Two major classes of neurons are responsible for maintaining the balance between excitation and inhibition in the brain. While excitatory projection neurons send “go” signals through the neurotransmitter glutamate, inhibitory local circuit interneurons send “stop” signals through the neurotransmitter gamma-amino butyric acid (GABA). To form GABA, GABAergic interneurons produce glutamate decarboxylase (GAD), the rate-limiting enzyme converting glutamate into GABA. It has recently become clear that glutamatergic and GABAergic synapses utilize fundamentally different mechanisms1. GABAergic interneuron diversity has recently been classified into groups based on morphology, gene expression and electrophysiology2. Given such diversity, it would be expected that GABAergic interneurons have multiple regulatory functions beyond that of a simple on/off switch3. It has recently become clear that GABAergic interneurons have diverse functions such as neuronal proliferation, migration and differentiation during development, and temporal synchrony and refinement of local cortical circuits4–8. Therefore, loss of the “stop” signal from altered GABAergic interneuron transmission would ultimately be expected to result in abnormal brain function.
In support of this idea, altered GABAergic regulated circuits have been implicated in different neurological disorders such as schizophrenia, autism, Tourette’s syndrome, Rett syndrome, and epilepsy. In epilepsy, decreased inhibition resulting from mutations in genes encoding ion channels or GABA receptors can cause uncontrolled neuronal firing9,10. In addition, disruption of Dlx1 and Arx1 homeodomain transcription factors critical for GABAergic interneuron migration during development causes epilepsy in mice and humans, respectively11,12. A consistent finding among schizophrenic patients is reduction in prefrontal cortex GAD67, the enzyme required for GABA synthesis13. Also, single nucleotide polymorphisms14 in the 5′ regulatory region of the GAD1 gene that codes for GAD67 have been linked to childhood onset schizophrenia. In addition, it has been proposed that increased hypermethylation of the GAD1 promoter may cause decreased GAD67 expression in schizophrenic patients15,16.
In autism spectrum disorders (ASD)17, it has been proposed that reduced minicolumn width in specific cortical layers may result from altered GABAergic function18–20 ultimately disrupting connectivity. It has also been proposed that Rett syndrome, an ASD caused by mutations in the methyl CpG-binding protein MECP2 in humans, may result from altered imprinting of the Dlx5 gene, a member of a family of transcription factors critical for GABAergic interneuron differentiation21. Importantly, MECP2 mutant mice carrying an exon 3 deletion display decreased inhibitory cortical activity22. Therefore, multiple reports support the idea that altered GABAergic function can cause a variety of neurological diseases.
Although single genetic loci have been linked to complex mental disorders such as autism and schizophrenia, these diseases are thought to result from the interaction of multiple genes and/or environmental factors. Evidence that a complex mental disorder can result from mutation of a single gene came from studies on Rett syndrome, an ASD that specifically affects neurons and causes Rett specific behavioral phenotypes23–25. Despite the fact that Mecp2 is a single gene, its ability to bind a common DNA modification still supports the idea that multiple gene targets are involved in the etiology of complex mental disorders. While the identification of MECP2 established a link between aberrant epigenetic modification and a complex mental disorder, the mechanism by which MECP2 mutations specifically affect neurons and cause Rett-specific behavioral phenotypes is still not clear.
Increasing evidence in the literature supports a role for non-protein coding RNAs (ncRNAs) in neurological disease. ncRNAs are functional RNA molecules, such as transfer RNA, ribosomal RNA, snoRNA, microRNA, siRNA, piRNA, and long non-coding RNA. While large-scale genomic studies reveal that ncRNAs are abundantly expressed, studies on individual ncRNAs reveal novel roles in transcriptional regulation and DNA methylation control26–28. In addition to multiple recent reviews, a comprehensive review of ncRNAs specifically involved in retinal development has recently been published29. Table 1 describes some of the known non-coding RNAs with possible implications in neurological disorders. Specifically, an anti-sense beta-secretase 1 RNA (BACE1-AS) stabilizes BACE1 RNA, resulting in elevated amyloid-beta protein in Alzheimer’s patients30. In this case, BACE1-AS functions through a post-transcriptional feed-forward mechanism. Anti-sense nitric oxide synthetase (anti-NOS) negatively regulates neuronal NOS, implicating anti-sense regulation as a modulator of long term memory formation31. In schizophrenia and affective disorders, DISC2, an anti-sense RNA to DISC1, is implicated in regulating these neural disorders32.
Table 1.
Long non-coding RNAs and neurological disorders
| Long Non-coding RNA | Disease | Significance | Function | Refs |
|---|---|---|---|---|
| BACE1-AS (anti-sense BACE1) | Alzheimer’s disease | increased expression in Alzheimer’s disease | enhances beta-secretase-1 (BACE1) mRNA stability, an important enzyme in Alzheimer’os disease | 30 |
| Evf-2 (anti-sense Dlx6) | GABA neuropathies | potentially implied in GABA neuropathies | transcriptional regulator of Dlx 5 and 6 expression, required for GABAergic interneuron development | 43,44 |
| SCA8 (or ATXN8OS) | Spinocerebellar Ataxia 8 (SCA8) | induced expression associated with neurodegenerative disease Spinocerebellar Ataxia 8 | may contribute to neurodegeneration through the alteration of RNA binding protein associations | 45 |
| anti-NOS (anti-sense nNOS) | long-term memory disorders | expression associated with improper long-term memory formation | negatively regulates the enzyme neuronal nitric oxide synthase (nNOS), crucial for the formation of long-term memory | 31 |
| DISC2 (anti-sense DISC1) | schizophrenia | expression is disrupted in schizophrenia | may be an anti-sense regulator of DISC1, essential for neuronal development | 32,46 |
| BC200 | Alzheimer’s disease | increased expression in Alzheimer’s brains | translational regulator targeted to somatodendritic domains of neurons, may affect long-term synaptic plasticity | 36 |
| BC1 | Fragile X Syndrome | associated with fragile X syndrome | binds fragile X protein (FMRP), required for FMRP-mediated inhibition of translation at the synapse | 37,38 |
| Tmevpg1 | Theiler’s virus induced neurological disease | positionally cloned candidate associated with susceptibility to Theiler’s virus induced neurological disease | may control the cytokine interferon gamma expression | 47 |
| PSZA11q14 (anti-sense DLG2) | schizophrenia | decreased expression in schizophrenia | anti-sense regulation of DLG2, involved in the assembly of NMDA receptors | 41 |
| ST7OT (anti-sense to ST7) | autism | associated with autism in one patient | possible regulator of ST7 gene | 42 |
| LIT1 (anti-sense KvLQT1) | Beckwith-Wiedemann Syndrome (BWS) | disrupted expression in BWS | anti-sense negative regulation of KvLQT1, a gene implicated in BWS | 48,49 |
| Peg8 | BWS | increased expression in BWS | regulates the expression of IGF2, associated with BWS | 50 |
| IPW | Prader-Willi Syndrome (PWS) | not expressed in PWS | regulates imprinted, paternally expressed genes found at location 15q11-q13, which is altered in PWS | 51 |
| Prion-associated RNAs | Prion Disease | expression may be associated with Prion Disease | may stimulate prion protein conversion, the infectious agent of prion disease | 52,53 |
| H19 | BWS | disrupted expression in BWS | possible regulator of the imprinting of chromosome 11p15.5 | 54 |
| ZNF127AS (anti-sense ZNF127) | PWS | disrupted expression in PWS | may regulate the imprinting of ZNF127, a gene altered in PWS | 55 |
| UBE3A antisense (antisense UBE3A) | Angelman Syndrome (AS) | increased or decreased expression levels in AS | regulates the imprinting of UBE3A, a gene implicated in AS | 56 |
The BC1 ncRNA and its primate form BC200 are not transcribed as anti-sense to their targets, but function as translational regulators. Both BC200 and BC133,34 are targeted to somatodendritic domains of neurons, and thought to be involved in synaptic plasticity. In support of this hypothesis, mice lacking BC1 RNA show decreased exploratory behavior and increased anxiety35. BC200 RNA is upregulated in Alzheimer’s disease36, whereas BC1 RNA has been shown to directly bind the fragile X syndrome protein (FMRP) affecting translational repression37,38. However, binding of BC1 RNA to FMRP is controversial and has recently been challenged39. Evidence from the challenging group suggests that BC1 represses translation by inhibiting the RNA unwinding activity of eukaryotic initiation factor 4A (eIF4A)40.
The SZ-1 RNA has been proposed to be an anti-sense regulator of DLG-2, controlling functional assembly of N-methyl-D-aspartate receptors41. In autism, a patient with a breakpoint in 7q31 raises the possibility of the involvement of another opposite strand RNA, ST7OT, a suppressor of tumorigenicity (ST742).
While many of these ncRNAs function at the post-transcriptional level, very few mechanisms utilized by anti-sense or opposite-strand ncRNAs at the transcriptional or epigenetic level have been defined. Only a small group of long-polyadenylated RNAs with known transcriptional or epigenetic regulatory activity has been identified (Tables 2 and 3), recently reviewed in Khalil et al. 2009. How such aberrant regulation might cause complex mental disorders is a topic of intense investigation.
Table 2.
Transcription-regulating long non-coding RNAs
| Long noncoding RNA | Function | Refs | |
|---|---|---|---|
| Specific Transcription Factors | SRA | forms a ribonucleoprotein complex with steroid hormone receptors (SHRs) to co-activate transcription | 57,58 |
| Evf-2 | recruits Dlx, a homeodomain transcription factor, and MECP2 to key intergenic DNA regulatory elements, regulating Dlx5 and Dlx6 expression | 43,44 | |
| HSR-1 | in response to heat shock, allows for the trimerization of the heat shock factor-1 (HSF-1), which then binds to the translation- elongation factor 1A (eIF1A) to initiate heat shock protein expression | 59 | |
| RNA upstream of CCND1 | forms a complex with the RNA-binding protein, TLS, facilitating the repression of CCND1 by the chromatin binding protein (CBP) and p300 | 60 | |
| LXRBSV | acts as a transcriptional co-activator with liver X receptor (LXR)-β to enhance receptor- mediated transactivation | 61 | |
| General Transcription Factors | 7SK | forms a complex with hexamethylenes bisacetamide-induced protein-1 (HEXIM1), which then binds to PTEFb, thereby preventing transcriptional elongation by RNA polymerase II | 62–64 |
| RNA upstream of DHFR | creates a triplex structure in the core promoter of DHFR, blocking the binding of TFIID and repressing transcription | 65 | |
| RNAP II | Alu Elements | binds to RNA polymerase II, blocking transcription | 66 |
Table 3.
Long non-coding RNA and chromatin modification
| Chromatin Modifying Complex | Long Non-coding RNA | Function | Refs |
|---|---|---|---|
| Polycomb chromatin remodeling complex | HOTAIR | recruits to the HoxD locus to silence gene expression | 67 |
| Tsix/RepA | RepA recruits the Polycomb complex to the X chromosome to induce heterochromatin formation and repress gene expression; Tsix inhibits this interaction | 68 | |
| Kcnq1ot1 | recruits the Polycomb complex and the G9a histone modifying complex to the Kcnq1 domain to silence gene expression | 69 | |
| Ash1 | TRE transcripts | possible recruitment to Ultrabithorax (Ubx), a Drosophila homeotic gene, or cis- acting repression of transcription | 70,71 |
| Histone methyltransferase MLL1 | Hoxb5/6as, Evx1as | associate with MLL1 and trimethylated H3K4 histones, suggesting a role in epigenetic regulation | 72 |
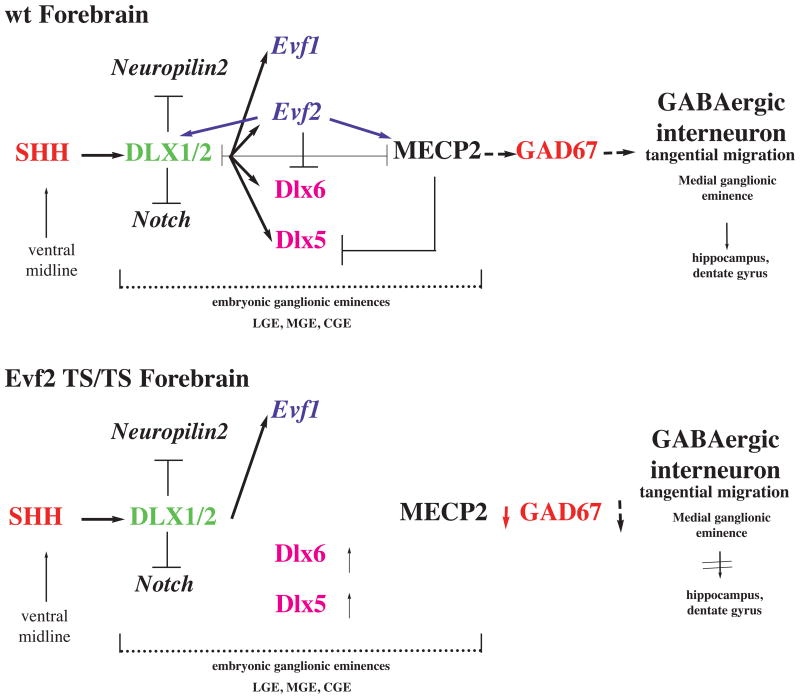
In this symposium, evidence was presented supporting that developmental control of Dlx genes by Evf2, a transcription-regulating ultraconserved ncRNA (trucRNA43), affects the number of GABAergic interneurons in the hippocampus (summarized in Figure 1)44. It was also shown that the Evf2 ncRNA recruits both positive (Dlx) and negative (MECP2) transcription factors to key DNA regulatory elements that control balanced Dlx 5 and 6 gene expression during embryonic brain development44. Further, data showed that Evf2 mouse mutants have reduced numbers of GABAergic interneurons in the early postnatal hippocampus and dentate gyrus. This is the first functional evidence linking a noncoding RNA transcriptional mechanism to MECP2 and GABAergic interneuron development, with possible relevance to an etiology for an ASD.
Figure 1. Evf-2 ncRNA dependent gene regulation and GABAergic interneuron development.
Modified from Bond et al. (2009, Fig S3) showing SHH (sonic hedgehog protein), MECP2 (DNA methyl-binding protein 2), GAD67 (glutamate decarboxylase, also GAD1), Dlx (vertebrate homologues of distalless, Drosophila homeodomain-containing transcription factor), Evf1 and 2, embryonic ventral forebrain ncRNAs, LGE (lateral ganglionic eminence), MGE (medial ganglionic eminence), CGE (caudal ganglionic eminence) embryonic structures that are the sources of adult GABAergic interneurons in the cortex, hippocampus, dentate gyrus and olfactory bulbs.
Given that GABAergic interneuron activity in the brain controls multiple higher functions, and altered GABA activity, as discussed above, has been linked to complex mental disorders3, it will be important to determine how commonly anti-sense or opposite-strand RNAs throughout the genome are responsible for recruitment of specific and/or general transcription factors. The ability to demonstrate that aberrant non-coding RNA-dependent epigenetic regulation can cause complex mental disorders would have important consequences to future investigations of these disorders. Specifically, any potential RNA regulators of genes involved in neuronal function including development, plasticity, dendritic branching, axonal transport, and signal transduction, would be studied and considered potential candidates in causing complex mental disorders. This demonstration would broadly impact studies on normal gene regulation in neuronal and non-neuronal cells and would also directly influence how genetic studies of complex mental disorders are investigated. Specifically, geneticists would not only focus on potential disease-causing candidates in protein-coding regions, but also on the expression and sequence of non-coding RNA transcripts, as well as DNA methylation profiles. As a result, RNAs like Evf2 that subtly regulate genes important to specific neural activities may be identified as the cause of some subset of complex mental disorders, yielding specific therapeutic targets.
Development of greater target specificity is critical to replacing present drugs that prevent or activate neural activity affecting multiple targets with detrimental side effects. Ultimately, our goal in discovering the exact mechanism responsible for a specific neurological disease or mental disorder is to develop specific drug targets that would rescue these disorders. Therefore, a major impact on drug development for long-term treatment and cures for complex mental disorders could potentially arise from developing novel RNA regulators.
Acknowledgments
This work was supported by the American Recovery and Reinvestment Act, NICHD RHD05650AZ to JDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008;11(9):1044–52. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- 2.Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72(1):1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 4.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 5.Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002;36(6):989–91. doi: 10.1016/s0896-6273(02)01136-4. [DOI] [PubMed] [Google Scholar]
- 6.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293(5532):1159–63. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 7.Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13(1):25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- 8.Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Physiol. 2009;587(Pt 9):1873–9. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31(2):184–9. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 10.Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- 11.Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8(8):1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32(3):359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- 13.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 14.Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10(6):581–8. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- 15.Costa E, Grayson DR, Guidotti A. Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention? Mol Interv. 2003;3(4):220–9. doi: 10.1124/mi.3.4.220. [DOI] [PubMed] [Google Scholar]
- 16.Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase 67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci U S A. 2005;102(35):12578–83. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26(26):6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31(6):537–43. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- 19.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 20.Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77(3):377–88. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet. 2005;37(1):31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- 22.Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102(35):12560–5. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 24.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56(3):422–37. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88(4):471–81. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 26.Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451(7175):202–6. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattick JS. A new paradigm for developmental biology. J Exp Biol. 2007;210(Pt 9):1526–47. doi: 10.1242/jeb.005017. [DOI] [PubMed] [Google Scholar]
- 28.Prasanth KV, Spector DL. Eukaryotic regulatory RNAs: an answer to the ‘genome complexity’ conundrum. Genes Dev. 2007;21(1):11–42. doi: 10.1101/gad.1484207. [DOI] [PubMed] [Google Scholar]
- 29.Rapicavoli NA, Blackshaw S. New meaning in the message: noncoding RNAs and their role in retinal development. Dev Dyn. 2009;238(9):2103–14. doi: 10.1002/dvdy.21844. [DOI] [PubMed] [Google Scholar]
- 30.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, 3rd, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14(7):723–30. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korneev SA, Straub V, Kemenes I, Korneeva EI, Ott SR, Benjamin PR, O’Shea M. Timed and targeted differential regulation of nitric oxide synthase (NOS) and anti-NOS genes by reward conditioning leading to long-term memory formation. J Neurosci. 2005;25(5):1188–92. doi: 10.1523/JNEUROSCI.4671-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millar JK, James R, Brandon NJ, Thomson PA. DISC1 and DISC2: discovering and dissecting molecular mechanisms underlying psychiatric illness. Ann Med. 2004;36(5):367–78. doi: 10.1080/07853890410033603. [DOI] [PubMed] [Google Scholar]
- 33.Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991;88(6):2093–7. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Iacoangeli A, Popp S, Muslimov IA, Imataka H, Sonenberg N, Lomakin IB, Tiedge H. Dendritic BC1 RNA: functional role in regulation of translation initiation. J Neurosci. 2002;22(23):10232–41. doi: 10.1523/JNEUROSCI.22-23-10232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewejohann L, Skryabin BV, Sachser N, Prehn C, Heiduschka P, Thanos S, Jordan U, Dell’Omo G, Vyssotski AL, Pleskacheva MG, et al. Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav Brain Res. 2004;154(1):273–89. doi: 10.1016/j.bbr.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104(25):10679–84. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalfa F, Adinolfi S, Napoli I, Kuhn-Holsken E, Urlaub H, Achsel T, Pastore A, Bagni C. Fragile X mental retardation protein (FMRP) binds specifically to the brain cytoplasmic RNAs BC1/BC200 via a novel RNA-binding motif. J Biol Chem. 2005;280(39):33403–10. doi: 10.1074/jbc.M504286200. [DOI] [PubMed] [Google Scholar]
- 38.Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112(3):317–27. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 39.Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1 RNA and the fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2008;105(2):734–9. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin D, Pestova TV, Hellen CU, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol. 2008;28(9):3008–19. doi: 10.1128/MCB.01800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polesskaya OO, Haroutunian V, Davis KL, Hernandez I, Sokolov BP. Novel putative nonprotein-coding RNA gene from 11q14 displays decreased expression in brains of patients with schizophrenia. J Neurosci Res. 2003;74(1):111–22. doi: 10.1002/jnr.10752. [DOI] [PubMed] [Google Scholar]
- 42.Vincent JB, Petek E, Thevarkunnel S, Kolozsvari D, Cheung J, Patel M, Scherer SW. The RAY1/ST7 tumor-suppressor locus on chromosome 7q31 represents a complex multi-transcript system. Genomics. 2002;80(3):283–94. doi: 10.1006/geno.2002.6835. [DOI] [PubMed] [Google Scholar]
- 43.Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20(11):1470–84. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bond AM, VanGompel MJW, Sametsky EA, Clark MF, Savage JC, Disterhoft JD, Kohtz JD. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nature Neuroscience. 2009;12:1020–1027. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mutsuddi M, Marshall CM, Benzow KA, Koob MD, Rebay I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14(4):302–8. doi: 10.1016/j.cub.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 46.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Clair DM, Muir WJ, Blackwood DH, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–23. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 47.Vigneau S, Rohrlich PS, Brahic M, Bureau JF. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J Virol. 2003;77(10):5632–8. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horike S, Mitsuya K, Meguro M, Kotobuki N, Kashiwagi A, Notsu T, Schulz TC, Shirayoshi Y, Oshimura M. Targeted disruption of the human LIT1 locus defines a putative imprinting control element playing an essential role in Beckwith-Wiedemann syndrome. Hum Mol Genet. 2000;9(14):2075–83. doi: 10.1093/hmg/9.14.2075. [DOI] [PubMed] [Google Scholar]
- 49.Mitsuya K, Meguro M, Lee MP, Katoh M, Schulz TC, Kugoh H, Yoshida MA, Niikawa N, Feinberg AP, Oshimura M. LIT1, an imprinted antisense RNA in the human KvLQT1 locus identified by screening for differentially expressed transcripts using monochromosomal hybrids. Hum Mol Genet. 1999;8(7):1209–17. doi: 10.1093/hmg/8.7.1209. [DOI] [PubMed] [Google Scholar]
- 50.Okutsu T, Kuroiwa Y, Kagitani F, Kai M, Aisaka K, Tsutsumi O, Kaneko Y, Yokomori K, Surani MA, Kohda T, et al. Expression and imprinting status of human PEG8/IGF2AS, a paternally expressed antisense transcript from the IGF2 locus, in Wilms’ tumors. J Biochem. 2000;127(3):475–83. doi: 10.1093/oxfordjournals.jbchem.a022630. [DOI] [PubMed] [Google Scholar]
- 51.Wevrick R, Francke U. An imprinted mouse transcript homologous to the human imprinted in Prader-Willi syndrome (IPW) gene. Hum Mol Genet. 1997;6(2):325–32. doi: 10.1093/hmg/6.2.325. [DOI] [PubMed] [Google Scholar]
- 52.Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate prion protein conversion. Nature. 2003;425(6959):717–20. doi: 10.1038/nature01979. [DOI] [PubMed] [Google Scholar]
- 53.Supattapone S. Prion protein conversion in vitro. J Mol Med. 2004;82(6):348–56. doi: 10.1007/s00109-004-0534-3. [DOI] [PubMed] [Google Scholar]
- 54.Sparago A, Cerrato F, Vernucci M, Ferrero GB, Silengo MC, Riccio A. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat Genet. 2004;36(9):958–60. doi: 10.1038/ng1410. [DOI] [PubMed] [Google Scholar]
- 55.Jong MT, Gray TA, Ji Y, Glenn CC, Saitoh S, Driscoll DJ, Nicholls RD. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8(5):783–93. doi: 10.1093/hmg/8.5.783. [DOI] [PubMed] [Google Scholar]
- 56.Runte M, Kroisel PM, Gillessen-Kaesbach G, Varon R, Horn D, Cohen MY, Wagstaff J, Horsthemke B, Buiting K. SNURF-SNRPN and UBE3A transcript levels in patients with Angelman syndrome. Hum Genet. 2004;114(6):553–61. doi: 10.1007/s00439-004-1104-z. [DOI] [PubMed] [Google Scholar]
- 57.Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O’Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97(1):17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 58.Lanz RB, Razani B, Goldberg AD, O’Malley BW. Distinct RNA motifs are important for coactivation of steroid hormone receptors by steroid receptor RNA activator (SRA) Proc Natl Acad Sci U S A. 2002;99(25):16081–6. doi: 10.1073/pnas.192571399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440(7083):556–60. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454(7200):126–30. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto K, Ishida E, Matsumoto S, Shibusawa N, Okada S, Monden T, Satoh T, Yamada M, Mori M. A liver X receptor (LXR)-beta alternative splicing variant (LXRBSV) acts as an RNA co-activator of LXR-beta. Biochem Biophys Res Commun. 2009;390(4):1260–5. doi: 10.1016/j.bbrc.2009.10.132. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414(6861):322–5. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- 63.Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 2001;414(6861):317–22. doi: 10.1038/35104575. [DOI] [PubMed] [Google Scholar]
- 64.Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell. 2003;12(4):971–82. doi: 10.1016/s1097-2765(03)00388-5. [DOI] [PubMed] [Google Scholar]
- 65.Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445(7128):666–70. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 66.Mariner PD, Walters RD, Espinoza CA, Drullinger LF, Wagner SD, Kugel JF, Goodrich JA. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell. 2008;29(4):499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 67.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750–6. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D, Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32(2):232–46. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 70.Petruk S, Sedkov Y, Riley KM, Hodgson J, Schweisguth F, Hirose S, Jaynes JB, Brock HW, Mazo A. Transcription of bxd noncoding RNAs promoted by trithorax represses Ubx in cis by transcriptional interference. Cell. 2006;127(6):1209–21. doi: 10.1016/j.cell.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanchez-Elsner T, Gou D, Kremmer E, Sauer F. Noncoding RNAs of trithorax response elements recruit Drosophila Ash1 to Ultrabithorax. Science. 2006;311(5764):1118–23. doi: 10.1126/science.1117705. [DOI] [PubMed] [Google Scholar]
- 72.Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, Gardiner BB, Askarian-Amiri ME, Ru K, Solda G, Simons C, et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18(9):1433–45. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]