Abstract
Ongoing monitoring of neuroleptic-induced extrapyramidal side effects (EPS) is important to maximize treatment outcome, improve medication adherence and reduce re-hospitalization. Traditional approaches for assessing EPS such as parkinsonism, tardive akathisia, or dyskinesia rely upon clinical ratings. However, these observer-based EPS severity ratings can be unreliable and are subject to examiner bias. In contrast, quantitative instrumental methods are less subject to bias. Most instrumental methods have only limited clinical utility because of their complexity and costs. This paper describes an easy-to-use instrumental approach based on handwriting movements for quantifying EPS. Here, we present findings from psychiatric patients treated with atypical (second generation) antipsychotics. The handwriting task consisted of a sentence written several times within a 2 cm vertical boundary at a comfortable speed using an inkless pen and digitizing tablet. Kinematic variables including movement duration, peak vertical velocity and the number of acceleration peaks, and average normalized jerk (a measure of smoothness) for each up or down stroke and their submovements were analyzed. Results from 59 psychosis patients and 46 healthy comparison subjects revealed significant slowing and dysfluency in patients compared to controls. We observed differences across medications and daily dose. These findings support the ecological validity of handwriting movement analysis as an objective behavioral biomarker for quantifying the effects of antipsychotic medication and dose on the motor system.
1. Introduction
Neuroleptic medications have been the mainstay for treating psychotic illness for over 50 years. While neuroleptics improve the lives of schizophrenic patients, the occurrence of neuroleptic-induced extrapyramidal side effects (EPS) with increasing dosage often imposes limits on the dosage actually required to treat the disease. Even after the emergence of a second generation of antipsychotics, EPS continue to cause concern (Miller et al., 2008), particularly in vulnerable populations, such as the elderly (Caligiuri et al., 2000).
Ongoing monitoring of EPS is important to maximize treatment outcome, improve medication adherence and reduce re-hospitalization. Effective management of EPS begins with early detection, and eventually, prevention. Early detection of EPS requires sensitive and reliable measurement. Traditional means of assessing EPS rely upon observer judgments of severity, but these subjective ratings suffer from low reliability, even after the required extensive training, and are insensitive to mild subclinical abnormalities (Lohr & Caligiuri, 1992; Caligiuri, 1997). Different examiners show different average judgments of the same patients, resulting in examiner bias. To overcome these limitations, investigators have developed instruments for quantifying EPS (e.g., load cells, strain gauges, accelerometers, and electromyograms). While these instruments enjoyed appeal in research settings, they have not been adopted for routine clinical or bedside use. The main reason is because these procedures require levels of technical expertise not always available in clinical settings. Currently, no techniques for the quantitative and objective measurement of EPS severity are available that can be easily used by neurologists, psychiatrists, and other practitioners in the clinical setting.
One such approach to quantifying drug-induced motor side effects involves the analysis of handwriting movements. Haase (1961) was the first to demonstrate a relationship between clinical effectiveness of neuroleptic mediation and EPS using handwriting analysis. Haase noted that as neuroleptic dosage increased, patients showed parkinsonism; their handwriting slowed (bradykinesia) and decreased in size, resembling the micrographia observed in Parkinson’s disease. The use of handwriting movements to assess EPS has been a focus of research primarily in Europe (Haase, 1978; Gerken et al., 1991; Künstler et al., 1999, 2000). However, the results have been mixed. For example, Gerken et al. (1991) used movement size (expressed by the area encompassed by handwriting) in schizophrenic patients for predicting treatment response. In their small sample of patients, they observed reductions in handwriting size in three of the nine treatment responders and in nine of the 12 treatment non-responders, suggesting that handwriting movement size was unable to predict treatment response. Künstler et al. (2000) used single photon emission tomography to examine the relationship between handwriting area and dopamine D2 receptor occupancy in schizophrenic patients before and after treatment with drugs (haloperidol, clozapine, or risperidone). They reported a highly significant linear relationship between D2 receptor occupancy and reduction in handwriting area. In a second study of 10 schizophrenic patients who received medication for the first time, Regenthal et al (2005) reported positive correlations between D2 receptor occupancy, plasma level of risperidone and its active metabolite 9-hydroxyrisperidone and reduction in handwriting area in previously drug-free patients. While none of the patients exhibited clinically observed EPS, the authors concluded that analysis of handwriting movements might be well-suited for evaluating neurological side effects of neuroleptic medications because of their sensitivity to D2 receptor occupancy.
The purposes of the present study were to test whether handwriting kinematic measures show greater impairments for some atypical antipsychotic medication than others and whether the severity of impairment is related to daily dose. Additionally, we aimed to compare the medication and dose effects on handwriting kinematics with those for traditional observer-based EPS severity ratings.
2. Methods
2.1 Subjects
This study involved a multi-site parallel group design. Subjects were recruited and tested at three sites including: San Diego, CA; Minneapolis, MN; and Indianapolis, IN. The study was carried out in accordance with the 1964 Declaration of Helsinki and all subjects signed institution-approved informed consent prior to participating. Subjects from each site received the same clinical evaluation and a computer-controlled handwriting motor test in the same order, using the same procedures. The original cohort consisted of 113 psychosis patients and 46 healthy comparison subjects. Patients were excluded for the following reasons: treatment with multiple antipsychotics including conventional agents (n=17); treatment with an anticholinergic medication (n=18); off antipsychotic at the time of testing (n=11); insufficient clinical or medication data (n=8). Thus, the final group consisted of 59 psychosis patients with active psychotic illness.
The mean (sd) age of the patient group was 50.55 years (8.72), which was higher than the mean for the healthy comparison subjects of 42.21 years (9.30) (t=4.70; p<0.01). We do not assume that the group difference we found could be explained as an aging effect. For example, Teeken et al. (1996) found that most age-related slowing is observed in discrete aiming movement tasks, while rapid, reciprocal arm movement tasks, comparable to continuous handwriting, show no significant slowing across this age range. The patient group consisted of 43 males and 16 females, whereas the healthy comparison group comprised 14 males and 32 females. The male: female ratio for the two subject groups was different (χ2 = 18.77; p<0.001). Similar to the aging effect, gender shows mainly an effect on discrete movements but no effect on reciprocal movements, which is comparable to continuous handwriting (Teeken et al., 1996). In spite of this evidence that the age and gender differences between groups is expected to have little effect, additional statistical tests were performed to examine any effects of these demographic variables on the handwriting movements.
2.2 Clinical Characteristics of Study Patients
Patients met DSM-IV criteria for either schizophrenia (n=45) or schizoaffective disorder (n=14). Study patients underwent clinical movement disorder assessment using the Abnormal Involuntary Movement Scale (AIMS; Guy et al., 1976) for tardive dyskinesia, the Simpson-Angus EPS scale (SAEPS; Simpson and Angus, 1970) for drug-induced parkinsonism, and the Barnes Akathisia Scale (BAS; Barnes, 1989) for akathisia. Severity of positive and negative symptoms of psychosis were rated using the Positive and Negative Symptom Scale (PANSS; Kay et al., 1987).
Fifty-one of the 59 patients were treated with a single atypical antipsychotic: aripiprazole (n=10); risperidone (n=17), quetiapine (n=9), olanzapine (n=10), ziprasidone (n=3) or clozapine (n=2). The remaining eight patients were on two atypical antipsychotics (risperidone in seven of the eight patients plus another atypical antipsychotic). Antipsychotic dose for each of these medications was converted to risperidone equivalent dose based on conversion table published in a consensus report (Kane et al., 2003). For cases treated with more than a single antipsychotic, the equivalent doses for all antipsychotics were summed to yield the net equivalent dosage. Table 1 shows the statistics of the examiner assessments and the equivalent dosage for the group of the 59 patients.
Table 1.
Patient Characteristics (n=59)
| Clinical Variable | Mean (sd) |
|---|---|
| Positive and Negative Symptom Scale (PANSS), Total score | 63.98 (17.49) |
| Positive Symptom Score from PANSS | 15.75 (5.81) |
| Negative Symptom Score from PANSS | 16.69 (5.77) |
| Barnes Akathisia Scale (BAS), Global Score | 1.13 (1.16) |
| Abnormal Involuntary Movement Scale (AIMS), Total Score | 3.19 (2.92) |
| Simpson-Angus EPS (SAEPS), Total Score | 4.84 (3.74) |
| Average Daily Dose, mg/day Risperidone Equivalents | 4.85 (3.26) |
2.3 Kinematic Variables of Handwriting
Handwriting movements were quantified using a commercial digitizing tablet and MovAlyzeR software (NeuroScript, LLC; Tempe, AZ, USA). We used a non-inking pen with a Wacom UD 9×12 digitizing tablet (30 cm × 22.5 cm, RMS accuracy 0.01 cm). Sampling rates were either 100 Hz or 200 Hz due to tablet driver updates in some sites during the course of the study. Data processing took individual sampling rates into account so that kinematic features are independent of sampling rate. The tablet was attached to a MS Windows laptop computer running MovAlyzeR software.
The data reported herein were collected as part of a larger study of handwriting kinematics in psychosis patients. The complete handwriting battery included 15 different writing patterns varying in vertical size and pattern complexity for both dominant and nondominant hands and normal and high speeds. The full battery of writing patterns included: 1) cursive loops, 2) continuous circles 3) a complex cursive loop sequence, and 4) a sentence, “Today is a nice day”. All tasks were repeated 3 times1 each at 1, 2, and 4 cm vertical stroke heights except the sentence and the high-speed circles which were produced only at the 2-cm vertical stoke size. The subjecvts performed all replications of one task before moving to the next task. The sequence of tasks was random. The duration of the handwriting test was about 20 minutes. For the purpose of this study, we report only the results from the sentence task. Subjects viewed only the tablet and because we used an inkless pen, the handwriting trace was not visible to the subject. The resultant handwriting traces were visible only to the examiner. Subjects were prevented from viewing the recorded trace to minimize any distracting effects of visual feedback on movement speed and smoothness. Data collection began when the pen tip came in contact with the tablet and ended when the pen was lifted for more than 3 seconds.
The X and Y coordinates were low-pass filtered at 8 Hz using a sinusoidal transition band of from 3.5 to 12.5 Hz (Teulings et al., 1984). Movements were then segmented into successive up and down strokes using interpolated vertical-velocity zero crossings. The basic unit of movement we are studying is the stroke. Each sentence produced approximately 60 vertical strokes depending upon writing style. The initial down stroke per trial was discarded. Only the first 14 remaining strokes were adopted in this analysis. These strokes correspond generally to the writing of “Today” until the middle of the “y”. Therefore, there were no large between-word movements.
The number of strokes per letter varies per writing styles. While some subjects developed writing styles that require more strokes per letter than others, we do not assume it will affect the group differences due to the moderately large sample size. It is possible that differences between cursive and handprint writing style could lead to differences in kinematic variables such as stroke duration, peak velocity or writing fluency. However, we assume that hand printers and cursive writers per group are proportionally spread across all subject groups in our large sample. Therefore, the more frequent pen lifts in handprint and its dysfluencies should not be confounded with groups.
Pen lifts, during writing a word are considered part of the motor program. Pen lifts higher than about 1 cm above the tablet will cause the digitizing tablet to loose samples. This will manifest itself in the raw data as a discontinuity which could jeopardize filtering and stroke-feature estimation. Therefore, we applied a discontinuity-detection algorithm which fills in an estimated number of samples based on the average pen speed enabling us to substitute the estimated number of missing samples. These discontinuities appeared to occur rarely, though, as most participants did not introduce discontinuities. Therefore, we do not think these discontinuities will affect the groups differently.
We focused on the vertical movement component only as this is the main movement component in Western cursive handwriting and handprint. For each segmented stroke vertical size, duration (DUR), absolute peak vertical velocity (PVV), and number of vertical acceleration peaks (APK) were calculated and similarly for the primary submovement (Meyer et al., 1988) DURsub, PVVsub, and APKsub. The primary submovement begins where the stroke begins and ends where the vertical velocity changes from decelerating to accelerating for the first time after the velocity peak. The primary submovement is comparable to the initial, ballistic phase of the up or down stroke. Acceleration peaks in the primary submovement occur thus before the velocity peak while the total number of acceleration peaks can occur before or after the velocity peak. In addition, handwriting smoothness was quantified by calculating the normalized jerk averaged (ANJ) per stroke (Teulings et al., 1997). Normalized jerk is unitless as it is normalized for stroke duration and length. ANJ was calculated using the following formula:
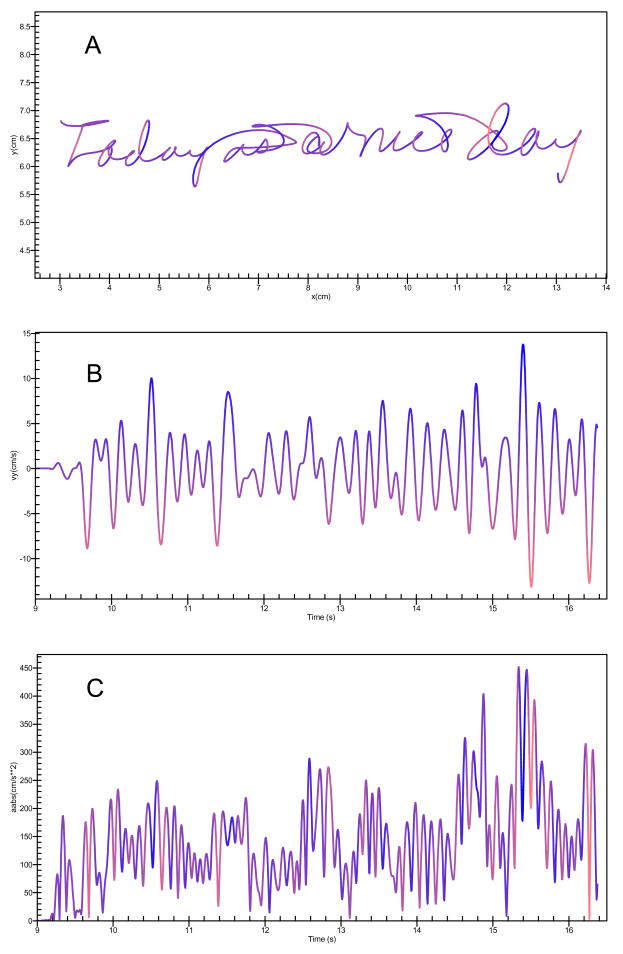
Longer segment durations, and lower peak velocities are reflective of slow movements or bradykinesia whereas higher ANJ scores and increased number of acceleration peaks per segment are indicative of dysfluent writing movements or dyskinesia. The estimation of the handwriting kinematic variables was conducted automatically. The computer program had no knowledge of the subject’s medication or EPS status and ran without intervention by the examiner. Samples of the handwritten sentence, segmentation markings and velocity derivative are shown in Figure 1.
Figure 1.
(A) Handwriting sample of the sentence “Today is a nice day” showing the raw sample with horizontal (x, cm) and vertical (y, cm) dimensions (A), corresponding vertical velocity (cm/s) over time (seconds) (B), and corresponding absolute acceleration (abs(cm/s**2) over time (seconds) (C). Airstrokes where the pen moves above the digitizer are also recorded and processed are also shown.
2.4 Statistical Analyses
2.4.1. Instrument Reliability
We examined the consistency of the handwriting kinematic variables using Cronbach’s α (Cronbach, 1951). A reliability coefficient across the three trials was obtained for each variable from the 46 healthy subjects. High inter-trial coefficients indicate that the trials are measuring the same underlying construct.
2.4.2 Cross-Sectional Group Analyses
To reduce data, features per stroke or primary submovement and per trial were averaged across all strokes or primary submovements, respectively, and across all trials per condition. The primary analyses to test differences between the patient and control groups involved one-tailed t-tests with critical significance level α <0.05 as we had definite expectations about the direction of the differences. The prevalence of abnormality on handwriting measures was determined by identifying the percentage of patients with scores that exceeded the upper 95th percentile for DUR, ANJ, APK or lower 5th percentile for PVV (and submovements where appropriate) of the non-patient standard group. Mean stroke size was analyzed to ensure that subject groups did not differ in the overall pattern of handwriting and that the groups performed the task as instructed.
Correlational analyses were performed to examine concurrence with traditional EPS severity rating scales, severity of psychopathology, and age. Because of the gender disparity between the patients and healthy comparison subjects, we examined any gender effects.
2.4.2 Effects of Antipsychotic Medication and EPS Status
Patients were subgrouped based on their primary antipsychotic medication into four groups: aripiprazole (n=11), risperidone (n=24), quetiapine (n=9), and olanzapine (n=10). The five patients treated with ziprasidone or clozapine were excluded from this medication-type analysis because of their small sample sizes. Two-way ANOVAs were performed to test main effects and interactions of EPS status (both based on handwriting measures and clinical rating scales) and medication group. For EPS status, patients were considered either normal or abnormal when at least one of the following rules applied: 1) based on the handwriting movement variables, subjects who exceeded the upper 95th percentile of the normal mean for DUR, ANJ, and APK or the lower 5th percentile for PVV were classified for the purpose of this analysis as abnormal; 2) based on the clinical severity scales, patients who received a global score greater than 1 on the BAS, a total score greater than 3 on the AIMS, or a total score greater than 3 on the SAEPS were classified for the purpose of this analysis as abnormal. The decision to use a total score of 3 or greater on the clinical ratings for operationally defining the presence of TD or EPS was based on the desire in the present study to include a sufficient number of patients with at least mild TD or EPS into the analysis. Correlational analyses were performed to evaluate relationships between the scores on the handwriting kinematic measures and EPS severity with antipsychotic dose. As the scores for the three clinical severity scales were not normally distributed, we used non-parametric analyses (Spearman rank order correlation and Kruskal-Wallis ANOVA).
3. Results
3.1 Instrument Reliability
Table 2 shows the results of the instrument reliability testing where each of the replications is considered an independent test. With the possible exception of the calculation of the APKsub, all of the handwriting kinematic variables exhibited high repeatability (with Cronbach’s α coefficients raging from 0.76 to 0.95). The relatively low coefficient for the APKsub (0.57) indicates that this variable may be measuring a multidimensional rather than a unidimensional latent construct.
Table 2.
Reliability estimates across trial replications for the seven handwriting kinematic variables for 46 healthy subjects
| Handwriting Kinematic Variables | Cronbach’s α |
|---|---|
| Duration/Stroke in ms (DUR) | 0.81 |
| Duration/Primary submovement in ms (DURsub) | 0.85 |
| Absolute Peak Vertical Velocity/Stroke in cm/s (PVV) | 0.94 |
| Absolute Peak Vertical Velocity/Primary submovement in cm/s (PVVsub) | 0.94 |
| Average Normalized Jerk/Stroke (ANJ) | 0.77 |
| Number of Vertical Acceleration Peaks/Stroke (APK) | 0.76 |
| Number of Vertical Acceleration Peaks/Primary submovement (APKsub) | 0.57 |
3.2 Patient versus Control Group Effects
Table 3 shows the descriptive statistics for each handwriting kinematic variable for the 46 healthy comparison subjects and 59 medicated psychosis patients. Patients exhibited significantly longer mean DUR and DURsub, lower PVV, higher ANJ, and greater APK and APKsub scores than the controls, but did not differ for the PVVsub measure.
Table 3.
Group Comparisons for the Handwriting Kinematic Variables
| Handwriting Kinematic Variables | Healthy Comparison Subjects (N=46) | Medicated Psychosis Patients (N=59) | Student’s t* |
|---|---|---|---|
| DUR | 174 (49) | 228 (68) | −4.51 (p≤0.0001) |
| DURsub | 157 (34) | 198 (53) | −4.56 (p≤0.0001) |
| PVV | 7.55 (1.91) | 6.40 (2.27) | 2.76 (p≤0.01) |
| PVVsub | 0.24 (0.19) | 0.30 (0.19) | −1.61 (p=ns) |
| ANJ | 19.73 (9.97) | 36.30 (27.03) | −3.95 (p≤0.005) |
| APK | 1.65 (0.68) | 2.16 (0.48) | −3.42 (p≤0.001) |
| APKsub | 1.45 (0.48) | 1.82 (0.60) | −3.38 (p≤0.001) |
Key: DUR = Duration of pen movement in ms; PVV = peak vertical velocity of pen movement strokes in cm/sec; ANJ= Average normalized jerk, a measure of movement smoothness; APK= the number of acceleration peaks/stroke; sub = primary submovement.
for all statistical comparisons, degrees of freedom = 103.
Results indicated that patients treated with atypical antipsychotic medications compared with healthy comparison subjects exhibited greater DUR (t=4.51; df=103; p<0.0001) and DURsub (t=4.56; df=103; p<0.0001), lower PVV (t=−2.76; df=103; p<0.01), increased ANJ (t=3.95; df=103; p<0.005), increased APK (t=3.42; df=103; p<0.001) and APKsub (t=3.38; df=103; p<0.001).
Based on the 5th and 95th percentile scores from the healthy comparison subjects’ mean score, 31 patients (52.5%) scored in the abnormal range on at least one measure. Of the seven measures, 12 patients (20.3%) had abnormal DUR, 18 (30.5%) had abnormal DURsub, 10 (16.9%) had abnormal PVV, 14 (23.7%) had abnormal ANJ scores, 8 (13.6%) had abnormal APK, and 8 (13.6%) had abnormal number of APKsub. PVVsub scores were normal for all patients.
As expected, both groups performed the test with the same writing sizes. Based on mean stroke size, both groups performed the handwriting task similarly, as expected. The mean (sd) vertical stroke size for the 46 healthy subjects was 0.67 cm (0.16 cm) while the mean stoke size for the 59 patients was 0.68 cm (0.17cm). These means were not statistically different (t=0.01; df=103; p>0.10) and suggest that group differences in other kinematic variables are not likely due to any difference in overall vertical stoke size of the handwriting movements.
3.3. Effects of Diagnosis: Schizophrenia versus Schizoaffective Disorder
The possibility exists that differences in treatment between schizophrenia (SZ) and schizoaffective (SA) patients could contribute to subgroup effects on the handwriting task. SZ patients were treated with significantly higher doses of antipsychotic than SA patients with mean (sd) risperidone equivalents of 5.34 (3.53) mg/day versus 3.25 (1.30) mg/day, respectively (t=2.16; df=57; p<0.05). This difference was consistent with group differences in severity of psychosis (based on PANSS total), with mean (sd) PANSS score of 67.2 (16.3) versus 53.1 (17.7), respectively (t=2.69; df=57; p<0.01). Interestingly, while SZ and SA patients did not differ on SAEPS total score, SZ patients exhibited significantly higher AIMS scores (mean=3.86; sd=2.87) than SA patients (mean=0.75; sd=1.48; t=3.61; df=57; p<0.001). SA and SZ patients also differed on two of the seven handwriting kinematic variables. SA patients exhibited significantly higher PVVsub values (mean =0.40 cm/s; sd=0.26) than SZ patients (mean =0.27 cm/s; sd=0.15; t=2.35; df=57; p<0.05) and lower APKsub values (mean = 1.52; sd=0.43) than SZ patients (mean=1.91; sd=0.62; t=2.18; df=57; p<0.05).
3.4 Age and Gender Effects
We evaluated the effects of age and gender on handwriting kinematics. As anticipated, the results failed to show a significant effect of age on any of the kinematic variables in this study. This is not to say that age may not be an important factor in the study of handwriting movements; however given the relatively narrow age range of our subjects, age was not a measurable factor. Regarding gender, ANOVA results revealed significant effects of gender for PVVsub and ANJ only (with males having higher velocities and less smoothness than females, which is not surprising); however there was no group × gender interaction indicating that the gender differences were the same for both groups of subjects.
3.5 Medication Effects
Two analyses were performed with the medication data: correlational analyses to evaluate the relationship between daily dose and performance on the clinical motor and handwriting kinematic variables and a one-way ANOVA for the medication group factor (aripiprazole, risperidone, quetiapine, and olanzapine) for each of the clinical motor variables and the handwriting kinematic variables. These results are shown in Table 4.
Table 4.
Medication Dosage and Medication Type Effects upon Clinical Scores and Handwriting Kinematic Variables
| Correlation with Daily Risperidone Equivalent Dose | Main Effect of Medication | Post-Hoc Tests 1 | |
|---|---|---|---|
| Clinical Observations | |||
| BAS | r=0.29 (p<0.05) * | Χ2=2.79 (ns)** | |
| AIMS | ns* | Χ2=1.07 (ns)** | |
| SAEPS | ns* | Χ2=3.28 (ns)** | |
| Handwriting Kinematics | |||
| DUR | r=0.70 (p<0.05) 2 | F3,50=3.46 (p<0.05) | R>Q (p<0.01) |
| DURsub | ns | F3,50=2.93 (p<0.05) | R>Q (p<0.01) |
| PVV | ns | F=1.00 (ns) | |
| PVVsub | ns | F=1.46 (ns) | |
| ANJ | r=0.86 (p<0.001) 2 r=0.66 (p<0.01) 3 r=0.48 (p<0.001) 4 |
F=1.25 (ns) | |
| APK | r=0.35 (p<0.05) 4 r=0.55 (p<0.01) 3 |
F=2.38 (ns) | |
| APKsub | r=0.28 (p<0.05) 4 r=0.50 (p<0.01) 3 |
F=1.74 (ns) |
Spearman rank order correlation coefficient
Kruskal-Wallis nonparametric ANOVA
R=risperidone, Q=quetiapine, p-value based on LSD tests.
Aripiprazole patients only (n=11)
Risperidone patients only (n=24)
All medicated patients (n=59)
ns= non-significant (p>0.05)
Results indicated that performance on several handwriting kinematic variables correlated with the daily equivalent dose and type of antipsychotic medication. Dose of aripiprazole was associated with slowing and more dysfluencies of the movement as expressed by an increase in DUR (r=0.70; p<0.05) and an increase in ANJ (r=0.86; p<0.001); however, dose of risperidone was mainly associated with the dysfluency measures such as increased ANJ (r=0.66; p<0.01) and higher APK (r=0.55; p<0.01) and APKsub (r=0.50; p<0.01) scores. Among the three clinical severity scales, only severity of akathisia (BAS) was associated with daily antipsychotic dose (rs = 0.29; p<0.05).
Significant differences were observed between patients treated with risperidone versus quetiapine for the handwriting kinematic variable DUR (LSD test; p≤0.01) and DURsub (LSD test; p≤0.01) measures only (see Table 4). Risperidone-treated patients had greater DUR (259 ± 84 ms) and greater DURsub (218 ± 62 ms) compared with patients treated with quetiapine (183 ± 24 ms and 162 ± 18 ms, respectively). In contrast, the three clinical severity scales did not show differences between patients treated with the four main atypical antipsychotics.
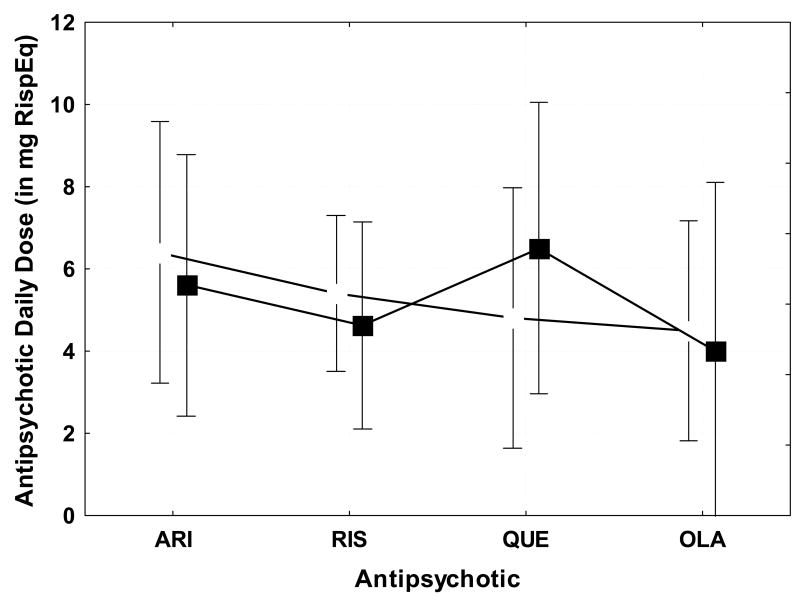
Analysis of the interaction effects revealed that patients with abnormally high ANJ were also the patients who were treated with higher doses of antipsychotic than those with normal ANJ for aripiprazole and risperidone, but not for quetiapine or olanzapine (F3,46 = 4.88; p<0.005). The mean daily risperidone equivalent dose (with 95% confidence intervals) for patients with normal versus abnormal ANJ scores for each of the four main antipsychotic groups (aripiprazole, risperidone, quetiapine, olanzapine) is shown in Figure 2. Figure 3 shows the mean daily risperidone equivalent dose (with 95% confidence intervals) for patients with versus without TD based on the AIMS. Comparing these figures highlights the greater sensitivities of the handwriting kinematic measure versus the clinical dyskinesia measures to detect differences in patients treated with different antipsychotics.
Figure 2.
Average daily dose in mg risperidone-equivalent for the patients with normal average normalized jerk (ANJ) scores (open boxes) and with abnormal ANJ scores (filled boxes) for patients treated with various atypical antipsychotic medications including: aripiprazole (ARI), risperidone (RIS), quetiapine (QUE), olanzapine (OLA). Patients with abnormal ANJ are also the patients with higher dosages of aripiprazole and risperidone. In contrast, patients treated with quetiapine or olanzapine did not show an association between higher dose and abnormal ANJ. P-values are for dosage differences between patients with normal versus abnormal ANJ.
Figure 3.
Average daily dose in mg risperidone-equivalent for patients with tardive dyskinesia (TD)(filled boxes) and non-TD patients (open boxes) treated with various atypical antipsychotic medications: aripiprazole (ARI), risperidone (RIS), quetiapine (QUE), olanzapine (OLA). Dosages for patients with or without TD were comparable for all medications studied.
3.6 Relation Between Movement Abnormalities and Psychopathology
Due to the overlap in movement and affective characteristics observed in schizophrenia and schizoaffective disorder, particularly with regard to poverty of movement, we examined whether performance of the handwriting task could have been influenced by psychopathology or whether observer severity ratings could be influenced by negative or positive symptoms of psychosis. Correlational analyses were performed to examine potential associations between the three observer-based rating scales (AIMS, SAEPS, BAS) and the seven handwriting kinematic variables and three psychopathology scores (PANSS total score, positive symptom and negative symptom subscale scores). We found that scores on all three clinical rating scales were associated with severity of psychopathology. Severity of akathisia (based on BAS global score) was associated with positive symptom severity (r=0.37; p<0.01) and total PANSS score (r=0.36; p<0.01). Severity of tardive dyskinesia (based on AIMS total score) was associated with negative symptom severity (r=0.29; p<0.05). Severity of EPS (based on SAEPS total score) was associated with negative symptom severity (r=0.32; p<0.05). Of the seven handwriting kinematic variables, only peak vertical velocity was associated with psychopathology with coefficients of r=0.31 (p<0.05), r=0.35 (p<0.01), and r=0.39 (p<0.01) for positive symptom, negative symptom, and total PANSS scores, respectively.
4. Discussion
The present study revealed several novel findings with respect to handwriting movement for patients treated with atypical antipsychotics. First, in contrast to healthy individuals, medicated patients exhibited abnormalities on several kinematic features of handwriting including movement duration, peak vertical velocity, smoothness, and number of acceleration peaks. Abnormalities were observed at the level of the individual stroke as well as submovements within a stroke. Second, we found that 52.5% of the patients scored in the abnormal range on at least one handwriting measure.
Third, in contrast to observer-based severity ratings of parkinsonism, tardive dyskinesia or akathisia, we found that severity of handwriting movement abnormality was positively associated with daily dose. This observation further strengthens our earlier observations based on a small sample size of risperidone-treated patients (Caligiuri et al., 2009). Daily dose was associated with increased movement duration and dysfluency of the handwriting movement especially in the aripiprazole and risperidone-treated patients. To our knowledge, this is the first study to demonstrate that patients treated with aripiprazole are more impaired than those treated with quetiapine or olanzapine on measures of motor function. This is also the first study to demonstrate that severity of the motor impairment increases with dose of aripiprazole. Collectively, the findings of the present study indicate that the measure of handwriting kinematics is an objective behavioral biomarker of the effects of antipsychotic medication on the motor system.
When handwriting movements were first considered as a means of assessing neuroleptic response, they were used to identify the optimal dose. This was known as the “neuroleptic threshold” (Haase 1961; 1978) and appeared valid for conventional antipsychotics such as haloperidol or fluphenazine. As conventional antipsychotics were gradually replaced by second-generation medicines, the concept of a neuroleptic threshold based on a measure of parkinsonism became less useful. With the exception of risperidone (Katz, et al 1999; Tandon & Jibson, 2002) second generation antipsychotics do not appear to produce EPS in a dose-dependent manner (Marder et al., 2003; Weiden, 2007). The present study confirms that risperidone and aripiprazole show dose-dependents effects on motor function. Several of our quantitative handwriting movement measures were highly correlated with daily dose, particularly for aripiprazole and risperidone. In contrast, clinically derived measures of EPS (parkinsonism, tardive dyskinesia, or akathisia) were not associated with daily dose across the four atypical antipsychotics studied. This is consistent with previous literature. Our findings of a significant relationship between dose of aripiprazole and severity of handwriting movement abnormalities underscore the importance of careful assessment in establishing a dose-response relationship for EPS in the newer atypical antipsychotics. Moreover the present findings suggest that antipsychotics may impart differences in handwriting movements that extend beyond the conventional versus atypical dichotomy as suggested by some (Tarsy et al., 2002; Weiden, 2007; Yang et al., 2007).
The findings of the present study support the notion that atypical antipsychotics represent a heterogeneous class of medications. For example, both handwriting measures and clinical severity ratings for parkinsonism indicated greater impairment among aripiprazole-treated patients than patients treated with either olanzapine or risperidone. This is consistent with a classification of atypical antipsychotics based on dopamine D2 binding affinity (Arnt, 1998; Kapur and Remington, 2001). In this classification, aripiprazole and risperidone are considered to have high dose-related D2 binding affinity, quetiapine low affinity, and olanzapine intermediate affinity. Based on data presented in Figure 2, our measure of pen movement smoothness during sentence writing may serve as a proxy measure of dopamine D2 binding affinity for atypical antipsychotics.
The present finding that handwriting movements were more impaired in patients treated with aripiprazole compared to olanzapine or quetiapine and that severity of impairment was dose-dependent were surprising given what is known about this drug’s partial D2 receptor agonism. Aripiprazole’s lower 5-HT2:D2 affinity ratio and higher D2 receptor affinity compared to olanzapine or quetiapine has been used to argue for minimal EPS liability with aripiprazole (Davies et al., 2004; Chrzanowski et al., 2006; Kessler, 2007). However, traditional observer ratings used to assess EPS in these studies were not likely to be sufficiently sensitive to detect differences between aripiprazole and other atypical antipsychotics. Another possible explanation for the paradoxical finding of impaired handwriting movements with aripiprazole may be that these patients were being treated with aripiprazole in order to manage pre-existing EPS and that at the time of the handwriting movement study, their EPS was unresolved. Nonetheless, the results concerning the higher incidence of EPS in aripiprazole-treated patients should be cautiously described as preliminary, as no history is presented of patients’ medication with typical or atypical antipsychotics before participating in this study, and no data were available concerning washout periods from treatment with typical antipsychotics.
Gallucci et al. (1997) observed an association between handwriting duration consistency and medication type in their schizophrenia patients. They found that handwriting movements among the schizophrenic patients were less efficient, less consistent, and tended to show larger stroke amplitudes than healthy subjects. With regard to effect of medication, patients on atypical antipsychotics (n=9) had more consistent movement durations compared to conventional antipsychotics (n=5). While the present study excluded patients on conventional antipsychotics, our finding that movement duration may be an important component in distinguishing the motor effects of different antipsychotics is consistent with the Gallucci et al. finding.
Handwriting kinematic variables such as movement speed, movement time, and smoothness (or conversely jerkiness) are core features of parkinsonism and dyskinesia that characterize drug-induced EPS. There was no expectation that the handwriting kinematic and observer ratings would show strong concurrent validity because of the many differences between the two approaches, including, but not limited to: 1) continuous versus ordinal data; 2) emphasis on rigidity (SAEPS) versus bradykinesia (handwriting); 3) observations of passive involuntary movement (AIMS) versus performance-based measure (handwriting).
While there were differences in the extent to which the instrumental and observer-based assessments were sensitive to differences in EPS liability among atypical antipsychotics and dose, these differences do not necessarily suggest that one approach is better than the other or that use of the handwriting measures leads to an over-estimation of EPS. The handwriting measures demonstrated that patients treated with risperidone exhibited more severe impairment than patients treated with olanzapine on the handwriting measures. In contrast, clinical severity ratings did not reveal such differences between medications. Moreover, performance on several handwriting measures (See Table 4) was significantly associated with daily dose; whereas among the three observer-based rating scales, only akathisia severity was associated with dose. The insensitivity of the clinical EPS ratings to detect differences between commonly prescribed atypical antipsychotic medications is likely due to the subjectivity and non-linearity of these scales. One other possibility is that while the SAEPS emphasizes clinical signs of rigidity, the handwriting approach is designed to measure movement speed, timing, and smoothness. It may be that differences in EPS liability among the various atypical antipsychotic medications may manifest as liability for bradykinesia and dyskinesia and not rigidity. Nonetheless, both approaches may be necessary to fully characterize the side effect profiles associated with different antipsychotic mechanisms of action. However, when the need calls for objective quantitative assessment, the instrumental approach would be most desirable.
The possibility exists that the observed abnormalities on our handwriting task could reflect some cognitive-motor disturbance associated with schizophrenia and not drug-induced EPS. In our preliminary study (Caligiuri et al., 2006) we reported that both schizophrenia patients with antipsychotic-induced EPS and patients with Parkinson’s Disease (PD) exhibited impaired movement velocities and velocity scaling on a handwriting task. Furthermore, the schizophrenia patients, but not the PD patients exhibited abnormalities in movement smoothness. This apparent dissociation between idiopathic PD and antipsychotic-induced EPS in schizophrenia on a single measure of pen movement smoothness raised the question whether we were detecting effects of diagnosis or EPS using our quantitative handwriting procedure. The results of the present study indicated that only peak vertical velocity (PVV) was associated with severity of psychopathology. The positive association between pen movement speed and severity of positive and negative symptoms of psychosis may be a reflection of suboptimal treatment. This notion would be consistent with the neuroleptic threshold hypothesis (Haase 1961; 1978). However the lack of an association between PVV and dose argues against this explanation for the present association between handwriting kinematics and psychosis symptoms. Thus, the putative relationship between performance on the handwriting task and psychopathology remain unclear. Further research will be needed to clarify the complex relationship between motor function, antipsychotic dose, and symptoms in patients with psychosis.
This study has some limitations. The cross-sectional study design did not permit testing whether a particular antipsychotic or dose caused the handwriting impairment. At this stage of the research, the findings are limited to provocative associations between atypical antipsychotics and handwriting movement impairment. Longitudinal studies will be needed to address the question of causality more directly. As noted above, we do not know if prior medication history or chronic pre-existing EPS contributed to the present findings of specific medication effects. Furthermore, this study enrolled too few patients treated with conventional antipsychotics as monotherapy to be able to form a comparison group. Inclusion of a conventional antipsychotic treatment group could help address questions regarding the EPS liability of atypical antipsychotics when assessed by handwriting movements. While our initial goal was to evaluate whether a handwriting movement test can provide useful information about the various antipsychotic medications and dose, additional research will be necessary to address whether handwriting movement analyses carry any predictive information about risk of EPS or benefits of switching antipsychotics. As noted above, we were unable to collect data on treatment duration or treatment history to address this limitation. However the primary purpose of the research was to demonstrate the feasibility and sensitivity of handwriting kinematic analyses for quantifying EPS in psychosis patients. Larger systematic studies will be needed to address the question EPS liability among different antipsychotics. The present study only suggests that handwriting measures may be more sensitive than observer severity ratings in detecting potential differences across commonly prescribed antipsychotic agents. Nonetheless, the present study of handwriting kinematics in patients treated with various atypical antipsychotics (e.g. risperidone and possibly aripiprazole) revealed highly robust effects of medication and dose previously not reported.
Acknowledgments
This research was supported by NIH grant R44 MH073192. Authors wish to acknowledge Todd May (San Diego), James Tacklind and Jean Russell (Minneapolis), and Nabeel Yehyawi, Craig Dike, Jeremy Davis, and Dave Bertram (Indianapolis) for their contributions in recruiting and assessing subjects and data management.
Footnotes
One of the three study sites administered 5 trials. As with the sites that administered only 3 trials, trials were averaged. We can assume the mean values were unaffected by the number of trials and that there were no systematic differences between groups on the number of trials administered.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnt J. Pharmacological differentiation of classical and novel antipsychotics. International Clinical Psychopharmacology. 1998;13 (suppl 3):S7–S14. doi: 10.1097/00004850-199803003-00002. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP. Instrumental measurement of tardive dyskinesia. In: Yassa R, Nair NPV, Jeste DV, editors. Neuroleptic-induced Movement Disorders: A Comprehensive Survey. Cambridge University Press; 1997. pp. 241–258. [Google Scholar]
- Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs and Aging. 2000;17:363–384. doi: 10.2165/00002512-200017050-00004. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Ruck RK. Scaling of movement velocity: a measure of neuromotor retardation in individuals with psychopathology. Psychophysiology. 1998;35:431–437. [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Human Movement Science. 2006;25:510–22. doi: 10.1016/j.humov.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr JB. Handwriting Movement Analyses for Monitoring Drug-Induced Motor Side Effects in Schizophrenia Patients Treated with Risperidone. Human Movement Science. 2009 doi: 10.1016/j.humov.2009.07.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowski W, Marcus R, Torbeyns A, Nyilas M, McQuade R. Effectiveness of long-term aripiprazole therapy in patients with acute relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology. 2006;189:259–266. doi: 10.1007/s00213-006-0564-3. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Davies M, Sheffler D, Roth B. Aripiprazole: a novel atypical antipsychotic drug with a unique robust pharmacology. CNS Drugs Review. 2004;10:317–336. doi: 10.1111/j.1527-3458.2004.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci RM, Phillips JG, Bradshaw JL, Vaddadi KS, Pantelis C. Kinematic analysis of handwriting movements in schizophrenic patients. Biological Psychiatry. 1997;41:830–833. doi: 10.1016/S0006-3223(96)00544-6. [DOI] [PubMed] [Google Scholar]
- Gerken A, Wetzel H, Benkert O. Extrapyramidal symptoms and their relationship to clinical efficacy under perphenazine treatment. A controlled prospective handwriting-test study in 22 acutely ill schizophrenic patients. Pharmacopsychiatry. 1991;24:132–137. doi: 10.1055/s-2007-1014456. [DOI] [PubMed] [Google Scholar]
- Guy W. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS. ECDEU Assessment Manual for Psychopharmacology, rev. Rockville, MD: National Institute of Mental Health; 1976. pp. 534–537. 1976. DHEW Pub. No. (ADM. 76-338) [Google Scholar]
- Haase HJ. Extrapyramidal modification of fine movements: a “conditio sine qua non” of the fundamental therapeutic action of neuroleptic drugs. Review Canadian Biology. 1961;20:425–49. [PubMed] [Google Scholar]
- Haase HJ. The purely neuroleptic effects and its relation to the “neuroleptic threshold”. Acta Psychiatrica Belgium. 1978;78:19–36. [PubMed] [Google Scholar]
- Kane JM, Leucht S, Carpenter D, Docherty JP Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The Expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders: Introduction, methods, commentary, and summary. Journal of Clinical Psychiatry. 2003;64(Supplement 12):5–19. [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biological Psychiatry. 2001;50:873–83. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia. A randomized, double-blind trial. Risperidone Study Group. Journal of Clinical Psychiatry. 1999;60:107–115. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;32:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kessler RM. Aripiprazole: What is the role of dopamine D2 receptor partial agonism? American Journal of Psychiatry. 2007;164:1310–1312. doi: 10.1176/appi.ajp.2007.07071043. [DOI] [PubMed] [Google Scholar]
- Künstler U, Hohdorf K, Regenthal R, Seese A, Gertz HJ. Diminution of hand writing area and D2-dopamine receptor blockade: Results from treatment with typical and atypical neuroleptics. Nervenarzt. 2000;71:373–379. doi: 10.1007/s001150050571. [DOI] [PubMed] [Google Scholar]
- Künstler U, Juhnhold U, Knapp WH, Gertz HJ. Positive correlation between reduction in handwriting area and D2 dopamine receptor occupancy during treatment with neuroleptic drugs. Psychiatry Research. 1999;90:31–39. doi: 10.1016/s0925-4927(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Caligiuri MP, Edson R, Lavori P, Adler LA, Rotrosen J, Hitzemann R. Treatment predictors of extrapyramidal side effects in patients with tardive dyskinesia: results from Veterans Affairs Cooperative Study 394. Journal of Clinical Psychopharmacology. 2002;222:196–200. doi: 10.1097/00004714-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Caligiuri MP. Quantitative instrumental assessment of tardive dyskinesia: A review. Neuropsychopharmacology. 1992;6:231–239. [PubMed] [Google Scholar]
- Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, Saha A, Ali M, Iwamoto T. Aripiprazole in the treatment of schizophrenia. Safety and tolerability in short-term, placebo controlled trials. Schizophrenia Research. 2003;61:123–136. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JEK. Optimality in human motor performance: Ideal control of rapid aimed movements. Psychological Review. 1988;95:340–370. doi: 10.1037/0033-295x.95.3.340. [DOI] [PubMed] [Google Scholar]
- Miller del D, Caroff SN, Davis SM, Rosenheck RA, McEvoy JP, Saltz BL, Riggio S, Chakos MH, Swartz MS, Keefe RS, Stroup TS, Lieberman JA Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE Investigators) Extrapyramidal side-effects of antipsychotics in a randomized trial. British Journal of Psychiatry. 2008;193:279–88. doi: 10.1192/bjp.bp.108.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenthal R, Kunstler U, Hesse S, Sabri O, Preiss R. D2 dopamine receptor occupancy, risperidone plasma level and extrapyramidal motor symptoms in previously drug-free schizophrenic patients. International Journal of Clinical Pharmacology and Therapeutics. 2005;43:370–378. doi: 10.5414/cpp43370. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. 1970;212 (Suppl 44):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Annals of Clinical Psychiatry. 2002;14:123–129. doi: 10.1023/a:1016811222688. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Baldessarini RJ, Tarazi FI. CNS Drugs. Vol. 161. 2002. Effects of newer antipsychotics on extrapyramidal function; pp. 23–45. Erratum in: CNS Drugs 2003;17, 202. [DOI] [PubMed] [Google Scholar]
- Teeken JC, Adam JJ, Paas FGWC, Van Boxtel MPJ, Houx PJ, Jolles J. Effect of age and gender on discrete and reciprocal aiming movements. Psychobiology and Aging. 1996;11:195–198. doi: 10.1037//0882-7974.11.2.195. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Maarse FJ. Digital recording and processing of handwriting movements. Human Movement Science. 1984;3:193–217. [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Coordination of fingers, wrist, and arm in Parkinsonian handwriting. Experimental Neurology. 1997;146:159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Weiden PJ. EPS profiles: the atypical antipsychotics are not all the same. Journal of Psychiatric Practice. 2007;131:13–24. doi: 10.1097/00131746-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Yang SY, Kao Yang YH, Chong MY, Yang YH, Chang WH, Lai CS. Risk of extrapyramidal syndrome in schizophrenic patients treated with antipsychotics: A population-based study. Clinical Pharmacology and Therapeutics. 2007;81:586–94. doi: 10.1038/sj.clpt.6100069. [DOI] [PubMed] [Google Scholar]