Abstract
Cocaine alters brain function from the early days of development throughout the entire life of an individual. Since the first preclinical research on cocaine sensitization was published, sex differences in response to the drug in adult rats have been noted. With the appearance of reports on “crack babies” during the 1980’s, sex differences in response to prenatal (developmental) exposure have been identified in both clinical and preclinical reports. Cocaine administered during early development in the rat produces wide-spread alterations in function which depend on the timing of drug administration as well as the sex of the animal. In males, the response patterns following postnatal day (PND) 11–20 cocaine administration (equivalent to the late prenatal period in humans) are quite similar to those seen following prenatal exposure (equivalent to the first half of pregnancy in humans). There is a general decrease in dopaminergic (DA) markers and reactivity perhaps due to the uncoupling of the D1 receptor from its second messenger system. While similar changes in D1 uncoupling are seen in females, behavioral and metabolic responses to drug challenges generally show increases in DA responsivity (except adolescents) perhaps due to the activational effects of estrogen and/or decreases in serotonin (5-HT) mediated regulation of DA function. We have found that a significant factor in the hyper-responsivity of the female is the role of the testing environment and the responses to stress which can obscure underlying neurochemical dysregulation. Whether parallel factors are operational in adult males and females is currently under investigation.
Keywords: Psychostimulants, Pregnancy, Behavior, Brain Function
Cocaine alters brain function from the early days of development throughout the entire life of an individual. The classical view is that cocaine acts through inhibition of the reuptake of dopamine (DA), serotonin (5-HT) and norepinephrine (NE) from the synapse suggesting that the presence of the transporters within synapses is a prerequisite for the action of cocaine. In development, however, cocaine’s actions as a local anesthetic (inhibiting Na channels) and as a vasoconstrictor (altering oxygen and nutrient delivery to developing brain), undoubtedly play more prominent roles in cocaine’s effects than later in development after the appearance of the transporters on developing neurites. Since the normal course of neural development relies heavily on the activity within the neural elements, perturbation of normal activity by any means has the potential to permanently alter the course of development.
Since the first preclinical research on cocaine sensitization was published [37], sex differences in response to the drug in adult rats have been noted. Research into the mechanisms of these differences, however, focused primarily on amphetamines with the work of Robinson, Becker, Stewart and others. Following the appearance of reports on “crack babies” during the 1980’s, sex differences in response to prenatal (developmental) cocaine exposure have been identified in both clinical and preclinical reports. Now hundreds of clinical and preclinical studies have reported that while cocaine use during pregnancy can be damaging to the developing brain, the effects can be subtle and are not the same in both males and females. Several clinical studies have found that males are more often adversely affected by prenatal cocaine exposure than females [3] [11] [12]. However, in the preclinical arena, while males are clearly affected, females are not spared. For the last 20 years, our laboratory has studied the effects of cocaine on neurobehavioral development and has amassed a sizable data base which includes a wide range of sex differences in the acute and chronic effects of cocaine on brain function, behavior and neurochemistry. These sex differences encompass many cognitive and behavioral domains and vary depending on when the drug is administered more so than on the dose of drug administered. However, we and others have primarily studied only sub-toxic doses of cocaine. To address the issue of “when” an exposure occurs, we present a brief discussion of what occurs in the developing brain and the timing of events in brain development in relation to day of birth in humans and rodents.
Critical periods of cocaine exposure
The most recent information equates the maturational state of the rat’s brain on the day of birth to approximately 14 to 16 weeks gestation in human [9]. Therefore, events which occur during the first half of pregnancy in human occur during prenatal life in the rat and events which occur during the last half of pregnancy in human occur during the early postnatal period in the rat. Cell division and migration for each brain region occur at unique genetically programmed times during the first half of pregnancy in humans (prenatal in the rat). Neurite extension, synaptogenesis, synaptic loss and apoptosis occur during the latter half of pregnancy in humans and during the postnatal period in the rat. However, synapse retention and neurite pruning are dependent on multiple factors including the level of activity in the neurons and synapses. Alterations in action potentials during this active period of development have profound effects on the synaptology, functional responses and even the cytoarchitecture of the developing circuits [26]. Since cocaine acts primarily through altering synaptic activity, we hypothesized that it would have the most profound effects on brain functional development if given during the postnatal period, this period of highly active development of neuronal function and architecture. Therefore, we administered cocaine during three periods of development: prenatally, from gestation day 8 to 22, early postnatal, from postnatal day 1–10, a period of neuropil expansion and synapse formation, or late postnatal, from day 11–20, a period of neuropil retraction and synaptic pruning. For the prenatal dosing, we administered cocaine at 30 or 60 mg/kg/day between gestation day (G) 8–22 via gastric intubation. For postnatal dosing, we administer cocaine directly to the pup at 25 or 50 mg/kg/day subcutaneously. We typically dose during one of these developmental periods and then examine behavior, brain function and neurochemistry at one of three ages: weanling or postnatal day (PND) 21, adolescent or PND 40–45, adult or PND 60. In addition, we have recently examined the effects of repeated cocaine administration to adult male and female rats.
Effects of period-specific cocaine exposure on brain metabolic activity: Prenatal exposure
We see very different effects on brain metabolic function in adult male and female rats exposed to cocaine during the three developmental periods (Table 1). The striking aspect of these results is that prenatal cocaine exposure had very little effect in adult females while postnatal cocaine exposure resulted in a stimulated metabolism in females in specific regions which varied depending on the period of drug administration (PND 1–10 or 11–20). Prenatal cocaine results in decreases in brain metabolism in males and no changes in females when measured in exposed adults [17]. Even weanling rats that have been exposed prenatally to cocaine show the same sexual dimorphism in metabolic responses [18]. Examination of pyramidal cell branching in cortex of 21 day old rats exposed to cocaine prenatally shows that males exhibit a reduction in dendritic branching, a finding which supports the reduction in metabolism while females show the opposite pattern, an increase in dendritic branching compared to control females (Dow-Edwards & Miller, unpublished). This increase in branching in females suggests a greater degree of connectivity in cortex which may support metabolism in the normal range.
Table 1. Summary of functional studies by exposure period.
2DG studies @ 60 days of age- no drug challenge
| Prenatal | Postnatal 1–10 | Postnatal 11–20 | ||||
|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| Cortex | 0/7 | 2/7↓ | 1/7↑ | 1/7↓ | 5/7↑ | 1/7↑ 1/7↓ |
| Motor | 0/5 | 1/5↓ | 3/6↑ | 0/6 | 4/5↑ | 0/5 |
| Sensory | 0/7 | 1/7↓ | 3/6↑ | 0/6 | 1/8↑ | 1/8↑ |
| Limbic | 0/11 | 5/11↓ | 9/14↑ | 0/14 | 5/14↑ | 1/14↓ |
| Hypothal | 0/9 | 5/9↓ | 0/9 | 0/9 | 2/8↑ | 0/8 |
Brain metabolic activity using the 2-Deoxyglucose (2DG) method at 60 days of age with no drug challenge. Cocaine was administered during one of three developmental periods and results expressed as number of statistically significant differences/total number of regions analyzed within each functionally defined grouping (e.g., cortex, motor etc). The arrow indicates the direction of the change. Clearly males and females exhibit very different responses to cocaine when given during the three phases of brain development. [Hypothal = hypothalamic regions]
While prenatal cocaine exposure dampens metabolism in the brains of male rats when measured at 21 and 60 days of age, there appears to be minimal effects on metabolism when measured at 41–45 days of age [50]. Adolescent animals show differences in non-drug stimulated behaviors compared to older and younger age groups [48]. Interestingly in the rat striatum there is a peak in dopamine D1 and D2 receptors at PND 40 such that the concentration of these receptors is higher than at PND 25 or 60 [49]. It is well established that adolescent mice and rats exhibit basal hyperactivity compared to younger or older ages and their responsivity to psychostimulant administration is dampened during this age period [5] [29] [48]. Additionally adolescent animals show different interactions with the environment compared to adults and these interactions have been shown to be altered by prenatal cocaine [35] [54]. Therefore, assessment of brain metabolism during the adolescent period might be expected to yield different results compared to other ages based solely on the unique behavioral, pharmacologic and neurochemical responsivity of adolescent rats.
Postnatal cocaine exposure; the latter half of human pregnancy
For several years our laboratory has focused on the effects of cocaine during the postnatal 11–20 period because this is the period when the forebrain and particularly the cortex exhibits maximal plasticity. Synapses are maintained or lost depending on the degree of activation they experience during this period and cocaine administration during this period increases glucose metabolism (and presumably synaptic activity) especially in females [22]. That is, the subchronic effects of cocaine on glucose metabolism were similar to the effects of the same treatment when studied in adulthood (Table 1). Males receiving cocaine during PND 11–20 showed few effects whereas females showed wide ranging increases in metabolism [19] [22]. Therefore, in contrast to prenatal exposure, cocaine administration during a period equivalent to the late prenatal period in humans produced increases in metabolism which are permanent primarily in females.
Metabolism following drug challenges
We have also examined the response to various drug challenges in developmentally exposed rats. The effects of prenatal cocaine exposure on methylphenidate (MPD, Ritalin, a psychostimulant like cocaine) response was examined in adolescent rats and found to be within the control range in most regions [50] (Table 2). On the other hand, metabolic responses to repeated amphetamine in adults exposed to cocaine during PND 11–20 are enhanced [32] or to the D1, D5 agonist SKF82958 are dampened (Table 2) [30]. These data suggest that PND 11–20 cocaine may result in an enhancement of the dopamine terminal fields and subsequent reduction or down regulation of the postsynaptic elements such that presynaptic effects overshadow the postsynaptic effects.
Table 2.
Summary of functional studies using drug challenges
| Cocaine exposure | age @ study | challenge drug | sex | results | ref |
|---|---|---|---|---|---|
| Prenatal G 8–22 | 41–44 d | MPD 10mg/kg | M& F | no effects | [50] |
| Postnatal 11–20 | 60 d | SKF82958 5 mg/kg | M | blunted response | [30] |
| Postnatal 11–20 | 125 d | amphetamine 2 mg/kg | M& F | inc response-motor sys | [32] |
Cocaine exposure periods were gestation day (G) 8 to 22 or postnatal days 11–20. Challenge drugs are MPD = methylphenidate administered acutely; SKF82958, a D1,D5 agonist, administered acutely; amphetamine sulfate administered subchronically (7 days). The results indicate how developmental cocaine exposure altered the response to the challenge drug compared to the control group.
Period-specific cocaine exposure alters behavioral responses to pharmacologic challenges: Sex specific effects following prenatal exposure
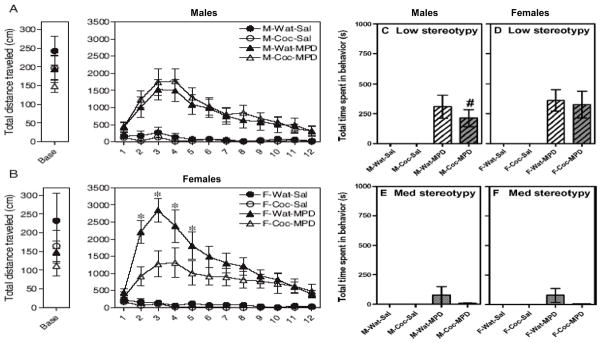
Cocaine administered from gestation day 8–22 @ 60 mg/kg/day produced sex-specific behavioral responses to 10 mg/kg MPD when measured at PND 41–44 [51]. Locomotion in female control rats that received MPD was significantly higher than that in all the other groups that received MPD as well as saline (Fig 1, A and B). However, prenatal cocaine produced a dampening of the locomotor response to MPD but only in females (Fig 1B). Since psychostimulants like MPD can produce stereotyped behavior (repeated seemingly nonsensical behavior) which competes with locomotor activity, we also quantified stereotyped behaviors using videotaped recordings during the time that the locomotor activity was being recorded. Male rats that received prenatal cocaine showed a significant decrease in the amount of time spent in low intensity stereotypy compared to the control group. Low intensity stereotypy (repeated head movements with locomotion) in female rats that received MPD was similar between prenatal treatments (Fig. 1, C and D). Medium intensity stereotypy (more intense head movements with or without locomotion) which had a low occurrence overall was not significantly different across prenatal treatment or sex but showed an average of 92 % reduction in the prenatal cocaine groups compared to the prenatal controls given the same dose of MPD (Fig. 1, E and F).
Figure 1.
Locomotor activity and stereotyped behavior after acute injection of either saline or methylphenidate (MPD). Panel A shows adolescent male rats and Panel B shows adolescent female rats that were exposed to prenatal cocaine or water (controls) and then challenged with saline or MPD. Baseline activity was collected during 20 min and illustrated as an average for that period. Panels C and D show Low intensity stereotypy (repeated head movements with locomotion) in the same male and female rats. Panels E and F show Medium intensity stereotypy (more intense repeated head movements with or without locomotion) in the same male and female rats. White bars illustrate water pretreated rats and gray bars represent cocaine pre-treated rats. N= 10–12 rats per group. Bars represent sem and * represents significantly different from all other groups. # represents significantly different from prenatal control males that also received MPD. Modified from Torres-Reveron & Dow-Edwards Neurotoxicology and Teratology, 2006; 28: 165–172.
These results show that prenatal cocaine exposure produces sexually dimorphic responses to MPD during the adolescent period. Our data suggest that the neuroanatomic substrates which mediate these decreases in the behavioral responses to MPD are different in males versus females since prenatal cocaine diminishes locomotor response only in females and stereotyped behavior only in the males. One possible explanation for these differences is that developing dopaminergic neurons of female rats mature before those of males [4]. Consequently a similar prenatal cocaine exposure in males and females will result in a longer exposure for dopaminergic striatal cells in females due to this earlier maturation. Locomotor activity is associated with mesolimbic DA (nucleus accumbens) function and stereotypy is associated with nigrostriatal (caudate-putamen) function. Therefore, we hypothesized that brain imaging studies would show sexually dimorphic changes in function that correlate with the sexually dimorphic behavioral changes. (see coupling of behavior and metabolism below).
Sex specific effects following postnatal exposure
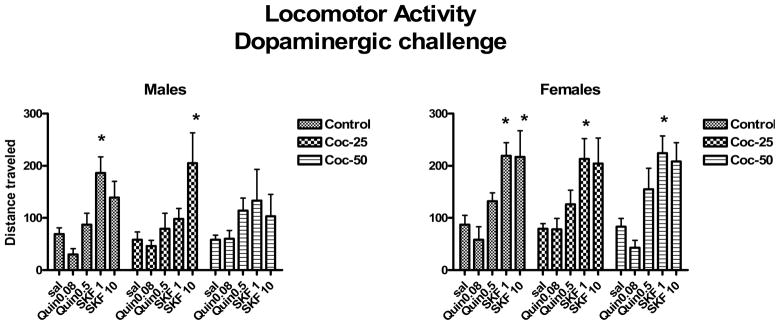
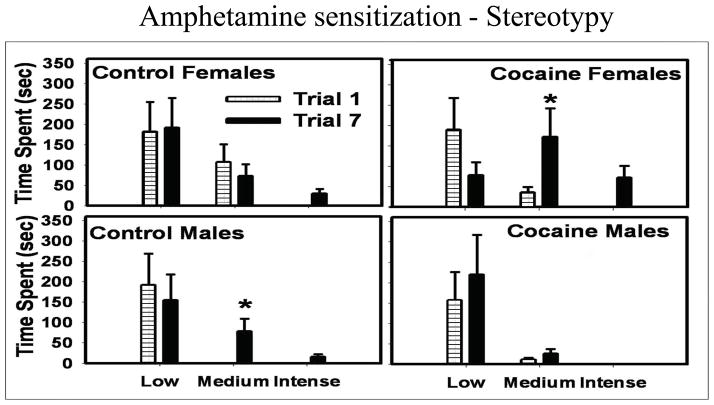
We have completed many studies in which we have examined sex differences in response to cocaine administration during PND 11–20 including adult behavioral responses to challenge with dopamine and serotonin agonists. A large study of rats receiving either vehicle or cocaine at 25 or 50 mg/kg during PND 11–20 demonstrated that cocaine exposure reduced responses to the D1, D5 agonist SKF82958 in adult males but had little effect in females (Fig 2) [16]. Responses to the D2, D3, D4 agonist, quinpirole, did not appear to be altered. Other studies show that PND 11–20 cocaine decreased dopamine transporter (DAT) expression in ventral mesencephalon and preprodynorphin expression in nucleus accumbens shell suggesting a dampening of function in dopaminergic circuits in exposed adult males (Table 3). Females were not examined in these studies [43]. Therefore, although the metabolic alterations in males were fairly subtle in adults following postnatal cocaine treatment (Table 1), the behavioral responses to a variety of dopaminergic challenges were dampened and several markers for the dopaminergic systems were reduced. Females on the other hand show increased responses to amphetamine challenge following PND 11–20 cocaine (Fig 3, Table 3). An assessment of the coupling of the D1 receptor to its’ second messenger system using neuronal membrane preparations incubated with dopamine and immuno-precipitated with antibodies directed against Gαs/olf or Gαi showed that PND 11–20 cocaine had no effect on D1 responsivity in striatum but reduced coupling in the frontal cortex [55]. Interestingly, there were no sex differences in this measure and D2 receptor coupling was normal in males and females in both regions. Therefore, the increased pharmacologic responses to indirect and direct DA agonists observed in females receiving cocaine postnatally in all likelihood reflect dysregulation of sub-cortical regions such as the striatum, which are intact for D1 coupling. A dampening of responses in males following direct DA agonists may reflect dysregulation in other non-striatal regions which were not examined for DA receptor coupling. In addition, an enhanced responsivity to the environment or testing situation appears to contribute to the enhanced behavioral responses observed in females since brain metabolic studies conducted in sensory-attenuated environments in females show decreases in metabolism rather than increases as seen in sensory-stimulating environments [20].
Figure 2.
Locomotor activity following challenge with dopaminergic drugs: Quinpirole (Quin), a selective D2, 3, 4 agonist, or SKF82958 (SKF) a selective D 1,5 agonist. Quinpirole was administered at 0.08 or 0.5 mg/kg ip (Quin .08 or Quin .5) and SKF 82958 at 1 or 10 mg/kg ip (SKF 1 or SKF 10). Saline dosing is indicated as sal. Data for adult males and females that had been injected with vehicle (control), cocaine at 25 mg/kg/day (Coc-25) or cocaine at 50 mg/kg/day (Coc-50) during postnatal days 11–20. * indicates significantly different from the response to saline within the group. Mean + sem
Table 3.
Effects of Developmental Cocaine Exposure on Behavior & Neurochemistry by Sex
| Prenatal G8-22# | Postnatal 11-20ψ | ||
|---|---|---|---|
| males | females | males | females |
| ↓ D1 responses | ↓ D1 responses | Dopamine system | |
| ↑ D2 responses | ↑ D2 responses | ↓ D1 loco | ↑ D1 sniffing |
| ↓ MPD-stim stereo | ↓ MPD-stim loco | ↓ amphet sensitization | ↑ amphet sensit |
| No Δ acoustic strl | ↓ acoustic strl | ↓ apo sensitization | no Δ apo sensit |
| No Δ ppDyn mRNA | ↓ ppDyn mRNA | ↓ DAT mRNA vMes | ND |
| ↓ ppDyn mRNA acc | ND | ||
| ↓ DA-stim acoustic startle | no Δ acoustic strl | ||
| Serotonin system | |||
| ↑ quipazine response | ↓ quipazine response | ||
| ↑ 8-OH-DPAT response | no Δ DPAT response | ||
| ↑ 5HT transporter | no Δ 5HT transporter | ||
| Cognitive Behavior | |||
| ↑ errors on RAM | ↑ errors on RAM | ||
| ↓ errors on RAM in retest | ↑ errors on RAM in retest | ||
Data from PND 1–10 cocaine administration are not shown. Doses of cocaine administered are 60mg/kg/day (via intragastric administration) prenatally or 25 or 50mg/kg/day sc postnatally.
ND indicates“not done”
indicates behaviors/neurochemistry measured either at 41–44 days of age or adulthood;
indicates behaviors/neurochemistry measured in adulthood.
Abbreviations: stim = stimulated; strl = startle; ppDyn mRNA = preprodynorphin mRNA; loco = locomotor activity; MPD = methylphenidate; amphet = amphetamine sulfate; apo = apomorphine; DAT = dopamine transporter; vMes = ventral mesencephalon; Stereo = stereotyped activity; Acc = N. Accumbens; RAM = radial arm maze; No Δ = no change, ↑ = increase, ↓ = decrease
Figure 3.
Amphetamine sensitization in adult male and female rats exposed to cocaine at 50 mg/kg during postnatal days 11–20. Data for males and females on the first day of treatment (trial 1) compared to that on day 7 (trial 7) after daily doses of 2 mg/kg amphetamine with the intensities of stereotyped behavior shown along the X axis. * indicates significant difference from day 1 value within group. Females treated with cocaine show greatly enhanced stereotyped behavior toward the end of treatment while cocaine produced the opposite effect in males. Mean + sem
Studies utilizing 5-HT agonists to assess behavioral effects show that PND 11–20 cocaine treatment produced increased responses to 8-OH-DPAT (a 5-HT1A agonist) and quipazine, a 5-HT3 agonist, and increased expression of the 5-HT transporter in dorsal raphe in males [15] [14]. Females treated with cocaine during the same period of development, on the other hand, show decreased responses to quipazine and no change in 8-OH-DPAT response or in the density of the 5-HT transporter. Since the forebrain dopaminergic regions are densely innervated by 5-HT neurons which in normal individuals maintain homeostatic control over behavioral output, one would expect a reduction in dopaminergic function as seen in males to be associated with a relative increase in 5-HT function. The classical studies administering 6 OHDA during the neonatal period provide an extreme example of the re-organization in brain that occurs with a decrease in DA and an increase in 5-HT innervation [6]. The opposite pattern of reorganizing appears to occur in females; an increase in dopaminergic function and a reduction in 5-HT function which would be consistent with the behavioral and brain metabolic patterns we observe. Therefore, each sex appears to exhibit a unique pattern of DA-5-HT reorganization following PND 11–20 cocaine administration.
One study of cognitive behavior in adult rats exposed to cocaine during PND 11–20 showed that both male and female exposed rats show increased errors on the 8 arm radial arm maze (RAM) when tested in a single room [31]. These results support the uncoupling of the D1 receptor from its second messenger system in frontal cortex. However, following a change in context, treated males actually made fewer errors and treated females made more errors than their respective controls again suggesting that context affects males and females differently.
Coupling of behavior and metabolism
Since glucose metabolism reflects both excitatory and inhibitory processes, it is difficult to interpret changes (or lack of changes in metabolism) without relating metabolism to behavior. Therefore, we examined the correlations between glucose metabolism and locomotor activity or stereotyped behaviors occurring at the same time that metabolism was determined in the study of prenatal cocaine and responses to methylphenidate (MPD) in adolescent rats [50]. Since locomotion is classically thought to be mediated by the nucleus accumbens and stereotypy by the dorsal striatum (caudate-putamen), we wanted to see if the prenatal treatments altered the pattern of correlations of metabolism in these regions with each behavior. That is, rates of brain metabolism in structures within the mesolimbic system which contains the n. accumbens and the nigrostriatal system which contains the d. striatum were correlated with locomotor activity and stereotyped behavior during the 15 min following the 2DG administration, within individual animals using the Pearson-Product-Moment correlation. Postnatal treatments (MPD or saline challenge) were collapsed such that correlations within prenatal treatment and sex grouping were assessed for significance. We found that the patterns of correlations between each behavior and metabolism in the various brain regions differed greatly depending on which prenatal treatment group and gender was under consideration (Table 4). Generally the groups treated with cocaine prenatally show a greater number of significant correlations between metabolism and behavior than do the control groups. While the range in values contributing to the correlation often relates to the magnitude of the correlations (r value), the range in values is not the major factor responsible for the difference in pattern of correlations here because the behavioral responses to MPD are dampened in the prenatal cocaine group. If anything, the controls showed the greatest range of responses to MPD and thus should have shown a greater number of significant correlations compared to the prenatal cocaine treated group. This was not the case.
Table 4.
Correlation of behavior and glucose metabolism in the mesolimbic and nigrostriatal circuits in rats exposed to cocaine during the prenatal period or controls
| Vehicle | Cocaine | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| Circuit | Locomotion | Stereotypy | Locomotion | Stereotypy | Locomotion | Stereotypy | Locomotion | Stereotypy |
| Mesolimbic | 1/17* | 6/17 | 0 | 1/17 | 4/17 | 11/17 | 0 | 10/17 |
| Nigrostriatal | 5/26 | 23/26 | 1/26 | 1/26 | 23/26 | 26/26 | 4/26 | 25/26 |
Table shows significant number of correlations between specific behaviors (locomotion or stereotypy) and rates of glucose metabolism in specific regions of the mesolimbic and nigrostriatal circuits measured simultaneously. Rats had been exposed to cocaine at 60 mg/kg/day or vehicle during gestation days 8–22 and were tested for behavioral and metabolic responses to saline or methylphenidate at 5 mg/kg on approximately postnatal day 44. Both saline and methylphenidate challenged rats were included in the Pearson Product Moment analysis.
Numerator is number of regions that showed significant correlations with the behavior listed in the column. Denominator is the number of regions assessed in either the mesolimbic (17 regions) or nigrostriatal (26 regions) circuit.
Together, the patterns of correlations of metabolism and behavior suggest that prenatal treatment with cocaine produces a tighter coupling of metabolism and behavior than that observed in control rats. A reasonable explanation for this observation is that prenatal cocaine produced a dysregulation of excitation and inhibition secondary to the imbalance in function of dopamine D1-like and D2-like receptors observed in prenatally cocaine exposed animals. This imbalance may be due to the uncoupling of the D1 receptor from its second messenger system in the striatum [23] [28] [53][56], an effect which does not appear to be sex-specific (and differs from the effect of postnatal cocaine administration which results in D1 uncoupling in the prefrontal cortex). In normal males, the homeostatic balance of the D1 and D2 receptors results in coupling between metabolism and behavior in 40% of the regions analyzed. In cocaine-treated males, however, the loss of homeostasis makes one system (the D2 system in this case) directly related to the behavioral output through coupling of metabolism and behavior. Cocaine exposed males show robust coupling of metabolism and behavior (75% of regions) especially in the nigrostriatal circuit (Table 4). Also, in females, the loss of D1 coupling following prenatal cocaine results in robust coupling of metabolism and behavior particularly in the nigrostriatal system. In spite of the reduced locomotor response to MPD in cocaine exposed females, there were few structures showing significant correlations between metabolism and behavior. These data suggest that locomotor activity especially in females is regulated by multiple structures within the forebrain and extended amygdala that were not examined for this correlational analysis. In conclusion, prenatal cocaine exposure can produce subtle effects in brain function which are not evident during the adolescent period when measured by conventional brain functional assays alone, but rather become evident when interpreted in the context of behavior, producing sex-specific differences in the coupling of metabolism and behavioral output.
Sex differences in adult animals
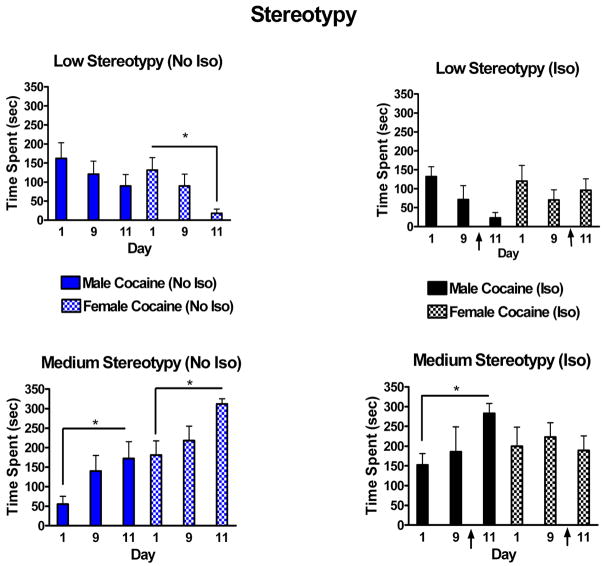
Many authors have reported that adult females are more responsive to cocaine than adult males, sensitize more readily than males to cocaine and self-administer greater quantities of cocaine than males (e.g. [2] [37] [40]) Based on the work of others, we conducted a brain functional imaging study of adult females and males receiving cocaine at 15 mg/kg for 11 days. The day prior to the functional study, all rats were anesthetized for catheter placement. Results showed that males and gonadectomized female rats had enhanced behavioral responses and brain metabolic activity following repeated cocaine administration while changes in intact females were substantially less prominent [13]. Curious about this result, we conducted a series of studies wherein we administered cocaine at 15mg/kg/day for 11 days and subjected half of the rats to anesthesia using isoflurane as we had in the previous experiment. However, no surgery was actually performed. This experiment also showed that isoflurane dampened the expression of sensitization in females not in males (Figure 4) [46]. Since isoflurane has potent effects on the GABA and glutamate systems, we began a series of studies to examine the sex differences in response to GABA agonists and positive modulators following single doses of cocaine or following cocaine-induced sensitization. Following a single dose of cocaine, diazepam, a positive allosteric modulator of the GABA receptor complex, dampened cocaine-stimulated locomotion similarly in male and female rats [44]. Also, gaboxadol (GBX), a GABAA agonist (also known as THIP), dampened locomotion similarly in males and females following cocaine administration. However, we have only examined a limited range of doses for these drugs.
Figure 4.
Sensitization in male and female rats following chronic treatment with cocaine (15 mg/kg/day). On day 10, half of the rats received a dose of isoflurane (↑) and the other half were “sham anesthetized”. Behavior quantified from video tapes on days 1, 9 and 11 indicate that all groups showed intensification of stereotypy from day 1 to 9. Isoflurane, however, dampened this trend in female rats only. * indicates significant difference from day 1 value (paired t test). Adapted from Siegal & Dow-Edwards 2009 [46].
We are currently examining the effects of GBX on cocaine-induced sensitization and have found that high doses decrease locomotion in both males and females while stereotypy was only dampened in females [45]. Thus, the repeated cocaine may alter GABA receptor subtypes in the dorsal striatum, the region which mediates stereotyped behavior, differently in males and females. Studies on the sex differences in the GABAergic system are rare. Females have been reported to have higher densities of GABAergic neurons in the striatum compared to males [36] and sex differences in GABA receptor densities during the early postnatal period have been associated with differences in susceptibility to seizures [39].
Discussion
The basis for the sex difference in response to cocaine is generally believed to be due to estrogen. Estrogens alter multiple phases of neuronal proliferation, apoptosis and differentiation through their receptors which are transcription factor members of the steroid hormone/retinoic acid receptor superfamily. Estrogens inhibit apoptosis and increase cortical cell proliferation [41] [52]. Estrogens promote neuritogenesis and synaptogenesis during development [34] and have multiple interactions with neurotrophins [33][47]. Since estrogens are well known to stimulate spine outgrowth in neurites [10], the increase in neurites and presumably synapses in females may provide an expansive network for cocaine to stimulate and promote synaptic retention. The activational effects of estrogens, which can be demonstrated by gonadectomy and steroid replacement studies in adults, are well known to mediate the effects of psychostimulants [1] [7] [8] [21] [24] [42]. In addition, psychostimulants increase levels of allopregnanolone which along with estrogen has many effects on GABA and other neurotransmitter systems [38]. Also, the timing of the DA/5HT innervations into forebrain is different in female and male. Females undergo maturation earlier. Females also have more dopamine transporter (DAT) than males in the striatum. Differences continue into the postnatal period with females showing a decrease in 5-HT at PND30 while males do not show this change until PND60 [27]. Therefore, genetically programmed sex differences in the ontogeny of the nervous system and the activational effects of hormones on the brain provide a complex platform on which cocaine can act.
Summary
Cocaine administered either prenatally or postnatally produces wide-spread alterations in function which depend on the timing of drug administered as well as the sex of the animal. In males, the response patterns following PND 11–20 cocaine administration (equivalent to the late prenatal period in humans) are quite similar to those seen following prenatal exposure (equivalent of the first half of pregnancy in humans). There is a general decrease in DA markers and reactivity perhaps due to the uncoupling of the D1 receptor from its second messenger system. Similar changes in D1 uncoupling following prenatal cocaine are seen in females, and this may be responsible for the decrease in behavioral responses to psychostimulants etc. Postnatal (or late prenatal in human) exposure in females results in behavioral and metabolic responses to drug challenges that generally show increases in DA responsivity perhaps due to the effects of estrogen and/or decreases in 5-HT mediated regulation of DA function. We have found that a significant factor in the hyper-responsivity of the female is the testing environment [20] which can obscure underlying neurochemical dysregulation. In addition, underlying sex differences in GABA and glutamate systems which have been largely unexplored undoubtedly contribute to the sex differences in response to developmental and adult cocaine exposure.
Acknowledgments
- National Institute on Drug Abuse
- DA04118, DA10990, DA019348, P50-DA024584
NIMH MH066852
NIDA for supply of methylphenidate and cocaine
Annelyn Torres-Reveron, PhD
Jeremy Weedon, PhD
Ning Zhao, MD
April Jackson, BS
Nora Siegal, BS
Stacy Stephenson, AA
Maiko Iijima, MA
Dothlyn Dunkley, MS
Susan Melnick, PhD
Yamit Busidan, DO
Lucille Grullon, BS
Mariya Kreymerman, BS
Onika Murray, BS
DLAR
Abbreviations
- 2DG
2 deoxyglucose
- 6 OHDA
6 hydroxydopamine
- 5HT
serotonin
- DA
dopamine
- PND
postnatal day
- NE
norepinepherine
- DAT
dopamine transporter
- ADHD
attention deficit hyperactivity disorder
- G
gestation day
- MPD
methylphenidate
- GABA
gamma amino butyric acid
- GBX
gaboxadol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- 2.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- 4.Beyer C, Pilgrim C, Reisert I. Dopamine Content and Metabolism in Mesencephalic and Diencephalic Cell Cultures: Sex Differences and Effects of Sex Steroids. J Neurosci. 1991;11:1325–1333. doi: 10.1523/JNEUROSCI.11-05-01325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: A behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown P, Gerfen CR. Plasticity within striatal direct pathway neurons after neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK 1/2 map kinase pathway. J Comp Neurol. 2006;498:415–430. doi: 10.1002/cne.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camp DM, Becker JB, Robinson TE. Sex differences in the effects of gonadectomy on amphetamine-induced rotational behavior in rats. Behav Neural Biol. 1986;46:491–495. doi: 10.1016/s0163-1047(86)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Camp DM, Robinson TE. Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stress. Behav Brain Res. 1988;30:69–88. doi: 10.1016/0166-4328(88)90009-5. [DOI] [PubMed] [Google Scholar]
- 9.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 11.Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Chiodo L, Sokol RJ. Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dow-Edwards D, Dasari VM, Menendez R, Lugo-Escobar N, Torres-Reveron A, Segarra AC. Alterations in brain glucose metabolism following repeated cocaine in adult rats: sex and gonadal status. Neuroscience Abstract. 2005 [Google Scholar]
- 14.Dow-Edwards DL. Modification of acoustic startle reactivity by cocaine administration during the postnatal period: comparison with a specific serotonin reuptake inhibitor. Neurotoxicol Teratol. 1996;18:289–296. doi: 10.1016/s0892-0362(96)90029-x. [DOI] [PubMed] [Google Scholar]
- 15.Dow-Edwards DL. Preweaning cocaine administration alters the adult response to quipazine: Comparison with fluoxetine. Neurotoxicol Teratol. 1998;20:133–142. doi: 10.1016/s0892-0362(97)00095-0. [DOI] [PubMed] [Google Scholar]
- 16.Dow-Edwards DL, Busidan Y. Behavioral responses to dopamine agonists in adult rats exposed to cocaine during the preweaning period. Pharm Biochem Behav. 2001;70:23–30. doi: 10.1016/s0091-3057(01)00582-2. [DOI] [PubMed] [Google Scholar]
- 17.Dow-Edwards DL, Freed LA, Fico TA. Structural and functional effects of prenatal cocaine exposure in adult rat brain. Dev Brain Research. 1990;57:263–268. doi: 10.1016/0165-3806(90)90052-z. [DOI] [PubMed] [Google Scholar]
- 18.Dow-Edwards DL, Freed-Malen LA, Gerkin LM. Sexual dimorphism in the brain metabolic response to prenatal cocaine exposure. Dev Brain Res. 2001;129:73–79. doi: 10.1016/s0165-3806(01)00184-5. [DOI] [PubMed] [Google Scholar]
- 19.Dow-Edwards DL, Freed-Malen LA, Hughes HE. Long-term alterations in brain function following cocaine administration during the preweanling period. Dev Brain Research. 1993;72:309–313. doi: 10.1016/0165-3806(93)90198-j. [DOI] [PubMed] [Google Scholar]
- 20.Dow-Edwards DL, Frick GS. Neurobehav Teratol Soc Abstract. 1996. Context-dependent effects of developmental cocaine exposure. [Google Scholar]
- 21.Forgie ML, Stewart J. Sex differences in amphetamine-induced locomotor activity in adult rats: role of testosterone exposure in the neonatal period. Pharmacol Biochem Behav. 1993;46:637–645. doi: 10.1016/0091-3057(93)90555-8. [DOI] [PubMed] [Google Scholar]
- 22.Frick GS, Dow-Edwards DL. The effects of cocaine on cerebral metabolic function in periweanling rats: the roles of serotonergic and dopaminergic uptake blockade. Dev Brain Research. 1995;88:158–170. doi: 10.1016/0165-3806(95)00094-t. [DOI] [PubMed] [Google Scholar]
- 23.Friedman E, Wang HY. Prenatal Cocaine Exposure Alters Signal Transduction in the Brain D1 Dopamine Receptor System. Ann N Y Acad Sci. 1998;846:238–247. [PubMed] [Google Scholar]
- 24.Glick SD, Hinds PA. Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol. 1984;99:119–121. doi: 10.1016/0014-2999(84)90442-4. [DOI] [PubMed] [Google Scholar]
- 25.Hansen-Trench LS, Segar TM, Barron S. Neonatal cocaine and/or ethanol exposure: Effects on a runway task with suckling reward. Neurotoxicol Teratol. 1996;18:651–657. doi: 10.1016/s0892-0362(96)00130-4. [DOI] [PubMed] [Google Scholar]
- 26.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–54. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns JM, Lubin DA, Lieberman JA, Lauder JM. Developmental effects of prenatal cocaine exposure on 5-HT1A receptors in male and female rat offspring. Dev Neurosci. 2002;24:522–530. doi: 10.1159/000069363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang HY, Friedman E, Levitt P. In Utero Cocaine-Induced Dysfunction of Dopamine D1 Receptor Signaling and Abnormal Differentiation of Cerebral Cortical Neurons. J Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laviola G, Wood RD, Kuhn C, Francis R, Spear L. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exper Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- 30.Melnick SM, Dow-Edwards DL. Blunted metabolic response to SKF 82958 in the mesolimbic system following preweaning cocaine treatment. Brain Res Dev Brain Res. 2003;143:253–259. doi: 10.1016/s0165-3806(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 31.Melnick SM, Kubie JL, Laungani R, Dow-Edwards DL. Impairment of spatial learning following preweaning cocaine exposure in the adult rat. Neurotoxicol Teratol. 2001;23:445–451. doi: 10.1016/s0892-0362(01)00157-x. [DOI] [PubMed] [Google Scholar]
- 32.Melnick SM, Torres-Reveron A, Dow-Edwards DL. Preweaning cocaine exposure alters brain glucose metabolic rates following repeated amphetamine administration in the adult rat. Dev Brain Research. 2004;153:127–134. doi: 10.1016/j.devbrainres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Miranda R, Sohrabji F, Singh M, Toran-Allerand CD. Nerve growth factor (NGF) regulation of estrogen receptors in explant cultures of the developing forebrain. J Neurobio. 1996;31:77–87. doi: 10.1002/(SICI)1097-4695(199609)31:1<77::AID-NEU7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Miranda R, Sohrabji F, Toran-Allerand CD. Interactions of estrogen with the neurotrophins and their receptors during neural development. Horm Behav. 1994;28:367–375. doi: 10.1006/hbeh.1994.1033. [DOI] [PubMed] [Google Scholar]
- 35.Molina VA, Wagner JM, Spear LP. The behavioral response to stress is altered in adult rats exposed prenatally to cocaine. Physiol Behav. 1994;55:941–945. doi: 10.1016/0031-9384(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 36.Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I, Pilgrim C. Sex differences in densities of dopaminergic fibers and GABAergic neurons in the prenatal rat striatum. J Comp Neurol. 1992;323:299–304. doi: 10.1002/cne.903230212. [DOI] [PubMed] [Google Scholar]
- 37.Post RM, Lockfeld A, Squillace KM, Contel NR. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- 38.Quinones-Jenab V, Minerly AC, Niyomchia T, Akahvan A, Jenab S, Frye C. Progesterone and allopregnanolone are induced by cocaine in serum and brain tissues of male and female rats. Pharmacol Biochem Behav. 2008;89:292–297. doi: 10.1016/j.pbb.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Ravizza T, Friedman LK, Moshé SL, Velísková J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 40.Robinson TE. Psychopharmacology (Berl) Vol. 84. 1984. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats; pp. 466–475. [DOI] [PubMed] [Google Scholar]
- 41.Sawada H, Ibi M, Urushitani M, Honda K, Nakanishi M, Akaike A, Shimohama S. Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB. 2000;14:1202–1214. doi: 10.1096/fasebj.14.9.1202. [DOI] [PubMed] [Google Scholar]
- 42.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- 43.Shi X, Dow-Edwards DL, Hurd Y. Perinatal cocaine decreased the expression of prodynorphin mRNA in nucleus accumbens shell in the adult rat. Molec Brain Res. 1998;62:82–85. doi: 10.1016/s0169-328x(98)00218-6. [DOI] [PubMed] [Google Scholar]
- 44.Siegal N, Dow-Edwards D. The role of GABAA receptors in sex differences in cocaine-stimulated locomotion. CPDD. 2009 Abstract 592406. [Google Scholar]
- 45.Siegal N, Dow-Edwards D. Effect of GABAA Agonist Gaboxadol on Cocaine-Induced Locomotion and Stereotypy. Soc Neurosci. 2009 Abstract 448.19. [Google Scholar]
- 46.Siegal N, Dow-Edwards DL. Isoflurane anesthesia interferes with the expression of cocaine-induced sensitization in female rats. Neurosci Lett. 2009;464:52–56. doi: 10.1016/j.neulet.2009.07.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh M, Meyer E, Millard W, Simpkins J. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic functions in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- 48.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 49.Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens 42. Brain Res Dev Brain Res. 2009;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 50.Torres-Reveron A, Dow-Edwards DL. Correlations of brain glucose metabolic rates and behavior in adolescent rats exposed to prenatal cocaine and subsequent methylphenidate administration. NBTS. 2005 Abstract 60. [Google Scholar]
- 51.Torres-Reveron A, Dow-Edwards DL. Prenatal Cocaine Dampened Behavioral Responses to Methylphenidate in Male and Female Adolescent Rats. Neurotoxicol Teratol. 2006;28:165–172. doi: 10.1016/j.ntt.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Wade SB, Oommen P, Conner WC, Earnest DJ, Rajesh C. Overlapping and divergent actions of estrogen and the neurotrophins on cell fate and p53-dependent signal transduction in conditionally immortalized cerebral cortical neuroblasts. J Neurosci. 1999;19:6994–7006. doi: 10.1523/JNEUROSCI.19-16-06994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang HY, Runyan S, Yadin E, Friedman E. Prenatal Exposure to Cocaine Selectively Reduces D1 Dopamine Receptor-Mediated Activation of Striatal Gs Proteins. J Pharmacol Exp Ther. 1995;273:492–498. [PubMed] [Google Scholar]
- 54.Wood RD, Spear LP. Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci. 1998;112:419–431. doi: 10.1037//0735-7044.112.2.419. [DOI] [PubMed] [Google Scholar]
- 55.Zhao N, Wang HW, Dow-Edwards D. Cocaine exposure during the early postnatal period diminishes medial frontal cortex Gs coupling to dopamine D1-like receptors in adult rat. Neuroscience Letters. 2008;438:159–162. doi: 10.1016/j.neulet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhen X, Torres C, Wang HY, Friedman E. Prenatal Exposure to Cocaine Disrupts D1A Dopamine Receptor Function Via Selective Inhibition of Protein Phosphatase 1 Pathway in Rabbit Frontal Cortex. J Neurosci. 2001;21:9160–9167. doi: 10.1523/JNEUROSCI.21-23-09160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]