Abstract
With the goal of rational design of systemic gene delivery system and efficient transfection of capillary endothelial cells forming the blood-brain barrier (BBB), we synthesized several short polyamines with reducible disulfide backbones for pDNA packaging, internalization and consequent release from endosomal compartments. The synthetic cationic polymers prepared from short linear PEI (pLPEI), triethylenetetramine (pTETA), and spermine (pSPE), demonstrated very low toxicity, good condensation capacity, and high levels of pDNA protection, producing small particulate nanoformulations. Mild reduction of the disulfide backbone allowed complete release of pDNA from these polyplexes. In vitro transfection of murine brain capillary endothelial bEnd.3 cells with pSPE, pTETA, and pLPEI polyplexes was 2.3–4.9 times more effective compared with the non-degradable LPEI 22kDa reagent (ExGen500) in the presence of serum. Their transfection ability was noticeably decreased following inhibition of the cellular reduced glutathione (GSH). After cellular uptake of biodegradable polyplexes, a disperse distribution of labeled pDNA in the cytoplasm of transfected cells was observed in contrast to ExGen500. Based on these polyamines, novel multifunctional polyplexes have been developed for efficient nuclear delivery of pDNA by co-application of NLS-peptide and PEG-modified intercalating conjugates. Significant increase of nuclear accumulation was observed, and the transfection of bEnd.3 cells was additionally enhanced nearly 2-fold, demonstrating 8.5-, 6.3- and 3.7-fold better levels for pLPEI, pTETA, and pSPE, respectively, compared to ExGen500. Following brain-specific targeting, these safe and effective polyplexes may be converted into systemic nanocarriers for gene delivery and transfection of the BBB.
Keywords: gene delivery, blood-brain barrier, non-viral transfection, biodegradable polyamines, nuclear targeting, NLS peptide
1. Introduction
Designing a safe non-viral gene delivery system which would be nontoxic and efficient for systemic administration is essential for a variety of medical applications. In recent years, cationic polymers have gained a certain importance as prospective gene delivery vehicles and competitors of sometimes unsafe viral vectors. Many cationic polymers could efficiently condense pDNA into compact particles and facilitate cellular uptake of nanosized complexes. Their efficiency was strongly associated with cytotoxicity and depended upon the molecular weight of the polycations. Successful non-viral gene delivery required a compromise between transfection efficiency and cytotoxicity. Besides cytotoxicity, polycations with a high-molecular mass may restrict pDNA release from polyplexes and hamper the ability of pDNA to escape endosomes and be delivered into the nuclei for efficient transgene expression. A reasonable solution was proposed by using biodegradable polycations. Various hydrolysable cationic polymers have been investigated, such as poly(β-amino esters) [1–3], poly(amino esters) [4, 5], and poly(amido amines) [6, 7]. However, the hydrolysis or enzymatic cleavage of these polycations usually requires many days to complete. By contrast, disulfide bonds can be degraded inside endosomes and in the cytoplasm in the presence of intracellular reduced glutathione, GSH [8, 9]. The concentration of extracellular GSH is typically 100–1000 times lower than intracellular GSH and the structural integrity of polymers with a disulfide backbone could be well-maintained in the extracellular environment as well as in the blood. This feature makes disulfide bonds a preferable choice when developing biodegradable nanocarriers for systemic gene delivery. In this study, we synthesized several short biodegradable polyamines and studied them as functional components of advanced polyplexes for transfection of brain capillary endothelial bEnd.3 cells. Compared to an efficient non-biodegradable polycationic transfecting reagent, ExGen500, these biodegradable polycations showed superior transfection ability and much lower cytotoxicity in this in vitro model of the BBB and in other cellular systems [10].
A major barrier for non-viral gene delivery is the nuclear envelope. Nuclear proteins move from the cytoplasm into the nuclei actively through the size-restricted nuclear pores using nuclear localization signals (NLS) and nuclear transport receptors [11]. In rapidly-dividing cells, pDNA is able to enter the nuclei during mitosis, but in slow-dividing endothelial cells, it is a very rare event, and the large exogenous pDNA molecules must be assisted to move across the cytoplasm into the nuclei. Although transfected pDNA can escape endosomes and accumulate in the perinuclear area due to an enhanced buffering capacity of many polycations, various approaches have been evaluated to further stimulate the nuclear entry of pDNA including application of NLS peptide [12, 13]. Recently, we described a novel class of NLS-PEG-tris-acridine intercalating conjugates for direct non-covalent pDNA modification, which were capable to significantly enhance the expression of luciferase-encoding plasmid mediated by lower than usual amounts of both lipo- and polyplexes [14]. Pre-formulation of pDNA with small amounts of NLS conjugates enhanced nuclear accumulation of pDNA and improved transgene expression in slow-dividing mouse brain capillary endothelial bEnd.3 cells.
The BBB is a physiological barrier to protect the neurons from potential toxic agents in the blood. Brain capillary endothelial cells, the major constitutive component of the BBB, express a variety of drug efflux transporters that selectively and efficiently restrict entrance of many substances, including chemotherapeutic drugs, into the brain [15]. Therefore, the efficacy of many therapies against brain diseases is largely decreased. Application of systemic transfection of the BBB for transient and selective inhibition of certain drug efflux transporters could significantly improve drug accumulation and efficacy of chemotherapy in the brain [16].
2. Materials and Methods
2.1. Materials
All solvents and reagents, except those which are specifically mentioned, were purchased from Sigma-Aldrich (St. Louis, MO) at the highest available quality grade and used without purification. Both gWIZ-Luc (6.7 kb) and gWIZ-GFP (5.7 kb) plasmids were originally purchased from Gene Therapy System (San Diego, CA), propagated in E. coli (DH5-α), and isolated using a QIAGEN Giga Endo-free plasmid purification kit (Valencia, CA). Plasmid DNA integrity and topology were analyzed by agarose gel electrophoresis.
2.2. Synthesis and characterization of biodegradable polycations
Equimolar amounts of N,N′-cystaminebisacrylamide (CBA) and corresponding polyamines, linear polyethylenimine (LPEI, MW 473), triethylenetetramine (TETA) and spermine (SPE), were dissolved in a minimal volume of methanol containing 10% water and stirred in tightly-closed vials under argon overnight at 60°C. An additional amount of polyamine (20%), dissolved in 1 mL of methanol, was added to viscous solutions, and the reaction was continued for another 3 h at 65°C. The reaction mixtures were diluted with 1M HCl to pH 5 and water to obtain homogeneous solutions and dialyzed (MWCO 2,000 Da) several times against deionized water. The polymeric products, pLPEI, pTETA and pSPE, were concentrated in vacuo and lyophilized. The typical yields varied from 40 to 75%. The nitrogen content of obtained polyamines was calculated from elemental analysis data (M-H-W Laboratories, Phoenix, AZ). The amount of protonated amino groups was measured by potentiometric titration with 0.01M HCl in 0.2M sodium chloride. The buffering capacity of polyamines was calculated based on the equation:
where ΔVHCl is the volume of 0.1 M HCl solution required for the polymer solution’s pH change from 7.4 to 5, and Nmol is the total amount of amine groups in the dissolved polyamines. Average molecular weight of the obtained polyamines was determined using a membrane osmometer, Osmomat 090 (Gonotec, Berlin, Germany) (Table 1).
Table 1.
Physico-chemical characteristics of synthesized polycations
| Polycation | Block MW | Measured MW (linearity R2)a | n° of blocks (chargeable aminogroups) | Nitrogen (total N) content, μmol/mgb | Protonated groups at pH7.0(5.0) μmol/mgc | Buffering capacity, % |
|---|---|---|---|---|---|---|
| pLPEI | 473 | 5,200 (0.96) | 8 (88) | 4.41 | 0.50 (0.75) | 20.9 |
| pTETA | 143 | 2,630 (1.00) | 7 (28) | 1.48 | 0.29 (0.41) | 17.4 |
| pSPE | 200 | 2,450 (0.99) | 6 (24) | 1.31 | 0.29 (0.40) | 15.7 |
Determined by membrane osmometry in water at 25°C;
Based on elemental analysis;
Obtained by potentiometric titration with 0.1N HCl in 0.2M sodium chloride solution.
2.4. Preparation of biodegradable polyplexes
Different amounts of polyamines (2 mg/mL in PBS) were added to 45 μg of pDNA in 200 μL of 20 mM HEPES buffer and incubated for 30 min at 25°C. When necessary, pDNA was initially pre-mixed with different amounts of NLS-PEG5000-tris-acridine (10 mg/mL) and incubated for 30 min at 25°C. The particle size and ζ-potential of these complexes were measured by dynamic light scattering using a Zetasizer Nano ZS90 (Malvern Instruments, UK). All measurements were performed in triplicate (PBS, 25 °C).
2.5. Electrophoresis mobility assay
High-molecular weight complexes of biodegradable polyamines with pDNA were prepared at varying N/P ratios from 5:1 to 30:1 was analyzed by an electrophoresis mobility assay. The samples were divided into two groups. One of these groups was treated with 25mM of dithiothreitol (DTT) for 30 min at 25°C. The treated and untreated samples were then loaded on 0.8% (w/v) UltraPure agarose gel (Invitrogen, Carlsbad, CA) and analyzed by electrophoresis at 120V for 35 min in 1×TAE buffer. After electrophoresis, the gel was stained by ethidium bromide and subjected to image analysis.
2.6. Resistance of polymer/pDNA complexes to DNase I
Polyplexes (N/P = 15) were exposed to DNase I (1 U/μg pDNA) at 37 °C for different time intervals in DNase reaction buffer (Promega, Pittsburgh, PA). The incubation was stopped on ice and by the addition of 10 μL of Stop-buffer (Promega) and 30 μL of heparin sodium solution (5000 USP units/mL, American Pharmaceutics Partners, Schaumburg, IL). The samples were loaded on 0.8% agarose gel and analyzed by electrophoresis as described above.
2.7. Cell line culture
Murine brain capillary endothelial cell line bEnd.3 was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultivated up to 27–29 passages in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 2% penicillin-streptomycin in a humidified incubator containing 5% CO2 at 37 °C.
2.8. In vitro transfection
All transfection experiments were performed in the presence of 10% FBS. Unless otherwise mentioned, bEnd.3 cells were seeded in 96-well plates with an initial density of 7 × 103 cells per well 24 h prior to transfection to obtain 80% confluency upon transfection. The transfection complexes containing 0.5 μg of pDNA were added to each well and incubated for 4 h at 37 °C. Then, the medium was replaced with fresh full medium and cells were incubated for an additional 40 h. For the cells pre-treated by D, L-buthionine sulfoxamine (BSO), different amounts of BSO solution (20 mM in PBS) were added to wells 18 h prior to transfection. Generally, pDNA was first incubated with NLS-PEG5000-tris-acridine conjugate for 30 min at 25°C, and then mixed with a polycation to form final transfection complexes. Luciferase expression level was measured using a Bio-Tek FLx800 microplate fluorescence reader and a firefly luciferase assay kit (Biotium, Hayward, CA). A calibration curve was plotted for luciferase at various concentrations. Protein content was measured by BCA assay (Pierce Biotechnology, Rockford, IL). All experiments were performed in 5 parallels and the results were expressed as ng of luciferase per mg of protein.
2.9. Confocal microscopy analysis
pDNA was labeled with CX-Rhodamine by the Label IT Tracker intracellular nucleic acid localization kit (Mirus Bio Corporation, Madison, WI) according to the manufacturer’s instructions. bEnd.3 cells were seeded on 8-chamber culture slides (BD Biosciences, Bedford, MA) with an initial cell density of 4 × 104 per chamber 24 h prior to transfection. The transfection complexes containing 1.4 μg of rhodamine-labeled pDNA were added to the cells as described above. After transfection, cells were washed by PBS and fixed by 4% (w/v) paraformaldehyde for 15 min on ice. Then SlowFade gold with DAPI solution (Invitrogen) was applied to the cells. The slides were carefully sealed and observed under an LSM 510 laser confocal scanning microscope (Carl Zeiss Inc., Germany).
gWIZ-GFP plasmid DNA which encodes green fluorescent protein was also used to evaluate the transfection efficiency of the polymeric gene delivery carriers. The transfection was conducted under identical procedure as mentioned above. After 44 h of further incubation, the cells were fixed by 4% paraformaldehyde and observed at 488nm.
2.10. Cytotoxicity assay
Cytotoxicity of transfection complexes in bEnd.3 cells was evaluated by thiazolyl blue tetrazolium bromide (MTT) assay. Briefly, bEnd.3 cells were seeded in 96-well plates with an initial density of 7 × 103 per well 24 h prior to the transfection. DNA/polycation complexes were incubated with cells using the exact same procedure as in the transfection. Then, 20 μL of the MTT solution (5 mg/mL) was added to each well and formazan crystals were allowed to form for 4 h. The medium was carefully removed and 200 μL of dimethylsulfoxide (DMSO) was added to each well. The plate was then incubated for 15 min at 37 °C and the absorbance of formazan was measured at 570 nm using a Model 680 microplate reader (BioRad, Hercules, CA). The experiments were performed in 5 parallels and relative cell viability was expressed as percentage viability by comparison to untreated control cells.
2.11. Statistical analysis
Experiment results are presented as the means ± SEM. Statistical significance was determined by the paired sample’s t-test using SPSS 13.0 software (SPSS Inc., Chicago, IL). In all cases, P < 0.05 was considered as statistically significant.
3. Results
3.1. Synthesis and characterization of biodegradable polycations
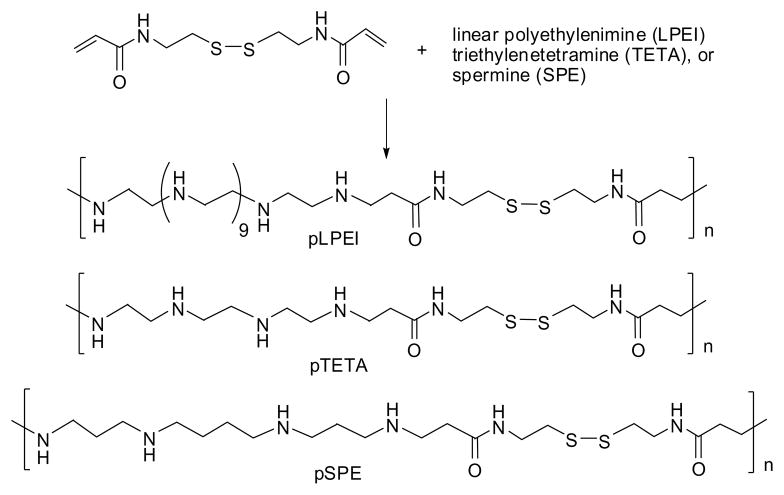
Biodegradable polycations pLPEI, pTETA and pSPE with disulfide backbones have been synthesized by the Michael addition reaction using N,N′-cystamine bisacrylamide (CBA) as shown in Scheme 1. The reaction proceeded smoothly in aqueous methanol at 60°C overnight with the formation of products having MW > 2,000 Da with yields up to 65%. In addition to linear oligomers, a formation of partially branched products is possible because of some activity of secondary amino groups, as it was previously reported [17, 18]. Polyamine oligomers 6–8 units long usually formed during this reaction based on molecular weight measurements. The amount of protonated amino groups at physiological pH determined by potentiometric titration was equal in molar content to about 11–22% of the total nitrogen content (Table 1). All of these polycations demonstrated a significant buffering capacity, the feature required for efficient endosomal escape of pDNA.
Scheme 1.
3.3. Properties of polycation/pDNA complexes
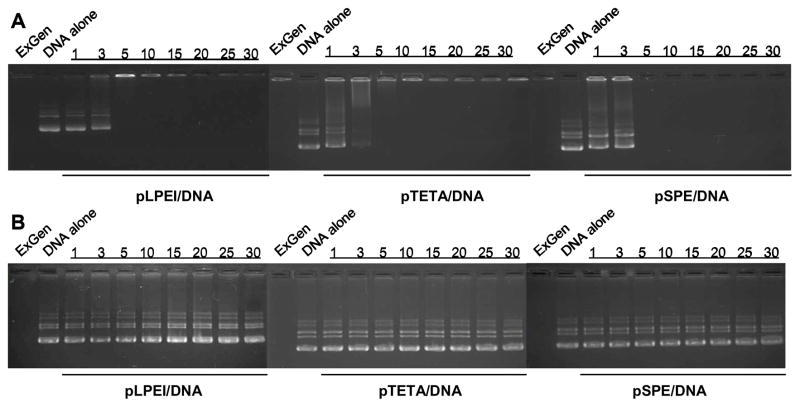
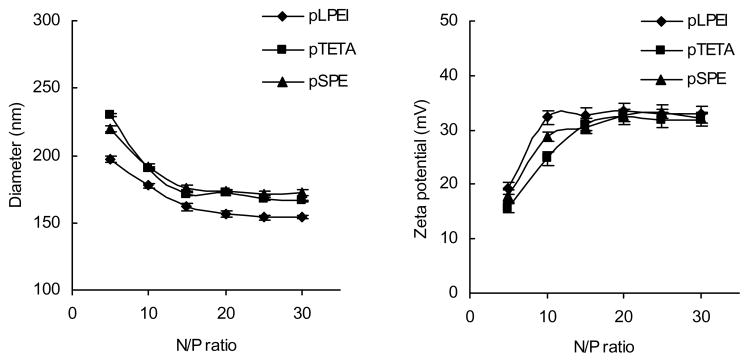
A gel retardation assay showed that all of the obtained biodegradable polyamines demonstrated ability to form stable complexes with pDNA, resulting in full immobility of pDNA complexes at a N/P ratio as low as 5 (Fig 1A). When these complexes were treated with 25 mM DTT before electrophoresis, their ability to form stable complexes with pDNA was completely lost, and the released pDNA migrated similarly to an intact pDNA. In comparison, ExGen500 (non-degradable linear PEI 22kDa) still tightly bonded pDNA and held it in the well (Fig 1B). Plasmid DNA was effectively condensed into nanosized complexes by biodegradable polycations. Fig 2A shows that the diameter of nanocomplexes gradually decreased from 200–250 nm to 160–180 nm at N/P ratio from 5 to 15 and did not show significant changes thereafter. Similarly, the overall surface charge of nanocomplexes was gradually increased from 15–20 mV (N/P ratio 5) to 30–35 mV (N/P ratio 15) and then stabilized (Fig 2B).
Figure 1.
(A) Biodegradable polyamines efficiently condense pDNA and affect electrophoresis mobility in agarose gel. DNA was incubated with either ExGen500 (N/P ratio = 5), or polyamines (N/P ratio is shown above) for 30 min at 25°C. Then the complexes containing 0.5μg of pDNA were loaded on 0.8% (w/w) agarose gel and analyzed by electrophoresis. (B) Reductive treatment by DTT (25mM, 30 min, 25°C) released pDNA from biodegradable polyplexes.
Figure 2.
Particle size (A) and surface charge (ζ-potential, B) of biodegradable polyplexes prepared in 20 mM HEPES solution. The results are shown as means ± SEM (n = 5).
3.4. Protective ability of biodegradable polycations
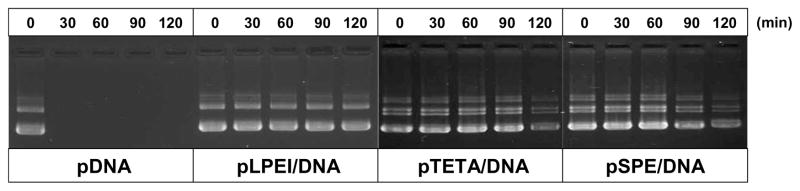
To evaluate protection of the complexed pDNA against enzymatic degradation, a DNase I digestion assay was conducted. Plasmid DNA complexed with biodegradable polymers was kept intact after 120 min of incubation with DNase I (Fig 3). On the contrary, free pDNA was completely digested at the same treatment within 30 min.
Figure 3.
Biodegradable polycations protect pDNA from enzymatic degradation. Polyplexes (N/P = 15) were treated by DNase I (1U/μg pDNA) at 37°C for different time intervals. 10 μL aliquots containing 0.5 μg of pDNA were taken at each time point and examined by 0.8% (w/v) agarose gel electrophoresis.
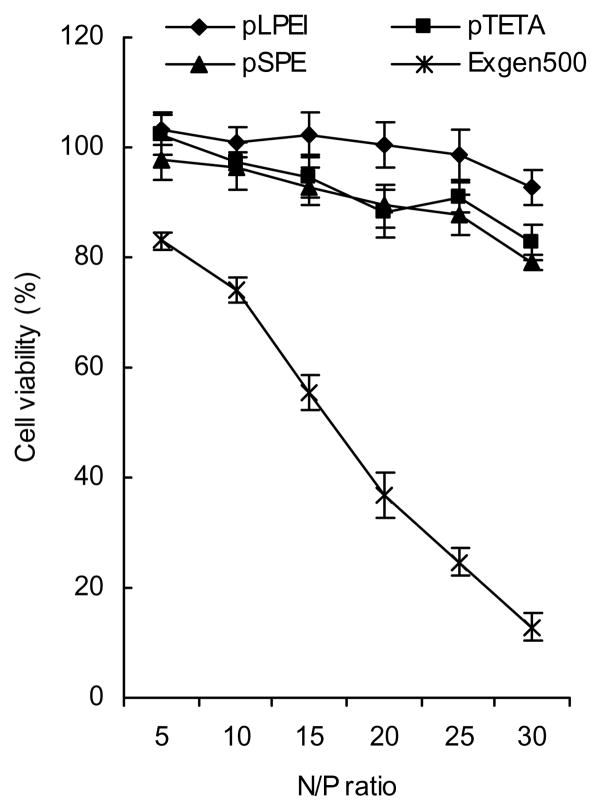
3.5. Cytotoxicity evaluation
Biodegradable polyamines were found to be much less toxic to bEnd.3 cells than non-biodegradable ExGen500. As shown in Figure 4, the viability of bEnd.3 cells treated by these polycations at concentrations corresponding to the effective transfection range (N/P ratio 10–20) was comparable to non-treated cells. By contrast, the relative cell viability of ExGen500-treated cells was sharply decreased within this interval. The observed IC50 values for NLS-PEG5000-tris-acridine conjugate and the hybrid pLPEI polyplex measured in bEnd.3 cells after 24h-treatment were equal to 6±1 and 0.4 mg/mL, respectively. These values were 4000 and 20 times higher than the working concentrations used in transfection experiments.
Figure 4.
Cytotoxicity of biodegradable polyplexes in bEnd.3 cells. The cells were treated by polyplexes using exactly the same procedure as used in the transfection study; and cytotoxicity was measured by an MTT assay. Relative cell viability was calculated by comparison with the untreated cells. Results are presented as means ± SEM (n = 5).
3.6. In vitro transfection
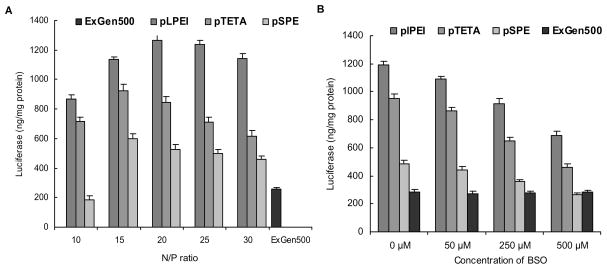
All transfection experiments with bEnd.3 cells were performed with luciferase-encoding pDNA in the presence of 10% serum (FBS). ExGen500 was used as a positive control at N/P ratio 6. All three biodegradable pLPEI, pTETA and pSPE-containing polyplexes demonstrated 4.9-, 3.6- and 2.3-fold higher transfection ability, respectively, than ExGen500 with the highest values observed at N/P ratios between 15 and 20 (Fig. 5A).
Figure 5.
(A) Transfection of bEnd.3 cells with biodegradable polyplexes of luciferase-encoding pDNA. Cells were incubated in 96-well plates with transfection complexes containing 0.5μg of pDNA for 4 h at 37°C in the presence of 10% FBS. The luciferase expression level and protein content were determined 40 h post-transfection. Results are presented as means ± SEM (n = 5). *, P < 0.05; **, P < 0.005; ***, P < 0.001. (B) Effect of BSO on luciferase expression levels. The bEnd.3 cells were pre-treated with BSO and transfected as mentioned previously. NS: no significance; **, P < 0.005; ***, P < 0.001.
When bEnd.3 cells were pre-incubated with BSO, a GSH inhibitor, before transfection, the luciferase expression level of biodegradable polyplexes was decreased in a dose-dependent manner. While 50 μM BSO didn’t result in a significant decline in the luciferase level for all three polyplexes, 500 μM of BSO sharply decreased the transfection activity of pLPEI to 57.4% (p < 0.0001), pTETA to 48.5% (p < 0.0001) and pSPE to 54.1% (p < 0.0001) of maximal observed values (Fig. 5B). By contrast, the transfection ability of non-degradable ExGen500 was not affected at all BSO concentrations (p > 0.05).
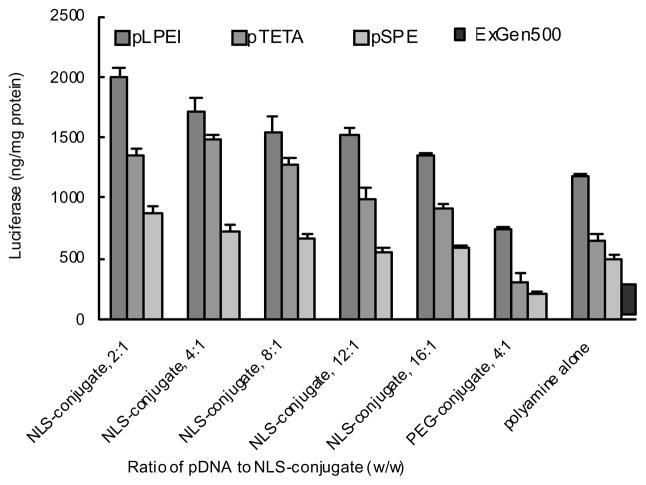
The transfection ability of biodegradable polyamines at their optimal N/P ratios was additionally enhanced when the pDNA was complexed with the nuclear targeting NLS-PEG5000-tris-acridine conjugate. The highest luciferase expression level was observed when the weight ratio of pDNA to NLS-conjugate was from 4:1 to 2:1 (w/w), accounting for an additional 1.7-, 2.3-, and 1.7-fold increase in the luciferase expression for pLPEI, pTETA and pSPE, respectively (Fig 6A). Totally, these polyplexes of pDNA complexed with NLS-PEG5000-tris-acridine were 8.5, 6.3 and 3.7-fold more effective compared to “gold standard” ExGen500. By contrast, in control experiments with polyplexes containing an equivalent amount of non-modified PEG5000-tris-acridine instead of NLS-PEG5000-tris-acridine conjugate, the observed luciferase expression was decreased for all three of the studied polyplexes.
Figure 6.
Transfection ability of biodegradable multifunctional polyplexes. Luciferase-encoding pDNA was pre-mixed with different amounts of NLS-PEG5000-tris-acridine conjugate and complexed with corresponding biodegradable polyamines at N/P ratio 15. Cells were incubated in 96-well plates with the transfection complexes containing 0.5μg of pDNA for 4 h at 37°C in the presence of 10% FBS. The luciferase expression level and protein content were determined 40 h post-transfection. Results are presented as means ± SEM (n = 5). *, P < 0.05; **, P < 0.005; ***, P < 0.001.
3.7. Intracellular localization of pDNA
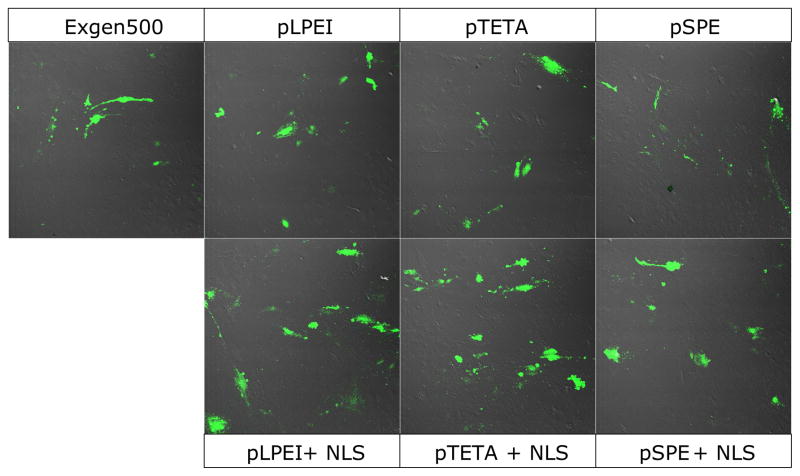
The transfection ability of biodegradable polyplexes alone and with the NLS-PEG5000-tris-acridine conjugate was evaluated using green fluorescent protein (GFP) expression. Confocal microscopy showed enhanced GFP expression in bEnd.3 cells transfected by biodegradable polyplexes compared to ExGen500 (Figure 7). Furthermore, the incorporation of NLS-PEG5000-tris-acridine in polyplexes resulted in an additional increase of the number of GFP expression-positive cells.
Figure 7.
GFP expression in bEnd.3 cells following the transfection by biodegradable polyplexes. The GFP pDNA, with or without NLS-PEG5000-tris-acridine conjugate, was complexed with biodegradable polyamines and added to the cells in 24-well plate. Cells were incubated for 4 h at 37°C in the presence of 10% FBS and 40 h later were examined with a laser scanning confocal microscope.
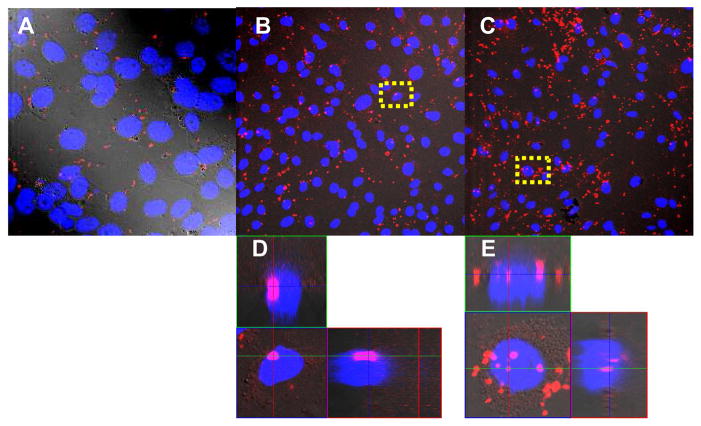
To investigate the intracellular fate of pDNA encapsulated in biodegradable polyplexes, pDNA was labeled with CX-Rhodamine and used for transfection of bEnd.3 cells. We evaluated only one type of polyplexes showing the highest transfection ability in bEnd.3 cells. Biodegradable pLPEI also demonstrated a much stronger ability than ExGen500 to deliver labeled pDNA into the cells. In addition, there was more pDNA observed in the nuclei of transfected cells or in the perinuclear region. It is important to note a difference in red cytoplasmic fluorescence pattern between cells transfected by ExGen500 and pLPEI polyplex-transfected cells. While mostly dotted accumulation in endosomes was found in cells transfected by ExGen500, more diffuse red fluorescence was observed in the cytoplasm of cells transfected by pLPEI-polyplex alone or with NLS-PEG5000-tris-acridine conjugate (Fig. 8). Additionally, in the presence of the conjugate, more red fluorescence was found in the cells and nuclei.
Figure 8.
Intracellular and intranuclear accumulation of RX-Rhodamine labeled pDNA after transfection with ExGen500 (A), pLPEI alone (B), or with NLS-PEG5000-tris-acridine conjugate (C). bEnd.3 cells were transfected with 1.4 μg of pDNA at N/P ratio 6 (ExGen500) and 15 and NLS/DNA ratio 0.25 for 4 h at 37°C in the presence of 10% FBS. Cells were supplemented with fresh complete medium and incubated overnight. After fixation, the cells were imaged with a laser scanning confocal microscope. Lateral images were taken in the Z-stack mode (D and E). Nuclear staining: DAPI.
4. Discussion
Development of non-viral vectors receives tremendous attention in the hope to find a substitute for viral vectors. In polyplexes, large supercoiled pDNA is compacted through electrostatic interactions to form stable particles for efficient delivery and cellular uptake [19]. One of the most efficient transfection reagents, PEI, demonstrated an ability to assist in pDNA release from endosomes into the cytoplasm [20]. Significant cytotoxicity is the major pitfall of PEI and its derivatives [21]. Earlier studies have shown that both transfection-ability and cytotoxicity are closely related to the molecular weight of PEI [22, 23]. Several biodegradable poly(amidoamines) have been synthesized by the Michael addition reaction and investigated as transfection reagents [17, 18]. These polymers showed lower cytotoxicity than non-biodegradable PEI. Another polyamine, spermine, is a natural polycation with excellent DNA-compacting ability. Spermine-based polymers can evidently serve also as less toxic substitutes of conventional transfection reagents.
In this study, we synthesized biodegradable polycations pLPEI, pTETA and pSPE for formulation of nontoxic polyplexes and compared their transfection efficacy with one of the most potent in vitro transfection reagent, ExGene500. These virtually non-toxic polyplexes can serve as a background for development of efficient systemic gene delivery systems. The reducible disulfide backbone of polyamines can facilitate pDNA release from polyplexes degrading in the presence of intracellular GSH. Our results also show that all used, biodegradable polyamines could efficiently condense pDNA and form stable nanoformulations with diameters ranging from 160 to 250nm. Remarkably, the difference in particle size between the biodegradable polyplexes seems to correlate with the length and the charge-density of cationic monomers. As shown in Fig 2A, at the same N/P ratio, the particle size of pLPEI-polyplexes was always smaller than that of pTETA- and pSPE-polyplexes. A possible explanation could be that pLPEI has a higher charge-density and condenses pDNA more efficiently through the formation of smaller complexes. The biodegradable polyamines could efficiently condense pDNA already at an N/P ratio as low as 5 (Fig 1A). The biodegradable polyplexes were stable in physiological solution. However, they completely and rapidly dissociated, releasing free pDNA, by adding a reductive agent, DTT (Fig 1B). These results demonstrate that introduction of disulfide bonds in a polyamine backbone can make the complexes sensitive to a reductive intracellular environment. Evidently, this feature also makes them much less cytotoxic than non-biodegradable ExGen500. On the other hand, these polyamines protect pDNA from enzymatic degradation in the extracellular environment, making them safe and efficient transfection reagents for systemic gene delivery.
Our results show that all three types of synthesized biodegradable polymers are capable to transfect bEnd.3 cells with a higher efficacy than commercial transfection agent ExGen500. It is also important to note that these improved transfection results were obtained in the presence of serum-containing media and for the BBB endothelial cells, which generally exhibit intrinsic resistance to exogenous transfection and are quite sensitive to the cytotoxicity of common transfection agents. Moreover, these conditions better correspond to conditions of systemic drug administration, which is the only way to efficiently reach the BBB in vivo. Among the biodegradable polyamines, transfection ability was positively correlated with buffering capacity. Primary amino groups protonated at physiological pH are functionally responsible for binding and unpacking pDNA [24], while secondary amino groups become protonated in endosomes and can promote osmotic swelling and rupture [25]. Proper combination of these amino groups and biodegradable disulfide bonds in polymeric gene carriers plays an important role in preventing gene-delivery bottlenecks. Read et al showed that intracellular levels of GSH affected delivery of pDNA complexed with reducible polycations [26]. Although reductive treatment by DTT resulted in the fast and complete release of pDNA from all synthesized biodegradable polyamines (Fig 1B), the real significance of disulfide bond reduction by GSH on transfection was further demonstrated by adding a GSH inhibitor, BSO, to the cells prior to transfection. Fig 5B shows that the observed levels of luciferase expression decreased in a dose-dependent manner with the addition of BSO. In addition, the fact that the ExGen500-mediated transfection was not affected demonstrated that the observed inhibition was not caused by the toxicity of BSO. A distinct difference in the intracellular release of fluorescently-labeled pDNA from complexes observed in bEnd.3 cells (Fig. 8) further proves the mechanism of disulfide bond reduction inside the cells. More dispersed red fluorescence was found inside the cells after transfection by biodegradable pLPEI, while only red dots were observed in cells transfected by ExGen500. This finding is quite consistent with the previous report, which showed a similar dispersed fluorescence in transfected cells [17].
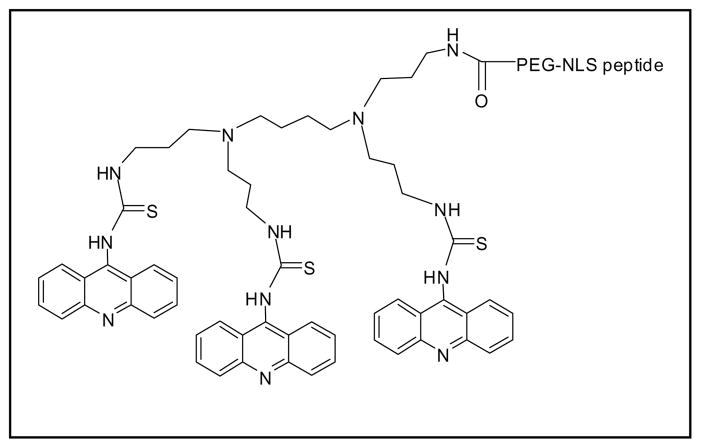
Endosomal escape and nuclear entry of pDNA are two major barriers that need to be overcome developing a safe and effective non-viral gene delivery system. A potential strategy to break both of these barriers is to fabricate a single nanocarrier that has multiple functional components facilitating at first the endosomal escape and, then, the nuclear translocation of DNA. Recently, we demonstrated that a small amount of NLS-PEG-tris-acridine conjugate could significantly enhance the in vitro transfection ability of pDNA encapsulated in lipoplexes formed by Lipofectin2000 or polyplexes formed by ExGene500 (Fig. 9) [10, 14].
Figure 9.
Chemical structure of NLS-peptide-PEG5000-tris-acridine conjugate
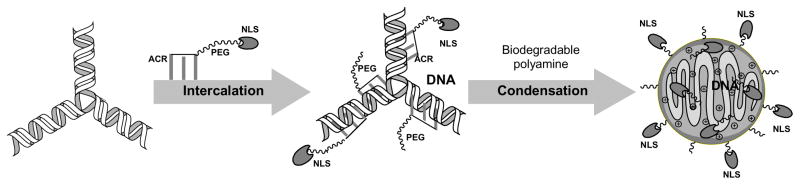
In this study, we demonstrated that pre-mixing of NLS-PEG-tris-acridine conjugate with pDNA before preparation of polyplexes with biodegradable polyamines can significantly enhance transfection efficacy biodegradable polyplexes in brain capillary endothelial bEnd.3 cells. As shown in Scheme 2, initially, supercoiled pDNA forms complex with intercalating conjugate and, then, is condensed by short biodegradable polyamines into compact particles surrounded with PEG corona. These particles demonstrated high aggregational stability during a prolonged incubation in physiological solution. Only a small amount of the conjugate was required to markedly improve the transfection efficacy, specifically, approximately 20–30 NLS-peptides per pDNA molecule (Fig. 5). On the contrary, addition of an equal amount of non-modified PEG5000-tris-acridine conjugate demonstrated no positive effect on transfection. Simultaneous application of the NLS-PEG-tris-acridine conjugate enhanced nuclear accumulation in bEnd.3 cells as illustrated in Fig 8. Further optimization of this system through introduction of brain-targeting peptide or mAb molecules is currently under way.
Scheme 2.
5. Conclusion
In conclusion, we have demonstrated that a novel gene delivery system prepared from short biodegradable polyamines and NLS-PEG intercalating conjugate can efficiently transfect brain capillary endothelial cells of the BBB in the presence of serum. These low-molecular-weight polycations showed negligible cytotoxicity and high transfection efficacy in cellular model of the BBB, murine bEnd.3 cells. Cellular accumulation, endosomal escape, and nuclear transport of pDNA have been markedly enhanced by combination of biodegradable polyamines with nuclear-targeting NLS-PEG-tris-acridine conjugate in compact polyplexes. These nanoformulations may serve as novel promising gene delivery vehicles for systemic applications, including targeted in vivo delivery of genetic material in the BBB.
Acknowledgments
The financial support from National Institute of Neurodegenerative Diseases and Stroke (R01 NS050660 for S.V.V.) is gratefully acknowledged. The authors thank Janice Taylor and James Talaska of the Confocal Laser Scanning Microscope Core Facility (University of Nebraska Medical Center) supported by the Nebraska Research Initiative and the Eppley Cancer Center for providing assistance with confocal microscopy. We are grateful to Arin Zeman for excellent technical assistance and Trevor Gerson for his help in the revision of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson DG, Lynn DM, Langer R. Semiautomated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem, Int Ed Engl. 2003;42:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 2.Lynn DM, Amiji MM, Langer R. pHresponsive polymer microspheres: rapid release of encapsulated material within the range of intracellular pH. pH. Angew Chem, Int Ed Engl. 2001;40:1707–1710. [PubMed] [Google Scholar]
- 3.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 4.Lim YB, Kim SM, Suh H, Park JS. Biodegradable, endosome disruptive, and cationic network-type polymer as a highly efficient and nontoxic gene delivery carrier. Bioconjug Chem. 2002;13:952–957. doi: 10.1021/bc025541n. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Wu D, Ma Y, Tang G, Wang S, He C, Chung T, Goh S. Novel poly(amino ester)s obtained from Michael addition polymerizations of trifunctional amine monomers with diacrylates: safe and efficient DNA carriers. Chem Commun (Camb) 2003;20:2630–2631. doi: 10.1039/b309487a. [DOI] [PubMed] [Google Scholar]
- 6.Lavignac N, Lazenby M, Foka P, Malgesini B, Verpilio I, Ferruti P, Duncan R. Synthesis and endosomolytic properties of poly(amidoamine) block copolymers. Macromol Biosci. 2004;4:922–929. doi: 10.1002/mabi.200400093. [DOI] [PubMed] [Google Scholar]
- 7.Richardson SC, Pattrick NG, Man YK, Ferruti P, Duncan R. Poly(amidoamine)s as potential nonviral vectors: ability to form interpolyelectrolyte complexes and to mediate transfection in vitro. Biomacromolecules. 2001;2:1023–1028. doi: 10.1021/bm010079f. [DOI] [PubMed] [Google Scholar]
- 8.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 9.Saitoa Go, Swansonb JA, Lee K-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 10.Vinogradov SV, Zhang H, Mitin A, Warren G. Intercalating conjugates of PEG with nuclear localization signal (NLS) peptide. Polym Prepr (ACS) 2008;49:434–435. [PMC free article] [PubMed] [Google Scholar]
- 11.Gorlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 12.Pouton CW, Wagstaff KM, Roth DM, Moseley GW, Jans DA. Targeted delivery to the nucleus. Adv Drug Deliv Rev. 2007;59:698–717. doi: 10.1016/j.addr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi Takehiko, Hamzavi Ramin, Nielsen PE. Targeted Delivery of Plasmid DNA into the Nucleus of Cells via Nuclear Localization Signal Peptide Conjugated to DNA Intercalating Bis- and Trisacridines. Bioconjugate Chem. 2006;16:1112–1116. doi: 10.1021/bc050093f. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Mitin A, Vinogradov SV. Efficient transfection of blood-brain barrier endothelial cells by lipoplexes and polyplexes in the presence of nuclear targeting NLS-PEG-tris-acridine conjugates. Bioconjug Chem. 2009;20:120–128. doi: 10.1021/bc8003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 16.Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr Drug DeliVery. 2007;4:324–333. doi: 10.2174/156720107782151241. [DOI] [PubMed] [Google Scholar]
- 17.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjug Chem. 2008;19:626–633. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjug Chem. 2006;17:1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 19.Mislick Kimberly A, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Natl Acad Sci USA. 1996;93:12349–12354. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boussif O, Zanta MA, Behr JP. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther. 1996;3:1074–1080. [PubMed] [Google Scholar]
- 21.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor receptor mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 26.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan LWA, Seymour A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]