mean fluorescence intensity. Data from one of three independent experiments with similar results are shown.
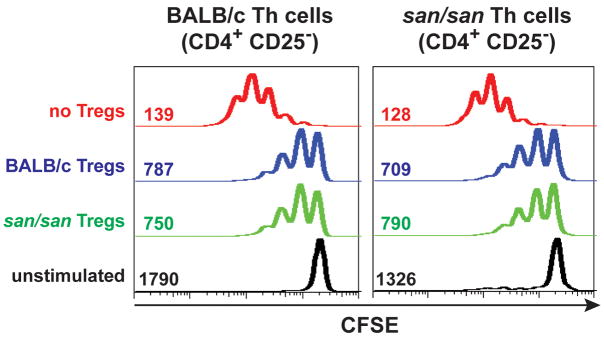
To examine whether the Roquinsan/san mutation has a direct effect on the ability of Treg cells to mediate suppression in vitro, we compared CD4+ CD25+ T cells from BALB/c Roquinsan/san mice with those from BALB/c mice for their ability to suppress proliferation of CD4+ T cells following stimulation with anti-CD3 and antigen presenting cells. The BALB/c and Roquinsan/san Treg cells inhibited the proliferation of CD4+ CD25− T cells to equivalent degrees (Fig. 2). Moreover, CD4+ CD25− T cells from Roquinsan/san and BALB/c mice were equally susceptible to suppression by CD4+ CD25+ T cells obtained from either BALB/c or Roquinsan/san mice (Fig. 2). Thus, the Roquinsan/san mutation does not appear to affect the intrinsic capacity of CD4+ CD25+ Treg cells to mediate suppression, as assessed by this in vitro T cell proliferation assay.
Figure 2.
TS1.Roquinsan/san Th cell proliferation is inhibited by Treg cells in vitro. These graphs are representative profiles of CFSE-labeled CD4+ CD25− cells from the indicated mice after three days of culture stimulated with CD3. The numbers are the corresponding geometric mean fluorescence of the data from the shown experiment. Data from one of four independent experiments with similar results are shown.
3.2. The in vivo model for anti-chromatin B cell activation
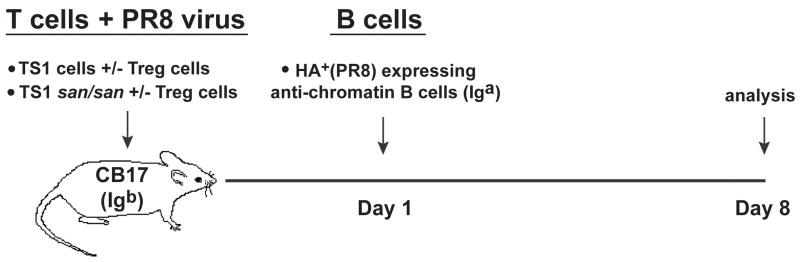
We established a transgenic CD4+ T cell and autoreactive B cell third-party transfer model in which the lymphocytes and antibody production can be tracked in an immmunocompetent mouse (Fig. 3) [3]. Anti-HA Th (CD4+ CD25−) cells were obtained from TS1 or TS1.Roquinsan/san mice that recognize the S1 peptide from the HA (hemagglutinin) protein of the PR8 influenza virus [18]. The anti-HA Treg (CD4+ CD25+) cells were purified from the TS1 × HA28 mice, in which Treg cells are heavily enriched for anti-HA specificity [19]. The CD4+ T cells with or without Treg cells were transferred into CB17 mice (Igb allotype) along with PR8 virus to initiate their activation (Fig. 3).
Figure 3.
Experimental model where CB17 (Igb) mice received CD4+ CD25−, TS1 or TS1.Roquinsan/san, and/or CD4+ CD25+ Treg cells from TS1 × HA28 mice and influenza virus at day 0 to prime the T cells. The following day, PR8 HA anti-chromatin B cells (Iga) were transferred from sd-VH3H9.HACII.Igκ−/− donors. Spleen cells from recipient CB17 mice were analyzed on day 8.
As a source of anti-chromatin B cells, we used site-directed-(sd)-VH3H9 kappa deficient (κ−/−) mice [3]. To provide cognate CD4+ T cell help for anti-chromatin B cells using the anti-HA T cells, the sd-VH3H9.Igκ−/− mice were bred to an additional transgenic lineage that expresses the PR8 HA as a neo-self antigen under the control of the MHC class II promoter (HACII) [25]. In this case, the PR8 HA is expressed by class II bearing cells, including B cells [26].
3.3. Treg cells are unable to fully block TS1.Roquinsan/san Th cell help for anti-chromatin B cells
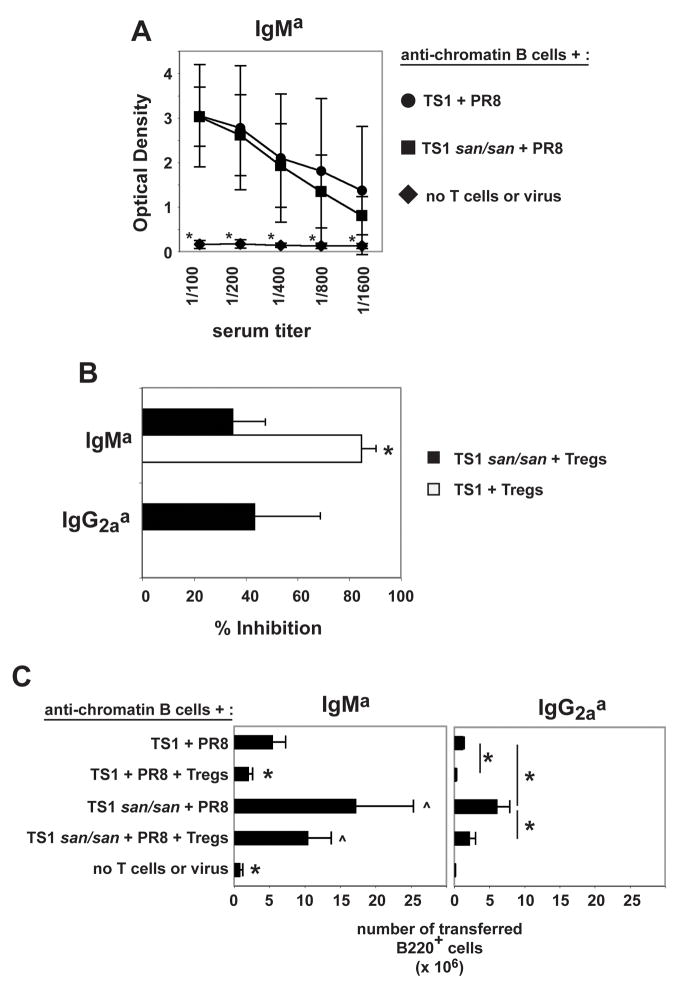
We evaluated the ability of the TS1 or TS1.Roquinsan/san Th cells to provide help for autoantibody responses with or without Treg cells by examining the anti-chromatin Iga serum antibody levels from recipient mice eight days post-transfer. Th cells from TS1 and TS1.Roquinsan/san mice were both able to drive anti-chromatin IgMa antibody production to a similar extent (Fig. 4A). While the Treg cells significantly reduced the anti-chromatin IgMa antibody production induced by TS1 Th cells, the decrease in anti-chromatin IgMa production with Treg and TS1.Roquinsan/san Th cells was much less pronounced (Fig. 4B). Unlike TS1 Th cells, TS1.Roquinsan/san Th cells were able to induce anti-chromatin IgG2aa antibody production, which was suppressed by the addition of Treg cells to a comparable degree as the IgM response (Fig. 4B and data not shown). Thus, Treg cells were less able to suppress anti-chromatin antibody production induced by CD4+ CD25− T cells from TS1.Roquinsan/san mice than from TS1 mice (Fig. 4B).
Figure 4.
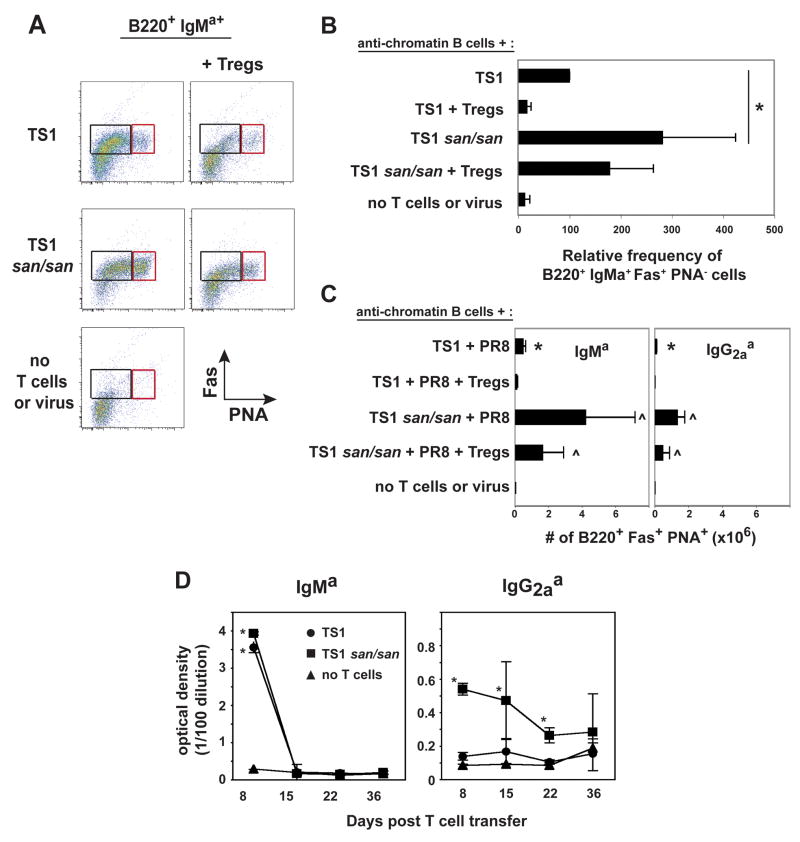
TS1 and TS1.Roquinsan/san T cells induce anti-chromatin antibodies at day 8. Treg cells completely block this in the context of TS1 T cells, but only partially block with TS1 Roquinsan/san T cells. A) The graph shows the average optimal density +/− the standard deviation of the indicated serum titer of three independent experiments. (*) indicates that the serum titer of the group is significantly different compared to all other groups (n=3). B) The graphs show the average percent inhibition +/− the standard deviation of the 1/100 diluted serum anti-chromatin IgMa from mice that received TS1 + Treg cells (white) or TS1.Roquinsan/san Th + Treg cells (black). The % inhibition of IgG2aa anti-chromatin by Treg cells is shown for the TS1 Roquinsan/san T cells only as the TS1 did not induce IgG2aa anti-chromatin antibodies. (*) indicates that the serum titer of the group is significantly different compared to all other groups (n=3). C) The graphs show the average IgMa and IgG2aa B cell recovery +/− the standard deviation in the spleen. (*) denotes significant difference to all groups (IgMa graph) or the specified groups (IgG2aa graph) (p < 0.05). The (^) marked groups are significantly different from all other groups but not from each other (n=3). No IgG1a B cells were detected in any of the groups tested (data not shown).
TS1.Roquinsan/san Th cells also resulted in the recovery of more IgMa and IgG2aa transferred B cells as compared to mice that received TS1 Th cells (Fig. 4C). Treg cells again resulted in a significant reduction of IgMa B cell numbers in the context of TS1 Th cells. Notably, although there were modest (but not significant) reductions in the recovery of IgMa B cells in mice that received both TS1.Roquinsan/san Th and Treg cells relative to those that only received TS1.Roquinsan/san Th cells, this number was comparable to the number of IgMa B cells in mice that received TS1 Th cells in the absence of Treg cells (Fig. 4C). Likewise, the number of IgG2aa B cells was significantly reduced in the presence of Treg cells in both TS1 and TS1.Roquinsan/san recipient mice. However, in the case of TS1.Roquinsan/san recipient mice, the frequency of IgG2aa B cells in mice that received Treg cells was comparable to those in mice that received TS1 Th cells without Treg cells (Fig. 4C). Thus, Treg cells can decrease the frequency of anti-chromatin B cells in mice receiving Th cells from both backgrounds, but the TS1.Roquinsan/san T cells retain a greater capacity to support anti-chromatin B cell activation.
3.4. Th cells from TS1.Roquinsan/san mice induce an anti-chromatin germinal center phenotype but not long-lived antibody production
One of the prominent features of Roquinsan/san mice is the formation of spontaneous germinal centers (Fig. 1A) [15]. We determined if anti-chromatin B cells participated in these reactions by counting the number of transferred B cells that co-expressed PNA and Fas (Fig. 5A and C). Mice that received TS1.Roquinsan/san Th cells had a higher number of IgMa and IgG2aa germinal center B cells compared to mice that received TS1 Th cells (Fig. 5C). Additionally, the IgMa B cells from mice that received the TS1.Roquinsan/san Th cells had a greater frequency of cells that were Fas+ in the absence of PNA, a phenotype of activated B cells [27], compared to mice that received TS1 Th cells (Fig. 5A left and right box, 5B). Treg cells significantly reduced the number of IgMa and IgG2aa germinal center B cells in mice that received TS1 cells (Fig. 5C). On the other hand, mice that received TS1.Roquinsan/san Th cells and Treg cells did not have a significant drop in germinal center B cells, although the numbers were reduced (Fig. 5C). TS1.Roquinsan/san Th cells with or without the addition of Tregs resulted in more germinal center anti-chromatin B cells than TS1 Th cells (Fig. 5C).
Figure 5.
At day 8 mice that received TS1.Roquinsan/san Th cells have more anti-chromatin B cells with a germinal center phenotype. A) Representative dot plots of the splenic B220 + IgMa+ cells that express the germinal center markers, PNA and Fas. The right box shows the cells that have a germinal center phenotype (PNA+ Fas+) and the left box indicates the anti-chromatin B cells that are activated without a germinal center phenotype, PNA− Fas+ (n=3). B) The graph shows the average +/− standard deviation of the relative percentage of the B220+ IgMa+ PNA− Fas+ population in the spleen. The percentage of B220+ IgMa+ PNA− Fas+ cells in the TS1 group was set at 100. The other groups were determined by the following formula: (% B220+ IgMa+ PNA− Fas+ in a non-TS1 group/% B220+ IgMa+ PNA− Fas+ in the TS1 group)*100. (*) indicates a significant difference (n=3). C) The graphs show the average IgMa and IgG2aa germinal center (B220+ PNA+ Fas+) B cell recovery +/− the standard deviation in the spleen. (*) indicates significant difference compared to all groups (p < 0.05). The (^) marked groups are significantly different from all other groups but not from each other (n=3). D) Long term serum anti-chromatin antibody production. The graphs shows the O. D. +/− the standard deviation of the serum at the 1/100 titer from the indicated groups after immunization. (*) indicates significantly different compared to mice that did not receive Th cells or virus (n=3).
Since the TS1.Roquinsan/san Th cells induced a greater germinal center response, we wanted to determine whether they would also promote long-term serum autoantibody production. However, similar to TS1 cells, TS1.Roquinsan/san cells did not promote long-lasting anti-chromatin IgMa or IgG2aa serum antibody production (Fig. 5D). These data suggest that the failure of anti-chromatin B cells to become long-term plasma cells in this system may be intrinsic to the B cells themselves, rather than being due to some inadequacy of T cell help.
3.5. TS1.Roquinsan/san Th cells maintain high levels of ICOS in the presence of Treg cells
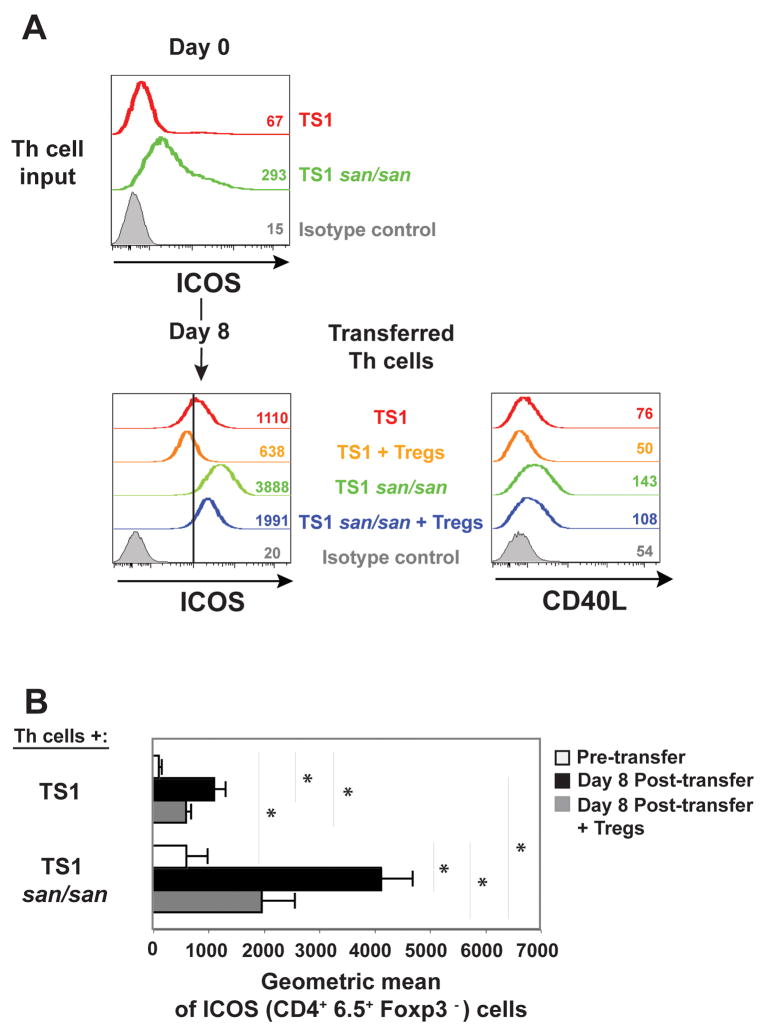
TS1.Roquinsan/san Th cells expressed higher levels of ICOS relative to TS1 Th cells ex vivo (Fig. 6A), like what was observed for the non-TCR transgenic Roquinsan/san Th cells (Fig. 1C) [15]. By day eight post in vivo transfer, the TS1 Th (CD4+ Foxp3−) cells had upregulated ICOS expression but when Treg cells were included in the transfer this upregulation was more modest (Fig. 6A and 6B). TS1.Roquinsan/san Th cells also increased ICOS levels by day eight but to a more dramatic extent (Fig. 6A and 6B). This too was diminished in the context of Treg cells, but nevertheless remained well above the expression on TS1 Th cells in the absence of Treg cells (Fig. 6A and 6B). CD40L expression showed a similar pattern, being highest in TS1.Roquinsan/san Th cells and only slightly diminished in the context of Treg cells (Fig. 6A). TS1.Roquinsan/san Th cells and TS1 Th cells express CD4 at similar levels (data not shown).
Figure 6.
ICOS and CD40L expression on the transferred CD4+ Th cells. A) ICOS expression on CD4+ Foxp3− cells pre-transfer (day 0) as well as ICOS and CD40L expression at day 8 post-transfer in the spleen on the indicated T cell populations. The numbers are the geometric mean fluorescence of the lines in the graph shown. For a reference point the black vertical line marks 1000 on the x axis of the histogram showing ICOS expression. The data are from one experiment that is representative of three independent experiments. B) The graph shows the average geometric mean for surface ICOS expression +/− the standard deviation on the CD4+ 6.5+ FoxP3− cells at either day 0 or day 8 (n=3).
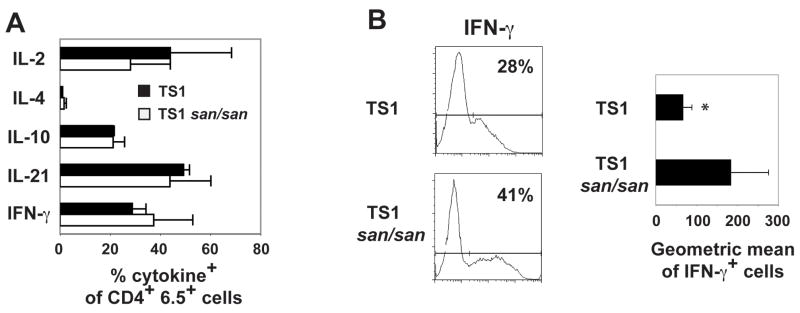
It was also possible that TS1.Roquinsan/san Th cells are able to provide efficient help for anti-chromatin B cell responses because the sanroque mutation leads to increased cytokine production. However, no differences were found in the frequency of TS1 versus TS1.Roquinsan/san Th cells that synthesized a variety of cytokines following stimulation (Fig. 7A). There was a difference between the two groups in that the IFN-γ+ cells stained more brightly in the TS1.Roquinsan/san Th cells than the TS1 Th cells (Fig. 7B). The increased mean fluorescence intensity for the IFN-γ+ TS1.Roquinsan/sanTh cell population was consistent with a previous report that showed that Roquinsan/san CD4+ T cells produced more IFN-γ compared to T cells from a control mouse [15].
Figure 7.
Intracellular cytokine production. A) The graph represents the average percent positive of the indicated cytokine +/− the standard deviation in the CD4+ 6.5+ T cell population (IL-2, n=3; IL-4, n=3; IL-10, n=2; IL-21, n=3; IFN-γ, n=3) at day 8 post-transfer. B) Representative histogram of IFN-γ and the graph shows the average geometric mean +/− the standard deviation of the IFN-γ+ cells from all experiments (n=3).
4. Discussion
Autoreactive B cells are kept in check by multiple mechanisms [28]. Some B cells are eliminated from the repertoire by cell death or receptor editing in the bone marrow, while others, including anti-chromatin B cells, can be found in the periphery albeit with a reduced life-span [29]. Importantly, anti-chromatin B cells can be rescued and induced to secrete autoantibodies by the administration of T cell help [3]. Thus, another mechanism to control autoreactive B cells is to regulate the availability of CD4+ T cell help.
Previous studies have shown that one mechanism for regulating the ability of CD4+ T cells to provide help for autoreactive B cells is the counterbalancing influence of Treg cells [3]. However, whether the susceptibility of autoreactive CD4+ T cells to Treg cell activity varies with phenotypic differences between Th cell populations has been unknown. We demonstrated here that the sanroque mutation altered the balance of activation and regulation of responding CD4+ T cells such that the ability of Treg cells to inhibit autoantibody production was substantially impaired. Thus, whereas Treg cells caused significant reductions in B cell survival, autoantibody production, and anti-chromatin germinal center B cell formation induced by TS1 Th cells, they were much less able to inhibit TS1. Roquinsan/san Th cell activity. This did not appear to be due to an intrinsic failure of Roquinsan/san Th cells to respond to Treg cells; the in vitro proliferation of Roquinsan/san CD4+ T cells could be suppressed by Treg cells as efficiently as could wild-type CD4+ T cells. Moreover, autoantibody levels, B cell survival, and anti-chromatin germinal center B cell formation induced by TS1.Roquinsan/san Th cell did appear to be reduced in mice that also received Treg cells, even though in several cases this reduction was not statistically significant. The key impact of the sanroque mutation was instead to enhance the overall ability of the Th cells to provide help for autoantibody response, such that even in the context of Treg cell activity, the TS1.Roquinsan/san Th cells that were not being blocked in their ability to provide B cell help. Interestingly, changes in other E3 ligases, such as Cbl-b or TRAF6, can also cause Th cells to become refractory to Treg cell suppression but the mechanism remains unknown [30, 31].
It was noteworthy that CD4+ T cells from Roquinsan/san mice exhibit many of the properties that are typically used to characterize TFH cells, including high ICOS levels, CXCR5 expression, and follicular localization (Fig. 1) [15]. Indeed, a prominent cells is their facility in providing help for B cells [13]. When characteristic of TFH TS1.Roquinsan/san Th cells were used as the source of help for anti-chromatin B cells there was enhanced anti-chromatin B cell recovery, germinal center development, and isotype switching compared to that induced by TS1 Th cells. Significantly, even though TS1.Roquinsan/san Th cells were able to enhance the formation of anti-chromatin germinal center B cells, there was no evidence of long-lived anti-chromatin antibody production. The data suggest that anti-chromatin B cells, which normally have a reduced lifespan [32], may be intrinsically blocked from developing into long-term plasma cells. Other mouse models have also described negative selection at this stage where autoreactive B cells participate in germinal centers but do not go on to the memory or long-lived plasma cell compartments [33, 34].
It is possible that the elevated levels of ICOS found on TFH cells might compromise their ability to be effectively regulated by Treg cells as we suggest for Roquinsan/san Th cells. In this regard, it was recently shown that a fraction of Treg cells transferred into immunodeficient mice can become TFH cells and promote germinal center development in the Peyer’s patches even in the presence of the remaining non-converted Treg cells [35]. These data suggest the possibility that TFH cells and Roquinsan/san Th cells are partially resistant to Treg cell suppression. Overall, these data reinforce the role of ICOS as a critical modulator of T-B cell interactions and show that mutations affecting this pathway can override or modulate B cell tolerance mechanisms that are exerted by Treg cells.
Acknowledgments
First, we thank Dr. Carola Vinuesa, Dr. Christopher Goodnow, and the Australian Phenome Bank at the Australian National University, Canberra, ACT 2601, Australia for generously providing the Roquinsan/san mice. Secondly, we thank Daniel Hussey, David Ambrose, and J. S. Faust from the Wistar Flow Cytometry Facility. Finally, we thank Dr. Audrey Y. Park for critical review of the manuscript. J. E. is supported by the National Institutes of Health (AI32137 and AR47913) and A. J. C. by the National Institutes of Health (AI24541). Additional support for J. E. and A. J. C. are provided by the National Cancer Institute (CA 10815) and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fields ML, Hondowicz BD, Wharton GN, Adair BS, Metzgarm MH, Alexander ST, Caton AJ, Erikson J. The regulation and activation of lupus-associated B cells. Immunological Reviews. 2005;204:165–183. doi: 10.1111/j.0105-2896.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 2.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. Journal of Experimental Medicine. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo S-j, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 4.Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+CD25+ Regulatory T Cells Inhibit the Maturation but Not the Initiation of an Autoantibody Response. J Immunol. 2005;175:4255–64. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- 5.Sekiguchi D, Jainandunsing S, Fields M, Maldonado M, Madaio M, Erikson J, Weigert M, Eisenberg R. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-dsDNA B cells. Journal of Immunology. 2002;168:4142–53. doi: 10.4049/jimmunol.168.8.4142. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 7.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 8.Fields ML, Nish SA, Hondowicz BD, Metzgar MH, Wharton GN, Caton AJ, Erikson J. The influence of effector T cells and Fas ligand on lupus-associated B cells. J Immunol. 2005;175:104–11. doi: 10.4049/jimmunol.175.1.104. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 10.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–9. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 11.Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. 2001;166:3659–62. doi: 10.4049/jimmunol.166.6.3659. [DOI] [PubMed] [Google Scholar]
- 12.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–5. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 13.King C, Tangye SG, Mackay CR. T Follicular Helper (T(FH)) Cells in Normal and Dysregulated Immune Responses. Annu Rev Immunol. 2008 doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 14.Hutloff A, Buchner K, Reiter K, Baelde HJ, Odendahl M, Jacobi A, Dorner T, Kroczek RA. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3211–20. doi: 10.1002/art.20519. [DOI] [PubMed] [Google Scholar]
- 15.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–8. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–5. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, Hutloff A, Giles KM, Leedman PJ, Lam KP, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 18.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. Journal of Experimental Medicine. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan MS, Riley MP, von Boehmer H, Caton AJ. Anergy and suppression regulate CD4(+) T cell responses to a self peptide. Eur J Immunol. 2000;30:136–44. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 21.Reed AJ, Riley MP, Caton AJ. Virus-induced maturation and activation of autoreactive memory B cells. J Exp Med. 2000;192:1763–74. doi: 10.1084/jem.192.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hondowicz BD, Batheja AO, Metzgar MHAJP, Perng OA, Willms S, Caton AJ, Erikson J. Efficient help for autoreactive B-cell activation requires CD4+ T-cell recognition of an agonist peptide at the effector stage. European Journal of Immunology. 2009;39:2377–2382. doi: 10.1002/eji.200939471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–76. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–41. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 26.Reed AJ, Noorchashm H, Rostami SY, Zarrabi Y, Perate AR, Jeganathan AN, Caton AJ, Naji A. Alloreactive CD4 T cell activation in vivo: an autonomous function of the indirect pathway of alloantigen presentation. J Immunol. 2003;171:6502–9. doi: 10.4049/jimmunol.171.12.6502. [DOI] [PubMed] [Google Scholar]
- 27.Mandik L, Nguyen K-AT, Erikson J. Fas receptor expression on B-lineage cells. Eur J Immunol. 1995;25:3148–3154. doi: 10.1002/eji.1830251124. [DOI] [PubMed] [Google Scholar]
- 28.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Seo S, Mandik-Nayak L, Erikson J. B Cell anergy and Systemic Lupus Erythematosus. Current Directions in Autoimmunity. 2003:6. doi: 10.1159/000066853. [DOI] [PubMed] [Google Scholar]
- 30.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–65. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 31.King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–92. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 32.Mandik-Nayak L, Seo S-j, Eaton-Bassiri A, Allman D, Hardy RR, Erikson J. Functional consequences of the developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. Journal of Immunology. 2000;164:1161–1168. doi: 10.4049/jimmunol.164.3.1161. [DOI] [PubMed] [Google Scholar]
- 33.Notidis E, Heltemes L, Manser T. Dominant, hierarchical induction of peripheral tolerance during foreign antigen-driven B cell development. Immunity. 2002;17:317–27. doi: 10.1016/s1074-7613(02)00392-8. [DOI] [PubMed] [Google Scholar]
- 34.Paul E, Lutz J, Erikson J, Carroll MC. Germinal center checkpoints in B cell tolerance in 3H9 transgenic mice. Int Immunol. 2004;16:377–84. doi: 10.1093/intimm/dxh035. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–92. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]