Abstract
Complete genome sequences were determined for two strains of avian paramyxovirus serotype 6 (APMV-6): the prototype Hong Kong (HK) strain and a more recent isolate from Italy (IT4524-2). The genome length of strain HK is 16236 nucleotide (nt), which is the same as for the other two APMV-6 strains (FE and TW) that have been reported to date, whereas that of strain IT4524-2 is 16230 nt. The length difference in strain IT4524-2 is due to a 6-nt deletion in the downstream untranslated region of the F gene. All of these viruses follow the “rule of six”. Each genome consists of seven genes in the order of 3’N-P-M-F-SH-HN-L5’, which differs from other APMV serotypes in containing an additional gene encoding the small hydrophobic (SH) protein. Sequence comparisons revealed that strain IT4524-2 shares an unexpectedly low level of genome nt sequence identity (70%) and aggregate predicted amino acid (aa) sequence identity (79%) with other three strains, which in contrast are more closely related to each other with nt sequence 94–98% nt identity and 90–100% aggregate aa identity. Sequence analysis of the F-SH-HN genome region of two other recent Italian isolates showed that they fall in the HK/FE/TW group. The predicted signal peptide of IT4524-2 F protein lacks the N-terminal first 10 aa that are present in the other five strains. Also, the F protein cleavage site of strain IT4524-2, REPR↓L, has two dibasic aa (arginine, R) compared to the monobasic F protein cleavage site of PEPR↓L in the other strains. Reciprocal cross-hemagglutination inhibition (HI) assays using post infection chicken sera indicated that strain IT4524-2 is antigenically related to the other APMV-6 strains, but with 4- to 8-fold lower HI tiers for the test sera between strain IT4524-2 and the other APMV-6 strains. Taken together, our results indicated that the APMV-6 strains represents a single serotype with two subgroups that differ substantially based on nt and aa sequences and can be distinguished by HI assay.
Keywords: Avian paramyxovirus, APMV-6, strain Italy, genome sequence
1. Introduction
Paramyxoviruses are pleomorphic, enveloped viruses containing a negative-sense, single-stranded RNA genome. These viruses have been isolated from a great variety of mammalian and avian species around the world, and in some cases from fish and reptiles (Lamb & Parks, 2007). Paramyxoviruses are classified under the family Paramyxoviridae, which includes two subfamilies, Paramyxovirinae and Pneumovirinae. The subfamily Paramyxovirinae is further divided into five genera: Morbillivirus (including measles [MeV] and canine distemper [CDV] viruses), Rubulavirus (including simian virus 5 [SV5, now also known as parainfluenza virus 5], mumps virus [MuV], and human parainfluenza virus [HPIV-2]), Respirovirus (including Sendai virus [SeV] and HPIV-1), Henipavirus (comprising Hendra virus [HeV] and Nipah virus [NiV]) and Avulavirus (comprising avian paramyxovirus [APMV] serotype 1, also known as Newcastle disease virus [NDV], and APMV serotypes 2 to 9). The subfamily Pneumovirinae comprises two genera: Pneumovirus (including human and bovine respiratory syncytial viruses [HRSV and BRSV]), and Metapneumovirus (comprising human and avian metapneumoviruses [HMPV and AMPV]) (Lamb et al., 2005).
The genomes of paramyxoviruses range from 13 to 19 kb and contain 6–10 genes that code for up to 12 different proteins. For the members of subfamily Paramyxovirinae, efficient genome replication depends on the total genome nucleotide (nt) length being an even multiple of six, known as ‘rule of six’ (Kolakofsky et al., 1998). The genome termini consist of extragenic regions, called the 3′-leader and 5′-trailer: the 3’leader region contains the genome promoter, and the trailer encodes the 3’ end of the antigenome containing the antigenome promoter. Each gene starts with a conserved gene start (GS) sequence and ends with a conserved gene end (GE) sequence. Transcription begins at the 3′-leader region and proceeds in a sequential manner by a start–stop mechanism that is guided by short, conserved gene-start (GS) and gene-end (GE) signals that flank each gene (Lamb & Parks, 2007). The genes are separated by non-coding intergenic sequences (IGS) that are conserved in length and sequence among the different gene junctions for some genera (Respirovirus, Morbillivirus, and Henipavirus) and are non-conserved in sequence or length for others (Rubulavirus, Avulavirus, Pneumovirus, and Metapneumovirus). All members of family Paramyxoviridae encode a nucleocapsid protein (N), a phosphoprotein (P), a matrix protein (M), a fusion protein (F), a haemagglutinin-neuraminidase (HN) or glycoprotein (G), and a large polymerase protein (L). Most members of subfamily Paramyxovirinae encode two additional proteins, V and W (or I, in case of genus Rubulavirus), from alternative open reading frames (ORFs) in the P gene that are accessed by RNA editing. In addition, members of the family Pneumovirinae and genus Rubulavirus contain a small gene designated SH, which encodes a small hydrophobic protein (SH).
Paramyxoviruses isolated from avian species fall into two distinct groups based on gene map and antigenic and sequence relationships: the APMVs of genus Avulavirus, and the avian metapneumoviruses of genus Metapneumovirus. The APMVs have been divided into nine different serotypes based on haemagglutination inhibition (HI) and neuraminadase inhibition (NI) assays (Alexander, 2003). NDV is an economically important disease of poultry and is the most studied member of the genus Avulavirus. Very little information is available about the molecular and biological characteristic and pathogenicity of APMV-2 through 9. APMV-2, −3, −6 and −7 have been associated with disease in domestic poultry (Zhang et al., 2007; Alexander & Collins, 1982; Warke et al., 2008a; Saif et al., 1997). APMV-3 and APMV-5 has been implicated in a severe pulmonary disease of birds (Jung et al., 2009; Nerome et al., 1978). The pathogenicity of the remaining APMV serotypes is not known. The APMV-6 virus causes mild respiratory disease and is associated with a drop in egg production in turkeys (Alexander, 1997). Experimental pathogenesis studies showed that APMV-6 was avirulent in chickens (Chang et al., 2001; Warke et al, 2008b). Recently, complete genome sequences have been determined for representative strains of all of the APMV serotypes except APMV-5: APMV-2 (Subbiah et al., 2008), APMV-3 (Kumar et al., 2008), APMV-4 (Nayak et al., 2008; Jeon et al., 2008), APMV-6 (Chang et al., 2001), APMV-7 (Xiao et al., 2009), APMV-8 (Paldurai et al., 2009) and APMV-9 (Samuel et al., 2009). This has substantially increased our understanding of the members of genus Avulavirus, but further studies are necessary to characterize the extent of diversity within the serotypes.
APMV-6 strain duck/HongKong/18/199/77 was first isolated from a domestic duck in Hong Kong (HK) in 1977 and is considered the prototype for the entire serotype (Shortridge et al., 1980). APMV-6 strain duck/Taiwan/Y1/99 (TW) was isolated from a domestic duck in Taiwan in 1999 (Chang et al., 2001). APMV-6 strain goose/FarEast/4440/2003 (FE) was isolated from a goose in the Far East of Russia in 2003 (GenBank accession No. EF569970). APMV-6 strains continue to be isolated from wild birds around the world (Warke et al., 2008a; Stanislawek et al., 2002). To date, the complete genome sequences of APMV-6 strains TW and FE have been determined.
APMV-6 is an atypical member of the genus Avulavirus in having a genome organization, 3’Leader-NP-P/V-M-F-SH-HN-L-5’Trailer, that includes an SH gene between F and HN genes, (Chang et al., 2001), which is not found in the other APMV serotypes sequenced to date. The biological function of the APMV-6 SH protein is not known. However, the SH proteins of SV5 and MuV appears to play an essential role in blocking the TNF-alpha-mediated apoptosis pathway (Wilson et al., 2006). The SH protein of HRSV has ion channel activity in planar lipid bilayers (Gan et al., 2008). HRSV from which the SH gene was deleted was fully viable in cell culture, but was slightly attenuated in mice and chimpanzees (Bukreyev et al., 1997 and Whitehead et al., 1999).
As noted, APMV-6 strains have been isolated from a wide range of avian species from different parts of the world. But little is known about the serological and genetic relationships among these strains. This information is important for understanding virus evolution and epidemiology and for the development of vaccines against these viruses. As a first step towards understanding the serological and genetic relationships among APMV-6 strains, we report the complete genome sequences of the APMV-6 prototype strain HK as well as a strain, duck/Italy/4524-2/07 (IT4524-2), that was isolated in Italy in 2007. We also determined sequences for the F, SH and HN genes of two more Italian strains, IT4526 and IT6895-1, that also were isolated in 2007, and compared the phylogenetic relatedness of all known APMV-6 strains to representative strains of the other APMV serotypes as well as members of other genera of family Paramyxoviridae. The results suggested that APMV-6 contains two subgroups that can be distinguished by sequence and antigenic comparisons.
2. Materials and Methods
2.1. Virus and cells
APMV-6/duck/Italy/4524-2/07 (IT4524-2), APMV-6 /duck/Italy/4526/07 (IT4526), and APMV-6/teal/Italy/6895-1/07 (IT6895-1) were kindly provided by Dr. Ilaria Capua (Istituto Zooprofilattico Sperimentale delle Venezie, Padova, Italy). APMV-6 /duck/HongKong/18/199/77 (HK) was received from the National Veterinary Services Laboratory, Ames, Iowa, USA. The viruses were propagated in 9-day-old embryonated specific pathogen free (SPF) chicken eggs. Infected allantoic fluid was harvested 3 days post-inoculation. The titer of the virus was determined by hemagglutination (HA) assay using 1% chicken RBC at room temperature. The following established cell lines: DF-1 chicken embryo fibroblast, African green monkey kidney (Vero), baby hamster kidney (BHK-21), Madin Darby Bovine Kidney (MDBK), Madin Darby Canine Kidney (MDCK), and human cervical carcinoma (HEp-2), were were grown in Dulbecco’s minimum essential medium (DMEM) containing 10% fetal bovine serum (FBS) and incubated at 37°C under 5% CO2. The cells were infected with 10−2 dilution of 28 HA units of egg-grown virus, with or without supplementation with 1 µg/ml trypsin (Invitrogen, USA) in serum-free DMEM. The infected cells were observed daily for 7 days for cytopathic effect (CPE) and HA activity of the cell culture supernatant.
2.2. Serological analysis
Antisera against APMV-6 strains HK and IT4524-2 were prepared by single infection of 2-weeks-old chickens via the intraocular (IO) and intranasal (IN) routes, mimicking natural infection. Briefly, three two-week old chickens of each group were infected with each virus (108 HAU) at separate time to avoid cross-infection. Two weeks after infection the chickens were bleed and sera were collected. The sera were heat-inactivated at 56 °C for 30 minutes and stored at −20 °C. HN-specific antibody titers in the serum samples were determined by HI assay using chicken erythrocytes as previously described (Alexander, 1997).
2.3. Virus RNA isolation and sequence analysis
Viral RNA was isolated from the allantoic fluid collected from virus-infected embryonated chicken eggs using RNeasy kit (QIAGEN, USA) according to the manufacturer's instructions. Most of the APMV-6 genome except the 3’ and 5’ termini was amplified into cDNA using (i) primers designed from the published APMV-6 strains TW (GenBank no. NC003043 and FE (GenBank no. EF569970), and (ii) consensus primers designed using the published APMVs sequences. All primers were commercially synthesized from Integrated DNA Technologies Inc, USA. Briefly, the first-strand cDNA was synthesized from viral RNA by Superscript II kit (Invitrogen) using random hexamers according to manufacturer’s instructions. PCR was performed using virus specific or consensus primers and Taq polymerase (Invitrogen). The PCR fragments were cloned into TOPO TA cloning kit (Invitrogen) and the clones were sequenced using vector primers. In addition, selected PCR products were purified by agarose gel electrophoresis and sequenced directly. The DNA sequencing was carried out using BigDye® Terminator v3.1 cycle sequencing kit (Applied Biosystems Inc, USA) in ABI 3130xl genetic analyzer. Every nt in the genome was sequenced at least three times and once directly from RT-PCR product without cloning, thus ensuring a consensus sequence.
2.4. Determination of the sequences of genome termini
The sequences of genome termini were determined by rapid amplification of cDNA ends (RACE) as previous described (Xiao et al., 2009). Briefly, for the 3’ end, viral genomic RNA was ligated with an adaptor 1 (5’-GAAGAGAAGGTGGAAATGGC GTTTTGG-3’, 5’-phosphorylated; 3’-blocked), (Li et al., 2005). The cDNAs were synthesized using adaptor-2 which is complementary to adaptor-1 (5’-CCAAAACGCCATTTCCACCTTC TCTTC-3’). The PCR was performed with adaptor-2 and a viral N-specific reverse primer. The sequence of the trailer region was determined using a L-specific forward primer Forward15773 (5’-GTAAGGAGACTAGTACCTCTGCTAGATAAGG-3’) for strain HK, and L 15193 forward primer (5’-CGCTATTACATCATGTGCTGTC-3’) for strain IT4524-2 for cDNA synthesis. The cDNA was subsequently poly dATP tailed using T4 terminal deoxy nucleotidyl transferase according to the manufacturer’s protocol (Invitrogen). The PCR was performed using specific forward primer which was used for RT and an oligo (dT) reverse primer (5’-ACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTV-3’) using the poly adenylated cDNA as template. The PCR products were cloned into the TOPO TA cloning vector and sequenced, and were also directly sequenced to ensure a consensus sequence.
2.5. Alignment and phylogenetic tree analyses
Sequence compilation and prediction of ORFs were carried out using the SeqMan and EditSeq programs in the Lasergene 6 (DNASTAR) software package (www.dnastar.com). The search for matching protein sequences in GenBank was done using the blastp program of the same package. Phylogenetic and molecular evolutionary analyses were conducted by using MEGA version 4.0 (Tamura et al., 2007).
2.6. Database accession numbers
The complete genome sequence of APMV-6 strains IT4524-2 and HK and nt sequences of F, SH and HN genes of strains IT4526 and IT6895-1, have been deposited in GenBank under accession no. GQ406232, EU622637, GQ406233 and GQ406234, respectively. Accession numbers for other Avulavirus sequences used in this study are given below: APMV-1 (NDV strain LaSota), AF077761; APMV-2, EU338414 (strain Yucaipa); APMV-3 (strain Netherland, Net) EU403085; APMV-4, EU877976 (strain Korea, KR) and FJ177514 (strain Hong Kong, HK); APMV-6, NC_003043 (strain TW), EF569970 (strain FE); APMV-7, FJ231524; APMV-8, FJ215863 (strain Delaware, Del) and FJ215864 (strain Wakuya, Wak) and APMV-9, EU910942.
3. Results
3.1. Growth characteristic of APMV-6 strains
APMV-6 prototype strain HK and the more-recently isolated strains IT4524-2, IT4526, and IT6895-1 each replicated to a titer of 258 HAU/ml in 9-day old embryonated chicken eggs. Since the growth characteristics and in vitro host spectrum of these strains were not known, we evaluated their replication, with and without added trypsin (1 µg/ml), in six established cell lines that each represent a different species of origin: chicken embryo fibroblasts (DF-1), African green monkey kidney (Vero) cells, baby hamster kidney (BHK-21) cells, Madin Darby Bovine Kidney (MDBK) cells, Madin Darby Canine Kidney (MDCK) cells, and human cervical carcinoma (HEp-2) cells. The production of virus was measured by HA assay (Table 1). Strains HK and IT4524-2 were able to replicate in Vero, MDCK, and MDBK cells in the absence of trypsin, with MDBK cells yielding the highest titers. The inclusion of trypsin resulted in a marginal (two-fold) increase in yield in MDCK and MDBK cells. These two APMV-6 strains also replicated in HEp-2, DF-1, and BHK-21 cells, but required trypsin and yielded lower titers. Strain IT4526 had the same pattern except that replication in Vero cells required trypsin whereas the yield in MDCK and MDBK cells was not increased with trypsin. The fourth strain, IT6895-1, replicated in only two cell lines and to lower final titers: MDBK cells, where trypsin was not required and did not increase the yield, and DF-1 cells, where trypsin was required. Thus, each of the four APMV-6 strains was able to replicate in at least one cell line without added trypsin, with some strains replicating in two or three lines without added trypsin. Inclusion of trypsin in these cases had either a marginal effect or no effect, indicating that replication indeed was substantially trypsin-independent. On the other hand, each of the four strains was strictly dependent on trypsin in at least three, and as many as five, of the six cell lines. The cell lines in which trypsin was required were also the ones that were less permissive. The viral cytopathic effects (CPE) that were observed involved rounding and detachment of cells, but there was no evidence of syncytia formation in any of the cell lines in the absence or presence of trypsin. Furthermore, these viruses failed to produce visible plaques under methylcellulose overlay in all six cell lines. The mean death times (MDT) of the four viruses in 9-day old embryonated chicken eggs were more than 168 h, indicating that they are avirulent for chickens and that, at least by this test, there was no evident difference in virulence among these four strains.
Table 1.
Replication of APMV-6 strains in cell lines
| Strain | Trypsin (1µg/ml) |
HA titer (HAU/ml) |
|||||
|---|---|---|---|---|---|---|---|
| HEp-2 | DF-1 | Vero | MDCK | MDBK | BHK21 | ||
| HK | − | − | − | 20 | 80 | 160 | − |
| + | 80 | 80 | 20 | 160 | 320 | 40 | |
| IT4524-2 | − | − | − | 40 | 40 | 160 | − |
| + | 80 | 160 | 40 | 80 | 320 | 40 | |
| IT4526 | − | − | − | − | 80 | 80 | − |
| + | 40 | 40 | 40 | 80 | 80 | 20 | |
| IT6895-1 | − | − | − | − | − | 80 | − |
| + | − | 20 | − | − | 80 | − | |
3.2. Serological relationship among APMV-6 strains
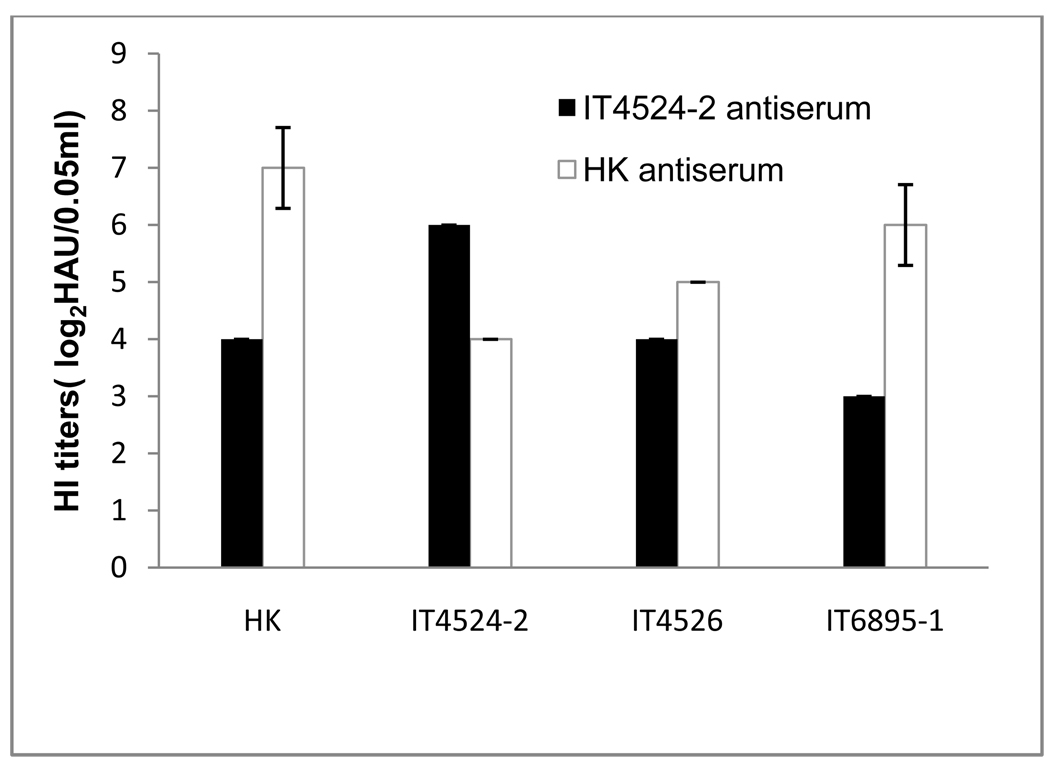
The serological relationship among the four APMV-6 strains was evaluated by HI assay using convalescent antisera that were raised against strain IT4524-2 or HK, by a single infection of chickens by the IN/IO route, mimicking natural infection. The HI assay showed that each of the antisera reacted with all four of the APMV-6 strains (Fig. 1), as would be expected. However, the four strains could be segregated into two groups based on reactivity. For example, against the HK strain, the HI titer of the homologous HK-specific antiserum was 8-fold higher than that of the heterologous IT4524-2-specific antiserum. Conversely, against the IT4524-2 strain, the HI titer of the homologous IT4524-2-specific antiserum was 4-fold higher than that of the heterologous HK-specific antiserum. Against the remaining two strains, IT4526 and IT6895-1, the HI titer of the HK-specific antiserum was 2- and 8-fold higher, respectively, than that of the IT4524-2-specific antiserum. These results suggested that there was a low level of antigenic variation among APMV-6 strains, with the HK, IT4526, and IT6895-1 strains representing one subgroup and the IT4524-2 strain representing a second subgroup.
Fig. 1.
Antigenic analysis of the indicated APMV-6 strains by a hemagglutination-inhibition (HI) assay using antisera specific to strains HK or IT4524-2. The anti-HK and IT4524-2 sera were collected 14 days following a single infection in 2-week old chickens.
3.3. Determination of genome sequences of APMV-6 strains HK and IT4524-2 and partial genome sequences for strains IT4526 and IT6895-1
We determined the complete genome sequences of APMV-6 strains HK and IT4524-2. In addition, for strains IT4526 and IT6895-1, we sequenced the region of the genome encoding the three surface glycoproteins (F-SH-HN). The genome of prototype strain HK was found to be 16,236 nt in length, which is the same as was previously reported for APMV-6 strains TW (Chang et al., 2001) and FE (GenBank no. EF569970). However, the genome of strain IT4524-2 was found to be 16,230 nt in length, which is 6 nt shorter than those of strains HK, TW and FE. The nt lengths of the genomes of strains HK and IT4524-2 are even multiples of six, as is the case with the previously reported sequences for the TW and FE strains. Thus, these viruses conform to the “rule of six”, which is a characteristic of all other members of subfamily Paramyxovirinae (Kolakofsky et al., 1998). As was the case with the previously reported TW and FE strains, APMV-6 strains HK and IT4524-2 have the gene order 3’leader-N-P-M-F-SH-HN-L-trailer5’. The partial sequence analysis of the IT4526 and IT6895-1 strains also indicated the presence of the SH gene. Thus, APMV-6 has the same gene order as the other APMV serotypes except that all of the APMV-6 strains that have been analyzed have in addition the SH gene located between the F and HN genes.
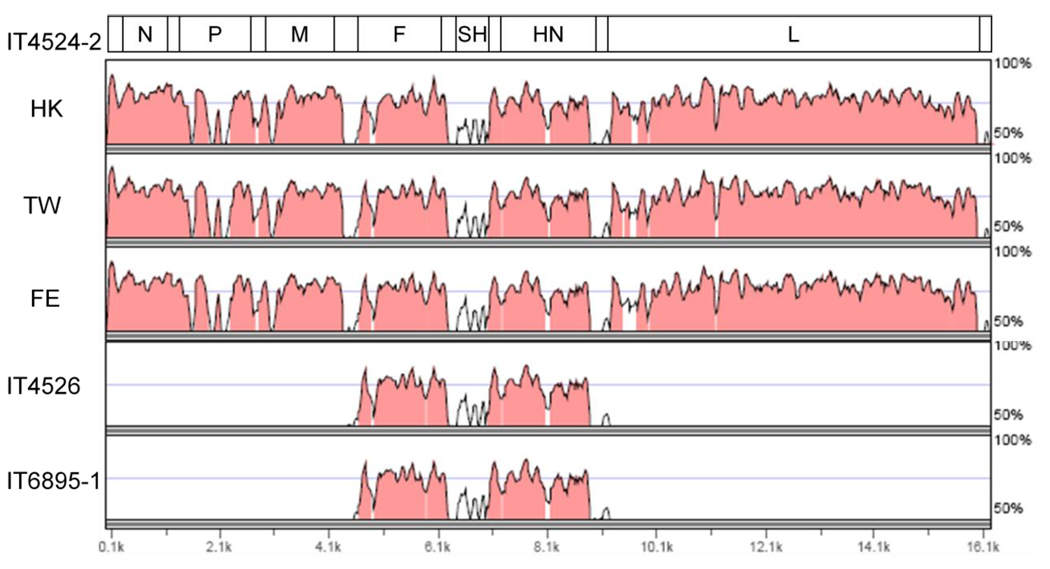
The complete genome of the prototype strain HK has 94% nt sequence identity with the previously-reported TW and FE strains, with an aggregate amino acid identity of 95% (Table 3). The TW and FE strains were previously found to be very closely related, with a nt sequence identity of 98% and an aggregate aa identity of 99%, However, these strains have only 70% nt and 79% aggregate aa sequence identity with strain IT4524-2. Thus, whereas these two strains have only 71% nt and 80% aggregate aa sequence identity with strain IT4524-2. Thus, whereas strains HK, TW, and FE are very closely related, strain IT4524-2 is somewhat distinct. This is consistent with the finding noted above that strain IT4524-2 was distinct antigenically. Alignment of the complete nt sequences of strain IT4524-2 with those of strains HK, TW, and FE showed that there were areas of substantial nt sequence differences that were distributed unevenly throughout the genome (Fig. 2). The regions of extensive divergence (less than 50% sequence identity) between strain IT4524-2 and the other APMV-6 strains were in the gene junction regions, including untranslated (UTR) gene sequences and IGS. The sequences of the ORFs that encode viral proteins had higher nt sequence identities (more than 75%) except for the SH gene (about 60%) and segments within the P gene. This comparison also revealed that the difference in length between strain IT4524-2 versus strains HK, TW, and FE is due to a 6-nt deletion in the downstream (3’ relative to mRNA) UTR of the F gene. An alignment of this UTR using the available sequences is shown in Fig. 3, and illustrates the extensive sequence divergence in untranslated regions of strain IT4524-2 compared to the other strains which, in contrast, form a closely related group.
Table 3.
Percent identities of APMV-6 viral proteins
| Strain | N | P | M | L | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HK | TW | FE | HK | TW | FE | HK | TW | FE | HK | TW | FE | ||
| IT4524-2 | 94.4 | 94.4 | 94.4 | 61.6 | 62.3 | 62.1 | 91.5 | 91.3 | 91.3 | 86.0 | 86.1 | 86.2 | |
| HK | 100 | 100 | 94.2 | 93.3 | 99.2 | 99.2 | 98.0 | 98.1 | |||||
| TW | 100 | 98.6 | 100 | 99.3 | |||||||||
| Strain | F | SH | HN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IT4526 | IT6895-1 | HK | TW | FE | IT4526 | IT6895-1 | HK | TW | FE | IT4526 | IT6895-1 | HK | TW | FE | |
| IT4524-2 | 85.5 | 85.3 | 85.5 | 85.7 | 85.7 | 55.6 | 56.6 | 54.9 | 55.6 | 55.6 | 81.4 | 80.9 | 81.1 | 80.6 | 80.9 |
| IT4526 | 99.3 | 98.4 | 98.9 | 99.3 | 99.3 | 90.8 | 97.9 | 98.6 | 98.7 | 97.2 | 97.9 | 98.2 | |||
| IT6895-1 | 98.4 | 98.9 | 99.3 | 90.1 | 97.2 | 98.6 | 96.9 | 97.6 | 98.2 | ||||||
| HK | 99.1 | 99.1 | 91.5 | 90.8 | 97.1 | 97.4 | |||||||||
| TW | 99.6 | 97.9 | 98.4 | ||||||||||||
Analyzed by Lasergene 6 with clustal W.
Fig. 2.
Nucleotide sequence alignment of the F mRNA 3’ UTRs of the six APMV-6 strains for which sequences are available. The sequences for strains IT4524-2 and HK are provided in full because they have little sequence identity. The other strain sequences are listed below, with nt identity to strain HK is indicated by dots. The unfilled rectangle on the left represents the stop codon of the F gene ORF, and the solid rectangle on the right represents the GE transcription signal of the F gene. The sequences are positive-sense, and the sequence numbers refer to the position in the UTR.
Fig. 3.
Global pairwise comparison of the complete sequence of the genome of strain IT4524-2 with complete (HK, TW, and FE) and partial (IT4526 and IT6895-1) sequences of other APMV-6 strains. Percent sequence identity (y-axis) is plotted versus position in the genome (x-axis). The comparisons with strains IT4526 and IT6895-1 involve only the F-SH-HN gene. The analysis was performed with the software mVISTA Limited Area Global Alignment of Nucleotides (LAGAN) (http://genome.lbl.gov/vista/index.shtml) (Brudno et al., 2003; Robinson et al., 2009).
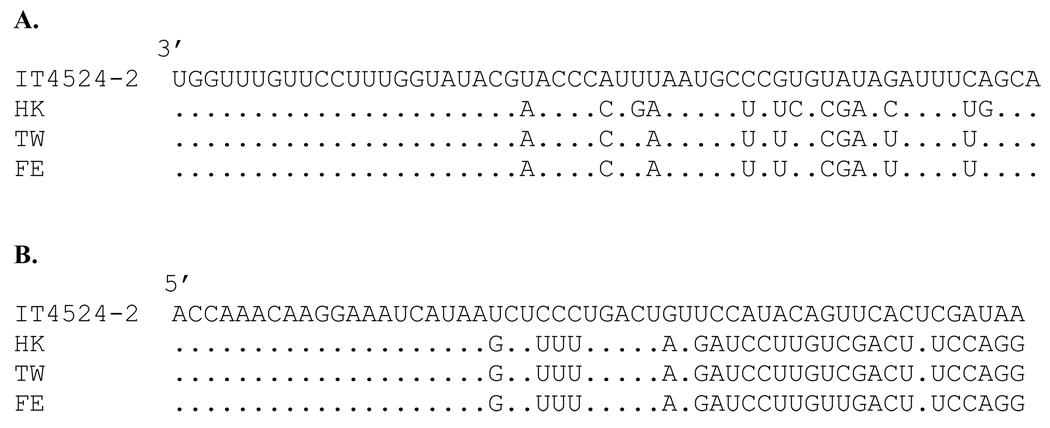
The 3’-leader sequences of APMV-6 strains HK and IT4524-2 consist of 55 nt, a length that is conserved among almost all the members of the subfamily Paramyxovirinae. The nt sequences of the leader region of strains HK and IT4524-2 differ at 13 out of 55 positions. In contrast, the sequence of the HK leader region differs from those of strains TW and FE at only four positions, and the sequences of these latter two strains are identical. Comparison of the leader sequences of all the four strains of APMV-6 showed that the 3’-terminal 22 nt were identical among the four strains (Fig. 4A). The lengths of the trailer regions of APMV-6 strains IT4524-2 and HK are 54 nt, which is same as strains TW and FE. The 5’-terminal 20 nt of the trailer region are identical among these four APMV-6 strains, but strain IT4524-2 differed from the other three strains at 25 of the remaining 34 nt (Fig. 4B). The trailer regions of strains HK and TW trailer regions are 100% identical and differ from that of strain FE at only one position.
Fig. 4.
Nucleotide sequence alignment of the leader (A) and trailer (B) regions of the indicated APMV-6 strains. Dots indicate identity with strain IT4524-2. Sequences are negative-sense.
The proposed gene-start (GS) signal sequence is highly conserved among APMV-6 strains (Table 2). The GS sequences of the P, F, HN and L genes of all four strains are 10 nt in length and have the identical sequence GAGGGGGAAG (positive-sense). The GS sequence of the M gene differed at a single position (underlined): GAGGGGGAAC. However, the GS of SH gene contained an apparent insertion of an additional G residue in the central run of G residues, which contains five C residues for the other genes but 6 for SH (underlined) GAGGGGGGAAG. For each gene, the sequence of the GS signal is identical among all of the APMV-6 strains for which sequences are available. The proposed gene-end (GE) signal sequences of the APMV-6 genes are also highly conserved (Table 2). The GE sequences of the N and SH genes conform to the sequence UUAAGA5–7, whereas those of the P, HN and L genes including that of the strain IT4524-2 F gene have a single nt difference (underlined): UUAAUA6. The GE sequences of the M and F genes are “UUAAUCA5–7, which contain an apparent insertion of a single nt (underlined) except that of the strain IT4524-2 F gene, but otherwise are identical to the GE signals of the P, HN, and L genes. For each gene (except for the strain IT4524-2 F gene), the sequence of the GE signal is most identical among all of the APMV-6 strains for which sequence is available.
Table 2.
Molecular features of genes of APMV-6 and their deduced protein products
| Gene | Strain | mRNA features (nt) |
Intergenic sequence (nt) |
Deduced protein (aa) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Total length |
Gene-start | 5′ UTR |
ORF | 3′ UTR |
Gene-end | ||||
| N | HK | 1571 | GAGGGGGAAG | 62 | 1389 | 90 | UUAAGAAAAAA | 7 | 465 |
| IT4524-2 | 1572 | GAGGGGGAAG | 62 | 1389 | 90 | UUAAGAAAAAAA | 6 | 465 | |
| TW | 1571 | GAGGGGGAAG | 62 | 1389 | 90 | UUAAGAAAAAA | 7 | 465 | |
| FE | 1571 | GAGGGGGAAG | 62 | 1389 | 90 | UUAAGAAAAAA | 7 | 465 | |
| P | HK | 1486 | GAGGGGGAAG | 43 | 1293 | 129 | UUAAUAAAAAA | 2 | 430 |
| IT4524-2 | 1486 | GAGGGGGAAG | 43 | 1293 | 129 | UUAAUAAAAAA | 2 | 430 | |
| TW | 1486 | GAGGGGGAAG | 43 | 1293 | 129 | UUAAUAAAAAA | 2 | 430 | |
| FE | 1486 | GAGGGGGAAG | 43 | 1293 | 129 | UUAAUAAAAAA | 2 | 430 | |
| P/V | HK | 1487 | GAGGGGGAAG | 43 | 807 | 627 | UUAAUAAAAAA | - | 268 |
| IT4524-2 | 1487 | GAGGGGGAAG | 43 | 816 | 618 | UUAAUAAAAAA | - | 271 | |
| TW | 1487 | GAGGGGGAAG | 43 | 807 | 627 | UUAAUAAAAAA | - | 268 | |
| FE | 1487 | GAGGGGGAAG | 43 | 807 | 627 | UUAAUAAAAAA | - | 268 | |
| P/W | HK | 1488 | GAGGGGGAAG | 43 | 534 | 901 | UUAAUAAAAAA | - | 177 |
| IT4524-2 | 1488 | GAGGGGGAAG | 43 | 474 | 961 | UUAAUAAAAAA | - | 157 | |
| TW | 1488 | GAGGGGGAAG | 43 | 534 | 901 | UUAAUAAAAAA | - | 177 | |
| FE | 1488 | GAGGGGGAAG | 43 | 534 | 901 | UUAAUAAAAAA | - | 177 | |
| M | HK | 1405 | GAGGGGGAAC | 103 | 1101 | 178 | UUAAUCAAAAAAA | 59 | 366 |
| IT4524-2 | 1404 | GAGGGGGAAC | 103 | 1101 | 178 | UUAAUCAAAAAA | 60 | 366 | |
| TW | 1405 | GAGGGGGAAC | 103 | 1101 | 178 | UUAAUCAAAAAAA | 59 | 366 | |
| FE | 1405 | GAGGGGGAAC | 103 | 1101 | 178 | UUAAUCAAAAAAA | 59 | 366 | |
| F | HK | 1836 | GAGGGGGAAG | 2 | 1668 | 144 | UUAAUCAAAAAA | 48 | 555 |
| IT4524-2 | 1830 | GAGGGGGAAG | 32 | 1638 | 138 | UUAAUAAAAAAA | 48 | 545 | |
| TW | 1836 | GAGGGGGAAG | 2 | 1668 | 144 | UUAAUCAAAAA | 49 | 555 | |
| FE | 1836 | GAGGGGGAAG | 2 | 1668 | 144 | UUAAUCAAAAAA | 48 | 555 | |
| IT4526 | 1836 | GAGGGGGAAG | 2 | 1668 | 144 | UUAAUCAAAAAA | 48 | 555 | |
| IT6895-1 | 1836 | GAGGGGGAAG | 2 | 1668 | 144 | UUAAUCAAAAAA | 48 | 555 | |
| SH | HK | 574 | GAGGGGGGAAG | 61 | 429 | 62 | UUAAGAAAAAA | 28 | 142 |
| IT4524-2 | 574 | GAGGGGGGAAG | 61 | 429 | 62 | UUAAGAAAAAA | 28 | 142 | |
| TW | 574 | GAGGGGGGAAG | 61 | 429 | 62 | UUAAGAAAAA | 29 | 142 | |
| FE | 574 | GAGGGGGGAAG | 61 | 429 | 62 | UUAAGAAAAAA | 28 | 142 | |
| IT4526 | 574 | GAGGGGGGAAG | 61 | 429 | 62 | UUAAGAAAAA | 29 | 142 | |
| IT6895-1 | 574 | GAGGGGGGAAG | 61 | 429 | 62 | UUAAGAAAAAA | 28 | 142 | |
| HN | HK | 2031 | GAGGGGGAAG | 40 | 1842 | 128 | UUAAUAAAAAA | 63 | 613 |
| IT4524-2 | 2031 | GAGGGGGAAG | 1842 | 128 | UUAAUAAAAAA | 63 | 613 | ||
| TW | 2031 | GAGGGGGAAG | 40 | 1842 | 128 | UUAAUAAAAAA | 63 | 613 | |
| FE | 2031 | GAGGGGGAAG | 40 | 1842 | 128 | UUAAUAAAAAA | 63 | 613 | |
| IT4526 | 2031 | GAGGGGGAAG | 40 | 1842 | 128 | UUAAUAAAAAA | 63 | 613 | |
| IT6895-1 | 2031 | GAGGGGGAAG | 40 | 1842 | 128 | UUAAUAAAAAA | 63 | 613 | |
| L | HK | 7017 | GAGGGGGAAG | 102 | 6726 | 168 | UUAAUAAAAAA | - | 2241 |
| IT4524-2 | 7017 | GAGGGGGAAG | 102 | 6726 | 168 | UUAAUAAAAAA | - | 2241 | |
| TW | 7017 | GAGGGGGAAG | 102 | 6726 | 168 | UUAAUAAAAAA | - | 2241 | |
| FE | 7017 | GAGGGGGAAG | 102 | 6726 | 168 | UUAAUAAAAAA | - | 2241 | |
For each APMV-6 strain, the IGS vary in length (from 2 to 63 nt, Table 2) and are not conserved from one gene junction to the next. Between APMV-6 strains, the IGS length at each junction is conserved except that there is a single nt difference in several cases due to variation in the proposed GE signal. For example, the IGS between the N and P genes of strain HK is 7 nt in length and its proposed GE signal is 11 nt long. In contrast, the IGS between the N and P genes of strain IT4524-2 is 6 nt in length because its proposed GE signal is 12 nt long. Thus, in these cases, the difference in IGS length lies in whether the final A residue of the GE signal indeed is part of the signal (as we have assigned it) or part of the following IGS. The nt sequences of the IGS of strain IT4524-2 had less than 50% nt identity with the corresponding IGS of the other APMV-6 strains. In contrast, among these other strains, the level of nt sequence identity between corresponding IGS was greater than 95% (data not shown).
3.4. The nucleocapsid protein (N) gene
The N gene of APMV-6 strains HK, IT4524-2, TW and FE is 1,389 nt in length and encodes a N protein of 465 aa (Table 2). The N protein of all the four strains contains an identical sequence segment, 324-FAPGNYPLMYSYAMG-338, that conforms to a motif (F-X4-Y-X3-Φ-S-Φ-A-M-G, where X is any aa and Φ is any aromatic aa) that has been identified in other members of the subfamily Paramyxovirinae and is thought to be responsible for N-N self assembly during genomic RNA binding (Yu et al., 1998; Morgan, 1991). In the case of APMV-6, the aa at positions 325 (alanine, A), 326 (proline, P), and 333 (tyrosine, Y) in the motif are conserved in all avulaviruses sequenced to date. The complete N protein of strain HK has 100% aa sequence identity with that of strains TW and FE, and a somewhat lower level of identity (94%) with that of strain IT4524-2 (Table 3).
3.5. The phosphoprotein (P) gene
The P gene of APMV-6 strains HK, IT4524-2, TW and FE is 1,293 nt long and encodes a P protein of 430 aa. The P protein of strain HK has an aa sequence identity of 94% and 93% with that of strain TW and FE, respectively, and a lower level of identity (62%) with that of strain IT4524-2 (Table 3). The P gene of strains HK, TW and FE contain a putative editing site AAAAAAGGG (negative-sense) at nt positions 461–472 of the P gene. The addition of a single G residue to the encoded mRNA would produce a V mRNA encoding a 268-aa V protein. In contrast, the putative editing site of strain IT4524-2 is at nt position 450–458 in the P gene. In addition, the V protein of strain IT4524-2 extends for 3 aa longer at the C-terminus than for the other strains, resulting in a V protein that is 3 aa longer other strains (271 aa, compared to 268 aa) (Fig. 5A). For all four strains, the V protein domain contains the conserved cysteine rich motif that is characteristic of most members of subfamily Paramyxovirinae. The carboxyl domain of the V protein of strain HK (aa positions 158 to 268 in the V protein) has 95% aa sequence identity with the corresponding domain of strains TW and FE, respectively, and a lower level of identity (50%) with that of strain IT4524-2. The addition of two G residues to the encoded mRNA in the P gene editing site would produce a W mRNA encoding a 177 aa W protein in the strains HK, TW and FE. The W mRNA of strain IT4524-2 encodes a shorter, 157 aa, W protein. Interestingly, the carboxyl domain of the W protein of strain IT4524-2 is only 4 aa which is 16 aa shorter than those of other three strains (Fig. 5B).
Fig. 5.
Amino acid sequence alignment of the C-terminal domain of the V proteins (A) and W proteins (B) of the indicated APMV-6 strains. Conserved cysteine (C) residues are underlined, dots indicate identity with strain IT4524-2, and gaps are indicated by dashes. The sequences are numbered according to the complete V and W proteins.
3.6. The matrix protein (M) gene
The M gene of APMV-6 strains HK, IT4524-2, TW and FE is 1,101 nt long and encodes a M protein of 366 aa. The M proteins of the four APMV-6 strains contain the sequence FPII at aa positions 22 to 25 that conforms to a “late motif” pattern (α-P-Φ-Φ, where α represents an aliphatic aa and Φ represents an aromatic aa), which is involved in virus assembly and budding in SV5 (Schmitt et al., 2005). The M protein of strain HK has an aa sequence identity of 99% with that of strains TW and FE, and a somewhat lower level of identity (92%) with strain IT4524-2 (Table 3).
3.7. The fusion protein (F) gene
The F gene of APMV-6 strains HK, IT4526, IT6895-1, TW and FE is 1,836 nt long (ORF, 1,668 nt) and encodes an F protein of 555 aa. In contrast, the F gene of strain IT4524-2 is 1,830 nt long (ORF, 1,638 nt) and encodes an F protein of 545 aa. The F protein of strain HK has a high level of aa sequence identity with the F proteins of strains TW (99%), FE (99%), IT4526 (98%), and IT6895-1 (98%), and a lower level of aa sequence identity (86%) with that of strain IT4524-2 (Table 3).
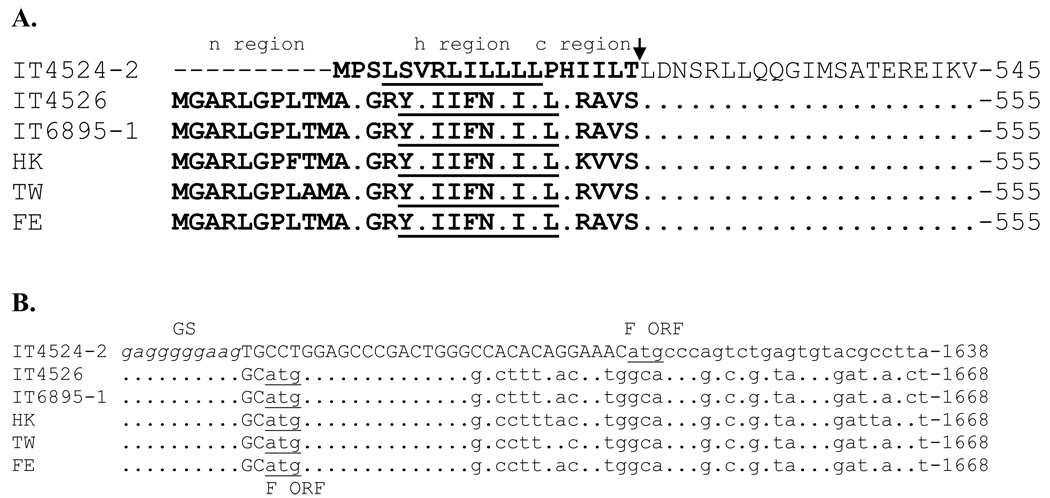
Amino acid sequence alignment showed that the F protein of strain IT4524-2 is 10 aa shorter at the N-terminus than those of the other strains (Fig. 6A), which accounts for the difference in length between these F proteins. This is due to a difference in the position of the ATG translational start codon in the respective ORFs: in strain IT4524-2, the start codon is located 33 nt downstream of the GS signal, whereas the start codon in each of the other strains is located 3 nt downstream of the GS signal (Fig. 6B). The signal peptide of the F protein of strain IT4524-2, as predicted by the Signal3.0 server (Bendtsen et al., 2004), is 19 aa in length, with cleavage occurring between positions 19 and 20 (T↓L). In contrast, the signal peptides of the other APMV-6 strain F proteins are 29 aa in length and are predicted to be cleaved between aa positions 29 and 30 (Fig. 6A). The aa sequence of the signal peptide of strain IT4524-2 has only a low level of sequence identity (26%) with those of the other strains, which amongst themselves share a higher level of identity (90–100%). The 29-aa signal peptide of APMV-6 strains HK, TW, FE, IT4526 and IT6895-1 has a polar N-terminus (n region), hydrophobic core (h region) and C-terminus (c-region) (Martoglio & Dobberstein, 1998). In contrast, the signal peptide of strain IT4524-2 does not contain the n-region because it is 10 aa shorter at the n-region. It is not known whether this has functional consequences for translocation into the endoplasmic reticulum.
Fig. 6.
Sequence alignments of the N-terminal region of the F protein (A) and 5’ end of the F mRNA (B) of the six APMV-6 strains. This illustrates an N-terminal truncation in the signal peptide of strain IT4524-2 compared to the others due to a difference in the position of the translational start site. (A) Amino acid sequence alignment of the N-terminal region of the F proteins of the indicated APMV-6 strains. The polar N-terminus (n region), hydrophobic core (h region, underlined) and C-terminus (c-region) of the signal peptide are indicated. The arrow indicates the predicted cleavage site of signal peptidase. Amino acid identity relative to strain IT4524-2 (top sequence) is indicated by dots; gaps in the IT4524-2 sequence compared to the others are indicated with dashes. (B) Nucleotide sequence alignment of the upstream end of the F mRNA of the indicated APMV-6 strains. The sequence is positive-sense, the GS signal is italicized, the remainder of the 5’ UTR is capitalized, and the ATG translational start sites are underlined. Nucleotide identity relative to strain IT4524-2 (top sequence) is indicated by dots.
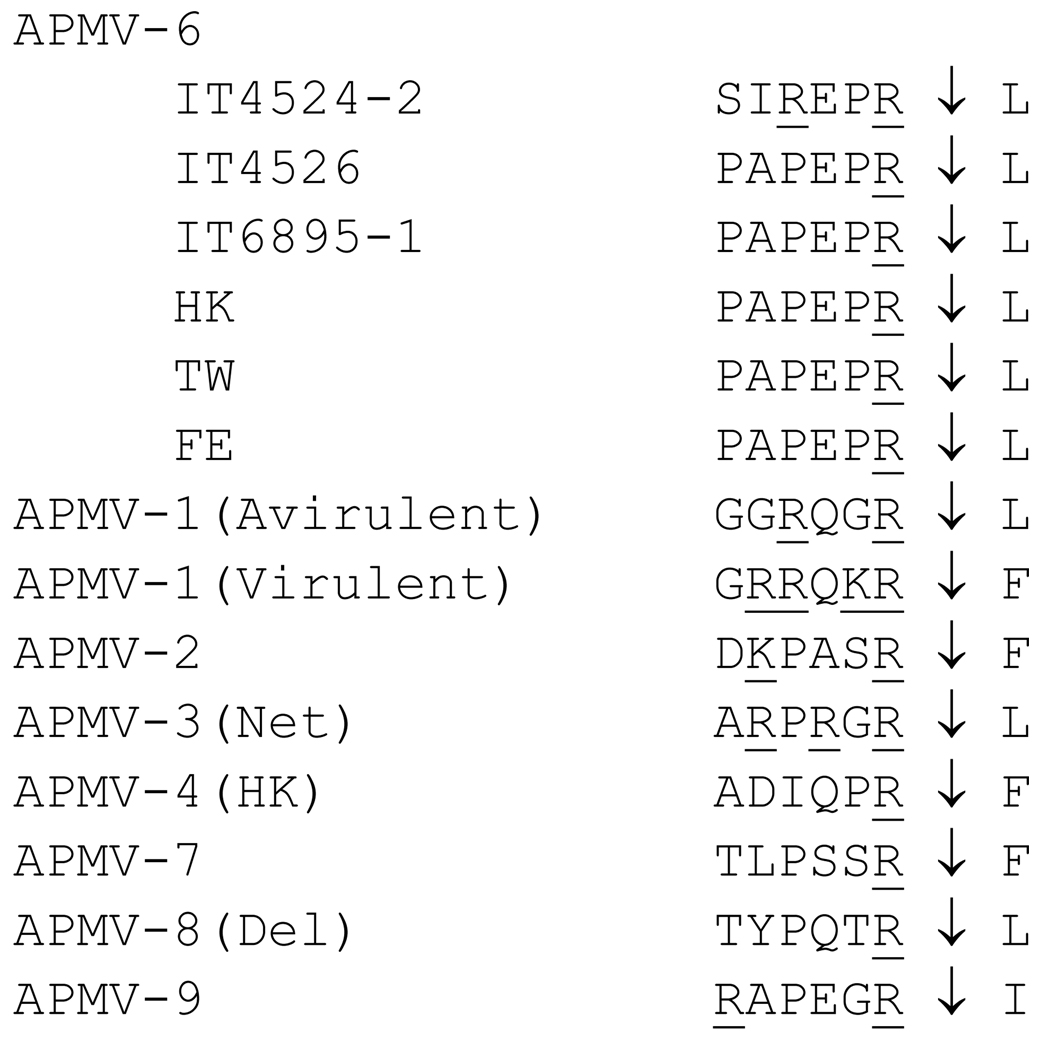
In the case of NDV, the cleavage sequence of the F protein is a critical factor for viral entry and pathogenesis. For APMV-6 strains TW, FE, IT4526, IT6895-1, and the prototype strain HK, the aa sequences spanning the cleavage site and adjacent upstream end of the F1 subunit are identical and contain a monobasic aa residue (arginine, R): PAPEPR↓L. In contrast, for strain IT4524-2, there are several aa differences including the presence of a second arginine residue at position −4 relative to the cleavage site (REPR↓L, Fig. 7). Despite the additional basic residue in strain IT4524-2, none of the cleavage sites determined to date for APMV-6 strains conforms to the preferred furin cleavage site (RX-R/K-R↓). In addition, the residue immediately following the cleavage site in each of the APMV-6 strains is leucine, whereas the presence of phenylalanine at this position is consistent with intracellular cleavage (Morrison et al., 1993). In comparison, virulent NDV strains have four basic aa followed by a flanking phenylalanine (RRQKR↓F) at the cleavage site, whereas avirulent NDV strains contain two basic aa followed by a flanking leucine residue (RQGR↓L) at the cleavage site (Lamb & Parks, 2007).
Fig. 7.
Alignment of the F protein cleavage site sequence of APMV-6 strain IT4524-2 with those of other APMVs. Basic aa were underlined and the cleavage position was indicated.
3.8. The small hydrophobic protein (SH) gene
The SH gene of all of these APMV-6 strains is 429 nt long and encodes an SH protein of 142 aa. The SH protein is predicted to be a transmembrane protein. The function of the APMV-6 SH protein is not known. The SH protein of strain HK has an aa sequence identity of 90% to 92% with those of strains TW, FE, IT4526, and IT6895-1, and a lower level of identity (55%) with strain IT4524-2 (Table 3).
3.9. The hemagglutinin-neuraminidase (HN) gene
The HN gene of all of these APMV-6 strains is 1,842 nt long and encodes an HN protein of 613 aa. The HN protein is predicted to be a type II integral membrane protein. The sequence motif NRKSCS that has been identified in other paramyxoviruses including other avulaviruses and is thought to be involved in sialic acid binding (Mirza et al., 1994) is present in avulavirus (Xiao et al., 2009). This motif also is present at aa positions 240 to 245 in the HN proteins of strains HK, IT4524-2, IT4526, IT6895-1 and FE, whereas the corresponding sequence in strain TW has a single aa difference (underlined): NRKSCN. The HN protein of strain HK has 97% aa sequence identity with all of these strains, except for 81% identity with that of strain IT4524-2 (Table 3).
3.10. The large polymerase protein (L) gene
The L gene of the APMV-6 strains HK, IT4524-2, TW and FE is 6,726 nt long and encodes a L protein of 2,241 aa. The motif QGDNQ present in L protein domain III of other nonsegmented negative strand RNA viruses is widely conserved and is thought to be involved in L protein transcriptional activity (Malur et al., 2002). This motif also is present at aa positions 776 to 780 in the L protein of APMV-6 strains HK, IT4524-2 and FE, whereas in strain TW the corresponding sequence has a single difference (underlined): QGDEQ. The L protein of strain HK has an aa sequence identity of 98% with those of strains TW and FE, and 86% with that of strain IT4524-2 (Table 3).
3.11. Evolutionary relatedness and genetic classification of the APMV-6 strains
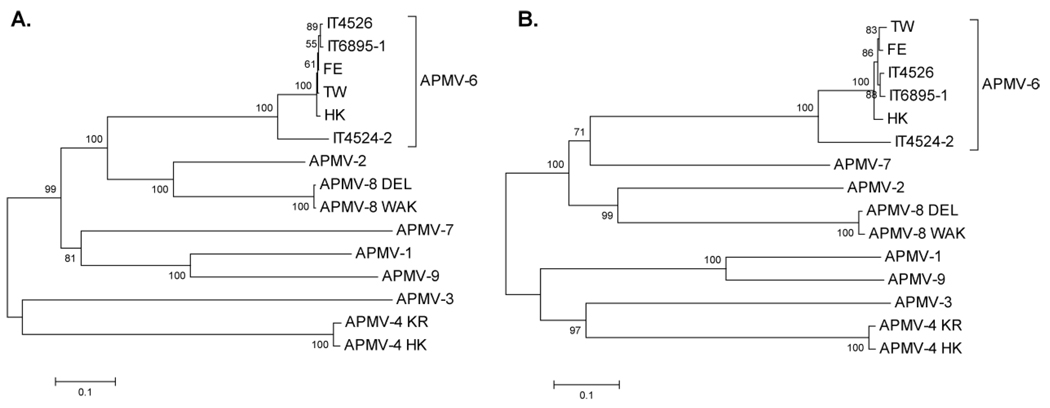
Phylogenetic trees were constructed based on aa sequence alignments of the F and HN proteins of the six APMV-6 strains for which sequences are available versus the cognate proteins of representatives of the other APMV serotypes, except APMV-5 for which sequences have not been reported (Fig. 8). The phylogenetic analysis clearly indicated that the APMV-6 strains analyzed in the present study, IT4524-2, IT4526, IT6895-1 and HK, are more closely related to the APMV-6 strains TW and FE than to other APMV serotypes, as would be expected. Furthermore, APMV-6 strains IT4526, IT6895-1, HK, TW, and FE are more closely related to each other than to strain IT4524-2 which is more distinct and appears to form a separate clade within APMV-6.
Fig. 8.
Phylogenetic trees based on the aa sequences of the F (A) and HN (B) proteins of the six strains of APMV-6 and representative strains of APMV serotypes 1–4 and 7–9. The trees were constructed by bootstrap analysis (1,000 replicates) using the neighbor-joining of the Poisson-corrected values for aa differences in the MEGA 4.0 phylogenetic analysis program (Tamura et al., 2007). All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons. The scale bar shows the number of substitutions per site. Bootstrap values are shown at the nodes.
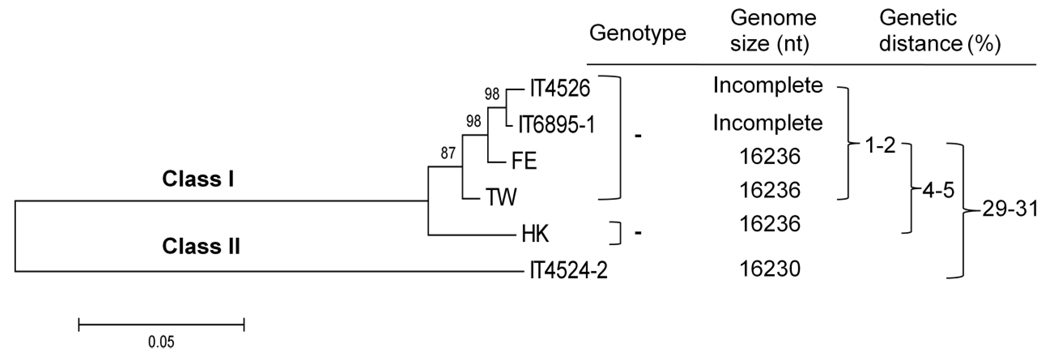
We also constructed a phylogenetic tree for the six APMV-6 strains based on a classification system for NDV that uses common genetic markers in the nt sequence of the F gene to classify NDV strains (Miller et al, 2009; Czegledi et al., 2006; Kim et al., 2007) (Fig. 9). This analysis indicated that the six APMV-6 strains could be divided into two classes: class II, which contains strain IT4524-2, and class I, which contains the other 5 strains. The comparative genetic distance between strain IT4524-2 and other APMV-6 strains was significant, with a range distance value of 29–31% (distance matrix not shown). Within class I, prototype strain HK has a range distance of 4–5% to other strains, compared to the value of 1–2% between the other strains (Fig. 9). This classification system should be helpful in grouping future APMV-6 isolates.
Fig. 9.
Phylogenetic tree based on the nt sequence of the F genes of the indicated APMV-6 strains, and proposed scheme for classification of APMV-6 strains. The unrooted tree was constructed by bootstrap analysis (1,000 replications) using the neighbor-joining of the Kimura-2-parameter method for nt differences in the MEGA 4.0 phylogenetic analysis program. All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons. Scale bar shows number of base substitutions per site. Bootstrap values are shown at the nodes. The percent genetic distances of complete F genes were computed by same program. The matrix of genetic distance among APMV-6 strains is not shown.
5. Discussion
APMVs constitute the genus Avulavirus and are divided into nine serotypes based on HI and NI assays. Among these serotypes, APMV-1 (NDV) is well characterized because it causes severe disease in poultry worldwide. A great deal of information is available on the antigenic and genetic relationships among APMV-1 strains isolated from different parts of the world. Recently, we and others have reported complete genome sequences for representative strains of APMV-2, 3, 4, 6, 7, 8 and 9. However, very little is known about the antigenic and genetic relationships among strains within serotypes 2 through 9. In this study, we have determined complete consensus genome sequences for the prototype APMV-6 strain HK, which was isolated from a domestic duck in Hong Kong in 1977, and strain IT4524-2, which was isolated from a duck in Italy in 2007. In addition, we determined consensus sequences for the F-SH-HN region of the genomes of two additional strains isolated in Italy in 2007, strains IT4526 and IT6895-1. The antigenic relationships among these four strains were evaluated using an HI assay, which is the principal standard for distinguishing between APMV serotypes. In addition, we evaluated the genetic relationships of these four strains with two other APMV-6 strains, TW and FE, for which complete genome sequences had previously been reported. This information will have implications for studies in pathogenesis and epidemiology and for the development of vaccines against APMV-6.
To evaluate the antigenic relationship among the four APMV-6 strains described in the present paper, we raised chicken antisera against strains HK and IT4524-2 separately by respiratory infection, mimicking a natural route of infection. Since serological responses tend to broaden over time and with repeated antigenic exposure, we (i) limited the immunization to a single infection, and (ii) collected serum samples at an early time point (14 days post infection). Our results showed the HI titer of the HK antiserum was 8-, 2-, and 8-fold higher against the homologous strain HK and strains IT4526 and IT6895-1, respectively, than was the HI titer of the IT4524-2-specific antiserum. Conversely, the HI titer of the IT4524-2-specific serum was 4-fold higher against the homologous strain IT4524-2 than that of the HK-specific antiserum. These results indicated an antigenic dimorphism that would be consistent with the existence of two antigenic subgroups within APMV-6, with strains HK, IT4526 and IT6895-1 belonging to one antigenic subgroup and strain IT4524-2 belonging to the second antigenic subgroup. The TW and FE strains were not evaluated in this assay, but their high degree of sequence relatedness to strains HK, IT4526 and IT6895-1, and divergence from strain IT4524-2, predicts that they would be grouped with the former three strains. In this regard, it will be of interest to evaluate additional APMV-6 strains and further evaluate these strains by neutralization assay.
The genome lengths of strains HK, TW and FE are 16236 nt compared to 16230 nt for strain IT4524-2. Among APMV-1 (NDV) strains, there are three genome sizes: 1) 15186 nt in early (>1930s) isolated strains, 2) 15192 nt in late (>1960s) isolated strains (due to a six nt insertion in the upstream of the N gene), and 3) 15198 nt (12 nt insertion in the P gene ORF) (Czegledi et al., 2006). These different genome sizes of NDV strains did not relate to the viral virulence, but seem to be related to the time (year) of virus isolation, with the genomes becoming progressively longer (Miller et al., 2009; Czegledi et al., 2006). However, the converse appeared to be the case for APMV-6, since strains HK, TW and FE were isolated in 1977, 1999 and 2003, respectively, whereas the shorter strain IT4524-2 was isolated in 2007. In any event, the genome lengths of strains HK, TW, FE and IT4524-2 follow the “rule of six”, indicating that this rule is a requirement for virus replication. The six nt difference for strain IT4524-2 was in the 3’ UTR of F mRNA. Interestingly, the 3’UTR of strain IT4524-2 F mRNA showed high level of nt sequence variation (37 to 40% nt identity) when compared with the corresponding sequence in other available APMV-6 strains. The UTRs of other viral genes also showed highly divergence. The UTRs of strain IT4524-2 genes shows a low level nt identities (37 to 56%), compared with those of other strains as high level identities (85 to 100%). The length of viral UTR can play an important role in virus replication and pathogenesis in paramyxoviruses. (Yan et al., 2009; Evans et al., 1990). Therefore, the difference in length of sequence of 3’UTR of IT4524-2 F mRNA might play a role of in virus replication and pathogenicity.
The genome of strain HK shared 94% nt identity with those of strains TW and FE, which in turn were 98% identical. In contrast, the genomes of strain IT4524-2 shared 70% nt sequence identity with strains HK, TW, and FE. With regard to the aggregate aa sequence, strain HK was 95% identical to strains TW and FE, which in turn were 97% identical. In contrast, strain IT4524-2 shared 79% aggregate aa identity with strains HK, TW, and FE. Thus, there was sequence dimorphism between these strains. Sequence analysis of the F-SH-HN genome region of strains IT4526 and IT6895-1 indicated that they were highly related to strains HK, TW, and FE. Taken together, this provided evidence for two subgroups, one containing strains HK, TW, FE, IT4526, and IT6895-1, and the other containing strain IT4524-2. This sequence dimorphism was completely consistent with the antigenic dimorphism noted above, and provides support for the existence of two APMV-6 subgroups. This subgroup difference could not be attributed to the time, place, or host of isolation, since both subgroups contained at least one strain isolated from ducks in Italy in 2007. It is noteworthy that the extent of sequence divergence between the two proposed subgroups of APMV-6 is greater than that between the two subgroups of HRSV, which share 81% nt sequence identity, or HMPV, which share 80% nt sequence identity (Biacchesi et al., 2003, Johnson et al., 1987). For all three viruses, the nt sequence divergence was the greatest in regions that do not encode protein: for example, the IGS between strains HK and IT4524-2 were less than 50% identical, similar to the value of 48% identity for the IGS between the two HPMV subgroups and 42% identity between the two HRSV subgroups (Biacchesi et al., 2003). When compared with the other serotypes of APMV, the genome of four APMV-6 strains had a nt sequence identity of 39% to 47% with APMV-1, 2, 3, 4, 7, 8, and 9. Thus, the divergence of nt sequence between the two proposed APMV-6 subgroups was substantially less than between either APMV-6 subgroup and the other APMV serotypes, as would be expected.
The APMV-6 is the only known virus in genus Avulavirus that encodes the additional SH protein, which is 142 aa in length for all six APMV-6 strains. Similar SH proteins also are present in the other paramyxoviruses: SV5 (44 aa), MuV (57 aa), HRSV (64 aa), human metapneumovirus (179 aa), and J-virus (179 aa) (Lamb & Parks, 2007). The SH protein of APMV-6 strain HK shares only 13 to 22% aa sequence identity with the SH protein of these other paramyxoviruses (analyzed by Lasergene 6 with Clustal V). Although the function of the APMV-6 SH protein is not known, the SH protein of HRSV is not necessary for virus viability (Whitehead et al., 1999) and the SH protein of SV5 might be involved in apoptosis (Wilson et al., 2006). Comparison of aa sequence relatedness of cognate proteins between strain IT4542-2 and other APMV-6 strains showed values of 81 to 94% aa identity, except for the P and SH proteins which are more divergent (62 and 55% aa identity, respectively). The extent of variability in the APMV-6 P proteins contrasts with that of the P proteins of the two subgroups of HMPV and HRSV, which are more highly conserved (85 and 90% aa identity, respectively) (Biacchesi et al., 2003). It is noteworthy that the C-terminal domain of the V protein has only 50% identity between strains HK and IT4524-2, and the complete V protein has only 54% identity. By analogy with other paramyxoviruses, the V protein is thought to play a role in blocking host type I interferon response. The finding that this pathogenesis factor is one of the more divergent proteins is surprising and raises the possibility that this may have consequences for pathogenesis. The SH proteins of the two subgroups of HMPV and HRSV are more highly conserved (59 and 72% aa identity, respectively) than for the two subgroups of APMV-6 (55% identity), which is consistent with the idea that there is greater divergence between the two APMV-6 subgroups than between those of HRSV and HMPV.
One interesting difference between APMV-6 strain IT4524-2 and other APMV-6 strains was observed in the F protein. The F protein of strain IT4524-2 has a signal peptide of 19 aa compared to a signal peptide of 29 aa in other APMV-6 strains. Signal peptides are usually 15 to more than 50 aa residues in length and contain a hydrophilic, positively charged N-terminal region (n region), a central hydrophobic domain of 7–10 residues (h region) and a C-terminal region (c region) containing the cleavage site for signal peptidase. The signal peptide is cleaved during the translocation of the secretory proteins across the lumen of the endoplasmic reticulum (Martoglio & Dobberstein, 1998). The n region of signal peptide of viral glycoprotein (like APMV F protein) was essential for protein processing and maturation, and the n region mutated virus was defective for viral infectivity in Foamy virus (Lindemann et al., 2001) and lymphocytic choriomeningitis virus (Schrempf et al., 2007). The signal peptide of the APMV-6 strain IT4524-2 F protein contains h and c regions, but lacks the n region. It will be of interest to investigate whether this has implications for processing and maturation compared to other APMV-6 strains.
Another difference between strain IT4524-2 and other APMV-6 strains was observed in the cleavage site of F protein. The aa sequence at the cleavage site of F protein plays a major role in NDV pathogenesis (Lamb and Parks, 2007). Virulent NDV strains have a multiple basic aa cleavage site R-X-K/R-R↓F, which is cleaved intracellularly by ubiquitous cellular furin-like proteases, and a phenylalanine (F) residue at the beginning of the F1 subunit, which also may play a role in facilitating cleavage (Morrison et al., 1993). The avirulent NDV strains have one or a few basic residues at the cleavage site and do not conform to the furin motif, and have a leucine (L) residue at the first position of F1 subunit. Interestingly, however, the putative cleavage sites of other APMV serotypes showed that the cleavage site sequences of some serotypes are not necessarily predictive of the protease activation phenotype (Xiao et al., 2009). The putative F protein cleavage site (PEPR↓L) of the APMV-6 strains HK, IT4526, IT6895-1, TW and FE contains a single basic residue while that of strain IT4524-2 contains dibasic residues. However, none of the sites conform to the furin cleavage site. Therefore it was surprising to find that each of these strains replicated in a trypsin-independent manner in at least one of the cell types that were tested and, in these cases, the addition of trypsin did not substantially increase replication. On the other hand, each of the strains required trypsin for replication in one or more of the tested cell types. Thus, there was unexpected diversity in the ability of various cell lines to cleave the various strains. In addition, in light of the fact that strains HK, IT4526, and IT6895-1 have the same cleavage site sequence, it was surprising that they did not exhibit exactly the same cleavage phenotype in all of the six tested cell lines. For example, strain IT6895-1 was trypsin-independent in only one cell line whereas strain HK was trypsin-independent in three lines. It may be that other residues in the F protein that differ between these viruses contributed to the cleavage phenotype. Strain IT4524-2, which had two basic residues in the cleavage sequence, had approximately the same cleavage as strain HK that contained only one basic residue, indicating that this difference did not significantly affect cleavability. On the basis of cleavage phenotype, it would not be possible to predict whether these strains would be virulent or not in vivo, since the cleavage phenotype varied with the cell line that was tested. However, evaluation of MDT in chicken eggs provided evidence of an avirulent phenotype for each of the strains.
In conclusion, complete genome sequences were determined for APMV-6 strains HK and IT4524-2. Comparison of the nt and predicted protein aa sequences among APMV-6 strains showed that the divergence between strain IT4524-2 and other APMV-6 strains was substantially greater overall than that between the two HMPV or the two HRSV subgroups. This grouping based on sequence relatedness was consistent with the antigenic analysis. This indicated that these APMV-6 strains represent two APMV-6 subgroups. We propose that the prototype strain HK and strains TW, FE, IT4526 and IT6895-1 represent one subgroup, and strain IT4524-2 represents a second subgroup. Given the rather substantial differences in the nt and aa sequences between the two APMV-6 subgroups, it was somewhat surprising that the extent of antigenic difference by HI test was modest. One possible explanation might be that the APMV-6 antigenic site in the HN protein that is involved in HI assay is more highly conserved between the two subgroups than the overall genome and aggregate amino acid sequences. Additional antigenic and genetic analysis involving additional APMV-6 strains is needed to further define the antigenic and genetic variability of APMV-6.
Acknowledgements
We thank Drs. Ilaria Capua and Isabella Monne at IZSV, Italy for helpful support and advice. We also thank Anandan Paldurai, Flavia Dias and Dianel Rockemann for their excellent technical assistance and help. “This research was supported by NIAID contract no.N01A060009 (85% support) and NIAID, NIH Intramural Research Program (15% support). The views expressed herein do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DJ. Newcastle disease and other avian Paramyxoviridae infections. In: Calnek BW, editor. Diseases of Poultry. Ames: Iowa State University Press; 1997. pp. 541–569. [Google Scholar]
- Alexander DJ. Avian Paramyxoviruses 2–9. In: Saif YM, editor. Diseases of poultry. 11th edn. Ames: Iowa State University Press; 2003. pp. 88–92. [Google Scholar]
- Alexander DJ, Collins MS. Pathogenecity of PMV-3/Parakeet/Netherland/449 /75 for chickens. Avian Pathol. 1982;11:179–185. doi: 10.1080/03079458208436091. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: Signal 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Biacchesi S, Skiadopoulos MH, Boivin G, Hanson CT, Murphy BR, Collins PL, Buchholz UJ. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- Brudno M, Do CB, Cooper GM, Kim MF, Davydov E, Green ED, Sidow A, Batzoglou S. LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 2003;13:721–731. doi: 10.1101/gr.926603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A, Whitehead SS, Murphy BR, Collins PL. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Hsieh ML, Shien JH, Graham DA, Lee MS, Shieh HK. Complete nucleotide sequence of avian paramyxovirus type 6 isolated from ducks. J Gen Virol. 2001;82:2157–2168. doi: 10.1099/0022-1317-82-9-2157. [DOI] [PubMed] [Google Scholar]
- Czeglédi A, Ujvári D, Somogyi E, Wehmann E, Werner O, Lomniczi B. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 2006;120:36–48. doi: 10.1016/j.virusres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Evans SA, Belsham G, Barrett T. The role of the 5' nontranslated regions of the fusion protein mRNA of canine distemper virus and rinderpest virus. Virology. 1990;177:317–323. doi: 10.1016/0042-6822(90)90486-b. [DOI] [PubMed] [Google Scholar]
- Gan SW, Ng L, Lin X, Gong X, Torres J. Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci. 2008;17:813–820. doi: 10.1110/ps.073366208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon WJ, Lee EK, Kwon JH, Choi KS. Full-length genome sequence of avain paramyxovirus type 4 isolated from a mallard duck. Virus Genes. 2008;37:342–350. doi: 10.1007/s11262-008-0267-4. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Jr, Olmsted RA, Prince GA, Murphy BR, Alling DW, Walsh EE, Collins PL. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987;61:3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A, Grund C, Müller I, Rautenschlein S. Avian paramyxovirus serotype 3 infection in Neopsephotus, Cyanoramphus, and Neophema species. J Avian Med Surg. 2009;23:205–208. doi: 10.1647/2008-022.1. [DOI] [PubMed] [Google Scholar]
- Kim LM, King DJ, Curry PE, Suarez DL, Swayne DE, Stallknecht DE, Slemons RD, Pedersen JC, Senne DA, Winker K, Afonso CL. Phylogenetic diversity among low-virulence newcastle disease viruses from waterfowl and shorebirds and comparison of genotype distributions to those of poultry-origin isolates. J Virol. 2007;81:12641–12653. doi: 10.1128/JVI.00843-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nayak B, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 3 reveals an unusually long trailer region. Virus Res. 2008;137:189–197. doi: 10.1016/j.virusres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Collins PL, Kolakofsky D, Melero JA, Nagai Y, Oldstone MBA, Pringle CR, Rima BK. Fauquet CM, editor. Family Paramyxoviridae. The Classification and Nomenclature of Viruses. The Eighth Report of the International Committee in Taxonomy of Viruses; Virus Taxonomy. 2005
- Lamb RA, Parks GD. In: Paramyxoviridae: the viruses and their replication, Fields Virology. 5th edn. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 1449–1496. [Google Scholar]
- Li Z, Yu M, Zhang H, Wang HY, Wang LF. Improved rapid amplification of cDNA ends (RACE) for mapping both the 5' and 3' terminal sequences of paramyxovirus genomes. J Virol Meth. 2005;130:154–156. doi: 10.1016/j.jviromet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Lindemann D, Pietschmann T, Picard-Maureau M, Berg A, Heinkelein M, Thurow J, Knaus P, Zentgraf H, Rethwilm A. A particle-associated glycoprotein signal peptide essential for virus maturation and infectivity. J. Virol. 2001;75:5762–5771. doi: 10.1128/JVI.75.13.5762-5771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind M, Shihmanter E. Antigenic relationships between avian paramyxoviruses. I. Quantitative characteristics based on hemagglutination and neuraminidase inhibition tests. Arch Virol. 1986;89:89–111. doi: 10.1007/BF01309882. [DOI] [PubMed] [Google Scholar]
- Malur AG, Gupta NK, De Bishnu P, Banerjee AK. Analysis of the mutations in the active site of the RNA-dependent RNA polymerase of human parainfluenza virus type 3 (HPIV3) Gene Expr. 2002;10:93–100. [PMC free article] [PubMed] [Google Scholar]
- Martoglio B, Dobberstein B. Signal sequences: more than just greasy peptides. Trends in Cell Biol. 1998;8:410–415. doi: 10.1016/s0962-8924(98)01360-9. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Decanini EL, Afonso CL. Newcastle disease: Evolution of genotypes and the related diagnostic challenges. Infect Genet. 2009;10:26–35. doi: 10.1016/j.meegid.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Mirza AM, Deng R, Iorio RM. Site-directed mutagenesis of a conserved hexapeptide in the paramyxovirus hemagglutinin–neuraminidase glycoprotein: effects on antigenic structure and function. J. Virol. 1994;68:5093–5099. doi: 10.1128/jvi.68.8.5093-5099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EM. Evolutionary relationships of paramyxovirus nucleocapsid-associated proteins. In: Kingsbury DW, editor. The Paramyxoviruses. New York: Plenum Press; 1991. pp. 163–179. [Google Scholar]
- Morrison T, McQuain C, Sergel T, McGtnnes L, Reitter J. The role of the amino terminus of F 1 of the Newcastle disease virus fusion protein in cleavage and fusion. Virology. 1993;193:997–1000. doi: 10.1006/viro.1993.1214. [DOI] [PubMed] [Google Scholar]
- Nayak B, Kumar S, Collins PL, Samal SK. Molecular characterization and complete genome sequence of avian paramyxovirus type 4 prototype strain duck/Hong Kong/D3/75. Virol. J. 2008;5:124. doi: 10.1186/1743-422X-5-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerome K, Nakayama M, Ishida M, Fukumi H. Isolation of a new avian paramyxovirus from budgerigar (Melopsittacus undulatus) J. Gen. Virol. 1978;38:293–301. doi: 10.1099/0022-1317-38-2-293. [DOI] [PubMed] [Google Scholar]
- Paldurai A, Subbiah M, Kumar S, Collins PL, Samal SK. Complete genome sequences of avian paramyxovirus type 8 strains goose/Delaware/1053/76 and pintail/Wakuya/20/78. Virus Res. 2009;142:144–153. doi: 10.1016/j.virusres.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmann T, Zeydanli MM, Herbst W, Kaleta EF. Isolation of a paramyxovirus-3 from turkeys with respiratory tract disease in Germany. Dtsch. Tierarztl. Wochenschr. 1991;98:138–141. [PubMed] [Google Scholar]
- Robinson CM, Shariati F, Zaitshik J, Gillaspy AF, Dyer DW, Chodosh J. Human adenovirus type 19: genomic and bioinformatics analysis of a keratoconjunctivitis isolate. Virus Res. 2009;139:122–126. doi: 10.1016/j.virusres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif YM, Mohan R, Ward L, Senne DA, Panigrahy B, Dearth RN. Natural and experimental infection of turkeys with avian paramyxovirus-7. Avian Dis. 1997;41:326–329. [PubMed] [Google Scholar]
- Sakai K, Mizutani T, Fukushi S, Saijo M, Endoh D, Kurane I, Takehara K, Morikawa S. An improved procedure for rapid determination of viral RNA sequences of avian RNA viruses. Arch Virol. 2007;152:1763–1765. doi: 10.1007/s00705-007-0999-9. [DOI] [PubMed] [Google Scholar]
- Samuel AS, Kumar S, Madhuri S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 9 and comparison with other paramyxoviruses. Virus Res. 2009;142:10–18. doi: 10.1016/j.virusres.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AP, Leser GP, Morita E, Sundquist WI, Lamb RA. Evidence for a new viral late-domain core sequence, FPIV, necessary for budding of a paramyxovirus. J. Virol. 2005;79:2988–2997. doi: 10.1128/JVI.79.5.2988-2997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf S, Froeschke M, Giroglou T, von Laer D, Dobberstein B. Signal peptide requirements for lymphocytic choriomeningitis virus glycoprotein C maturation and virus infectivity. J Virol. 2007;81:12515–12524. doi: 10.1128/JVI.01481-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge KF, Alexander DJ, Collins MS. Isolation and properties of viruses from poultry in Hong Kong which represent a new (sixth) distinct group of avian paramyxoviruses. J Gen Virol. 1980;49:255–262. doi: 10.1099/0022-1317-49-2-255. [DOI] [PubMed] [Google Scholar]
- Stanislawek WL, Wilks CR, Meers J, Horner GW, Alexander DJ, Manvell RJ, Kattenbelt JA, Gould AR. Avian paramyxoviruses and influenza viruses isolated from mallard ducks (Anas platyrhynchos) in New Zealand. Arch Virol. 2002;147:1287–1302. doi: 10.1007/s00705-002-0818-2. [DOI] [PubMed] [Google Scholar]
- Steward M, Samson A, C, Errington W, Emmerson PT. The Newcastle disease virus V protein binds zinc. Arch Virol. 1995;140:1321–1328. doi: 10.1007/BF01322759. [DOI] [PubMed] [Google Scholar]
- Subbiah M, Xiao S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 2 (strain Yucaipa) and comparison with other paramyxoviruses. Virus Res. 2008;137:40–48. doi: 10.1016/j.virusres.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Whitehead SS, Bukreyev A, Teng MN, Firestone CY, St Claire M, Elkins WR, Collins PL, Murphy BR. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73:3438–4342. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warke A, Appleby L, Mundt E. Prevalence of antibodies to different avian paramyxoviruses in commercial poultry in the United States. Avian Dis. 2008a;52:694–697. doi: 10.1637/8390-070308-RESNOTE.1. [DOI] [PubMed] [Google Scholar]
- Warke A, Stallknecht D, Williams SM, Pritchard N, Mundt E. Comparative study on the pathogenicity and immunogenicity of wild bird isolates of avian paramyxovirus 2, 4, and 6 in chickens. Avian Pathol. 2008b;37:429–434. doi: 10.1080/03079450802216645. [DOI] [PubMed] [Google Scholar]
- Wilson RL, Fuentes SM, Wang P, Taddeo EC, Klatt A, Henderson AJ, He B. Function of small hydrophobic proteins of paramyxovirus. J Virol. 2006;80:1700–1709. doi: 10.1128/JVI.80.4.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Paldurai A, Nayak B, Subbiah M, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 7 (strain Tennessee) and comparison with other paramyxoviruses. Virus Res. 2009;145:80–91. doi: 10.1016/j.virusres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Rout SN, Kim SH, Samal SK. Role of untranslated regions of the hemagglutinin-neuraminidase gene in replication and pathogenicity of newcastle disease virus. J Virol. 2009;83:5943–5946. doi: 10.1128/JVI.00188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Hansson E, Shiell B, Michalski W, Eaton BT, Wang LF. Sequence analysis of the Hendra virus nucleoprotein gene: comparison with other members of the subfamily Paramyxovirinae. J. Gen. Virol. 1998;79:1775–1780. doi: 10.1099/0022-1317-79-7-1775. [DOI] [PubMed] [Google Scholar]
- Zhang GZ, Zhao JX, Wang M. Serological survey on prevalence of antibodies to avian paramyxovirus serotype 2 in China. Avian Dis. 2007;51:137–139. doi: 10.1637/0005-2086(2007)051[0137:SSOPOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]