Abstract
Arachidonic acid derived endogenous electrophile 15d-PGJ2 has gained much attention in recent years due to its potent anti-proliferative and anti-inflammatory actions mediated through thiol modification of cysteine residues in its target proteins. Here, we show that 15d-PGJ2 at 1µM concentration converts normal mitochondria into large elongated and interconnected mitochondria through direct binding to mitochondrial fission protein Drp1 and partial inhibition of its GTPase activity. Mitochondrial elongation induced by 15d-PGJ2 is accompanied by increased assembly of Drp1 into large oligomeric complexes through plausible intermolecular interactions. The role of decreased GTPase activity of Drp1 in the formation of large oligomeric complexes is evident when Drp1 is incubated with a non-cleavable GTP analog, GTPγS or by a mutation that inactivated GTPase activity of Drp1 (K38A). The mutation of cysteine residue (Cys644) in the GTPase effector domain, a reported target for modification by reactive electrophiles, to alanine mimicked K38A mutation induced Drp1 oligomerization and mitochondrial elongation, suggesting the importance of cysteine in GED to regulate the GTPase activity and mitochondrial morphology. Interestingly, treatment of K38A and C644A mutants with 15d-PGJ2 resulted in super oligomerization of both mutant Drp1s indicating that 15d-PGJ2 may further stabilize Drp1 oligomers formed by loss of GTPase activity through covalent modification of middle domain cysteine residues. The present study documents for the first time the regulation of a mitochondrial fission activity by a prostaglandin, which will provide clues for understanding the pathological and physiological consequences of accumulation of reactive electrophiles during oxidative stress, inflammation and degeneration.
Introduction
Mitochondrial structure and function in cells is maintained by a delicate balance between fission and fusion events mediated by dynamin related GTPases [1]. Dynamins are large proteins with amino-terminal GTPase domain followed by middle domain and a GTPase effector domain (GED) [2]. Mitochondrial fission is mediated by a large dynamin related GTPase called dynamin related protein 1 (Drp1) likely through cooperation of mitochondrial outer membrane proteins Fis1 and Mitochondrial fission factor Mff [1]. Drp1 is located in the cytosol of mammalian cells and translocates to mitochondria at fission sites/foci where it couples GTP hydrolysis to membrane constriction and fission [3]. It has been reported that an increase in Drp1 oligomerization reflects an increase in the fission-competent rings formed before mitochondrial fragmentation [4]. Moreover, posttranslational modifications like phosphorylation and sumoylation of Drp1 were also shown to regulate mitochondrial shape changes [5]. Notwithstanding the regulation of Drp1 function by above mentioned posttranslational modifications, very little is known regarding the functional regulation of Drp1 by protein reactive electrophiles in vivo.
The lipid electrophile 15 deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), a cyclopentenone prostaglandin (CyPG), has been established as a modulator that is produced in vivo during resolution phase of inflammation [6]. In addition to its anti-inflammatory property, 15d-PGJ2 also exhibits diverse biological effects, including anti-proliferative and anti-viral activities. Prostaglandins are reported to be physiologically present in the body fluids in picomolar to nanomolar concentrations with local concentrations reaching low micromolar range at sites of acute inflammation [7]. However, the concentrations of reactive CyPGs are often underestimated several folds lower than that were originally produced because they form covalent adducts with proteins. CyPGs alter normal function of many proteins as well as their redox status by reacting with free cysteine thiols of cellular proteins via Michael addition. 15d-PGJ2 has been shown to either impair activities of I kappa B kinase, AP-1 family genes, NF-kappa B, thioredoxinand β-actin proteins or promote activities of H-Ras and PPARγ proteins through adduct formation with cysteine residues of these proteins. As 15d-PGJ2 exerts its biological effects at least in part through a reaction with cellular proteins, the identification of target molecules of 15d-PGJ2 may facilitate efforts to develop new strategies to deal with these electrophilic protein modulators. Here we demonstrate for the first time that 15d-PGJ2 interacts with the mitochondrial division protein Drp1 and causes an extensive mitochondrial networking and fusion.
Materials and Methods
Materials
Rat kidney proximal tubule cells (RPTC) and HeLa cells were grown in serum supplemented Ham's F-12/DMEM and MEM medium, respectively. All prostaglandins, GW9662, and T0070907 were from Cayman chemicals. Anti-HA (Sigma Aldrich) and α-Drp1 (BD Bioscience) mouse monoclonals, α-AIF rabbit polyclonal (Santa Cruz Biotechnology) and HRP-conjugated secondary antibodies to mouse and rabbit IgG (Jackson Immunoresearch Laboratories) were used. Nucleotides and ATP measurement reagents (Sigma Aldrich), MitoTracker Red (Invitrogen), MitoDsRed plasmid (Clontech), Fugene HD transfection reagent (Roche Applied Science) and site-directed mutagenesis kit (Stratagene) were used. Plasmids overexpressing Drp1 Wild-type and Drp1 K38A were a kind gift from Dr. Alex Van Der Bliek. All other reagents were of the highest grade available.
15d-PGJ2 treatment
RPTC were plated at 1–2 × 105 cells/cm2 in 35-mm collagen coated dishes. After overnight growth, cells were incubated with 20 µM of 15d-PGJ2 in fresh growth medium for different times. For each data point, triplicate cultures were prepared and data represents average of at least three independent experiments. All controls were treated with DMSO. HeLa cells were similarly treated with 15d-PGJ2 after growing cells overnight. 0.25 × 105 cells per cm2 were seeded in 35mm dishes for transfection and treatments were done within 20–24h after transfection. In some experiments, the thiol containing anti-oxidant NAC was added to the cells 1h before 15 d-PGJ2 treatment.
Mitochondrial morphology and Measurement of ATP
Mitochondrial morphology was observed either by stable expression of MitoDsRed plasmid in RPTC and HeLa or by staining with 250 nM of MitoTracker red. Confocal images were obtained on a confocal laser scanning microscope (Olympus FV-1000 or Zeiss LSM 510) at the Institutional Imaging Facility. To measure ATP, control and experimental cells were lysed in perchloric acid and ATP in cell extract was measured as described before [8].
Electron microscopy
Control and 15d-PGJ2 treated cells in a 35 mm dish were processed for electron microscopy. Cells were washed and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.4. Fixed cells were incubated with 1% osmium tetroxide (OsO4) for 1 hr at 4°C and after 20 min incubation at 4°C in 1% uranyl acetate; cells were washed thoroughly and further processed for electron microscopy.
Western blotting
Protein concentration was estimated with BCA reagent (Pierce Kit) using BSA as standard. Proteins were resolved by reducing SDS-PAGE in Xcell II mini cell on 4–12% and 10% NuPAGE gels using MOPS running buffer (Invitrogen). Proteins from the gel were electroblotted onto 0.2-µm PVDF membranes after electrophoresis and were analyzed by western blotting using appropriate primary and peroxidase-conjugated secondary antibodies.
Blue Native PAGE
Homogenization, fractionation of mammalian cells (HeLa and RPTC) for blue native PAGE (BN-PAGE) was done as described [9]. Briefly, cells were homogenized in diluted sucrose buffer (83 mM sucrose, 6.6 mM imidazole/HCl, pH 7.0) in a 1 ml dounce homogenizer and mitochondrial and microsomal pellet and cytosolic supernatant fractions were collected by differential centrifugation. Mitochondria were extracted in 20 µl of 1× Native PAGE sample buffer (Invitrogen) containing 1% Dodecyl maltoside (DDM) [9]. The 100,000×g supernatant containing the cytosol was further concentrated using a Nanosep 10 kDa cutoff membrane (Pall Corporation). 35 µg of cytosol was mixed with 1× Sample buffer containing 1% DDM. All samples were centrifuged at 50,000×g for 15 minutes and mixed with Coomassie blue G-250 dye before loading onto BN-PAGE gels (Invitrogen). Proteins resolved were transferred onto PVDF membranes, and subjected to immunoblotting for HA or Drp1. The sizes of the complexes were established on the basis of the mobility of marker proteins (GE Healthcare): thyroglobulin (669 kD), apoferritin (440 kD), catalase (232 kD) and BSA (67 kD).
Protein capture by biotinylated-15d-PGJ2
RPTCs were treated with 10 µM of biotinylated 15d-PGJ2 for 2h. Control and experimental cells were lysed in isotonic buffer containing 1% NP40 and biotinylated proteins were bound to MPG streptavidin beads (Pure Biotech LLC, Middlesex, NJ) following the manufacturer’s instructions. The presence of Drp1 in the bound proteins was detected by Western blotting.
GTPase Activity Assay
The cDNA for wild-type Drp1 cloned in pCAL-n-EK for the production of calmodulin-binding peptide (CBP)-Drp1 fusion protein was a kind gift from Dr. Craig Blackstone. The soluble CBP-tagged full length wild-type Drp1 was purified from Escherichia coli BL21 (DE3) using calmodulin affinity resin [10] and was dialyzed against buffer (20 mM HEPES, pH 7.2, 2 mM MgCl2, and 1 mM dithiothreitol). GTP hydrolysis by recombinant Drp1 was assayed using a simple, continuous, coupled GTP regenerating assay as described before [11] by measuring the depletion of NADH in a fluorescence reader (TECAN).
Results
15d-PGJ2 induces extensive mitochondrial elongation and mitochondrial dysfunction
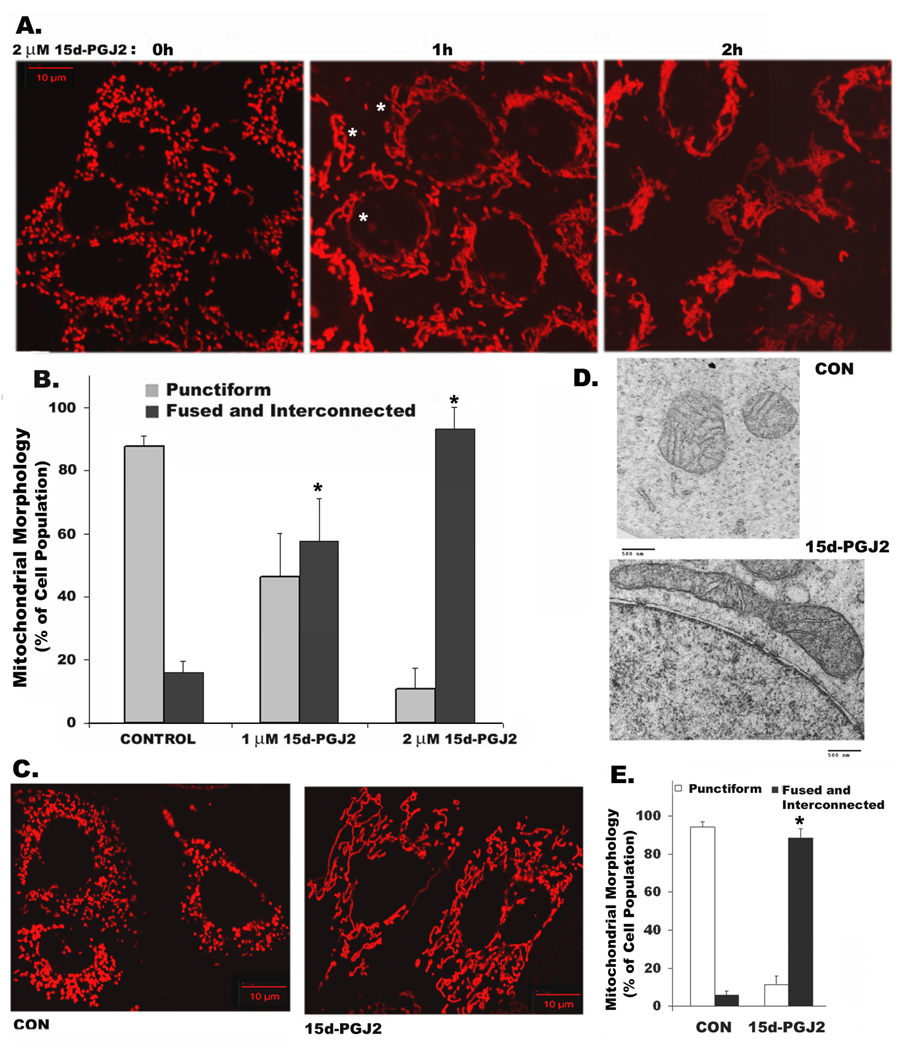
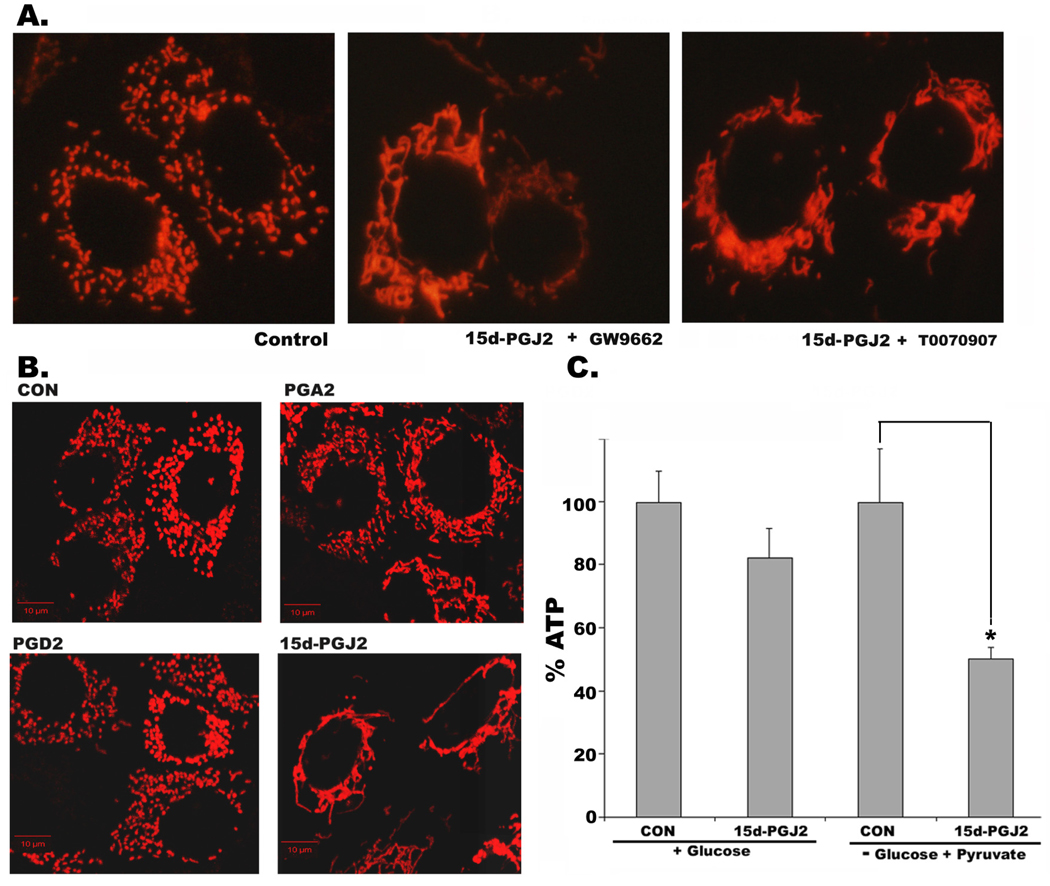
Treatment of RPTC with 15d-PGJ2 induced extensive mitochondrial elongation within 2h of treatment. Unlike HeLa cells, normal rat kidney proximal tubule cells or primary cultures of mouse proximal tubule cells (supplementary Fig.1) contain punctiform mitochondria (Fig.1A, left panel). The punctiform mitochondria fused into long tubular structures within 1h of 2 µM 15d-PGJ2 treatment (Fig. 1A, middle panel). In two hours, mitochondria became completely fused and interconnected (Fig. 1A, right panel). The number of cells with moderately long or fused and interconnected mitochondria increased from <20% in untreated cells (0h) to ~60% with 1µM and to ~90% with 2 µM of 15d-PGJ2 treatment (Fig 1B). In view of the ability of serum proteins to sequester 15d-PGJ2 and reduce its activity [12], we determined whether serum attenuated 15d-PGJ2 activity in RPTC. Not surprisingly, it required 10–20 fold higher concentration of 15d-PGJ2 (20 µM) in the presence of 10% serum to induce mitochondrial changes (Fig. 1C and E) compared to serum free medium (Fig. 1 A and B). To avoid the confounding effects of serum starvation on 15d-PGJ2 induced changes, rest of the experiments were performed in serum-containing medium. Mitochondrial elongation induced by 15d-PGJ2 was also confirmed by electron microscopy. As shown in Fig. 1D, untreated RPTC contain small round mitochondria, which became elongated when treated with 15d-PGJ2. Since 15d-PGJ2 is a natural ligand of nuclear receptor, PPARγ, we tested the effect of PPARγ antagonists, GW9662 and T0070907 on 15d-PGJ2 induced mitochondrial changes. Both the antagonists failed to prevent 15d-PGJ2 induced mitochondrial fusion (Fig. 2A) suggesting role of PPARγ independent events in mediating these changes. To test the specificity of 15d-PGJ2 on mitochondrial shape change, we tested other prostaglandins. Our results indicated that in contrast to 15d-PGJ2, either prostaglandin A2 (PGA2) or prostaglandin D2 (PGD2) or PGE2 (not shown) did not cause significant mitochondrial elongation (Fig. 2B). Since PPARγ independent effects of 15d-PGJ2 have been shown to be mediated by production of ‘ROS’, we ruled out the involvement of ROS by including ROS scavengers, TEMPOL and Trolox. Only thiol containing antioxidants NAC and NMPG blocked 15d-PGJ2 induced mitochondrial elongation (supplemental Figure 2). Furthermore, 15d-PGJ2 treatment also resulted in decreased production of ATP, which was more pronounced when cells were forced to use the mitochondrial pathway for ATP production in a medium devoid of glucose, containing pyruvate and 2-deoxy-glucose (Fig 2C). This result is consistent with the finding of Parone et al [13] indicating that lack of fission results in mitochondrial dysfunction.
Figure 1. 15d-PGJ2 induces extensive mitochondrial fusion and elongation.
Cells expressing MitoDsRed were observed by confocal fluorescence microscopy. Changes in mitochondrial morphology in RPTC from punctiform in control (0 h) to fused and interconnected structures (indicated by white asterisks) in cells treated for 1h or 2h with 2 µM 15d-PGJ2 in serum-free growth medium (A) or 20 µM 15d-PGJ2 for 2h in full growth medium containing 10% serum (C); Bar: 10 µm. Number of cells with punctiform or fused and interconnected mitochondrial structures were scored in control and 15d-PGJ2 treated at 1 and 2 µM concentrations for 2h (B) or 20 µM 15d-PGJ2 in serum containing medium (E). Data represent mean + s.d. (from triplicates, * Student’s t-test P<0.01). Electron microscopic images showing changes in mitochondrial structure in RPTC after treating with 20 µM 15d-PGJ2 for 0 and 2h (D) ; Bar: 500 nm.
Figure 2. 15d-PGJ2 induced changes in mitochondrial morphology are PPAR γ independent and are unique to 15d-PGJ2 that leads to decreased ATP production.
A) Effect of PPAR γ antagonists GW9662 or T0070907on 15d-PGJ2 induced mitochondrial remodeling in RPTC expressing MitoDsRed. B) Mitochondria morphology of RPTC treated with DMSO (CON) or 20 µM of PGA2, PGD2 or 15d-PGJ2 for 2h, observed by confocal microscopy as described above. C) Total cellular ATP levels in RPTC that were grown in regular medium or glucose-free medium containing pyruvate and 2-deoxy Glucose at 0h and 2h of treatment with 15d-PGJ2 (20 µM). Asterisk (*) represents Student’s t-test P<0.05 from three independent experiments. Bar: 10 µm.
Binding, inhibition of GTPase activity and induction of oligomerization of Drp1 by 15d-PGJ2
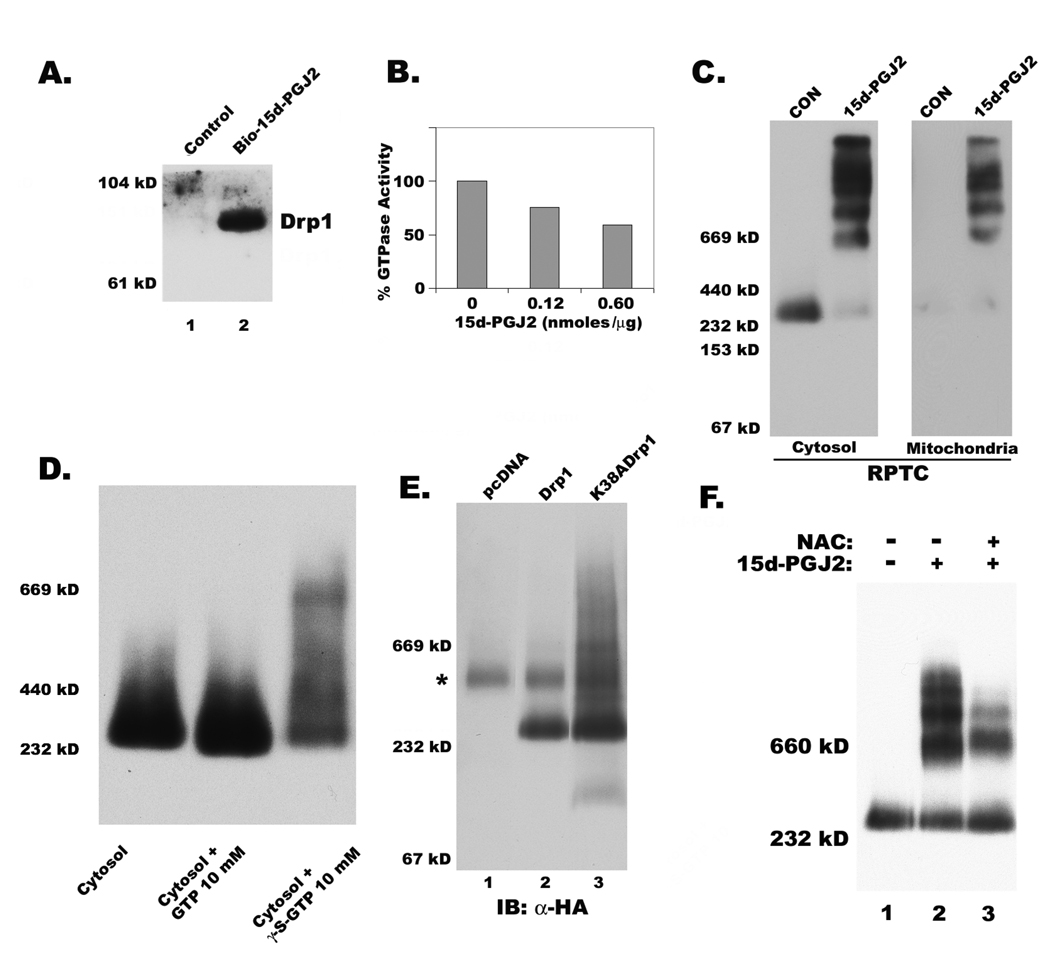
Since our immunoblots indicated possible modification of Drp1, but not other fission and fusion proteins, by 15d-PGJ2 (not shown), RPTC were treated with a biotinylated derivative of 15d-PGJ2. Proteins that were modified by the prostaglandin were purified by streptavidin conjugated beads were further analyzed. Data shown in Fig. 3A clearly indicated that 15d-PGJ2 was able to bind to Drp1. To test the effect of 15d-PGJ2 on intrinsic GTPase activity of Drp1 upon binding in vitro, we used recombinant full length Drp1 and observed significant inhibition of GTPase activity by the cyclopentenone prostaglandin (Fig. 3B). Since oligomerization of Drp1 is required for cooperative increase in GTPase activity necessary for mitochondrial fission, we tested the effect of 15d-PGJ2 on the formation of oligomeric complexes of Drp1.
Figure 3. Covalent binding by 15d-PGJ2 and inhibition of GTPase activity resulted in oligomerization of Drp1.
A) Western blot showing captured Drp1 by streptavidin beads after incubating RPTC with biotinylated 15d-PGJ2 (bio-15d-PGJ2) for 6h. B) Effect of 15dPGJ2 on GTPase activity of recombinant Drp1, indirectly measured by coupled assay of NADH oxidation as described in the experimental section. Data represents an average of two independent experiments. C) Oligomeric complexes of Drp1 in cytosol and mitochondria in RPTC treated with 15d-PGJ2 were observed by immunoblotting after BN-PAGE as described in methods section. D) Incubation with non cleavable GTP analog (GTPγS) but not GTP promotes Drp1 oligomerization in the cytosol. E) Immunoblotting of total cell lysates expressing HA-tagged wild-type Drp1 and mutant Drp1 (K38A) that is deficient in GTPase activity after separated by BN-PAGE. Asterisk (*) indicates a non-specific reactive band to α-HA antibody. F) Effect of thiol antioxidant N-acetyl cysteine (NAC) on Drp1 oligomerization induced by 15d-PGJ2.
Analysis of Drp1 from cytosolic and mitochondrial fractions of normal and DMSO treated cells indicated that it predominantly exists as a tetramer with a molecular weight of ~300 kD. 15d-PGJ2 treatment resulted in a change in the migration of Drp1 as several distinct high molecular weight oligomeric complexes that may reach the size of ~2×106 daltons (Fig. 3C). There is not only an increase in oligomerization of Drp1 in the cytosol but also translocation of these large oligomers into mitochondria (Fig. 3C). However, this increased oligomeric Drp1 in mitochondria failed to cause their fragmentation suggesting that these stabilized Drp1 oligomers may be devoid of fission activity. Therefore, we hypothesized that decreased GTPase activity of Drp1 may not affect its assembly into oligomeric complexes but prevent their disassembly required for mitochondrial fragmentation. To test this hypothesis, we incubated cytosol from untreated cells with a non-cleavable GTP analog, GTPγS. Interestingly, lack of GTP cleavage resulted in a shift in the mobility of tetrameric Drp1 into larger complexes in BN-PAGE gels (Fig. 3D). To further confirm our hypothesis that loss of Drp1 GTPase activity results in the stabilization of its oligomeric complexes due to failed disassembly, we expressed wild-type and GTPase inactive mutant forms of Drp1 in HeLa cells. Exogenously expressed HA-tagged wild-type Drp1 migrated as a tetramer with a molecular weight of ~300 kD by BN-PAGE (Fig. 3E). In contrast, the GTPase mutant of Drp1 (K38A) migrated as several oligomeric complexes of high molecular weight (Fig. 3E). Compared to 15d-PGJ2 treatment, the complexes of mutant Drp1 (K38A) were less distinct, with a smeared appearance (Fig. 4E). Moreover, NAC significantly inhibited the formation of Drp1 oligomers, thus confirming that 15d-PGJ2 interacts with free sulfhydryls of Drp1 to stabilize its oligomers (Fig. 3F).
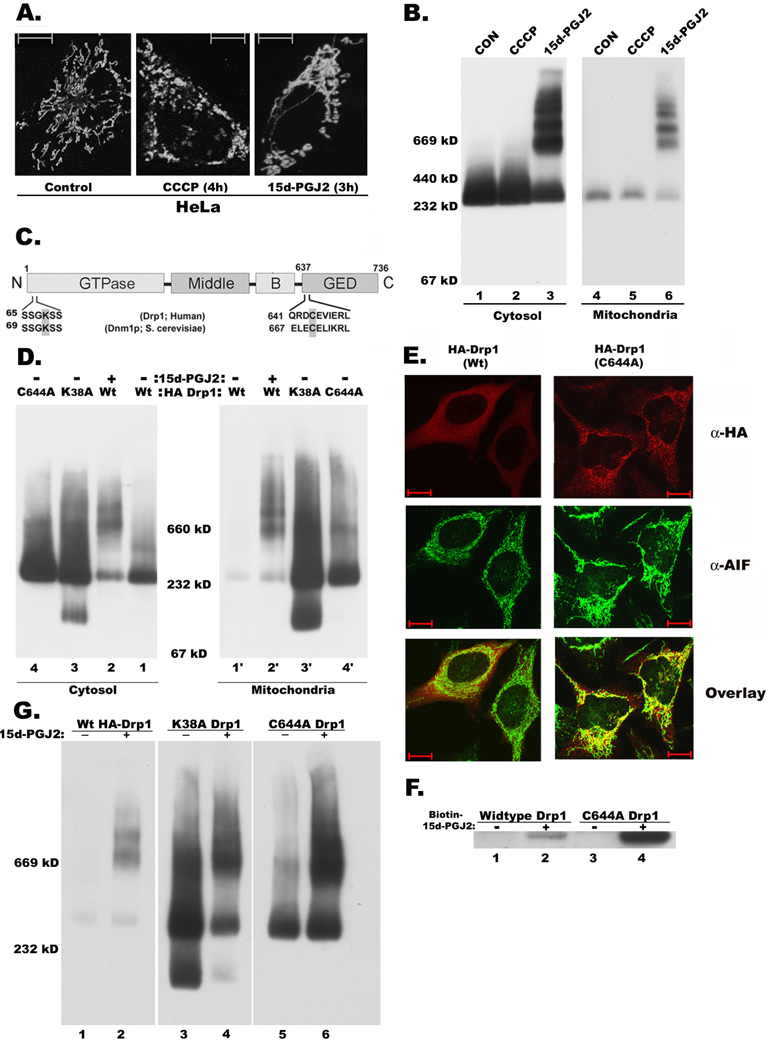
Figure 4. C644A GED domain mutation is similar to K38A GTPase inactive mutation in causing mitochondrial elongation.
A) Mitochondrial morphology of HeLa cells (preloaded with mitotracker red dye) treated with either 15d-PGJ2 for 3h or CCCP for 4h were observed by confocal fluorescence microscopy. B) Immunoblotting for Drp1 of HeLa cells that were treated with 15d-PGJ2 or CCCP as above. Cytosolic and Mitochondrial fractions were prepared as described in experimental section and were subjected to BN-PAGE and Western blotting. C) Cysteine residue in the GED domain of Drp1 is conserved from humans to Saccharomyces cerevisiae (Dnm1p). D) BN-PAGE analysis of cytosolic and mitochondrial fractions isolated from HeLa cells that were transfected with wild-type (HA-Drp1), GTPase mutant (HA-K38A Drp1) or GED mutant (HA- C644A Drp1) as described before. E) Cells that were transfected with HA-tagged wild-type (wt) or mutant (C644A) Drp1 were double immunostained for both Drp1 (α-HA; monoclonal) and AIF, a mitochondrial marker that were labeled with Cy3 and Alexa 488 conjugated secondary antibodies, respectively. Bar: 10µm. F) Capture of wild-type and C644A mutant Drp1 by biotin-15d-PGJ2. G) Oligomeric complexes of wildtype, K38A, C644A mutant Drp1 proteins in transfected HeLa cells that were treated with or without 15d-PGJ2.
Mutation of GED domain cysteine (C644A) induces formation of oligomeric Drp1 and its association with mitochondria
In general, mitochondria in untreated HeLa cells are relatively elongated compared to RPTC (Fig. 4A, left panel). However, treatment with 15d-PGJ2 resulted in further elongation and entanglement of mitochondria (Fig. 4A, right panel) and increased formation of high molecular weight oligomeric complexes of Drp1 in both cytosolic and mitochondrial cellular fractions (Fig. 4B). In contrast, treatment with carbonyl cyanide m-chlorophenylhydrazone (CCCP), a mitochondrial uncoupler, resulted in severe fragmentation of mitochondria (Fig. 4A, middle panel) without any change in the Drp1 oligomerization status or translocation (Fig. 4B). The single cysteine (644 Cys) residue in the GED domain of Drp1, conserved among Drp1 like proteins (Fig. 4C) but not among dynamins, was shown before to be the target of thiol reactive electrophiles [14]. To test the role of cysteine 644 in the maintenance of Drp1 structure and function, we substituted it with alanine through site-directed mutagenesis. While expression of wild-type Drp1 resulted in increased fragmentation of mitochondria in HeLa cells, expression of mutant proteins resulted in mitochondrial elongation (Fig. 4E). BN-PAGE analysis of cytosolic and mitochondrial fractions clearly indicated that in contrast to wild-type Drp1, both the mutants of Drp1 (K38A and C644A) showed more Drp1 oligomeric complexes both in the cytosol and mitochondria. While wild-type Drp1 predominantly appeared as cytosolic (Fig. 4D, lanes 1, 1’), the mutant forms increasingly localized to mitochondria in addition to cytosolic presence (Fig. 4D, lanes 1’, 3’, 4’). Similarly, 15d-PGJ2 treatment caused Drp1 oligomerization and increased mitochondrial association (Fig. 4D, lanes 1’, 2’). Immunofluorescence analysis of HeLa cells transfected with wild-type and C644A mutant of Drp1 by double staining for HA epitope of exogenous Drp1 and endogenous mitochondrial marker, AIF clearly supported our BN-PAGE data that while wild-type Drp1 is predominantly localized to the cytosol, C644A mutant is associated with mitochondria (Fig. 4E). To test whether the C644A further interacts with 15d-PGJ2 or not, HeLa cells expressing mutant Drp1 were incubated with biotinylated-15d-PGJ2. From the streptavidin pull-down assay, we observed binding of both wild-type and mutant (C644A) Drp1 to 15d-PGJ2. For reasons not known, the level of wild-type Drp1 expressed is much lower than mutant Drp1. The ability of C644A mutant to bind biotinylated-15d-PGJ2 (Fig. 4F) suggests that other cysteine residues in the middle domain of Drp1 may also be involved. Moreover, 15d-PGJ2 was able to super-oligomerize C644A and GTPase (K38A) mutants (Fig. 4G) based on the relative abundance of 669 kD oligomer over the ~300 kD tetramer suggests that covalent modification of middle domain cysteine/s by 15d-PGJ2 may stabilize Drp1 oligomers.
Discussion
This is the first report to show that 15d-PGJ2 induces novel changes in mitochondrial morphology through possible inactivation of Drp1. Our conclusion is based on the following findings: (a) 15d-PGJ2 induced mitochondrial elongation, (b) biotinylated-15d-PGJ2 was found to directly bind Drp1, (c) 15d- PGJ2 reduced GTPase activity and induced oligomerization of Drp1, (d) oligomerization of Drp1 also mimicked by non-cleavable GTP analog (GTPγS) or the GTPase inactive mutant of Drp1 (K38A) or GED cysteine mutant (C644A). Since mitochondrial morphology is regulated by a balance of fission and fusion protein activity, inactivation of the fission protein Drp1 by 15d-PGJ2 (Fig. 3) could shift the balance towards fusion, resulting in mitochondrial elongation. However, involvement of other fission or fusion proteins in prostaglandin mediated mitochondrial fusion cannot be completely excluded. Changes in mitochondrial morphology induced by 15d-PGJ2 were unique to this prostaglandin as other prostaglandins failed to do so (Fig. 2B). Our BN-PAGE results revealed the formation of high molecular weight oligomeric complexes of Drp1 in the cytosol and mitochondria within 2 h of 15d-PGJ2 treatment (Fig. 3C). It is interesting to note that mutation of cysteine residue (Cys644), the target for electrophile binding[14], also resulted in increased self assembly and mitochondrial association of Drp1, and mitochondrial elongation (Fig. 4) suggesting this residue to be a potential site for redox regulation. Like Dynamin, Drp1 forms higher order oligomeric structures that are sensitive to GTP [3, 16]. Consistent with GTP hydrolysis requirement, the non-cleavable GTP analog, GTPγS, accentuates oligomerization (Fig. 3D). Interestingly, inhibition of GTPase activity by mutagenesis or inactive GTP analog substitution only generated diffused oligomers. On the other hand, 15d-PGJ2 treatment resulted in the formation of distinct oligomers although GTPase activity was only partial inhibited. The reason for only 50% of GTPase activity being inhibited by 15d-PGJ2 is that in the absence of cysteine residues in the GTPase domain, covalent modification of GTPase effector domain (GED) will only reduce faster exchange of GDP-GTP required for higher activity but will not completely block GTPase activity. Mutations in GTPase domain such as K38A are known to completely inhibit GTPase activity of Drp1 [15]. Therefore, it is likely that 15d-PGJ2 may bind to additional sulfhydryls to stabilize oligomers formed by GTPase inhibition. There are 8 other cysteine residues in the middle domain that can be modified by 15d-PGJ2. In fact, C644A mutant showed not only binding to biotin-15d-PGJ2 (Fig.4F), but also super oligomerized in the presence of 15d-PGJ2 as shown by the relative abundance of 669 kD oligomer over the ~300 kD tetramer (Fig.4G). Our future investigations will include mutagenesis of all the 8 middle domain cysteine residues.
For dynamin and its related proteins, oligomerization appears to stimulate GTPase activity through conformational interactions between the GTPase and the GED domains [17] that causes the dissociation of oligomers. As per the recent models proposed for dynamin [18], inhibition of GTPase activity either by interfering with the GTPase domain or GED domain or both will result in the failed disassembly of Drp1 oligomeric complexes. In this regard, it is noteworthy that either 15d-PGJ2 treatment or overexpression of the GED C644A mutant resulted in the formation of high molecular weight oligomeric complexes and elongated mitochondria (Fig. 3 and Fig. 4). Similar formation of Drp1 oligomeric complexes by GTPase and GED mutants of Drp1 were reported by Zhu et al [15]. So far it has been reported that Drp1 is able to assemble and this assembly stimulates its GTPase activity [1]. The role of GTPase in disassembly of Drp1 appears to be more important than in assembly from our studies as well as from the studies described by Zhu et al [15]. Inhibition of 15d-PGJ2 induced Drp1 oligomerization by thiol antioxidant NAC may be due to protection of cysteine residues on Drp1 from possible modification by the prostaglandin.
Conclusion
This finding illustrates that Drp1 is susceptible to modification by reactive electrophiles formed from toxic chemicals and endogenous oxidant stress. Identification of proteins susceptible to such modification may provide novel insights to understand cellular mechanisms of adaptive response to oxidative stress and consequences of protein damage due to inflammation, injury, cellular redox alteration, toxicity or disease.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grant DK54472 and Morrison Trust Grant to P.S and National Institutes of Health grant DK37139 to M.A.V. We greatly acknowledge the help of Drs. Richard J. Youle, Alexander M. van der Bliek and Craig Blackstone for the constructs and/or cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annual review of biochemistry. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 2.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nature reviews. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 3.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santel A, Frank S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB life. 2008;60:448–455. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- 6.Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci U S A. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. Journal of periodontal research. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg JM, Saikumar P. Mitochondrial function. Methods in molecular medicine. 2003;86:351–371. doi: 10.1385/1-59259-392-5:351. [DOI] [PubMed] [Google Scholar]
- 9.Wittig I, Braun HP, Schagger H. Blue native PAGE. Nature protocols. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 10.Zhu PP, Patterson A, Lavoie B, Stadler J, Shoeb M, Patel R, Blackstone C. Cellular localization, oligomerization, and membrane association of the hereditary spastic paraplegia 3A (SPG3A) protein atlastin. J Biol Chem. 2003;278:49063–49071. doi: 10.1074/jbc.M306702200. [DOI] [PubMed] [Google Scholar]
- 11.Ingerman E, Nunnari J. A continuous, regenerative coupled GTPase assay for dynamin-related proteins. Methods in enzymology. 2005;404:611–619. doi: 10.1016/S0076-6879(05)04053-X. [DOI] [PubMed] [Google Scholar]
- 12.Oh JY, Giles N, Landar A, Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. The Biochemical journal. 2008;411:297–306. doi: 10.1042/bj20071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennehy MK, Richards KA, Wernke GR, Shyr Y, Liebler DC. Cytosolic and nuclear protein targets of thiol-reactive electrophiles. Chemical research in toxicology. 2006;19:20–29. doi: 10.1021/tx050312l. [DOI] [PubMed] [Google Scholar]
- 15.Zhu PP, Patterson A, Stadler J, Seeburg DP, Sheng M, Blackstone C. Intra- and intermolecular domain interactions of the C-terminal GTPase effector domain of the multimeric dynamin-like GTPase Drp1. J Biol Chem. 2004;279:35967–35974. doi: 10.1074/jbc.M404105200. [DOI] [PubMed] [Google Scholar]
- 16.Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappie JS, Acharya S, Liu YW, Leonard M, Pucadyil TJ, Schmid SL. An intramolecular signaling element that modulates dynamin function in vitro and in vivo. Mol Biol Cell. 2009;20:3561–3571. doi: 10.1091/mbc.E09-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux A, Antonny B. The long and short of membrane fission. Cell. 2008;135:1163–1165. doi: 10.1016/j.cell.2008.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.