Abstract
Changes in epithelial cell activity and the production of pro-inflammatory cytokines were examined utilizing an organotypic culture system as an in vitro model to study the effects of radiation on oral keratinocytes to simulate what is thought to occur in radiation-induced oral mucositis. Monolayer cultures of oral keratinocyte were irradiated by varying the dose. Cell injury was assessed using a colony forming efficiency (CFE) assay. Third passage oral keratinocytes were seeded onto AlloDerm® to form a 3D construct of an ex vivo produced oral mucosa equivalent (EVPOME) which was irradiated with 0, 1, 3 and 8 Gy. Formalin-fixed sections of the EVPOME were used for histology and immunohistochemistry to examine proliferative capacity. Epithelial cell viability of EVPOME was measured by MTT assay. Spent culture medium was used to determine post-radiation pro-inflammatory cytokine production. Basal cells became more swollen and pyknotic as radiation increased, implying loss of cell viability also determined by MTT assay. The number of Ki-67 immunopositive cells and CFE showed negative correlation with radiation, indicating loss of cell proliferative capacity. The production of pro-inflammatory cytokines, IL-1α and IL-8, tended to increase in a radiation dose dependent manner. The EVPOME lacking submocosal cellular components was a useful model.
Keywords: radiation-induced oral mucositis, in vitro assay system, organotypic culture, pro-inflammatory cytokine
Oral mucositis is a severe side effect for patients undergoing radiation and/or chemotherapy for head and neck tumors, clinically characterized by erythema, ulceration and pseudomembrane formation of the oral mucosa resulting in ulcers producing severe pain and discomfort that make dietary intake difficult 24. The severity of the signs and symptoms may increase patient morbidity and culminate in the cessation of cancer therapy treatment 20-22.
Monolayer cultures of oral keratinocytes are often used to assess the effects of ionizing radiation, but are two dimensional and do not represent accurately what is occurring in situ where the cells live in three dimensions1. Several investigators have utilized artificial reconstructed skin or mucosa substitutes for clinical or basic science studies to evaluate the functional aspects of mucosa and the cytotoxic effects to radiation7, 14, 15, 17. These systems utilized an irradiated xenogeneic feeder layer of cells and/or medium that contained animal serum and pituitary extract. These cells and/or supplements add an unknown element to the culture conditions through cross-contamination with prions and/or slow viruses, and the culture medium for the cells is not chemically well defined.
In response to these limitations the authors developed a tissue-engineered three-dimensional (3D) human stratified oral mucosa, an ex vivo produced oral mucosa equivalent (EVPOME) in a culture system devoid of animal serum products, pituitary extracts and a xenogeneic irradiated cell feeder layer10. EVPOME can be utilized as a more accurate representation of cellular behavior in situ to assess the effects of irradiation on cell behavior, cell kinetics and the release of pro-inflammatory cytokines.
Recent studies elucidated more complex mechanisms of oral mucositis in which dynamic multiple molecular and cellular events occur, the activation of transcription factors such as nuclear factor-κB (NF-κB) and the upregulation of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6)11, 21. Five biological stages of oral mucositis were defined: initiation, primary damage response, signaling amplification, ulceration and healing20. The present study was carried out to assess the effect of ionizing radiation on oral mucosa keratinocytes without submucosal cellular components and to examine the metabolisms of oral mucosa keratinocytes within the EVPOME during the early stages of development of oral mucositis. This was accomplished by exposing the EVPOME model to gradually increasing doses of radiation. Cell viability was then assessed using MTT assay for cellular metabolism5, 13. Cell proliferation was determined by quantifying the presence and expression of a cell proliferation antigen10. The release of pro-inflammatory cytokines, IL-1α and 8, was quantified in the culture medium before and after irradiation, as they are thought to effect the primary damage reaction/signaling amplification caused by ionizing radiation10, 20, 21, 25.
Materials and Methods
Monolayer culture of oral mucosa keratinocyte
Oral mucosa keratinocytes were obtained from surgically discarded oral mucosa (6 samples: 2 male and 4 female). Sample tissues were washed three times in Dulbecco's phosphate-buffer saline (PBS) (BD Whittaker, Walkersville, MD, USA) supplemented with 15 μg/ml gentamycin and 7.5 μg/ml fungizone (GIBCO/Invitrogen, Carlsbad, CA, USA). After trimming subcutaneous tissue and blood, samples were digested in 0.04% trypsin (Sigma, St. Louis, MO, USA) overnight at room temperature. 0.0125% trypsin inhibitor (Cascade Biologics, Portland, OR, USA) was used to neutralize the trypsin and the epidermal side of the mucosa was gently scraped with a scalpel to detach the mucosa keratinocytes. The cell suspension was filtered through a 240 μm nylon mesh, centrifuged at 1100 rpm for 5 min, resuspended in chemically defined serum-free medium (EpiLife®, Cascade Biologics) supplemented with 0.06 mM calcium concentration, 12.7 mg/ml gentamycin and 192 μg/ml fungizone (GIBCO/Invitrogen), and then seeded into flasks at an initial density of 8.0×105 cells/cm2. Cells were incubated at 37°C, 5% CO2 in humidified air. Medium was changed every other day. Once keratinocyte cultures reached 60-70% confluency, they were split and passaged with 0.025% trypsin/EDTA solution (Cascade Biologics), neutralized by defined 0.0125% trypsin inhibitor (Cascade Biologics), centrifuged, and resuspended at a seeding density of 8.0×104 cells/cm2. Passage three cells were used for assaying colony forming efficiency and making the survival curve for ionizing radiation.
Fabrication of EVPOME model
The development of human EVPOME was established according to the protocol described previously10. Briefly, passage three cells were used for fabrication of human EVPOME. Circular pieces of AlloDerm® (Lifecell, Branchburg, NJ, USA), 1.0 cm in diameter, were rehydrated by sterile PBS and the surface was coated with 1 μg/ml/cm2 collagen type IV (BD Biosciences, Flanklin Lakes, NJ, USA) at 4°C in a refrigerator overnight. Oral keratinocytes were seeded onto AlloDerm® at a density of 1.2–1.5×105/cm2 and cultured at a high calcium concentration of 1.2 mM under submerged condition for 4 days. The equivalent was raised to an air-liquid interface culture plate (Organogenesis Inc, Canton, MA, USA) with high calcium concentration and cultured for 7 additional days, for a total culture time of 11 days.
Ionizing irradiation of EVPOMEs
X-ray irradiation was performed using Therapax DXT300 X-ray radiation source (Pantak, Easthaven, CT, USA). The focus-culture plate distance was 50 cm and the dose rate was approximately 3 Gy/min. Control plates were sham-irradiated by placing them on the radiation table without irradiating. All EVPOMEs and monolayer cultures of oral mucosa keratinocyte were kept out of the incubator during irradiation (for approximately 15 min). The dose applied was 0, 1, 3 and 8 Gy in the form of X photons of 300 kV. These doses were determined by a pilot study. Irradiated and control EVPOMEs and monolayer cultures of oral mucosa keratinocyte were immediately returned to the incubator at 37°C in a humidified environment and cultured for an additional 24 h.
Spent culture media and EVPOMEs were taken for samples of each assay. Spent cultured media was taken and stored in a freezer at -20°C. All spent culture medium samples were processed through an ELISA at the same time to assess the presence of the pro-inflammatory cytokines, IL-1α and IL-8. EVPOME was cut in half and one piece was fixed with 10% formalin for histopathological examination and immunohistochemistry, while the other was used immediately for cell viability assay.
Colony forming efficiency (CFE) assay
CFE for cell proliferation, was determined by plating irradiated cells in a 60 mm culture dish at a density of 500 cells/cm2 using monolayer cultures of oral mucosa keratinocyte. Cells were irradiated with 0, 0.5, 1, 3, 5, and 8 Gy and cultured for 10-12 days. Colonies larger than 16 cells were counted under ×100 magnification with a phase contrast microscope, and each dose of CFE fraction was calculated using Equation (1):
| (1) |
Data were presented as mean ± SE.
Nonlinear regression analysis, using a linear-quadratic model of the cell survival curve, was used to calculate the cell survival fraction (SF) according to Equation (2):18
| (2) |
Where D represents the radiation dose, exp is the root of naturalized logarithm (Euler's constant) and α and β are the curve parameters derived from linear-quadratic analysis.
Histological findings
Formalin fixed EVPOMEs were embedded in paraffin and cut into 4 micron sections, mounted on glass slide, and stained with hematoxylin-eosin.
Ki-67 immunohistochemistry
After deparaffinization, sections to be stained for Ki-67 were placed in a pressure cooker (Nordicware, Minneapolis, MN, USA) with 0.01 M citrate buffer (pH=6.0) and treated for 20 min in a household microwave oven. After microwave treatment, sections were allowed to cool at room temperature and immersed in methanol containing 0.3% hydrogen peroxide (Sigma) to quench endogenous peroxidase. 1.5% horse normal serum in PBS was used for preventing non-specific protein binding for 30 min and sections were incubated with anti-human mouse Ki-67 antibody (Beckman Coulter, Miami, FL, USA) at a concentration of 1:150 for 24 h at 4°C. After incubation, biotinylated horse anti-mouse antibody (Vector laboratories. Burlingame, CA, USA) was applied to section for 30 min. Finally, sections were developed with 0.02% 3,3-diaminobenzidine (Dojindo, Gaithersburg, MA, USA) in 50 mM Tris-HCl solution (pH=7.6) containing 0.006% hydrogen peroxide (Sigma) and counterstained with Gill's hematoxylin. As a negative control, the primary antibody was replaced with PBS (pH=7.4).
Evaluation of the proliferation activity of irradiated keratinocytes in EVPOME
To quantify the proliferative activity of keratinocytes in EVPOME after ionizing radiation, the ratio of Ki-67 positive cell as a proliferative index (PI) was calculated. 500 basal keratinocytes and Ki-67 labeled basal keratinocytes were counted at a magnification of ×250 under a light microscope (ausJENA, Jena, Germany). Ki-67 positive ratio (PR) was determined by dividing the number of Ki-67 positive cells into 500 on each dose specimens. PI was calculated by dividing the PR of 1, 3 and 8 Gy into that of 0 Gy.
Cell viability assay
Cell viability was measured using a water-soluble MTS assay, modified from hydrophobic MTT, according to the procedure of Mosmann13. After irradiation to EVPOME, 150 μl of Cell Titer 96 Aqueous One Solution Reagent (PROMEGA, Madison, WI, USA) and 1.5 ml of fresh high calcium EpiLife® medium was added to each well of the irradiated EVPOME culture plate and incubated at 37°C for 4 h. Spent media were collected and applied to a 96-well plate. Optical density was measured at 492 nm using a Multiskan EX reader (Thermo Electron Corporation, woburn, MA, USA). Results were calculated from the ratio of the optical density for the irradiated and control samples.
Measurement of the amount of undetermined pro-inflammatory cytokines, IL-1 α and IL-8 in the spent medium
The amount of IL-1α and IL-8 was measured in spent medium using commercial based enzyme-linked immunosolvent assay kits (R&D systems, Minneapolis, MN, USA). Assay plates were read at 480 nm and analyzed using a Multiskan EX 96-well plate reader (Thermo Electron Corporation) and the results were given in pg/ml. Since the cell viability of EVPOME varied between the groups of different radiation doses, the amount of cytokines produced was normalized by the data from the MTT assay, which has been shown to correlate directly with the viable cell number in the authors' culture system16.
Statistical analysis
The results were represented as mean ± SD by duplicated experiments with three EVPOME samples. One-way analysis of variance (one-way ANOVA) test was carried out by PRISM (GraphPad software, San Diego, CA, USA) and p<0.05 was determined to be statistically significant. The nonlinear regression analysis and survival curve were also carried out by PRISM.
Results
CFE
From Equation (1), the CFE that was calculated for cells irradiated with 0.5, 1, 3, 5 and 8 Gy, was 0.818±0.07, 0.585±0.09, 0.16±0.06, 0.062±0.02 and 0.009±0.005, respectively. Using this data, the nonlinear regression analysis was carried out and doses of 50% and 90% colony forming, were calculated from equation (2) (1.4 and 3.7 Gy, respectively; Fig 1). Figure 2 shows the colony formation after irradiation. Colony formation decreased in direct relation to increase in dose. The number of oral mucosa keratinocytes decreased as the radiation dose increased. Cell morphology changed from a smooth and oval appearance to a stretched and irregular shape, indicating degenerative changes within the cells (Fig 2).
Figure 1.
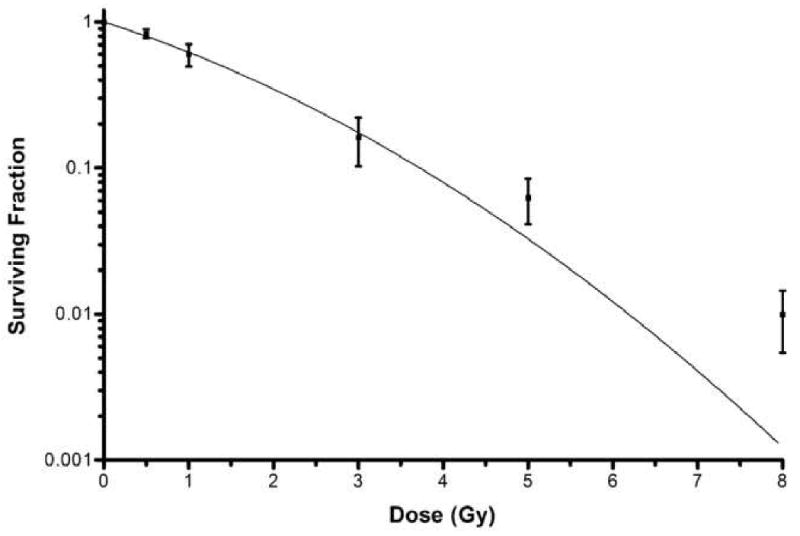
Linear regression analysis survival curve. The dose of 50% and 90% CFE was 1.4 and 3.7 Gy, respectively, from Equation (2).
Figure 2.
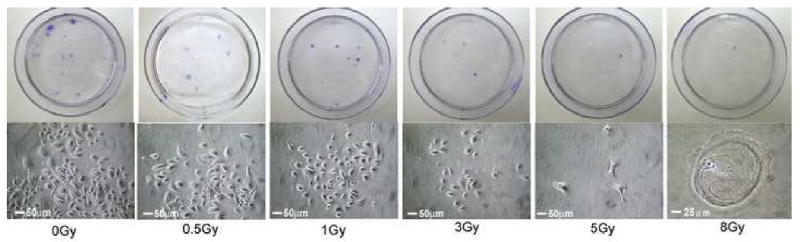
The colony forming and oral keratinocyte shape after X-ray irradiation. Colony formation decreased as radiation dose increased. The number of oral mucosa keratinocytes decreased in number as the radiation dose increased. Cell morphology changed from a smooth and oval appearance to a stretched and irregular shape, indicative of cellular degenerative changes.
Histological changes in irradiated oral keratinocytes in EVPOME
In the non-irradiated control EVPOME numerous intact and aligned cuboidal basal cells with densely stained nuclei were noted along the basement membrane. At 1 Gy of irradiation, keratinocytes within the EVPOME kept their basal cell-like arrangement along the basement membrane. At 3 Gy, the oral keratinocytes started to show faint hematoxylin staining of nuclei while at 3 Gy the cells presented as swollen shapes. At 8 Gy, the cells appeared to lose their regular arrangement along the basement membrane. Hematoxylin binds to the chromatin in the nuclei of cells4, 19, thus a faint nucleic stain of irradiated cells would imply that the structure of nucleosome was damaged by the ionizing radiation (Fig 3).
Figure 3.
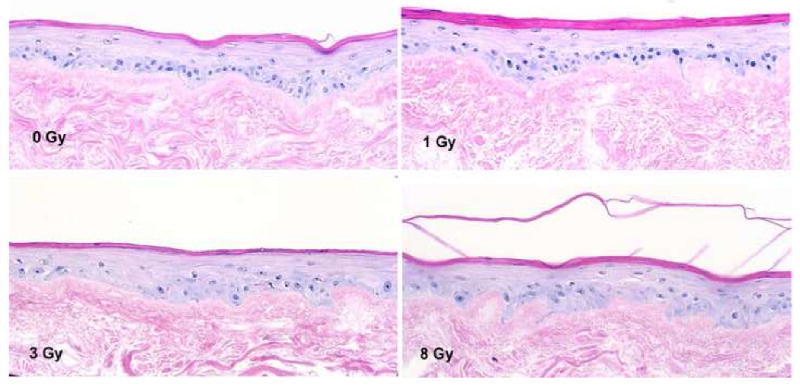
The histological change of EVPOMEs after ionizing radiation. At 0 Gy, the EVPOME appeared healthy with well aligned cells along the basement membrane. At 8 Gy, oral mucosa keratinocytes were aberrant in alignment and faint nuclear staining of the basal cells was seen. The basal cell had pyknoic nuclei. (HE stain, ×200)
Ki-67 immunohistochemistry
Ki-67 antigen is expressed within the nuclei of cells that are in a proliferating stage10 and is also observed in the basal layer cells of oral mucosa along the basement membrane. Ki-67 positive cells were seen at the basal epithelium on unirradiated EVPOME. Ki-67 positive cells gradually disappeared from the basal layer along the basement membrane in EVPOMEs as the radiation dose increased. At 8 Gy, few Ki-67 positive cells were observed within the basal layer and suprabasal regions of EVPOME (Fig 4).
Figure 4.
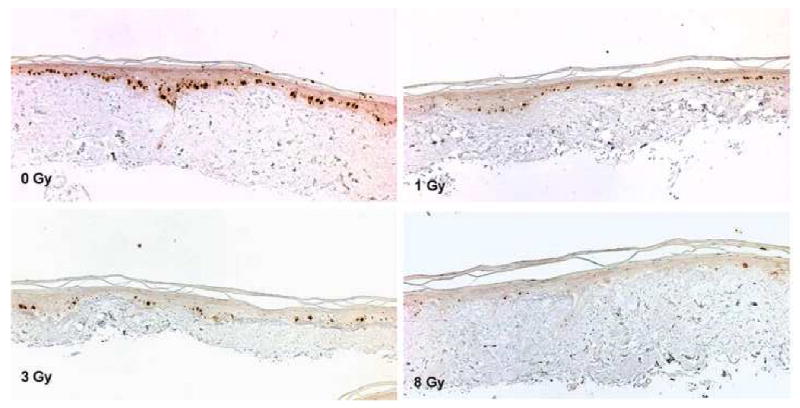
The result of Ki-67 immunohistochemistry. At 0 Gy, Ki-67 positive cells are mainly seen within the basal layer of cells. At 8 Gy, the number of positive cells dramatically decreased and they were scarce (×200).
Proliferation index (PI) on irradiated oral mucosa keratinocytes
Ionizing radiation dramatically affected the proliferative state of the oral keratinocytes in EVPOME. PI decreased as the radiation dose increased and nearly half of the oral keratinocytes in EVPOME had ceased proliferation at 1 Gy of irradiation (0.65±0.12). At 8 Gy, PI showed only 30% of oral keratinocytes maintained their proliferative activity 24 h after being irradiated (0.31±0.03). Statistically, there was a significant difference in all irradiated groups (p<0.05, Fig 5).
Figure 5.
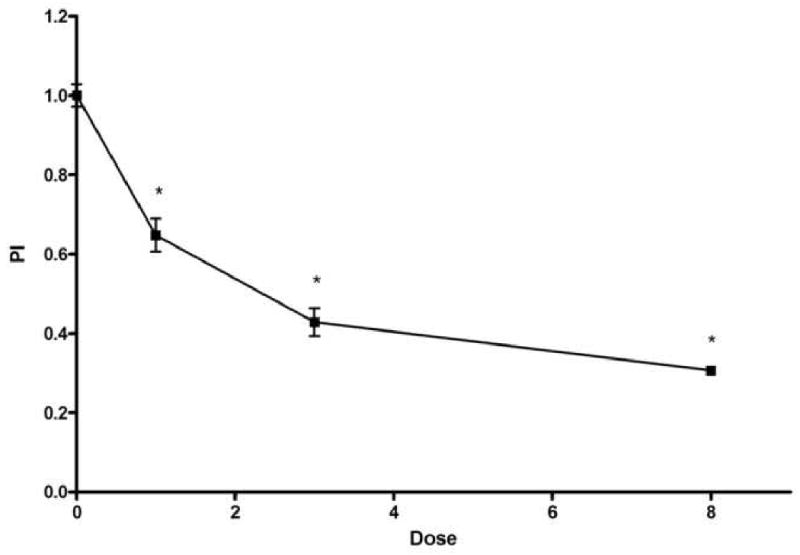
PI of Ki-67 positive basal keratinocytes. PI decreased as the radiation dose increased. Significant differences were seen at 1, 3 and 8 Gy (p<0.05). The x axis indicates the radiation dose and the y axis the PI. Each value represents mean ± SEM.
Cell viability
MTT assay of the oral keratinocytes in the EVPOME showed a decrease of cell viability directly with an increase in irradiation dose. At 8 Gy there was a statistical significance in the drop in cell viability (p<0.05). Cell viability was much higher, over 50% (55.87±2.82) at 8 Gy compared with the PI seen at the same dose (Fig. 5).
Assay of pro-inflammatory cytokines, IL-1α and IL-8 release
Pro-inflammatory cytokines are thought to play important roles in causing cell/tissue injury in radiation-induced oral mucositis so the authors measured the amount of undetermined pro-inflammatory cytokines released in spent media after they were irradiated11, 20, 21. At 1 and 3 Gy of irradiation, the release of IL-1α was slightly decreased (22.51±12.82 and 27.00±4.99 pg/ml, respectively), when compared with the unirradiated (control) EVPOME (27.84±5.66 pg/ml). The released IL-1α increased rapidly at 8 Gy of irradiation (72.30±10.72 pg/ml) and was statistically significant (p<0.01). The release of IL-8 also increased as the radiation dose increased. There were also significant differences at 8 Gy (551.56±44.45 pg/ml) (p<0.01, Table 1) compared with the control (237.46±17.91 pg/ml).
Table 1.
Pro-inflammatory cytokines, IL-1 alpha and IL-8, secretion, 24 h after irradiation (pg/ml)
| 0 Gy (Control) | 1 Gy | 3 Gy | 8 Gy | |
|---|---|---|---|---|
| IL-1 alpha | 27.84 ± 5.66 | 22.51 ± 12.82 | 27.00 ± 4.99 | 72.30 ± 10.72* |
| IL-8 | 237.46 ± 17.91 | 299.42 ± 34.56 | 308.79 ± 39.16 | 551.56 ± 44.45* |
Values are mean ± SEM of three independent experiments with three irradiated EVPOMEs samples for each radiation dose.
P<0.01 is level of significance for comparison of cytokine values from cultured media.
Discussion
The activation of transcription factors, particularly NF-κB, in all components of mucosa, followed by the increased production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 that mediate cell injury, play an important role in the development of oral mucositis11, 21. The aim of the present study was to examine the proliferative capacity and viability of epithelial cells and the production of pro-inflammatory cytokines to better understand the post-irradiation biological effects on oral keratinocytes. This in vitro model of radiation-induced oral mucositis allowed the authors to detect changes in cellular activities in cultured oral keratinocytes without submucosal cellular components.
Ionizing radiation-induced oral mucositis is a complicated process that involves the effects of irradiation on the keratinocytes and other cell populations in the submucosal tissue, such as, fibroblasts and endothelial cells20, 21, 24. The exact mechanisms of irradiation-induced oral mucositis are unknown. Part of the difficulty is the lack of a suitable in vitro model for oral mucositis. Investigators mostly use a monolayer cell culture system that only consist of highly proliferating cells and does not include cells in other states of development, such as differentiated cells, making this type of culture system suitable only for testing simple cell proliferation1.
The oral keratinocyte has the intracellular infrastructure to secrete chemokines such as VEGF, IL-1α and IL-8. Within the submucosal tissue, fibroblast and endothelial cells can also secrete chemokines3, 12. In systems that utilize a xenogeneic irradiated feeder layer of mouse fibroblasts this may cause confusion if one is mainly interested in the effects of irradiation on the keratinocyte. In addition, the use of fetal calf serum or pituitary extract added to the culture medium creates a poor chemically defined medium that does not allow accurate assessment of the release of the chemokines resulting directly from cellular injury due to the irradiation. The development of a 3D tissue engineered human oral mucosa in a serum-free, feeder layer-free system in a chemically defined medium10 allows a more controlled model for assessment of the effects of radiation on the oral keratinocytes.
In this study, monolayer culture was used to make a survival curve for irradiated oral keratinocytes. The linear-quadratic curve developed in this study is consistent with previous reports18. CFE decreased as the radiation dose increased but cell viability and secretion of pro-inflammatory cytokine did not match the magnitude of change with the radiation level (data not shown). The results using a monolayer culture system do not appear to give the results one would expect from a representative model for oral mucositis. There were changes in the cell morphology of the 3D EVPOME oral mucosa model with increases in radiation dose. The basal cells were severely damaged by the ionizing radiation with the development of pyknotic nuclei and a disarrangement of the basal cells along the basement membrane. These in vitro findings were similar to those seen in irradiation models of small animals6.
Ki-67 antigen is present in the S, G2 and M phases of the cell cycle. Cellular expression of Ki-67 is indicative of the proliferation phase of the cell8. In this study, the number of positive Ki-67 cells in the basal layer of EVPOME decreased in proportion to the increase in the radiation dose. This decrease in the expression of Ki-67 correlated directly with a decrease in the proliferative capacity of these cells. This suggests that the actively proliferating cells at the level of the basement membrane in the EVPOME had been seriously compromised with few cells noted in mitosis, eventually leading to decreased epithelial thickness. This is comparable with the phases of primary damage response/signal amplification seen in the in vivo response of oral mucositis20.
The authors also noted a decrease in cell viability with an increase in radiation dose but even with 8 Gy the cell viability remained over 50%. This might be a result of the MTT assay used which measures mitochondrial activities13. After radiation damaged to DNA, the cellular proliferative capacity was compromised but the cells were still able to carry out other metabolic activities while other cell functions were not injured. Ionizing radiation is thought to cause direct and indirect cell damage rather than immediate cell death in basal epithelial cells, which precipitate the onset of the cascade of extensive mucosa injury21. The authors' model could be beneficial for studying cell survival and metabolic activity rather than cell death due to sustained epithelial cell viability. Targeting mitochondrial activity affecting viability could be another approach to protect cells against irradiation9.
This is the first report to show pro-inflammatory cytokines, IL-1α and IL-8, showed an increase in their release into the culture medium as the radiation dose was increased. These results are similar to those reported to occur in animal models of oral mucositis23. IL-1α has been show to play a central role in the activation of keratinocytes, which initiate the inflammatory process3, 12, 25. IL-8 is also a potent chemoattractant for neutrophils and lymphocytes3. The authors have demonstrated that pro-inflammatory cytokines were released from oral keratinocytes in an organotypic model after irradiation. It has been shown that these pro-inflammatory cytokines are capable of further activation of additional transcription factors important in the development of oral mucositis11, 20. The fact that the IL-8 concentration was shown to be greater than IL-1α post-irradiation may be a novel way of modulating the cascade that leads to oral mucositis by controlling the release of specific pro-inflammatory cytokines.
In summary, the authors' in vitro EVPOME model of oral mucositis is similar in behavior to in vivo animal models using ionizing radiation. Since the EVPOME model consists only of oral keratinocytes, is devoid of a feeder layer of fibroblasts and is free of serum and pituitary extracts it is suitable for evaluating the kinetics of irradiated oral mucosa keratinocytes in vitro. This experiment model seems to reproduce the early phase of radiation-induced oral mucositis in vitro and may help to elucidate the mechanism(s) that may play a role in this phenomenon and help to assist in the therapeutic management of radiation-induced oral mucositis.
Figure 6.
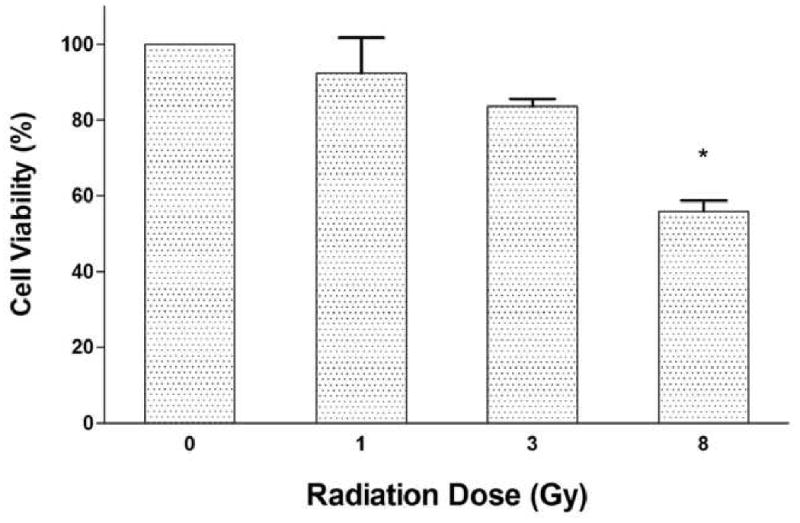
Result of the MTT cell viability assay. Cell viability decreased dose-dependently. Difference was statistically significant at 8 Gy (p<0.05). The x axis shows the radiation dose, the y axis shows the ratio of the viability. Each value is mean ± SEM.
Acknowledgments
This work was supported by a MUNN grant (G001847) from the University of Michigan and NIH Grant DE13417 (SEF).
Funding: MUNN grant (G001847) from the University of Michigan and NIH Grant DE13417 (SEF).
Footnotes
Declarations
Competing Interests: None declared
Ethical Approval: Not required
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 2.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 3.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 4.Bettinger C, Zimmermann HW. New investigations on hematoxylin, hematein, and hematein-aluminium complexes. Histochemistry. 1991;96:215–228. doi: 10.1007/BF00271540. [DOI] [PubMed] [Google Scholar]
- 5.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 6.Dorr W, Emmendorfer H, Haide E, Kummermehr J. Proliferation equivalent of ‘accelerated repopulation’ in mouse oral mucosa. Int J Radiat Biol. 1994;66:157–167. doi: 10.1080/09553009414551061. [DOI] [PubMed] [Google Scholar]
- 7.Doucet O, Robert C, Zastrow L. Use of a Serum-free reconstituted Epidermis as a Skin Pharmacological Model. Toxicology In Vitro. 1996;10:305–313. doi: 10.1016/0887-2333(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 8.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 9.Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 10.Izumi K, Song J, Feinberg SE. Development of a tissue engineered human oral mucosa: From the bench to the bed side. Cells Tissues Organs. 2004;176:134–152. doi: 10.1159/000075034. [DOI] [PubMed] [Google Scholar]
- 11.Logan RM, Stringer AM, Bowen JM, Yeoh ASJ, Gibson RJ, Sonis ST, Keefe DMK. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treatment Rev. 2007;33:448–460. doi: 10.1016/j.ctrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie RC, Sauder DN. The role of keratinocyte cytokines in inflammation and immunity. J Invest Dermatol. 1990;95:105S–107S. doi: 10.1111/1523-1747.ep12874955. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Nelson D, Gay RJ. Effects of UV irradiation on a living skin equivalent. Photochem Photobiol. 1993;57:830–837. doi: 10.1111/j.1751-1097.1993.tb09219.x. [DOI] [PubMed] [Google Scholar]
- 15.Ponec M. Skin constructs for replacement of skin tissues for in vitro testing. Adv Drug Deliv Rev. 2002;54(Suppl):S19–S30. doi: 10.1016/s0169-409x(02)00112-6. 1. [DOI] [PubMed] [Google Scholar]
- 16.Rakhorst HA, Tra WMW, Sluijs STPV, Hovius SER, Levendag PC, Kanaar R, Hofer SOP. Quantitative analysis of radiation-induced DNA break repair in a cultured oral mucosal model. Tissue Eng. 2006;12:3395–3403. doi: 10.1089/ten.2006.12.3395. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Izumi K, Tobita T, Nakanishi Y, Feinberg SE. Constitutive release of cytokines by human oral keratinocytes in an organotypic culture. J Oral Maxillofac Surg. 2009 doi: 10.1016/j.joms.2009.02.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slonina D, Hoinkis C, Dorr W. Effect of keratinocyte growth factor on radiation survival and colony size of human epidermal keratinocytes in vitro. Radiat Res. 2001;156:761–766. doi: 10.1667/0033-7587(2001)156[0761:eokgfo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Smith AA. Specific staining of tissue components with metal-hematoxylin complexes. Micron. 2002;33:95–103. doi: 10.1016/s0968-4328(00)00059-7. [DOI] [PubMed] [Google Scholar]
- 20.Sonis ST. The pathobiology of mucositis. Nat rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 21.Sonis ST. Pathobiology of oral mucositis: Novel insights and opportunities. J Support Oncol. 2007;9(suppl 4):3–11. [PubMed] [Google Scholar]
- 22.Stiff P. Mucositis associated with stem cell transplantation: current status and innovative approaches to management. Bone Marrow Transplant. 2001;27(Suppl):S3–S11. doi: 10.1038/sj.bmt.1702863. 2. [DOI] [PubMed] [Google Scholar]
- 23.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 24.Yeoh A, Gibson R, Yeoh E, Bowen J, Stringer A, Giam K, Logan R, Keefe D. Radiation therapy-induced mucositis: relationships between fractionated radiation, NF-κB, COX-1, and COX-2. Cancer Treatment Rev. 2006;32:645–651. doi: 10.1016/j.ctrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Zaghloul MS, Dorie MJ, Kallman RF. Interleukin-1 modulatory effect on the action of chemotherapeutic drugs and localized irradiation of the lip, duodenum, and tumor. Int J Radiat Oncol Biol Phys. 1993;26:417–425. doi: 10.1016/0360-3016(93)90959-y. [DOI] [PubMed] [Google Scholar]


